ABSTRACT
Advances in the novel approach to control ischemic heart disease and heart failure using stem cells or progenitor cells from bone marrow, mesenchyme, or myocardial tissue itself have demonstrated efficacy for increasing left ventricular function, decreasing infarct scar tissue, improving exercise tolerance and heart failure symptoms, and, in some studies, decreasing mortality and reducing rehospitalization for intractable angina or subsequent myocardial infarction. The most common techniques utilize injections of cells into the coronary vasculature or directly into specific areas of vulnerable myocardium. Although few adverse effects have been noted in clinical trials of these procedures, further clinical trials over the next decade should provide further advances in interventional techniques, ancillary supporting technologies to enhance cell regeneration, and applications in ischemic heart disease, cardiomyopathies, and cardiac genetic disorders.
Introduction
Major issues in the management of ischemic heart disease (IHD) and consequent left ventricular (LV) dysfunction and failure include regeneration of insufficient coronary vasculature and regeneration of cardiomyocytes to decrease morbidity and mortality. In addition to established interventional and supportive strategies to decrease ischemia and maintain systolic performance, the use of stem cells has been extensively studied in clinical trials to bolster established interventions. This review will focus on the development of stem‐cell utilization and possible future approaches to improve efficacy of this novel therapy.
Cardiac stem‐cell therapy has been focused on interventions in acute myocardial infarction (AMI), postinfarct cardiac support including ischemic cardiomyopathy, and refractory angina. Such therapy appears to protect the heart against ischemic injury and decrease the development of cardiac fibrosis.
Although the term stem cell has been utilized for this area of intervention, such cells include not only bone marrow stem cells, but more mature endothelial progenitor cells (EPCs), resident cardiac stem cells (CSCs), mesenchymal stem cells (MSCs), skeletal myoblasts, and embryonic stem cells. Stem‐cell therapy may lead to reversal of pathophysiologic changes in coronary heart disease (CHD) and heart failure (HF). It is utilized as an adjunct to coronary interventions to bolster vascular support of the myocardium and for regeneration of myocardial cells. Such vascular regeneration can provide relief from symptoms refractory to maximal antianginal agents. Additionally, gene therapy is being utilized to support generation and proliferation of stem cells.
Physiologic and Pathophysiologic Considerations
Human pluripotent stem cells have the capability of differentiating into coronary vascular endothelial cells or cardiomyocytes. The inflammatory reaction during AMI leads to mobilization of progenitor stem cells from bone marrow by neural and humoral signal activation and increased local production of chemoattractants in the myocardium. This may lead to intrinsic neovascularization of the myocardium as well as cardiomyocyte repopulation (Figure 1). However, high‐intensity inflammation may also lead to EPC dysfunction. For example, elevated C‐reactive protein levels inhibit EPC differentiation and survival by reducing EPC endothelial nitric oxide synthase (eNOS) messenger RNA expression1 and decreasing angiogenic activity.2 Therefore, depending upon the intensity of inflammatory stimulus, EPC activity may be increased3 or decreased.4 Acute myocardial infarction is usually associated with relatively low inflammatory activity.
Figure 1.
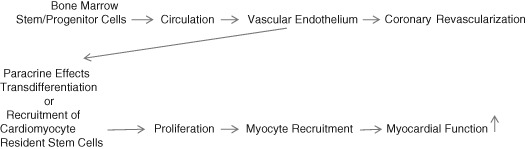
EPC administration into the coronary artery leads to engraftment into coronary endothelium. EPCs promote (1) revascularization of the coronary vessel. Some EPCs can transdifferentiate into cardiomyocytes or through paracrine effects causing (2) recruitment of resident cardiomyocyte stem cells that proliferate and regenerate myocardial tissue. Both effects can increase myocardial function. Abbreviations: EPC, endothelial progenitor cell.
Historical Perspective
Several approaches have been utilized for tissue regeneration. Therapeutic angiogenesis was initially evaluated by vascular endothelial growth factor (VEGF) injection producing collateral‐artery proliferation.5 This was followed by the generation of human stem cells in 1998.6, 7 In 1997, CD34+ marker progenitor cells from adult bone marrow were reported to differentiate ex vivo into an endothelial phenotype.8 Moreover, these isolated cells incorporated into new vessels in ischemic locations. The following year, these newly termed EPCs were found to circulate in the blood,9 and CSCs were described in human myocardium with the marker c‐kit + .10 In 2001, rapid mobilization of bone marrow CD34+ was reported after AMI.11
It had previously been assumed that adult cardiomyocytes could not be regenerated. However, an ingenious study using C14 labeling, based on human exposure to nuclear bomb tests in the early 1950s, demonstrated newly formed cardiomyocytes.12 Further, about 1% of cardiomyocytes were found to regenerate in younger adults, decreasing to 0.25% to 0.5% in older adults. In 2001, evidence was reported for human myocyte division after AMI.13 New cardiomyocytes were found to proliferate at the border of infarcted tissue. In 2004, CSCs were isolated from adult murine hearts as self‐adhered clusters of progenitor cells, termed cardiospheres.14 Recently, introduced cardiospheres were found to reduce infarct scar size and improve cardiac function after AMI.15
Characterization of Endothelial Progenitor Cells and Options for Expansion
In peripheral blood, several possible sources of EPCs may exist, in increasing maturity: (1) myeloid cells, which may mature into endothelial cells under cultivation; (2) rare hematopoietic stem cells; (3) other circulating progenitor cells; and (4) mature endothelial cells shed off arterial walls. However, EPCs are isolated and cultured primarily from bone marrow on the basis of surface markers such as CD34+, CD133+, and the endothelial marker protein vascular endothelial growth factor receptor 2 (VEGFR2). CD34+ is a marker of cells in later stages of maturity, and CD133+ marker cells are barely lineage committed. Circulating EPCs may also be used as biomarkers for cardiovascular disease or for enriching cells for angiogenesis.16 The number of circulating EPCs varies inversely with the presence of cardiovascular risk factors.17 After adjusting for other factors such as drug therapy and concomitant disease, increased EPC levels are independently associated with cardiovascular events and mortality.18
Therapeutic agents that appear to increase mobilization and activity of circulating EPCs include statins,19 angiotensin‐converting enzyme inhibitors,20 and peroxisome proliferator‐activated receptor–γ agonists (pioglitazone21; Table 1).
Table 1.
Stimulation of Increase in EPCs
| Intrinsic | Extrinsic |
| Inflammation | EPC administration |
| Oxidative stress | Statins |
| Exercise | ACEIs/ARBs |
| Estrogens | PPAR‐γ agonists |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; EPC, endothelial progenitor cells; PPAR‐γ, peroxisome proliferator‐activated receptor‐γ.
Principles of Utilization
Stem‐cell therapy is utilized to augment neovascularization of myocardium of cardiac tissue and vascular endothelium. Autologous stem cells are isolated from the iliac crest bone marrow, harvested ex vivo, enriched using a special centrifugation procedure, and subjected to quality‐control methods. In patients with AMI, this is usually accomplished 4 to 7 days after infarction. After enrichment, cells are injected in the cardiac catheterization laboratory into a coronary artery or directly into myocardium. Cells may be mobilized using gene therapy with VEGF or isolated cells treated with eNOS transcription to enhance migratory and neovascularization capacity.22
Other cell populations have been harvested from mesenchymal tissues, skeletal muscle (skeletal myoblasts), myocardium (resident CSCs), and embryonic tissue (embryonic stem cells), but these processes are still primarily in the developmental stages. However, MSCs have been shown to improve cardiomyocyte regeneration in phase I/II clinical trials.23
A standard percutaneous coronary angioplasty procedure is utilized with coronary infusion of EPCs multiple times with balloon inflation. This procedure allows sufficient time for the EPCs to come in contact with the microcirculation site. Injection into a coronary vein has not been successful. Location of specific myocardial areas of intervention utilizes an electromechanical mapping system (Noga XP cardiac navigation system; Biosense Webster, Diamond Bar, CA) that maps the ischemic, infarcted, or scarred site. For example, a voltage map might show adequate voltage at a site, but the simultaneous mechanical map might show poor contraction at the site, indicating hibernating myocardium (Figure 2). For intramyocardial injections, a catheter system is used with a retractable needle with Noga guidance, contact is made with the endothelium in or near nonviable myocardium, with injections of cells 1 cm apart. With hibernating myocardium, direct injection into the area may salvage the myocardium and improve angiogenesis.24 A trial of autologous EPCs under such conditions showed a 75% decrease in perfusion defects and improved left ventricular ejection fraction (LVEF) of ≥20%.25
Figure 2.
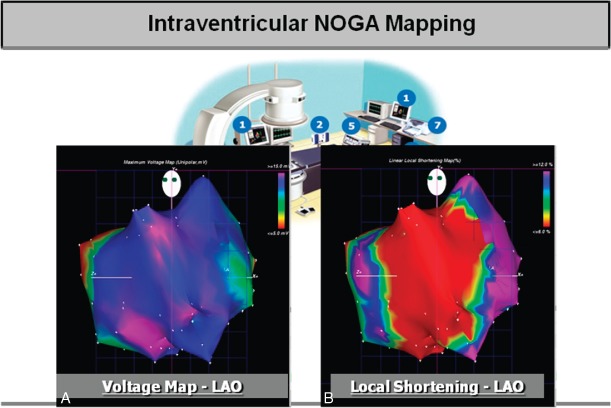
Simultaneous electromechanical mapping system for determining location of cell injection sites. Voltage map (A) showing normal voltage activity (blue). Mechanical activity map (B) showing absent local shortening (red). The superimposed information indicates hibernating but viable myocardium. Illustration courtesy of Dr. Gary L. Schaer, Division of Cardiology, Rush University Medical Center; and Biologics Delivery Systems Group. Abbreviations: LAO, left anterior oblique projection.
Endothelial progenitor cells can be enriched in vivo, ex vivo, and in local tissue, and modified by gene transfer. Stimulation of EPC production in bone marrow can be accomplished by using VEGF or granulocyte macrophage‐colony stimulating factor.26 Ex vivo, EPCs are placed in a culture medium for up to 7 days.
In targeted tissue area, local accumulation of transplanted EPCs can be expanded by local injection of factors such as stroma derived factor.27 Gene transfer has also been accomplished in animal models for dysfunctional cells, such as from diabetics, to enhance the angiogenic response.28
Activity of transplanted cells may be influenced by the cell type itself, the isolation and delivery method, and the utilization of enhancing factors. Cell dose levels have influenced the efficacy of myocardial tissue perfusion and long‐term reduction of infarct size.29 The baseline LVEF after AMI may influence the efficacy of improvement of function; an inverse correlation between LVEF and improvement after 4 months was found, with the best results with the lowest LVEF.30
Considerations in Intracoronary Interventions
Several concerns about vascular injury during and after percutaneous coronary intervention (PCI) impact upon the efficacy of intracoronary vasculogenesis therapy. Vascular injury resulting from PCI may lead to acute stent thrombosis, neointimal hyperplasia, or in‐stent restenosis. Endothelial progenitor cells can facilitate re‐endothelialization in sites of endothelial damage.9 Restenosis following PCI has been associated with decreased circulating EPCs with increased activity.31 Drug‐eluting stents have associated antiproliferating agents that may reduce vascular healing and decrease local functioning endothelium. To counteract this problem, studies are being focused on localizing EPC installation in animal models.31 Further studies are needed to determine a definitive role of supportive EPC injections in preventing angioplasty and stent complications.
Cardiomyocyte Regeneration
As indicated above, the heart has a low detectable regenerative capacity.32 Cardiac stem cells may derive from resident cardiomyocytes or from circulating stem cells reaching the myocardium after AMI. Cardiac stem cells themselves, expressing c‐kit+, the stem cell receptor factor, may not only generate cardiomyocytes, but also endothelial and mesenchymal cells in the heart.13 C‐kit + activation binds stem cell factor to its ligand.33 Using c‐kit + and cardiosphere‐derived cells via coronary artery injection in patients with postinfarction LV dysfunction, investigators have demonstrated increased LVEF and reduced scar size. The engrafted cells are presumed to activate endogenous systems for cardiac repair, mobilizing bone‐marrow EPCs and CSCs, which facilitates cardiomyocyte reentry. Stimulation of cytokines by these engrafted cells may lead to inhibition of apoptosis of cardiomyocytes, preventing ventricular remodeling and expansion of chamber size.13
Specific Conditions
Acute Myocardial Infarction
The relatively mild inflammatory reaction to AMI mobilizes stem cells and EPCs from bone marrow into the circulation. Local chemoattractants in the myocardium are produced that attract these cells. Resident CSCs proliferate in areas surrounding the infarcted myocardium. Circulating VEGFs increase. Mobilized stem cells and EPCs in the circulation reach a maximum within hours after an ischemic episode, and increased levels may persist for several months.17
Although meta‐analysis of clinical trials of stem/progenitor cells will be discussed below, several clinical trials deserve comment. Two studies in which intracoronary injections were utilized within a week of AMI, the randomized controlled trials (RCTs) Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR‐AMI; 204 patients) and Bone Marrow Transfer to Enhance ST‐Elevation Infarct Regeneration (BOOST; 60 patients), demonstrated improvement in LVEF in 4 to 6 months after cell transfer, but no change in left ventricular end‐diastolic volume (LVEDV).34, 35 Although the REPAIR‐AMI study found a sustained benefit after 12 months and a reduction in adverse cardiovascular events after 2 years,36 initial LV function benefit was not sustained in BOOST.29 The Autologous Stem Cell Transplantation in Acute Myocardial Infarction (ASTAMI) study of 100 patients also found no change in LVEF, infarct size or chamber dimensions, or adverse‐event rate up to 6 months after treatment.37 However, in the nonrandomized but controlled Clinical Benefit and Long‐Term Outcome After Intracoronary Autologous Bone Marrow Cell Transplantation in Patients With Acute Myocardial Infarction (BALANCE) study (124 patients), infarct size, mortality, and exercise capacity were improved after 5 years in the treatment vs control group.38
Thus, results demonstrate some efficacy in improving LVEF and exercise capacity, and decreasing infarct size and mortality, but results are mixed. With successful outcomes, LVEF has increased by approximately 5%.
Recovery After Acute Myocardial Infarction and Ischemic Cardiomyopathy
To support or improve LV function after AMI, 2 approaches to EPC/progenitor cell transfusions include early postinfarct intervention and intervention when LV function has decreased to the ischemic cardiomyopathy level. As indicated above, early intervention has improved LV function by approximately 5% vs controls. One small nonrandomized study of cell intervention in 18 “chronic MI” patients 5 months to 8 years post‐MI (Intracoronary Autologous Mononuclear Bone Marrow Cell Transplantation [IACT] Study) suggested subsequent smaller postinfarct area (−30%) and improved LVEF (15%) and wall motion in the peri‐infarct area.39
Several studies of intracoronary stem cell intervention in ischemic cardiomyopathy have shown improvement in LV function. One example is the Acute and Long‐Term Effects of Intracoronary Stem Cell Transplantation in 191 Patients With Chronic Heart Failure (STAR) study (391 patients including controls) in which intervention produced an increase in LVEF, decreased mortality and improved exercise capacity over 3 months to 5 years vs controls.40
A small study (8 ischemic cardiomyopathy patients) using intramyocardial injection of progenitor cells over 5 years after AMI with 1 year follow‐up demonstrated improved regional contractility around the myocardial scar and decreased LVEDV.41
As of 2013, 36 clinical trials of stem‐cell intervention for cardiomyopathy, mostly ischemic, had been reported, of which 12 were RCTs.43 Sources of transplanted cells included bone marrow progenitor cells or mononuclear cells, MSCs, CSCs, or skeletal myoblasts. An analysis of these studies indicated improvement in LVEF in 6 of 11 studies using bone marrow progenitor cells and in 11 of 15 studies using skeletal myoblasts. In all 36 clinical trials, the most common complications were ventricular dysrhythmias in trials of skeletal myoblasts.42 Only 2 studies used CSCs, and 1 study used MSCs.
In summary, the use of stem‐cell transplantation in patients with ischemic cardiomyopathy appears promising for improvement in LV function and possibly survival; and, importantly, the use of stem cells in cardiomyopathy provides the only alternative to cardiac transplantation itself in improving the cardiomyocyte population rather than stabilization of the cardiac function.
Intractable Angina
It is estimated that there are 850 000 patients in the United States with refractory angina, despite optimal medical therapy or mechanical intervention.43 A number of phase I/II clinical trials have been concluded or are underway to determine the efficacy of stem‐cell transplantation in alleviating anginal symptoms in this condition. Studies in animal models have indicated increase in capillary density with neovascularization produced by this technique. Early‐phase trials in patients with refractory angina have demonstrated variable effects on angina frequency as well as heart failure symptoms. For example, intramyocardial application of EPCs resulted in improvement in angina frequency and quality of life over 3 months.44 In the Prospective Randomized Trial of Direct Endomyocardial Implantation of Bone Marrow Cells for Treatment of Severe Coronary Artery Diseases (PROTECT‐CAD) trial of 28 patients with refractory ischemia, direct intramyocardial injection of bone marrow cells demonstrated improvement in anginal symptoms, LV function, exercise time, and New York Heart Association (NYHA) class.45 A randomized phase II study of 167 patients with refractory angina during electromechanical mapping of viable myocardial sites demonstrated improvement in angina symptoms and exercise tolerance over 6 to 12 months.43
Although phase III studies of intractable angina are awaited, it must be emphasized that pharmacologic intervention is frequently not optimal and that it is imperative that future clinical trials in this area focus on patients whose clinical course has demonstrated adequate course of antianginal treatment, whether by pharmacologic agents or mechanical intervention. The relative efficacy of intracoronary vs direct intramyocardial injection of stem cells also awaits evaluation.
Meta‐Analyses
A number of meta‐analyses have been published on cardiovascular stem‐cell studies. These include animal‐model studies and early‐ and late‐phase trials. There is, of course, some overlap in the studies included in various analyses. We have selected several meta‐analyses published between 2007 and 2014 to review briefly with further information in Table 2. Only patient studies are reviewed, emphasizing salient findings and publication dates indicated. Most of the studies evaluated were intracoronary interventions; a few were intramyocardial. In reviewing these meta‐analyses, one should bear in mind that there may considerable heterogeneity among studies for each analysis. For example, in the meta‐analysis by Abdel‐Latif et al,46 evaluations included RCTs and controlled cohort studies; number of patients varied between 20 and 204; cell injection from AMI or PCI was accomplished between 1 day and 81 months; the number of bone marrow cells used varied between 2 × 106 and 60 × 109; and bone marrow cells used included mononuclear cells, MSCs, and circulating progenitor cells. Within individual trials, 4 of 12 RCTs favored bone marrow treatment vs controls, and in the 6 cohort studies, 2 favored bone marrow treatment vs controls. This analysis of 18 studies (2007) involving 999 patients with IHD demonstrated improved LV function, reduced infarct scar size, and reduced LVEDV.46
Table 2.
Summary of Selected Meta‐Analyses of Clinical Stem‐Cell Studies: Intracoronary Injections Unless Otherwise Noted
| Author | Trials Design and Conditions Included | No. of Trials (No. of Patients) | Median F/U, mo | Results |
|---|---|---|---|---|
| Abdel‐Latif46 (2007) | RCT/Co; IHD | 18 (999) | 3–18 | ↑LVEF (3.7%), ↓scar size (−5.5%), ↓LVESV (−4.8 mL) |
| Zhao47 (2007) | RCT; IHD, IM/IC | 10 (422) | 3–6 | ↑LVEF (4.6%), ↓LVESVa (−0.4), ↓LVEDVa (−0.4) 6 mo |
| Lipinski48 (2008) | RCTs; AMI (<14 d) | 10 (698) | 6 | ↑LVEF (3.0%), ↓scar size (−5.6%), ↓ LVESV (−7.4 mL), ↓recur MI |
| Martin‐Rendon49 (2008) | RCTs; AMI (≤7 d) | 13 (811) | 4–6 | ↑LVEF (3.0%), ↓scar size (−3.5%), ↓ LVESV (−4.7 mL) |
| Zhang50 (2008) | RCTs; AMI (4–7 d) | 7 (660) | 3–18 | ↑LVEF (4.6%), ↓LVESV (−0.28), ↓CE (OR: 0.32), ↓rest/UA (OR: 0.59) |
| Brunskill51 (2009) | RCTs; AMI/IHD, IM/IC | 21 (1091) | 3–6 | IM > IC ↑LVEF (5.9%)b; increase sig. only with lower baseline LVEF in chronic ischemia |
| Jeevanantham52 (2012) | RCTs; AMI/IHD, IM/IC | 50 (2625) | ↑LVEF (4.0%), ↓scar size (−4.0%), ↓LVESV (−8.9 mL), ↓LVEDV (−5.2 mL) | |
| Delewi53 (2013) | RCT; AMI | 26c (1710) | 6–12 | ↑LVEF (3.9%), ↓scar size NS, ↓LVESV (−9.4 mL), ↓recur AMI (RR: 0.44), ↓read HF, UA (RR: 0.59) |
| Fisher54 (2014) | RCT; IHD/HF | 23 (1255) | Variable | ↓Mortality (RR: 0.28), 8 studies; ↑LVEF (−2.6%), 6 studies |
| Tian55 | RCT; IM | 11 (492) | 3–12 | ↑LVEF (4.9%), ↓LVESV (−10.7 mL) |
Abbreviations: AMI, acute myocardial infarction; CE, cardiac events; Co, nonrandomized cohort controlled studies; F/U, follow‐up; HF, heart failure; IC, intracoronary injection; IHD, chronic ischemic heart disease; IM, intramyocardial injection; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; MI, myocardial infarction; NS, not significant; OR, odds ratio; RCT, randomized controlled trial; read, readmission; recur, recurrent; rest, restenosis; RR, risk ratio; scar size, infarction or scar volume; UA, unstable angina.
IC injections unless otherwise noted.
Weighted mean differences.
IM delivery > IC delivery.
Analysis of 23 studies.
An analysis of 10 RCTs (2007) involving 422 participants with chronic CHD in combination with PCI or coronary bypass procedures found an increase in LVEF and reduced systolic and diastolic dimensions at 6 months postprocedure. There was a lack of adequate information about control groups and co‐interventions.47
An analysis of controlled trials with 14 days of AMI involving 10 studies, 698 patients (2007), with median 6‐month follow‐up with associated PCI, showed a 3% increase in LVEF, a reduction of infarct size of 5.6%, and a decrease in LV end systolic volume.48 A significant reduction in recurrence of AMI was also found. Of note, as with other meta‐analyses of study protocols, different cell types (eg, bone marrow cells, peripheral mononuclear cells), types of cell marker (eg, CD34+, CD133+), selected enrichment of cell colonies with G‐CSF vs none, time of intervention after AMI (1–12 days), and follow‐up (3–18 months) were variables that need to be considered in comparing individual study results.
An analysis of 13 trials of 811 participants with AMI (2008) showed improved LVEF and decreased cardiac volume and myocardial lesion area compared with controls.49 Improved LVEF related to infusion of cells within 7 days of AMI.
In 7 RCTs of 660 patients after AMI (2009), cell therapy from 4 to 7 days after AMI was superior to 24‐hour intervention in improving LVEF, reducing LV end volume, and decreasing the incidence of revascularization.50
In 21 clinical trials of 1091 patients with AMI and chronic IHD (2009), the route of delivery of transplanted cells had some bearing on outcome.51 Cardiac function was significantly improved with intramyocardial injection vs coronary infusion. Significant improvement in cardiac function was inversely related to baseline LVEF.
In 50 studies of 2625 patients receiving bone marrow transplants for IHD (2012), LVEF increased (5.5%, vs 3.3% in the placebo groups), and infarct size and LV diastolic and end‐systolic volumes decreased.52 At 1 year, the treatment groups showed a reduction in combined endpoints of death, recurrence of AMI, and revascularization procedures.
An analysis of 23 RCTs with 1710 participants with AMI (2013) showed improvement of LVEF at 6 months with further improvement at 12 months compared with noninjected controls.53 A reduction in silent myocardial ischemia and readmission for heart failure and unstable angina were found.
In 23 RCTs involving 1255 participants with chronic CHD and heart failure (2014), mortality and rehospitalization for heart failure were reduced over ≥12 months in 8 studies, but no clear benefits in other studies.54 Overall, LVEF and NYHA class were improved. No long‐term adverse events were found in the 19 studies evaluating this measure.
Finally, in 11 RCTs involving 492 participants with old prior AMIs (2014), using intramyocardial stem cells, LVEF was increased by 5% and LV systolic and diastolic volumes were reduced.55 Most of these studies involved associated coronary artery bypass grafting; however, 5 trials had a relatively small sample size, and LVEF was not significantly changed in these studies.
In summarizing the results of multiple meta‐analyses, we may draw the following conclusions: (1) intracoronary bone marrow cell treatment may lead to moderate improvement of LVEF at least in the short term with decreased LV volume; (2) there is some evidence for a decrease in early mortality, recurrent AMI, decreased angina attacks, increased exercise tolerance, and reduced hospitalization for heart failure symptoms; (3) direct intramyocardial injection of cells may have special benefit in hibernating myocardium; and (4) the route of injection, time of injection in the case of AMI, type of cell, volume of delivery, and use of ancillary cell‐enrichment methods all have a bearing on successful transplantation.
A caveat about reported meta‐analyses was described in a recent critique evaluating possible in‐trial or between‐trial discrepancies.56 In comparing 133 reports from 49 bone marrow stem cell clinical trials, >600 discrepancies among trials were found. For example, the number of discrepancies was correlated with purported increases in LVEF. General discrepancies included conflicts in protocol and follow‐up, contradiction between figures and numerical data, statistical errors, suppression of significant changes, reporting nonsignificant figures as significant or the opposite, misclassification of NYHA class (0 to −5!), or impossible numbers of patients, percentages, or summary statistics).
Other concerns about these meta‐analyses involve the lack of adequate clinical endpoints and prevention of cardiac remodeling, perhaps resulting from the relative paucity of large numbers of study subjects that would be needed for conclusive evidence of benefit. Therefore, there should be some caution about becoming too enthusiastic about the efficacy of stem‐cell intervention until studies with robust patient numbers and adequate long‐term follow‐up are accomplished.
The Future
Clinical phase III trials are underway to evaluate long‐term effects of transplanted stem/pluripotent cells. One area that is especially intriguing is the stimulation of endogenous CSCs to produce cardiomyocytes. The delivery of modified RNA encoding human VEGF may improve cardiac function and enhance long‐term survival based upon animal models.21
Expansion of the use of transplanted pluripotent cells to human disease models is now being evaluated. For example, Gurdon and Yamakana received the 2012 Nobel Prize in Medicine or Physiology for generating patient‐specific induced pluripotent stem‐cell cardiomyocytes for studying models for such conditions as familial hypertrophic cardiomyopathy, arrhythmogenic right ventricular dysplasia, and others.57
For stem cell/pluripotent cell therapy in general, future advances foreseen include further evaluation of priming of cells to increase therapeutic efficacy, technologic advances to support these therapies such as tissue engineering, and the use of combined strategies with gene therapy. More specifically, these advances would include modification of cells before transplantation such as encoding with VEGF, as indicated above, the use of drugs, small molecules, and plasmids.58 Biomaterials may enhance the transplanted cells within the myocardial environment. This could include bioactive signaling incorporated in the biomaterials to improve efficacy in the myocardium. An example of this is the use of insulin‐like growth factor–carrying nanofiber, which enhances CSC utilization in repair of cardiac injury.59 An example of tissue engineering is use of hypoxia‐tolerant tissue grafts to replace injured myocardium.60 Stromal cells in combination with progenitor cells may bolster their effects. Fibrin glue may be useful in sealing transplantation injection sites to decrease cell leakage.61 Contrast echocardiography can utilize microbubbles containing cells for transplant. Signal disruption of the microbubbles would deposit the cells at the site for transplantation.62 Coating stents with anti‐CD34+ antibodies could allow seeding of these stents with circulating EPCs.63 These would develop into endothelial cells covering the stent struts and the denuded vascular wall.
In transplantation of cardiospheres or myoblasts, use of antiapoptotic treatments of angiogenic growth factors before implantation may increase cell survival.64, 65
Thus, the future of stem cell/pluripotent cell transplantation is bright with the promise for the addition of myocardial and vascular regeneration for cardiac disease. Much further research and clinical‐trial exposition must be accomplished, however, before the impact of stem‐cell transplantation on cardiac regeneration and clinical efficacy can be amply demonstrated.
Conclusion
Over the next decade, much advance is expected in the use of stem/progenitor cells to supplement current standard techniques in decreasing morbidity, mortality, and symptomatology from major cardiac disease. These advances are predicated on regeneration of myocardial and vascular cells with resultant increases in cardiac function, decreases in infarct scar tissue, and increase in functional coronary vasculature. There is still considerable question about the clinical impact of stem‐cell studies in heart disease. There are still many questionable cause‐and‐effect relationships, such as the transdifferentiation of bone marrow cells into cardiomyocytes, for example.66, 67 At present, the clinical cardiologist in the catheterization laboratory should find this area a burgeoning field for research, and the practicing cardiologist outside the catheterization laboratory would find that keeping abreast of these advances would stimulate his or her interest in a novel area of patient care. A recent review in the Journal of Molecular and Cellular Cardiology provides further insights into this area and a more extended reference list.68 A further discussion of the discrepancies and contradictions of studies in stem‐cell therapy may be found in another recent publication.69
The author has no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Verma S, Kuliszewski MA, Li SH, et al. C‐reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C‐reactive protein and cardiovascular disease. Circulation. 2004;109:2058–2067. [DOI] [PubMed] [Google Scholar]
- 2. Suh W, Kim KL, Choi JH, et al. C‐reactive protein impairs angiogenic functions and decreases the secretion of arteriogenic chemo‐cytokines in human endothelial progenitor cells. Biochem Biophys Res Commun. 2004;321:65–71. [DOI] [PubMed] [Google Scholar]
- 3. Yao EH, Yu Y, Fukuda N. Oxidative stress on progenitor stem cells in cardiovascular diseases. Curr Pharm Biotechnol. 2006;7:101–108. [DOI] [PubMed] [Google Scholar]
- 4. Güven H, Shepherd RM, Bach RG, et al. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J Am Coll Cardiol. 2006;48:1579–1587. [DOI] [PubMed] [Google Scholar]
- 5. Takeshita S, Zheng LP, Brogi E, et al. Therapeutic angiogenesis: a single intra‐arterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. [DOI] [PubMed] [Google Scholar]
- 7. Thomson JA, Itskovitz‐Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts [published correction appears in Science. 1998;282:1827]. Science. 1998;282:1145–1147. [DOI] [PubMed] [Google Scholar]
- 8. Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 9. Shi Q, Rafii SS, Wu MH, et al. Evidence for circulating bone marrow–derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 10. Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:1–14. [DOI] [PubMed] [Google Scholar]
- 11. Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. [DOI] [PubMed] [Google Scholar]
- 12. Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. [DOI] [PubMed] [Google Scholar]
- 14. Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. [DOI] [PubMed] [Google Scholar]
- 15. Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere‐derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase I trial. Lancet. 2012;379:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–1189. [DOI] [PubMed] [Google Scholar]
- 17. Wojakowski W, Landmesser U, Bachowski R, et al. Mobilization of stem and progenitor cells in cardiovascular diseases. Leukemia. 2012;26:23–33. [DOI] [PubMed] [Google Scholar]
- 18. Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. [DOI] [PubMed] [Google Scholar]
- 19. Llevadot J, Murasawa S, Kureishi Y, et al. HMG‐CoA reductase inhibitor mobilizes bone marrow–derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Min TQ, Zhu CJ, Xiang WX, et al. Improvement in endothelial progenitor cells from peripheral blood by ramipril therapy in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2004;18:203–209. [DOI] [PubMed] [Google Scholar]
- 21. Wang CH, Ciliberti N, Li SH, et al. Rosiglitazone facilitates angiogenic progenitor cell differentiation toward endothelial lineage: a new paradigm in glitazone pleiotropy. Circulation. 2004;109:1392–1400. [DOI] [PubMed] [Google Scholar]
- 22. Sasaki K, Heeschen C, Aicher A, et al. Ex vivo treatment of bone marrow mononuclear cells with endothelial NO synthetase enhancer AVE9488 enhances their functional capacity for cell therapy. Proc Natl Acad Sci U S A. 2006;103:14537–14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chou SH, Lin SZ, Kuo WW, et al. Mesenchymal stem cell insights: prospects in cardiovascular therapy. Cell Transplant. 2014;23:513–529. [DOI] [PubMed] [Google Scholar]
- 24. Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5‐year follow‐up from the randomized controlled BOOST trial. Eur Heart J. 2009;30:2978–2984. [DOI] [PubMed] [Google Scholar]
- 25. Perin EC, Dohmann HF, Borojevic R, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. [DOI] [PubMed] [Google Scholar]
- 26. Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF gene. Nature. 1996;380:435–439. [DOI] [PubMed] [Google Scholar]
- 27. Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell‐derived factor‐1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. [DOI] [PubMed] [Google Scholar]
- 28. Iwaguro H, Yamaguchi J, Kalka C, et al. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–738. [DOI] [PubMed] [Google Scholar]
- 29. Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow‐up data from the randomized, controlled BOOST (Bone Marrow Transfer to Enhance ST‐elevation Infarct Regeneration) trial. Circulation. 2006;113:1287–1294. [DOI] [PubMed] [Google Scholar]
- 30. Miettinen JA, Ylitalo K, Hedberg P, et al. Determinants of functional recovery after myocardial infarction of patients treated with bone marrow–derived stem cells after thrombolytic therapy. Heart. 2010;96:362–367. [DOI] [PubMed] [Google Scholar]
- 31. Matsuo Y, Imanishi T, Hayashi Y, et al. The effect of senescence of endothelial cells on in‐stent stenosis in patients undergoing coronary stenting. Intern Med. 2006;45:581–587. [DOI] [PubMed] [Google Scholar]
- 32. Bergmann O, Jovinge S. Cardiac regeneration in vivo: mending the heart from within? Stem Cell Res. 2014;13:523–531. [DOI] [PubMed] [Google Scholar]
- 33. Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. [DOI] [PubMed] [Google Scholar]
- 34. Schächinger V, Erbs S, Elsässer A, et al; REPAIR‐AMI Investigators . Intracoronary bone marrow–derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. [DOI] [PubMed] [Google Scholar]
- 35. Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone‐marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. [DOI] [PubMed] [Google Scholar]
- 36. Murasawa S, Asahara T. Cardiogenic potential of endothelial progenitor cells. Ther Adv Cardiovasc Dis. 2008;2:341–348. [DOI] [PubMed] [Google Scholar]
- 37. Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. [DOI] [PubMed] [Google Scholar]
- 38. Yousef M, Schannwell CM, Köstering M, et al. The BALANCE Study: clinical benefit and long‐term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53:2262–2269. [DOI] [PubMed] [Google Scholar]
- 39. Strauer BE, Brehm M, Zeus T, et al. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT study. J Am Coll Cardiol. 2005;46:1651–1658. [DOI] [PubMed] [Google Scholar]
- 40. Strauer BE, Yousef M, Schannwell CM. The acute and long‐term effects of intracoronary stem cell transplantation in 191 patients with chronic heart failure: the STAR‐heart study [published correction appears in Eur J Heart Fail. 2013;15:360]. Eur J Heart Fail. 2010;12:721–729. [DOI] [PubMed] [Google Scholar]
- 41. Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse modeling. Circ Res. 2011;108:792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Losordo DW, Henry TD, Davidson C, et al. Intramyocardial autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Ramshorst J, Bax JJ, Beeres SL, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA. 2009;301:1997–2004. [DOI] [PubMed] [Google Scholar]
- 45. Tse HF, Thambar S, Kwong YL, et al. Prospective randomized trial of direct endomyocardial implantation of bone marrow cells for treatment of severe coronary artery diseases (PROTECT‐CAD trial). Eur Heart J. 2007;28:2998–3005. [DOI] [PubMed] [Google Scholar]
- 46. Abdel‐Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow–derived cells for cardiac repair: a systematic review and meta‐analysis. Arch Intern Med. 2007;167:989–997. [DOI] [PubMed] [Google Scholar]
- 47. Zhao Q, Ye X. Additive value of adult bone‐marrow‐derived cell transplantation to conventional revascularization in chronic ischemic heart disease: a systemic review and meta‐analysis. Expert Opin Biol Ther. 2011;11:1569–1579. [DOI] [PubMed] [Google Scholar]
- 48. Lipinski MJ, Biondi‐Zoccai GG, Abbate A, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta‐analysis of controlled clinical studies. J Am Coll Cardiol. 2007;50:1761–1767. [DOI] [PubMed] [Google Scholar]
- 49. Martin‐Rendon E, Brunskill SJ, Hyde CJ, et al. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–1818. [DOI] [PubMed] [Google Scholar]
- 50. Zhang S, Sun A, Xu D, et al. Impact of timing on efficacy and safety of intracoronary autologous bone marrow stem cells transplantation in acute myocardial infarction: a pooled subgroup analysis of randomized controlled trials. Clin Cardiol. 2009;32:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brunskill SJ, Hyde CJ, Doree CJ, et al. Route of delivery and baseline left ventricular ejection fraction, key factors of bone‐marrow‐derived cell therapy for ischaemic heart disease. Eur J Heart Fail. 2009;11:887–896. [DOI] [PubMed] [Google Scholar]
- 52. Jeevanatham V, Butler M, Saad A, et al. Adult bone marrow cell therapy improves survival and indices long‐term improvement in cardiac parameters: a systematic review and meta‐analysis. Circulation. 2012;126:551–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Delewi R, Andriessen A, Tijssen JG, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a meta‐analysis of randomised controlled clinical trials. Heart. 2013;99:225–232. [DOI] [PubMed] [Google Scholar]
- 54. Fisher SA, Brunskill SJ, Doree C, et al. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev. 2014;4:CD007888. [DOI] [PubMed] [Google Scholar]
- 55. Tian T, Chen B, Xiao Y, et al. Intramyocardial autologous bone marrow cell transplantation for ischemic heart disease: a systematic review and meta‐analysis of randomized controlled trials. Atherosclerosis. 2014;233:485–492. [DOI] [PubMed] [Google Scholar]
- 56. Nowbar AN, Mielewczik M, Karavassilis M, et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE) weighted regression and meta‐analysis. BMJ. 2014;348:g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Matsa E, Sallam K, Wu JC, et al. Cardiac stem cell biology: glimpse of the past, present, and future. Circ Res. 2014;114:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tongers J, Losordo DW, Landmesser U. Stem cell progenitor cell–based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J. 2011;32:1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Davis ME, Hsieh PC, Takahashi T, et al. Local myocardial insulin‐like growth factor 1 (IGF‐1) delivery with biotinylated peptic nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:8155–8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zimmermann WH, Melnychenko I, Wasmeier G, et al. Engineered heart‐tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. [DOI] [PubMed] [Google Scholar]
- 61. Terrovitis J, Lautamäki R, Bonios M, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intracardiac cell–derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ghanem A, Steingen C, Brenig F, et al. Focused ultrasound‐induced stimulation of microbubbles augments site‐targeted engraftment of mesenchymal stem cells after acute myocardial infarction. J Mol Cell Cardiol. 2009;47:411–418. [DOI] [PubMed] [Google Scholar]
- 63. Ward MR, Stewart DJ, Kutryk MJ. Endothelial progenitor cell therapy for the treatment of coronary disease, acute MI, and pulmonary arterial hypertension: current perspectives. Catheter Cardiovasc Interv. 2007;70:983–998. [DOI] [PubMed] [Google Scholar]
- 64. Menasché P. Cellular transplantation: hurdles remaining before widespread clinical use. Curr Opin Cardiol. 2004;19:154–161. [DOI] [PubMed] [Google Scholar]
- 65. Sakakibara Y, Nihimura K, Tambara K, et al. Prevascularization with gelatin microspheres containing basic fibroblast growth factor enhances the benefits of cardiomyocyte transplantation. J Thorac Cardiovasc Surg. 2002;124:50–56. [DOI] [PubMed] [Google Scholar]
- 66. Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. [DOI] [PubMed] [Google Scholar]
- 67. Balsam LB, Wagers AJ, Christensen JL, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. [DOI] [PubMed] [Google Scholar]
- 68. Pavo N, Charwat S, Nyolczas N, et al. Cell therapy for human ischemic heart diseases: critical review and summary of the clinical experiences. J Mol Cell Cardiol. 2014;75:12–24. [DOI] [PubMed] [Google Scholar]
- 69. Francis DP, Mielewczik M, Zargaran D, et al. Autologous bone marrow–derived stem cell therapy in heart disease: discrepancies and contradictions. Int J Cardiol. 2013;168:3381–3403. [DOI] [PubMed] [Google Scholar]


