ABSTRACT
Background
High‐sensitivity cardiac troponin T (hsTnT) is used in many countries, but is not available in the United States. Prior evidence has been viewed as inconclusive as to whether low cardiac troponin T (cTnT) concentrations detected with hsTnT are prognostically meaningful compared with fourth‐generation cTnT.
Hypothesis
The aim of this study was to assess the prognostic performance of low‐level cTnT elevations using the hsTnT assay compared with the assay (fourth‐generation) currently available in the United States.
Methods
We measured serum cTnT in 4160 patients with non–ST‐elevation acute coronary syndrome using both the hsTnT and fourth‐generation assays. Patients were stratified at the 99th percentile cut point for each assay.
Results
Patients with baseline hsTnT ≥14 ng/L (n = 3697) vs <14 ng/L were at higher 30‐day risk of cardiovascular death (CVD) or myocardial infarction (MI) (9.1% vs 1.9%, P < 0.0001). After adjusting for all other elements of the Thrombolysis In Myocardial Infarction risk score, hsTnT ≥14 carried a 5.2‐fold higher risk of CVD/MI (95% confidence interval [CI]: 2.6‐10.1, P < 0.0001). Low levels of hsTnT (14–50 ng/L) also revealed increased risk (CVD/MI: 6.4%, P = 0.002). Importantly, patients with negative fourth‐generation cTnT but positive hsTnT were at 4.5‐times higher risk of CVD/MI (95% CI: 1.9‐11.0, P = 0.0008) than patients with negative hsTnT. In contrast, patients with a negative hsTnT but positive fourth‐generation cTnT result had a lower rate of CVD/MI than with a positive hsTnT (1.3% vs 8.2%, P = 0.0005).
Conclusions
Low‐level increases in cTnT detected using the hsTnT assay identified patients at a meaningfully higher risk and who might otherwise be missed, and improves upon risk stratification using the cTnT assay currently available in the United States.
Introduction
Cardiac troponin (cTn) is a specific marker of myocardial injury1 and plays an important role in both the diagnosis and risk assessment of patients with suspected acute coronary syndromes (ACS).1, 2, 3, 4, 5 Troponin elevation, at any level with prior generation assays, has been associated with higher rates of death and myocardial infarction (MI) after ACS.6 However, as more sensitive assays have emerged, the proportion of patients with suspected ACS and positive troponin has increased substantially, and the prognostic significance, especially at a low level of cTn elevations, has drawn clinical debate.
New high‐sensitivity cTn assays have been reported to improve overall diagnostic accuracy in patients with suspected ACS.7, 8 A high‐sensitivity assay for cardiac troponin T (hsTnT) is clinically available and already used in many countries worldwide. This assay is not clinically available in the United States, where a prior fourth‐generation assay for cardiac troponin T (cTnT) remains in use. Clinicians have remained concerned that changing to a more sensitive assay may result in a greater proportion of patients with elevated cTnT with diminished prognostic value. The available evidence examining this concern has been viewed as inconclusive by regulatory authorities and many clinicians in the United States. Therefore, we investigated the prognostic performance of the hsTnT assay in direct comparison to the fourth‐generation cTnT assay in a large population of patients with suspected ACS. A particular focus of this investigation was to determine whether the low concentrations detected with hsTnT below the lowest range of the fourth‐generation cTnT assay are prognostically meaningful.
Methods
Patient Population
In this study, we pooled individual patient data from patients with non–ST‐elevation ACS enrolled in the biomarker substudies of the Early Glycoprotein IIb/IIIa Inhibition in Non‐ST‐Segment Elevation Acute Coronary Syndrome (EARLY‐ACS, N = 1718) and Otamixaban for the Treatment of Patients with Non–ST‐Segment Elevation Acute Coronary Syndrome (SEPIA‐ACS1 TIMI 42, N = 2442) trials. In both trials, patients were eligible if they were within 24 hours of having symptoms at rest lasting for at least 10 minutes, suggestive of ACS, and had at least 1 additional high‐risk feature.9, 10 Exclusion criteria for both have been published.10 In EARLY‐ACS, patients were randomly assigned in a 1:1 ratio to either the early, routine administration of eptifibatide or early placebo with delayed, provisional administration of eptifibatide after coronary angiography.9 In SEPIA‐ACS1 TIMI 42, patients were randomized to receive 1 of 5 doses of otamixaban vs heparin plus eptifibatide as described previously.10 The protocols (including the biomarker substudies) were approved by institutional review boards, and written consent was obtained from all patients.
Troponin Testing
Serum was isolated and stored at temperatures of −20 °C or colder and then shipped frozen to the Thrombolysis In Myocardial Infarction (TIMI) Clinical Trials Laboratory (Boston, MA) and stored at −80 °C or colder until testing. All baseline blood samples (N = 4160) from the 2 trials that were available for determination of cTnT using both the hsTnT assay (Roche Diagnostics, Indianapolis, IN) as well as the current commercial troponin T assay (TnT, fourth generation; Roche Diagnostics) were included in this analysis. Troponin testing was performed by individuals who were blinded to clinical events and treatment. The hsTnT assay is a sandwich immunoassay that uses 2 monoclonal antibodies that specifically recognize 2 epitopes on the central portion of the human cardiac troponin T protein.11 The hsTnT assay has a limit of the blank (signal in a blank cuvette) of 3 ng/L (0.003 µg/L), coefficient of variation of ≤10% at a limit of 13 ng/L (0.013 µg/L), and 99th percentile reference limit of 14 ng/L (0.014 µg/L).12 The fourth‐generation cTnT assay has a detection limit of 10 ng/L (0.01 µg/L), a 99th percentile reference limit of 10 ng/L (0.01 µg/L), and a coefficient of variation of ≤10% at a limit of 30 ng/L (0.03 µg/L).13 After all the testing was completed, a low‐end calibration issue for the hsTnT assay was identified by the manufacturer in the lot used for our analysis.14 For this reason, all data were recalibrated and blinded to clinical events in conjunction with Roche Diagnostics using methods previously described.15
We have previously measured hsTnI (ARCHITECT STAT hsTnI; Abbott Laboratories, Abbott Park, IL).16 This assay has a limit of the blank of 1.3 ng/L and a coefficient of variation of 10% at 3.0 ng/L and a 99th percentile reference limit of 26 ng/L.11, 17 Data for each of these assays were available in 4154 patients.
Endpoints
The primary endpoint of this analysis was cardiovascular death (CVD) or new or recurrent MI at 30 days. Each endpoint was adjudicated by an independent clinical events committee that was blinded to treatment allocation.
Statistical Methods
As recommended by current professional guidelines, the results of testing using the hsTnT assay were analyzed dichotomized at the 99th percentile reference limit of 14 ng/L.1 In addition, we evaluated categories of hsTnT based on the limit of the blank (3 ng/L), and quartiles of concentration ≥14 ng/L, including a low level of cTnT of 50 ng/L, which correlates with the concentration of the fourth‐generation assay at the level of the 10% coefficient of variation.18, 19 The prognostic performance at the 99th percentile was directly compared between the hsTnT assay and the fourth‐generation cTnT assay. Adjusted analyses were performed using the elements (except troponin) of a well‐validated risk model in ACS (TIMI risk score).20 Odds ratios (ORs) for clinical outcomes at 30 days were calculated by logistic regression analyses. Kaplan‐Meier estimates of the cumulative incidence at 30 days are reported. There were no significant interactions between the randomized therapies in the 2 trials and the association of hsTnT with outcome, and thus all analyses presented are the aggregate of the randomized groups. Supplemental analyses comparing the results with both the hsTnT and hsTnI assays are also reported. All analyses were performed by the TIMI Study Group using Stata version 9.2 (StataCorp LP, College Station, TX).
Results
Baseline Characteristics
Baseline characteristics for the 4160 patients with available hsTnT and fouth‐generation cTnT data are summarized in the Table 1. The baseline concentration of hsTnT was ≥14 ng/L in 89% of patients. Patients with baseline hsTnT ≥14 ng/L were older and more commonly male. They also were more likely to have diabetes mellitus, have a history of prior heart failure, and have a high TIMI risk score at the time of presentation. Patients with hsTnT ≥14 ng/L were more likely to undergo revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG).
Table 1.
Baseline Characteristics
| hsTnT <14 ng/L (n = 463) | hsTnT ≥14 ng/L (n = 3697) | P Value | |
|---|---|---|---|
| General characteristics | |||
| Age, y, mean | 60.3 | 64.1 | <0.0001 |
| Male, % | 58.3 | 70.5 | <0.0001 |
| Medical history | |||
| Hypertension, % | 70.5 | 72.2 | 0.43 |
| Hyperlipidemia, % | 59.4 | 56.6 | 0.25 |
| Smoker, % | 27.8 | 31.0 | 0.16 |
| DM, % | 26.5 | 31.9 | 0.017 |
| Previous MI, % | 19.3 | 26.5 | 0.0008 |
| Previous CHF, % | 4.5 | 8.1 | 0.007 |
| Presentation | |||
| Time from symptom onset, h | 13.2 | 13.2 | 0.89 |
| TIMI risk score, % | |||
| High | 15.2 | 35.9 | <0.0001 |
| Intermediate | 62.2 | 55.2 | |
| Low | 22.7 | 8.9 | |
| Therapy during hospitalization | |||
| Aspirin, % | 98.3 | 98.0 | 0.69 |
| β‐Blocker, % | 76.0 | 87.1 | <0.0001 |
| ACEI or ARB, % | 62.1 | 70.7 | 0.0002 |
| Statin, % | 82.5 | 85.5 | 0.09 |
| Heparin or LMWH, % | 46.8 | 73.4 | <0.0001 |
| Clopidogrel, % | 94.4 | 86.7 | <0.0001 |
| PCI, % | 48.8 | 59.7 | <0.0001 |
| CABG, % | 5.4 | 10.4 | 0.0007 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; CHF, congestive heart failure; DM, diabetes mellitus; hsTnT, high‐sensitivity cardiac troponin T; LMWH, low‐molecular‐weight heparin; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Prognostic Performance of hsTnT
99th Percentile Reference Limit
Compared with patients with hsTnT below the 99th percentile, patients with a baseline hsTnT concentration ≥14 ng/L had significantly higher rates of CVD/MI at 30 days (9.1% vs 1.9%, P < 0.0001), including CVD (2.2% vs 0.2%, P = 0.02) and MI (7.5% vs 1.7%, P < 0.0001) individually. After adjusting for the remaining elements of the TIMI risk score including age, coronary risk factors, prior coronary disease, recent aspirin use, refractory ischemia, and ST‐segment deviation, patients with an hsTnT concentration ≥14 ng/L were at a 5.2‐fold higher risk of CVD/MI (adjusted OR: 5.2, 95% confidence interval [CI]: 2.6‐10.1) compared to patients with hsTnT <14 ng/L (Figure 1). Moreover, the risks of CVD and MI were individually significantly higher (CVD: adjusted OR: 11.3, 95% CI: 1.5‐81.5; MI: adjusted OR: 4.7, 95% CI: 2.3‐9.5).
Figure 1.
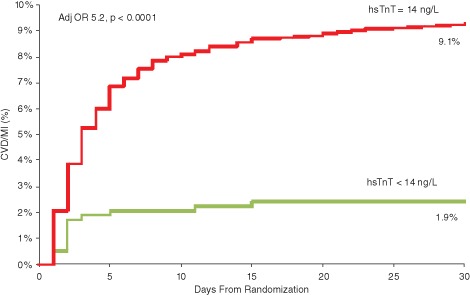
Kaplan‐Meier estimated cumulative incidence of cardiovascular death or myocardial infarction stratified by baseline high‐sensitivity cardiac troponin T (hsTnT) using the 99th percentile reference limit. Abbreviations: Adj OR, adjusted odds ratio; CVD, cardiovascular death; MI, myocardial infarction.
Analysis Across hsTnT Concentration Ranges
Of the 3697 patients with hsTnT ≥14 ng/L, 532 patients (14.4%) had low‐level elevations above the 99th percentile reference limit (14 ng/L) but <50 ng/L. Compared with patients with hsTnT <14 µg/L, patients with low‐level elevation (14 to <50 ng/L) had significantly higher rates of CVD (1.9% vs 0.2%, P = 0.04), MI (5.5% vs 1.7%, P = 0.003), and CVD/MI (6.4% vs 1.9%, P = 0.001). Such low‐level increases in hsTnT were independently associated with a 3.3‐fold higher rate of CVD/MI at 30 days compared to patients at <14 ng/L (adjusted OR: 3.3, 95% CI: 1.6‐7.0) (Figure 2).
Figure 2.
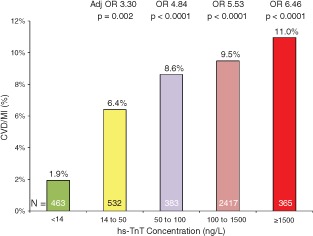
Cardiovascular death or myocardial infarction at 30 days stratified by baseline high‐sensitivity cardiac troponin T (hsTnT) concentration. Abbreviations: Adj OR, adjusted odds ratio; CVD, cardiovascular death; MI, myocardial infarction; OR, odds ratio.
Patients who had detectable levels of hsTnT (>3 ng/L) but were at or below the 99th percentile reference limit (14 ng/L) represented 10.6% of the total population (n = 440). When compared to the very small number of patients (n = 23) with undetectable levels of hsTnT (<3 ng/L), patients with detectable levels of hsTnT but below the 99th percentile had significantly higher rates of CVD/MI at 30 days (2.0% vs 0%, P < 0.0001).
Comparison With the Fourth‐Generation cTnT Assay
Examining results from both the hsTnT and the fourth‐generation cTnT assays, patients with an elevated hsTnT result were at higher risk of CVD/MI at 30 days regardless of the result with the fourth‐generation assay (Figure 3, top left). There were 231 patients (5.6%) in this high‐risk study population who had baseline troponin levels below the 99th percentile using the fourth‐generation assay, with a 30‐day rate of CVD/MI of 6.9%. Among these patients, a positive hsTnT identified 63% of patients as higher risk, with a 30‐day event rate of 8.2% vs 4.7% for the remainder (Figure 3, bottom). Those who had a fourth‐generation cTnT value at or above the 99th percentile had a 30‐day rate of CVD/MI of 8.4%. Interestingly, a negative hsTnT result identified 10% of patients with a low event rate despite a positive cTnT result with the older fourth‐generation assay (1.3%) (Figure 3, bottom). Therefore, examining the 2 groups with discordant hsTnT and fourth‐generation cTnT results revealed hsTnT to provide more accurate risk stratification (Figure 3, right). When formally comparing the C‐statistic of each assay when added separately to the TIMI risk score, there was significant improvement with hsTnT compared to the fourth‐generation cTnT assay (+0.043, P < 0.001).
Figure 3.
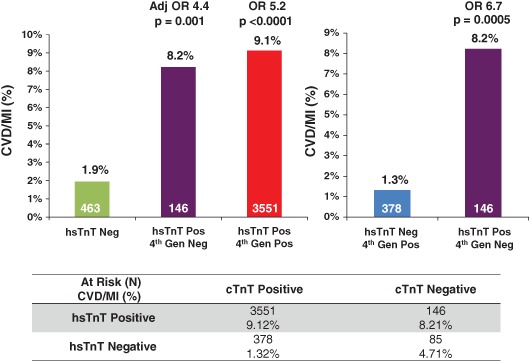
Cardiovascular death or myocardial infarction at 30 days stratified at the 99th percentile reference limits for the high‐sensitivity cardiac troponin T (hsTnT) and fourth‐generation cardiac troponin T (cTnT) assays. Abbreviations: Adj OR, adjusted odds ratio; CVD, cardiovascular death; Gen, generation; MI, myocardial infarction; Neg, negative; OR, odds ratio; Pos, positive.
Comparison With hsTnI
Overall, the proportion of patients with discordant hsTnT and hsTnI results was small (n = 200, 4.8%). Only 20 patients had a positive hsTnI and negative hsTnT result, precluding reliable estimation of the event rate. Notably, among patients with a positive hsTnT but negative hsTnI result (n = 180), the event rate was intermediate (5.0%) compared with the 30‐day rates of CVD or MI for those with concordant negative (2.0%) or concordant positive (9.3%) hsTnT and hsTnI results.
Discussion
Despite the established improved diagnostic accuracy of the hsTnT assay,7, 8 the clinical relevance of low elevations in cTnT continue to be debated. In this nested prospective evaluation of the prognostic performance of the hsTnT assay in more than 4100 patients with non–ST‐elevation ACS, we found that the hsTnT assay delivers strong prognostic capability at the guidelines‐based 99th percentile cut point. Moreover, low‐level elevation of hsTnT identified patients at more than 3‐fold higher short‐term risk of CVD or recurrent MI. Last, this hsTnT assay, which remains investigational in the United States, demonstrated enhanced prognostic performance compared with the current US commercial assay for cTnT.
These findings are important for understanding the potential clinical advantages of this hsTnT assay that is now used widely outside the United States. We found that not only did the hsTnT assay identify high‐risk patients not detected with the prior generation assay, but also correctly discriminated a population at low risk who were potentially falsely identified by a positive fourth‐generation assay result. We speculate that this finding may relate to the low‐end imprecision of the fourth‐generation assay around its 99th percentile. In contrast, by virtue of improved analytical performance in this range of concentration, the hsTnT assay also demonstrates enhanced overall prognostic performance.
In addition to the specific findings with respect to this emerging hsTnT assay, our results are of more general relevance to the interpretation of low‐level elevations of cTn using newer generation assays in patients with a high clinical suspicion for ACS. We have previously shown that abnormal results in the low range of concentration above the 99th percentile using so‐called current generation “sensitive” assays for cTn are indicative of poor prognosis.21 However, it has been uncertain as to whether this conclusion could be extrapolated to the emerging generation of high‐sensitivity assays.22 The finding that patients with low‐level elevations of hsTnT (14–50 ng/L) were at a greater than 3‐fold higher risk of cardiovascular death or MI at 30 days, underscores that such low‐level increases in cTn are clinically relevant using a high‐sensitivity assay. Despite recent data raising a question of whether the hsTnT assay meets expert consensus criteria for high sensitivity in a healthy reference population,14 our findings demonstrate clinical performance that was superior to the fourth‐generation cTnT assay and comparable to a high‐sensitivity assay for cTnI.
Our observations add to those from several important smaller prior studies. Among 1452 randomly selected patients with ACS enrolled in the Global Use of Strategies To Open Occluded Coronary Arteries IV trial (GUSTO‐IV), using samples collected late after presentation and initiation treatment (48 hours), Lindahl and colleagues showed persistent elevation of hsTnT in similarly identified patients at increased risk despite normal third‐generation cTnT result.23 Similarly, Ndrepepa et al showed that among 447 patients with ACS, elevation of hsTnT among patients with a negative fourth‐generation cTnT had similar long‐term rates of death (4 years) when compared to patients who were positive for both assays, and that a hsTnT ≥14 ng/L was an independent predictor of mortality at 4 years.24 Our robust study builds on these findings to show a clear relationship between hsTnT assessed at presentation with short‐term cardiovascular mortality and recurrent MI in a large, well‐characterized population. These findings offer additional clinical relevance due to the early sampling and short‐term outcomes that drive decision making during initial ACS management. Moreover, our study offers new information regarding the patients with a discordantly positive fourth‐generation assay result in the setting of a normal hsTnT.
It is also noteworthy that in our study of perceived high‐risk patients with a clinical diagnosis of ACS, a hsTnT value below the limit of the blank identified an unexpectedly low‐risk cohort. This observation lends additional support to diagnostic and triage strategies that use negative hsTnT results to select candidates who may not require inpatient evaluation and therapy. For example, the usefulness of hsTnT for a rapid rule‐out or rule‐in of MI in the emergency department was recently demonstrated by Reichlin and colleagues, who showed that hsTnT testing at the time of presentation and 1 hour later enabled MI to be excluded in 60% of the population, with 100% sensitivity and 100% negative predictive value.25
Limitations
This study was conducted among patients from clinical trials that enrolled patients with a high clinical suspicion of ACS with high‐risk features. Accordingly, the proportion of patients with negative troponin results was small, and the findings may not be generalizable to a broad population of patients presenting to the emergency department with chest pain. In addition, there is limited power to investigate the event rates and risk in patients with negative Tn concentrations. It should be noted, however, that the clinical prognostic impact may reasonably be expected to be even greater (in terms of the number of patients discriminated as low risk by hsTnT) with application in a broader population. In addition, the prognostic capability of the hsTnT assay for patients with low‐risk features or with atypical chest pain is not addressed in this study. It does reason, however, that the negative predictive value of the hsTnT assay might actually be higher in a group with a lower pretest probability of acute cardiac disease. Last, our data underwent recalibration due to an issue reported by the manufacturer after testing was completed as discussed in the Methods section. Recalibration, however, did not meaningfully alter any of the risk relationships that we have reported.
Conclusion
An assay for hsTnT that remains investigational in the United States performs robustly for risk stratification at the guidelines‐based 99th percentile decision limit. Moreover, low‐level increases in cTnT concentration detected using the hsTnT assay identify individuals at a clinically meaningful higher risk who might otherwise be missed using the currently available fourth‐generation assay for cTnT.
Drs. Sabatine and Morrow contributed equally to the oversight of this work.
hsTnT testing was supported by Roche Diagnostics.
The TIMI Study Group has received research grant support from Abbott, Amgen, AstraZeneca, Beckman Coulter, BG Medicine, BRAHMS, Bristol‐Myers Squibb, Buhlmann, Critical Diagnostics, Daiichi Sankyo Co Ltd, Eli Lilly and Co, GlaxoSmithKline, Merck and Co, Nanosphere, Novartis Pharmaceuticals, Ortho‐Clinical Diagnostics, Pfizer, Randox, Roche Diagnostics, Sanofi‐Aventis, Siemens, and Singulex.
MP Bonaca is an investigator and receives salary support from the TIMI Study Group. He has received remuneration for consulting from Roche.
P Jarolim has received research support from Daiichi Sankyo, Roche Diagnostics, AstraZeneca, Siemens, Abbott, and Merck. He has received remuneration for consulting from T2 Biosystems and Quanterix.
E Bohula‐May, N Deenadayalu, RP Giugliano, E Braunwald, and MS Sabatine are investigators and receive salary from the TIMI Study Group.
DA Morrow is an investigator and receives salary from the TIMI Study Group. He has received remuneration for consulting from Abbott, Beckman‐Coulter, Boehringher Ingelheim, Critical Diagnostics, Genentech, Gilead, Instrumentation Laboratory, Johnson & Johnson, Merck, Novartis, Roche Diagnostics, and Servier.
LK Newby has received consulting fees from Roche, see https://www.dcri.org/about‐us/conflict‐of‐interest.
J Grinstein, SA Murphy, MJ Conrad have no additional disclosures to report.
Research reported in this publication was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health under award number RC1HL099692 and contract number HHSN268201000033C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 2. Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac‐specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335:1342–1349. [DOI] [PubMed] [Google Scholar]
- 3. Heidenreich PA, Alloggiamento T, Melsop K, McDonald KM, Go AS, Hlatky MA. The prognostic value of troponin in patients with non‐ST elevation acute coronary syndromes: a meta‐analysis. Journal of the American College of Cardiology. 2001;38:478–485. [DOI] [PubMed] [Google Scholar]
- 4. Morrow DA, Cannon CP, Jesse RL, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53:552–574. [DOI] [PubMed] [Google Scholar]
- 5. Davis MB, Shafton A, Desai A, et al. Reliable exclusion of acute coronary syndrome among hospitalized patients with elevated troponin. Clin Cardiol. 2014;37:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. James SK, Armstrong P, Barnathan E, et al. Troponin and C‐reactive protein have different relations to subsequent mortality and myocardial infarction after acute coronary syndrome: a GUSTO‐IV substudy. J Am Coll Cardiol. 2003;41:916–924. [DOI] [PubMed] [Google Scholar]
- 7. Aldous SJ, Richards M, Cullen L, et al. Diagnostic and prognostic utility of early measurement with high‐sensitivity troponin T assay in patients presenting with chest pain. CMAJ. 2012;184:E260–E268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mills NL, Churchhouse AM, Lee KK, et al. Implementation of a sensitive troponin I assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA. 2011;305:1210–1216. [DOI] [PubMed] [Google Scholar]
- 9. Giugliano RP, White JA, Bode C, et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med. 2009;360:2176–2190. [DOI] [PubMed] [Google Scholar]
- 10. Sabatine MS, Antman EM, Widimsky P, et al. Otamixaban for the treatment of patients with non‐ST‐elevation acute coronary syndromes (SEPIA‐ACS1 TIMI 42): a randomised, double‐blind, active‐controlled, phase 2 trial. Lancet. 2009;374:787–795. [DOI] [PubMed] [Google Scholar]
- 11. Apple FS, Collinson PO; IFCC Task Force on Clinical Applications of Cardiac Biomarkers . Analytical characteristics of high‐sensitivity cardiac troponin assays. Clin Chem. 2012;58:54–61. [DOI] [PubMed] [Google Scholar]
- 12. Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high‐sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. [DOI] [PubMed] [Google Scholar]
- 13. Hermsen D, Apple F, Garcia‐Beltran L, et al. Results from a multicenter evaluation of the 4th generation Elecsys Troponin T assay. Clin Lab. 2007;53:1–9. [PubMed] [Google Scholar]
- 14. Apple FS, Jaffe AS. Clinical implications of a recent adjustment to the high‐sensitivity cardiac troponin T assay: user beware. Clin Chem. 2012;58:1599–1600. [DOI] [PubMed] [Google Scholar]
- 15. Hallermayer K, Jarausch J, Menassanch‐Volker S, et al. Implications of adjustment of high‐sensitivity cardiac troponin T assay. Clin Chem. 2013;59:572–574. [DOI] [PubMed] [Google Scholar]
- 16. Bohula May EA, Bonaca MP, Jarolim P, et al. Prognostic performance of a high‐sensitivity cardiac troponin I assay in patients with non‐ST‐elevation acute coronary syndrome. Clin Chem. 2014;60:158–164. [DOI] [PubMed] [Google Scholar]
- 17. Lipowsky C, Laird D, Workman R, et al. Development of a highly sensitive immunoassay for cardiac troponin I for the ARCHITECT i2000SR and i1000SR analyzers. Clin Chem. 2012;58:A5. [Google Scholar]
- 18. Twerenbold R, Jaffe A, Reichlin T, et al. High‐sensitive troponin T measurements: what do we gain and what are the challenges? Eur Heart J. 2012;33:579–586. [DOI] [PubMed] [Google Scholar]
- 19. Saenger AK, Beyrau R, Braun S, et al. Multicenter analytical evaluation of a high‐sensitivity troponin T assay. Clinica Chimica Acta. 2011;412:748–754. [DOI] [PubMed] [Google Scholar]
- 20. Antman E, Cohen M, Bernink P, et al. The timi risk score for unstable angina/non–st elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. [DOI] [PubMed] [Google Scholar]
- 21. Bonaca M, Scirica B, Sabatine M, et al. Prospective evaluation of the prognostic implications of improved assay performance with a sensitive assay for cardiac troponin I. J Am Coll Cardiol. 2010;55:2118–2124. [DOI] [PubMed] [Google Scholar]
- 22. Morrow DA, Antman EM. Evaluation of high‐sensitivity assays for cardiac troponin. Clin Chem. 2009;55:5–8. [DOI] [PubMed] [Google Scholar]
- 23. Lindahl B, Venge P, James S. The new high‐sensitivity cardiac troponin T assay improves risk assessment in acute coronary syndromes. Am Heart J. 2010;160:224–229. [DOI] [PubMed] [Google Scholar]
- 24. Ndrepepa G, Braun S, Schulz S, et al. Comparison of prognostic value of high‐sensitivity and conventional troponin T in patients with non‐ST‐segment elevation acute coronary syndromes. Clinica Chimica Acta. 2011;412:1350–1356. [DOI] [PubMed] [Google Scholar]
- 25. Reichlin T, Schindler C, Drexler B, et al. One‐hour rule‐out and rule‐in of acute myocardial infarction using high‐sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211–1218. [DOI] [PubMed] [Google Scholar]


