ABSTRACT
Background
Acute changes in high‐sensitivity troponin T (hs‐TnT) are induced by myocardial ischemia during exercise stress testing, but there are no reports of pharmacological stress testing.
Hypothesis
The pattern of troponin release by myocardial ischemia–induced pharmacological stress testing differs according to the ischemic burden in stable patients.
Methods
In total, 250 patients with suspected coronary artery disease underwent pharmacological stress magnetic resonance imaging (MRI). The amount and degree of myocardial ischemia on MRI and ischemic outcomes at 6 months were determined. hs‐TnT levels were measured at baseline and 1 and 3 hours after testing. The 6‐month clinical outcome was prespecified.
Results
Fifty‐one patients had moderate to severe myocardial ischemia (group A), and 199 patients had no or mild myocardial ischemia (group B). hs‐TnT levels were significantly higher in group A than B at baseline (11 vs 8 pg/mL, P = 0.016) and at 1 hour (12 vs 8 pg/mL, P = 0.009) and 3 hours after testing (12 vs 9 pg/mL, P = 0.012). Baseline hs‐TnT levels of ≥14 pg/mL showed a 43% sensitivity and 77% specificity in predicting moderate to severe ischemia by MRI (P = 0.03; area under the curve: 0.608, P = 0.017). Patients administered dobutamine had a higher acute change in hs‐TnT levels 3 hours after testing than did those administered adenosine (21 vs 0 pg/mL, P < 0.001). There was a trend toward a higher incidence of myocardial infarction in patients with baseline hs‐TnT levels of ≥14 pg/mL.
Conclusions
hs‐TnT levels are significantly higher in patients with moderate to severe than no or mild myocardial ischemia.
Introduction
Cardiac troponin levels reportedly rise and fall in normal marathon runners.1 Middleton et al2 studied the kinetics of cardiac troponin after marathon running in normal subjects and proposed that elevated troponin levels occur with heavy exercise without major coronary artery disease (CAD). Kurz et al3 studied 195 patients with CAD who underwent nuclear testing with single‐photon emission computed tomography. There was no correlation between the degree of myocardial ischemia and acute changes in high‐sensitivity troponin T (hs‐TnT) levels after nuclear testing.
Transient exercise stress test‐induced myocardial ischemia is associated with a quantifiable increase in circulating troponin levels detected by an ultrasensitive troponin I assay. However, no studies have investigated how pharmacological stress testing affects the quantitative detection of hs‐TnT levels in patients with and without ischemia. Therefore, this study aimed to determine hs‐TnT levels and their correlation with the amount and degree of myocardial ischemia in patients undergoing cardiac pharmacological stress magnetic resonance imaging (MRI).
Methods
The Siriraj Institutional Review Board approved the study. Investigations were in accordance with the Declaration of Helsinki. All patients gave written informed consent. This prospective cohort study enrolled 250 consecutive patients in whom CAD was suspected and who underwent pharmacological stress MRI between January 2010 and November 2013. Patients with clinically significant valvular heart disease, severe aortic stenosis, hypertrophic cardiomyopathy, pulmonary embolism, clinically significant pulmonary hypertension, chronic kidney disease, and severe left ventricular dysfunction with an ejection fraction of <30% were excluded. We also excluded patients who refused to participate in the study, which involved waiting for 3 hours after the test for blood sampling. Baseline clinical characteristics and the amount and degree of myocardial ischemia as shown by MRI were obtained.
Blood samples for determination of hs‐TnT levels were obtained at baseline and 1 and 3 hours after the pharmacological stress test. Serum hs‐TnT levels were measured by an immunoassay using electrochemiluminescence technology. This new assay was recently analytically validated. It has a lower limit of detection of 3 pg/mL and a coefficient of variation at the 99th percentile reference limit (13.5 pg/mL) of 9%, which meet the guidelines recommended by international cardiology societies.4
Cardiac MRI Protocol
All patients underwent cardiovascular MRI using a Philips Achieva XR 1.5‐T MR scanner (Philips Healthcare, Best, the Netherlands) equipped with a 32‐element coil. Global and regional myocardial function was assessed in long‐axis, 2‐chamber, 4‐chamber, multiple‐slice, and short‐axis views of the whole left ventricle. A standard retrospective electrocardiograph (ECG)‐gated cine balanced steady‐state free precession sequence was used with multi–breath holds for volumetric and functional ventricular assessment (spatial resolution = 1.5 × 1.5 × 8.0 mm, 10–12 slices, gap = 0 mm, sensitivity encoding factor = 2, repetition times (TRs) = 3.3 and 2.7 ms, echo times (TEs) = 1.6 and 1.3 ms, field of view = 270 × 320 mm, flip angle = 60°).
Stress Protocol
For adenosine stress perfusion MRI, intravenous adenosine was infused at 140 µg/kg/min for 3 minutes with continuous ECG monitoring, and blood pressure was measured every minute. During the last minute of adenosine infusion, 0.05 mmol/kg of intravenous gadolinium was injected at 4 mL/s followed by a 20‐mL 0.9% saline flush at 4 mL/s.
For evaluation of ischemia, three 8‐mm‐thick short‐axis slices were obtained at the basal, mid, and apical levels of the left ventricle with a single‐shot, prospectively gated, balanced, steady‐state free precession sequence (TR = 2.6 ms, TE = 1.3 mm, flip angle = 50°, acquisition voxel size = 2.55 × 2.55 × 8.0 mm, field of view = 320–350 mm). A saturation prepulse was applied prior to acquisition. A 90° preparation pulse with 100‐ms recovery time was used. Parallel imaging was used with a sensitivity encoding factor of 2.0 to 2.5.
Three‐dimensional late gadolinium enhancement (LGE) was performed 7 to 10 minutes after injection of 0.2 mmol/kg gadolinium. Multiple short‐axis slices at the same level as the functional study were obtained, and 2‐ and 4‐chamber views were acquired for LGE. The LGE images were acquired using a 3‐dimensional, segmented, gradient‐echo, inversion‐recovery sequence with TE = 1.25, TR = 4.1, flip angle = 15°, field of view = 270 × 320 mm, in‐plane resolution = 1.26 × 1.50 mm, slice thickness = 8 mm, and sensitivity encoding factor = 1.5.
For the dobutamine stress study, dobutamine was intravenously infused during 3‐minute stages at incremental doses of 10, 20, 30, and 40 µg/kg/min until ≥85% of the age‐predicted heart rate was reached. ECG and symptoms were monitored continuously, and blood pressure was measured every 3 minutes. If the target heart rate was not achieved at the peak dose of dobutamine infusion, atropine was administrated in 0.25‐mg increments to a maximal dose of 2.0 mg. Stress testing was discontinued when the target heart rate was achieved or when 1 of the following occurred: new wall motion abnormality in ≥2 adjacent segments, severe chest pain or dyspnea, a ≥40‐mm Hg decrease in systolic blood pressure, severe arterial hypertension of ≥220/120 mm Hg, or severe arrhythmia.
Perfusion defects that were present at stress, but not in LGE images, indicated myocardial ischemia. Perfusion in each segment was graded on a 3‐point scale (transmural ischemia index: 0 = normal, 1 = subendocardial ischemia, and 2 = transmural ischemia). All segmental scores were summed to produce a perfusion score (0–32) for each patient. Myocardial ischemia was estimated as a percentage of the total myocardium by dividing the perfusion score by 48 and multiplying by 100. An ischemic burden of <10% was considered as mild ischemia, ≥10% to <20% as moderate ischemia, and ≥20% as severe ischemia.5
Two cardiologists who were unaware of the biomarker results allocated the patients into 2 groups. Based on the amount and degree of ischemia on MRI, patients with moderate to severe ischemia were allocated to group A. Patients with no or mild ischemia were allocated to group B. The 6‐month clinical outcomes of myocardial infarction, recurrent angina, hospitalization from angina, revascularization, and death were prespecified.
Statistical Analysis
Continuous variables are expressed as median (minimum, maximum) or mean ± standard deviation. Comparison of continuous variables between groups was performed using the 2‐sample t test or the Mann‐Whitney U test. Nonparametric comparison (Kruskal‐Wallis test) was used for primary analysis. All statistics were performed with SPSS version 19.0 (IBM Corp., Armonk, NY).
Results
In total, 250 patients were enrolled in the study. Fifty‐one patients had moderate to severe myocardial ischemia (group A), and 199 had no or mild myocardial ischemia (group B). Almost all patients had chronic stable angina (Table 1). The main presentation was dyspnea on exertion, followed by atypical angina. Diabetes, a history of prior CAD, higher creatinine levels, and lower ejection fraction were more common in group A than B. Most patients underwent adenosine stress testing (96.1% in group A and 87.9% in group B, P = 0.089). The remaining patients underwent dobutamine stress testing. The type of pharmacological stress testing was not different between the 2 groups.
Table 1.
Baseline Demographics of Patients With Moderate to Severe Ischemia (Group A) and Patients With No or Mild Ischemia (Group B) as Shown by Magnetic Resonance Imaging
| Characteristics | Group A, n = 51 | Group B, n = 199 | P Value |
|---|---|---|---|
| Age, y | 65 ± 9 | 66 ± 11 | 0.521 |
| Male | 25 (49.0) | 80 (40.2) | 0.255 |
| Symptoms | |||
| Dyspnea on exertion | 20 (39.2) | 107 (53.8) | 0.064 |
| Typical angina | 14 (27.5) | 14 (7.0) | <0.001 |
| Atypical angina | 10 (19.6) | 40 (20.1) | 0.937 |
| Congestive heart failure | 5 (9.8) | 17 (8.5) | 0.783 |
| Asymptomatic | 7 (13.7) | 37 (18.6) | 0.415 |
| Diabetes mellitus | 27 (52.9) | 66 (33.2) | 0.009 |
| Hypertension | 42 (82.4) | 157 (78.9) | 0.584 |
| Dyslipidemia | 40 (78.4) | 146 (73.4) | 0.460 |
| Coronary artery disease | 15 (29.4) | 28 (14.1) | 0.010 |
| Prior CABG* | 3 (5.9) | 8 (4.0) | 0.700 |
| Smoker | 18 (35.3) | 61 (30.7) | 0.525 |
| Ejection fraction, % | 60 (30–88) | 68 (30–90) | 0.004 |
| Creatinine, mg/dL | 1.1 (0.5–3.0) | 1.0 (0.5–2.6) | 0.024 |
| Type of pharmacological stress test | |||
| Adenosine | 49 (96.1) | 175 (87.9) | 0.089 |
| Dobutamine | 2 (3.9) | 24 (12.1) | 0.089 |
Abbreviations: CABG, coronary artery bypass grafting.
Values are mean ± standard deviation, mean (range), or n (%).
hs‐TnT Levels at Baseline and After the Stress Test
hs‐TnT levels were significantly higher in group A than B at baseline (P = 0.016) and at 1 hour (P = 0.009) and 3 hours (P = 0.012) after pharmacological stress MRI. There was a trend toward a higher level of circulating hs‐TnT levels after pharmacological stress testing, but hs‐TnT levels between baseline and 3 hours did not meet the criteria of an acute change for detecting myocardial infarction (Table 2).
Table 2.
Comparison of hs‐TnT Levels Before, During, and After the Pharmacological Stress Test Among Patients With Moderate to Severe Ischemia (Group A) and Patients With No or Mild Ischemia (Group B) as Shown by Magnetic Resonance Imaging in All Patients, in the Adenosine Stress Test Group, and in the Dobutamine Stress Test Group
| All Patients, pg/mL | Group A, n = 51 | Group B, n = 199 | P Value |
|---|---|---|---|
| Baseline hs‐TnT levels | 11 (3–193) | 8 (3–81) | 0.016 |
| hs‐TnT levels at 1 hour | 12 (3–184) | 8 (3–75) | 0.009 |
| hs‐TnT levels at 3 hours | 12 (3–178) | 9 (3–378) | 0.012 |
| Adenosine Stress Test Group, pg/mL | Group A, n = 49 | Group B, n = 175 | P Value |
| Baseline hs‐TnT levels | 11 (3–193) | 7 (3–70) | 0.009 |
| hs‐TnT levels at 1 hour | 12 (3–184) | 7 (3–67) | 0.005 |
| hs‐TnT levels at 3 hours | 12 (3–178) | 7 (3–67) | 0.001 |
| Dobutamine Stress Test Group, pg/mL | Group A, n = 2 | Group B, n = 24 | P Value |
| Baseline hs‐TnT levels | 59 (7–112) | 12 (3–81) | 0.499 |
| hs‐TnT levels at 1 hour | 49 (20–78) | 14 (3–75) | 0.148 |
| hs‐TnT levels at 3 hours | 122 (111–133) | 48 (13–378) | 0.149 |
Abbreviations: hs‐TnT , high‐sensitivity troponin T.
Data are presented as median hs‐TnT level (range).
Cutoff Point of hs‐TnT Levels in Predicting Moderate to Severe Ischemia by MRI
Cutoff Point of hs‐TnT Levels at Baseline
hs‐TnT levels between the 2 groups were plotted as a box plot. The receiver operating characteristics (ROC) curve showed that baseline hs‐TnT levels of ≥14 pg/mL had a 43.0% sensitivity (30.5%–56.7%) and 77.4% specificity (71.0%–82.6%) in predicting moderate to large ischemia by MRI (P = 0.03). The ROC curve yielded an area under the curve of 0.608 (P = 0.017) (Figure 1).
Figure 1.
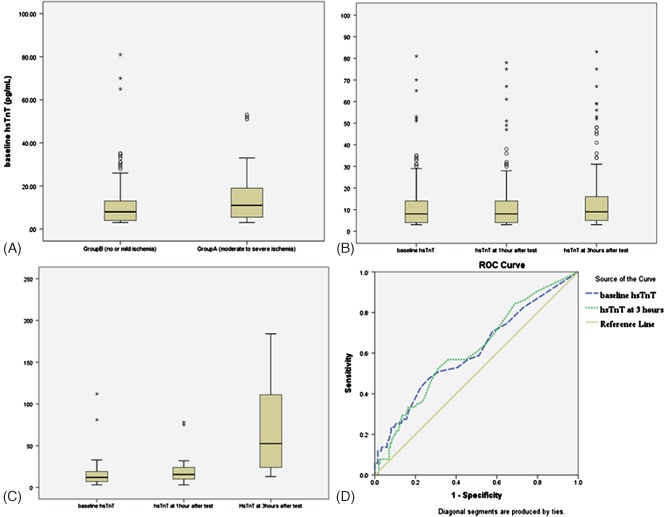
(A) Box plot of high‐sensitivity troponin T (hs‐TnT) values at baseline in the no‐ and mild‐ischemia group (group B) and in the moderate‐ to severe‐ischemia group (group A). (B) Box plot of hs‐TnT levels in individual subjects at baseline, 1 hour after the stress test, and 3 hours after the stress test. (C) Box plot of hs‐TnT levels in individual subjects who underwent the dobutamine stress test at baseline, 1 hour after the stress test, and 3 hours after the stress test. (D) Receiver operating characteristic (ROC) curve of baseline hs‐TnT levels ≥14 pg/mL and hs‐TnT ≥12 pg/mL, 3 hours after the stress test in predicting moderate to severe ischemia by magnetic resonance imaging. Abbreviations: ROC, receiver operating characteristic.
Cutoff Point of hs‐TnT Levels 3 Hours After Stress Test
The ROC curve showed that hs‐TnT levels of ≥12 pg/mL at 3 hours after the pharmacological stress test had a 52.9% sensitivity (40.0%–69.5%) and 68.3% specificity (61.5%–74.4%) in predicting moderate to large ischemia by MRI (P = 0.005). The ROC curve yielded an area under the curve of 0.614 (P = 0.012) (Figure 1).
Type of Pharmacological Stress Test and hs‐TnT Levels
Adenosine Stress MRI
Adenosine stress MRI was undertaken in 224 patients (49 in group A and 175 in group B). hs‐TnT levels were significantly higher in group A than B at baseline (11 vs 7 pg/mL, P = 0.009), 1 hour (12 vs 7 pg/mL, P = 0.005), and 3 hours (12 vs 7 pg/mL, P = 0.001) There was no acute change in hs‐TnT levels before and after adenosine stress MRI in either group (Table 2).
Dobutamine Stress MRI
Dobutamine stress MRI was undertaken in 26 patients (2 in group A and 24 in group B). hs‐TnT levels were similar in groups A and B at baseline (59 vs 12 pg/mL, P = 0.499) and at 1 hour (49 vs 14 pg/mL, P = 0.148), but they were not significantly different 3 hours after dobutamine stress testing in groups A and B (122 vs 48 pg/mL, P = 0.149). There was a trend toward an acute change between before and after dobutamine stress MRI (Table 2).
Effect of Different Pharmacological Stress Tests on hs‐TnT Release
We performed a paired‐samples t test to determine significant changes in hs‐TnT levels between baseline and 3 hours after the pharmacological stress test. There was no significant change in hs‐TnT levels in the adenosine stress test. In the dobutamine stress test, there was a trend toward hs‐TnT release, especially in group A. However, fewer patients underwent the dobutamine stress test than the adenosine stress test (2 vs 24, respectively); therefore, this difference was not significant. We then compared the delta change (3‐hour hs‐TnT–baseline hs‐TnT) between patients who received adenosine vs dobutamine as the pharmacological stress test. Patients who received dobutamine had a higher median acute change in hs‐TnT levels after the test than did those who received adenosine (21 vs 0 pg/mL, P < 0.001). These results emphasize the difference in pharmacological mechanisms of inducing ischemia and the effect of troponin release with different pharmacological stress tests (Tables 3 and 4).
Table 3.
Paired Sample Analysis of the Change in hs‐TnT Levels Between 3 Hours After the Stress Test and at Baseline Among Patients With Moderate to Severe Ischemia (Group A) and Patients With No or Mild Ischemia (Group B) as Shown by Magnetic Resonance Imaging
| Delta Change Between 3 Hours and Baseline | Total No.: Groups A/B | Group A | Group B | P Value |
|---|---|---|---|---|
| Delta change in all patients | 250: 51/199 | 0 (−15 to 104) | 0 (−6 to 373) | 0.558 |
|
Delta change in adenosine group |
224: 49/175 | 0 (−15 to 23) | 0 (−6 to 10) | 0.880 |
| Delta change in dobutamine group | 26: 2/24 | 63 (21 to 104) | 21 (−1 to 373) | 0.500 |
Abbreviations: hs‐TnT , high‐sensitivity troponin T.
Data are shown for all patients, those who underwent the adenosine stress test (adenosine group), and those who underwent the dobutamine stress test (dobutamine group).
Data are presented as median hs‐TnT level (range).
Table 4.
Paired Sample Analysis of the Change in hs‐TnT Levels Between 3 Hours After the Stress Test and at Baseline Among Patients Who Received Adenosine Versus Dobutamine as the Pharmacological Stress Test
| Delta Change Between 3 Hours and Baseline | Total No.: Adenosine/Dobutamine | Adenosine | Dobutamine | P Value |
|---|---|---|---|---|
| Delta change between adenosine vs dobutamine in all patients | 250: 224/26 | 0 (−15 to 23) | 21 (−1 to 373) | <0.001 |
| Delta change between adenosine vs dobutamine in group B | 199: 175/24 | 0 (−6 to 10) | 21 (−1 to 373) | <0.001 |
| Delta change between adenosine vs dobutamine in group A | 51: 49/2 | 0 (−15 to 23) | 62 (21 to 104) | 0.019 |
Abbreviations: hs‐TnT , high‐sensitivity troponin T.
Data are presented as median hs‐TnT level (range) and are shown for all patients, those with moderate to severe ischemia (group A), and those with no or mild ischemia (group B) as shown by magnetic resonance imaging.
Angiographic Data
Coronary angiography was performed after the stress test in 38 of 51 patients in group A and in 20 of 199 patients in group B. Among patients with angiographic data, 3‐vessel disease involvement was found significantly more often in group A than B (53% vs 15%, P = 0.005). Nonsignificant CAD was less likely to be found in group A than B (5% vs 35%, P = 0.006). The mean degree of stenosis was more severe in all coronary vessels in group A than B. In group A, the degree of stenosis was significantly higher in the left anterior descending artery (87.5% vs 65%, P = 0.031), left circumflex artery (80% vs 0%, P = 0.018), and right coronary artery (80% vs 15%, P = 0.033) than in group B.
Clinical Endpoints
Clinical Endpoints Based on hs‐TnT Levels 3 Hours After Stress Test
One death and 2 myocardial infarctions occurred at 6 months of follow‐up in patients with a baseline hs‐TnT level of ≥14 pg/mL, whereas no deaths or myocardial infarctions occurred in patients with a baseline hs‐TnT level of <14 pg/mL. More patients with a baseline hs‐TnT level of ≥14 than <14 pg/mL tended to develop future myocardial infarction. Composite endpoints based on baseline hs‐TnT levels of ≥14 pg/mL were not different between the 2 groups (22.4% vs 17.7%, P = 0.401). Logistic regression analysis adjusted for the severity of ischemia was not significant for each endpoint.
Clinical Endpoints Between Groups A and B
One death occurred at 6 months of follow‐up in group A. Patients in group A were more likely to develop recurrent angina (19 vs 7 patients, P < 0.001), hospitalization from angina (6 vs 2 patients, P = 0.001), revascularization (25 vs 10 patients, P < 0.001), and myocardial infarction (2 vs 0 patients). Death and myocardial infarction occurred in 1 patient in group A none in group B. The composite endpoints of recurrent angina, coronary revascularization, myocardial infarction, and death at 6 months were significantly higher in group A than in group B (34 [66.7%] vs 13 [6.6%] patients, P < 0.001).
Discussion
hs‐TnT levels were correlated with the amount and degree of myocardial ischemia as assessed by pharmacological MRI in stable patients. The peak hs‐TnT level did not exceed the upper limit of normal (>14 pg/mL), and the delta change was small. Baseline hs‐TnT levels were correlated with the amount of ischemia.
No acute change in hs‐TnT levels occurred between baseline and 3 hours after testing with adenosine. However, hs‐TnT levels 3 hours after the dobutamine stress test were significantly higher than baseline levels. The 2 different pharmacological stress tests (adenosine and dobutamine) had different effects on the level of hs‐TnT release after inducing ischemia.
A low hs‐TnT level in association with chronic stable CAD is common.6 Additionally, elevated hs‐TnT levels in patients with significant CAD by angiogram or thallium have been described.7, 8, 9, 10 In patients with clinically stable and angiography‐proven CAD, hs‐TnT levels correlate with CAD severity as assessed by the number of stenotic vessels and atherosclerosis plaque burden on computed tomography or coronary angiography.6, 11, 12
This study also showed that brief periods of ischemia caused release of low hs‐TnT levels. Previous studies have shown that troponin I may increase in proportion to the severity of myocardial ischemia,8 but this has not been shown with hs‐TnT.9 In several studies, low levels of hs‐TnT were associated with brief myocardial ischemia, such as after rapid atrial pacing, supraventricular tachycardia, strenuous endurance exercise, and exercise stress testing.8, 13, 14, 15 In a model of rapid atrial pacing, hs‐TnT levels were increased in patients with and without CAD, but the levels tended to be higher 3 hours after rapid pacing in patients with CAD.13 Liebetrau et al7 found that measurement of hs‐TnT levels during exercise stress testing improved the sensitivity and specificity of the exercise stress test in detecting significant CAD. However, all patients in their study underwent stepwise bicycle ergometry as an exercise stress test. No information on troponin release under pharmacological stress testing is available.
In our study, hs‐TnT levels increased in patients with and without documented CAD, but they tended to be higher 3 hours after the dobutamine stress test in patients with than without CAD. This finding could be explained by the different mechanisms of action of these 2 drugs. Although dobutamine increases the heart rate and contractility similar to exercise stress testing or rapid pacing, adenosine causes transient arterial vasodilatation in all vascular beds, causing a “steal phenomenon” and shunting the blood flow to a less resistant area.16, 17, 18
Several mechanisms have been described for troponin release due to myocardial ischemia. White19 described 5 other potential mechanisms of troponin release without myocardial necrosis. An increase in cellular wall permeability and cellular release of proteolytic troponin degradation products may be related to troponin release in the setting of myocardial ischemia. A rapid rise and fall of troponin within 24 hours of myocardial ischemia supports this possibility. Unfortunately, in our protocol, hs‐TnT levels were only measured 3 hours after inducing ischemia.
In patients with acute chest pain, resting hs‐TnT levels predict myocardial perfusion abnormalities.20 hs‐TnT levels are measured at the time of computed tomography angiography or approximately 4 hours after emergency department presentation with acute chest pain. hs‐TnT levels are correlated with reversible ischemia and the presence of mixed plaques. Elevated hs‐TnT levels may be caused by provocable ischemia in the presence and absence of significant CAD. In patients with acute chest pain, the presence of mixed plaques without significant stenosis may be explained by plaque rupture with distal embolization.
We found that patients with stable CAD had low hs‐TnT levels due to a brief period of ischemia. More importantly, even with a small quantifiable rise in hs‐TnT levels below established cutoffs for myocardial infarction, baseline hs‐TnT levels were correlated with the amount of ischemia on MRI and the extent and degree of CAD, as shown by the degree of stenosis and number of coronary vessels involved on coronary angiography. In patients with stable CAD, a small rise in baseline hs‐TnT levels is clinically meaningful because it is associated with the amount of ischemia, which represents the extent of coronary vessel involvement and degree of stenosis of CAD.
Study Limitations
This prospective cohort comprised patients with stable CAD undergoing pharmacological stress MRI in the outpatient setting. Patients who refused to participate were excluded. Measurement of hs‐TnT levels 24 hours after the stress test enabled observation of the rapid rise and fall pattern within 24 hours. This further confirmed our notion of ischemic‐induced release of hsTnT. However, our study was limited because it was performed in an outpatient setting. The majority of pharmacological stress tests were adenosine stress tests. The number of patients who underwent the dobutamine stress test was too small to draw any conclusions about different patterns of change in hs‐TnT between the adenosine and dobutamine stress tests.
Conclusion
In stable CAD, hs‐TnT levels can be released after a brief period of ischemia. The level of troponin release differs between adenosine and dobutamine stress tests. More importantly, even when there is a small quantifiable rise in hs‐TnT levels below the cutoff for myocardial infarction, baseline hs‐TnT levels are correlated with the amount of myocardial ischemia. Our findings provide a more comprehensive understanding of hs‐TnT in clinical practice. Physicians should not disregard a small rise in hs‐TnT levels, even below the cutoff for myocardial infarction, in patients with stable CAD.
Acknowledgments
The authors are grateful to Suthiphol Udomphunthurak for statistical analysis.
The Chalerm Prakiat Foundation, Faculty of Medicine Siriraj Hospital, funded this study.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Koller A. Exercise‐induced increases in cardiac troponins and prothrombotic markers. Med Sci Sports Exerc. 2003;35:444–448. [DOI] [PubMed] [Google Scholar]
- 2. Middleton N, George K, Whyte G, et al. Cardiac troponin T release is stimulated by endurance exercise in healthy humans. J Am Coll Cardiol. 2008;52:1813–1814. [DOI] [PubMed] [Google Scholar]
- 3. Kurz K, Giannitsis E, Zehelein J, et al. Highly sensitive cardiac troponin T values remain constant after brief exercise‐ or pharmacologic‐induced reversible myocardial ischemia. Clin Chem. 2008;54:1234–1238. [DOI] [PubMed] [Google Scholar]
- 4. Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high‐sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. [DOI] [PubMed] [Google Scholar]
- 5. Motwani M, Maredia N, Fairbairn TA, et al. Assessment of ischaemic burden in angiographic three‐vessel coronary artery disease with high‐resolution myocardial perfusion cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2014;15:701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Omland T, Pfeffer MA, Solomon SD, et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61:1240–1249. [DOI] [PubMed] [Google Scholar]
- 7. Liebetrau C, Gaede L, Dorr O, et al. High‐sensitivity cardiac troponin T and copeptin assays to improve diagnostic accuracy of exercise stress test in patients with suspected coronary artery disease [published online ahead of print April 3, 2014]. Euro J Prev Cardiol. doi: 10.1177/2047487314529691 [DOI] [PubMed] [Google Scholar]
- 8. Sabatine MS, Morrow DA, de Lemos JA, et al. Detection of acute changes in circulating troponin in the setting of transient stress test‐induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J. 2009;30:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roysland R, Kravdal G, Hoiseth AD, et al. Cardiac troponin T levels and exercise stress testing in patients with suspected coronary artery disease: the Akershus Cardiac Examination (ACE) 1 study. Clin Sci. 2012;122:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Falkensammer J, Gasteiger S, Stojakovic T, et al. Elevated baseline hs‐cTnT levels predict exercise‐induced myocardial ischemia in patients with peripheral arterial disease. Clin Chim Acta. 2012;413:1678–1682. [DOI] [PubMed] [Google Scholar]
- 11. Ndrepepa G, Braun S, Schulz S, et al. High‐sensitivity troponin T level and angiographic severity of coronary artery disease. Am J Cardiol. 2011;108:639–643. [DOI] [PubMed] [Google Scholar]
- 12. Laufer EM, Mingels AM, Winkens MH, et al. The extent of coronary atherosclerosis is associated with increasing circulating levels of high sensitive cardiac troponin T. Arterioscler Thromb Vasc Biol. 2010;30:1269–1275. [DOI] [PubMed] [Google Scholar]
- 13. Turer AT, Addo TA, Martin JL, et al. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol. 2011;57:2398–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Redfearn DP, Ratib K, Marshall HJ, et al. Supraventricular tachycardia promotes release of troponin I in patients with normal coronary arteries. Int J Cardiol. 2005;102:521–522. [DOI] [PubMed] [Google Scholar]
- 15. Mingels A, Jacobs L, Michielsen E, et al. Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin Chem. 2009;55:10–108. [DOI] [PubMed] [Google Scholar]
- 16. Meyer U, Schiffer W, Schulz FW, et al. Proceedings: coronary steal phenomenon in intracoronary infusion of adenosine, nitroglycerine, persantin, intensain and nifedipine [in German]. Z Kardiol. 1974;0(suppl 1):33. [PubMed] [Google Scholar]
- 17. Ertl G, Simm F, Wichmann J, et al. The dependence of coronary collateral blood flow on regional vascular resistances. Pharmacological studies with glyceryl trinitrate, adenosine and verapamil. Naunyn Schmiedebergs Arch Pharmacol. 1979;308:265–272. [DOI] [PubMed] [Google Scholar]
- 18. Verani MS. Pharmacological stress with adenosine for myocardial perfusion imaging. Semin Nucl Med. 1991;21:266–272. [DOI] [PubMed] [Google Scholar]
- 19. White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol. 2011;57:2406–2408. [DOI] [PubMed] [Google Scholar]
- 20. Ahmed W, Schlett CL, Uthamalingam S, et al. Single resting hsTnT level predicts abnormal myocardial stress test in acute chest pain patients with normal initial standard troponin. JACC Cardiovasc Imaging. 2013;6:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


