ABSTRACT
Background
Many factors are associated with no‐reflow (NRF) phenomenon in ST‐segment elevation myocardial infarction (STEMI), including plasma glucose, age, and pre–percutaneous coronary intervention (PCI) thrombus score. Initial clinical assessment would benefit from accurate NRF prediction. This study aimed to develop a simple scoring system to predict the risk of NRF in patients undergoing primary PCI with STEMI.
Methods
Baseline clinical and procedural variables were used for risk score development (the training dataset, n = 912) and validation (the test dataset, n = 864). Independent predictors of NRF from the multivariable model were assigned integer weights based on their coefficients and incorporated into a risk score. The discriminant ability of the score was tested by receiver operating characteristic analysis using the test dataset.
Results
The final model included 7 significant variables, which were age, pain‐to‐PCI time, neutrophil count, admission plasma glucose level, pre‐PCI thrombus score, collateral circulation, and Killip class. All these variables were then used to build a risk score in terms of the prediction of NRF. Receiver operating characteristic analysis demonstrated good risk prediction with a c statistic of 0.800 (95% confidence interval: 0.772‐0.826) in the test dataset.
Conclusions
In patients with STEMI treated by primary PCI, incidence of NRF phenomenon may be predicted with an acceptable accuracy based on a 7‐item simplified risk score.
Introduction
Primary percutaneous coronary intervention (PCI) has been shown to be the most effective reperfusion strategy in the treatment of acute myocardial infarction (AMI).1 Brisk Thrombolysis In Myocardial Infarction (TIMI) grade‐3 flow immediately after PCI in AMI is related to improved clinical outcomes.2 However, a sizable number of patients fail to restore optimal myocardial reperfusion, mostly because of no‐reflow (NRF) phenomenon. No‐reflow phenomenon is defined as suboptimal myocardial reperfusion through a part of coronary circulation without angiographic evidence of mechanical vessel obstruction.3 This phenomenon has been documented in >30% of AMI patients after thrombolysis or primary mechanical intervention.4 Also, in patients with ST‐segment elevation myocardial infarction (STEMI) treated by primary PCI, the NRF phenomenon is a strong predictor of both short‐term and long‐term mortality.5 Compared with those with adequate reflow, patients with NRF phenomenon tend to have higher incidences of death, myocardial infarction, and heart failure.6, 7 The etiology of NRF is not yet fully understood, but it is assumed to be of multifactorial origin. Previous studies have identified several factors associated with NRF phenomenon, including plasma glucose, age, and pre‐PCI thrombus score.8, 9, 10, 11 However, there is no screening tool to predict individual risk of developing NRF and to help health professionals make a decision on further intervention and select eligible patients into protocols focusing on mechanism and therapy of NRF. Therefore, we developed a score based on weighted clinical and angiographic characteristics to predict risk of NRF in patients with STEMI.
Methods
Study Population
We prospectively recruited patients age >18 years who had a STEMI of <15 hours' duration from onset of symptoms until arrival at the department of cardiology in 3 participating institutions. Those who had ≥1 of the following factors were excluded from this study: (1) patients undergoing coronary artery bypass grafting; (2) patients with mechanical complications (ventricular free wall rupture, ventricular septal rupture, and papillary muscle rupture with severe mitral regurgitation); and (3) patients undergoing thrombolysis. All patients signed the written informed consent. The study was approved by the University Human Ethics Committee.
Clinical Data Collection
Blood samples, including plasma glucose, white blood cell count, creatine kinase (CK), and cardiac troponins, were required to be obtained on admission. Detailed clinical data were obtained, including age, sex, Killip class on hospital admission, time to hospital admission, and medication before AMI. A 12‐lead electrocardiogram was acquired at the time of presentation.
Definitions
ST‐segment elevation myocardial infarction was defined as chest pain suggestive of myocardial ischemia for ≥30 minutes within the previous 15 hours, accompanied by >1‐mm (0.1‐mV) ST‐segment elevation in ≥2 contiguous leads and later confirmed by CK and CK‐MB increases and/or troponin increase. Cardiac symptoms lasting >30 minutes that occurred within 48 hours before the onset of infarction were defined as preinfarction angina. Severity of heart failure was assessed according to the Killip classification. Anterograde coronary flow in the infarct‐related artery (IRA) was graded based on the TIMI grading system.12 Thrombus score was graded as previously described by the TIMI Study Group.13 Myocardial blush grade was assigned as described by van 't Hof et al.14 Angiographic NRF can be defined as a TIMI flow grade <3 or 3 plus a myocardial blush grade 0–1.15
Primary Percutanous Coronary Intervention Procedure and Angiographic Analysis
Patients were pretreated with oral 300 mg aspirin, oral 300 mg clopidogrel, and intravenous (IV) 5000 IU unfractionated heparin. Use of a glycoprotein IIb/IIIa inhibitor (tirofiban, 10 µg/kg bolus followed by 0.15 µg/kg/min IV infusion) was left to the discretion of the primary operator. Primary PCI, including balloon predilatation and stent implantation, was performed only for IRA with TIMI flow grade ≤2. Intracoronary stents were implanted as a final treatment option when the anatomy was suitable. Low‐molecular‐weight heparin was injected subcutaneously in the subsequent 72 hours. The quantitative angiographic assessment was done by 2 independent observers on a validated quantitative coronary angiographic system, CMS 4.0 (Medis, Leiden, the Netherlands). Clinical investigators did not participate in the angiographic assessment.
Statistical Analysis
The dataset was randomly divided into 2 datasets using a computer‐generated random number: the training dataset for model development and the test dataset for validation of the developed NRF risk score.
In the training dataset, associations between each variable and NRF were first tested using univariate analysis. Clinical variables considered for entry into the multivariate predictive model included age, sex, and smoking status; presence/absence of diabetes mellitus, hypertension, preinfarction angina, and/or prior PCI; systolic blood pressure, diastolic blood pressure, heart rate, admission plasma glucose, CK‐MB, admission neutrophil counts, and B‐type natriuretic peptide; and time interval from pain to PCI, medication before AMI (aspirin, angiotensin‐converting enzyme inhibitors, statins, calcium channel blockers, and β‐blockers), and Killip classes. Also, the following angiographic variables were involved: IRA, initial TIMI flow grade, number of narrowed coronary arteries, stent diameter, total stent length, administration of tirofiban, use of aspiration thrombectomy, pre‐PCI thrombus score, collateral circulation grade, maximal inflation pressure, and the type of intervention. Receiver operating characteristic (ROC) curves were employed to identify the best cutoff point of each continuous variable in terms of the capability of prediction of the future incidence of NRF. The variables tested included age, time interval from pain to PCI, concentration of glucose, high‐sensitivity C‐reactive protein, neutrophil counts, and B‐type natriuretic peptide, each of which was then transformed into a binomial categorical variable based on the cutoff point identified.
Multiple backward stepwise logistic regression was performed to identify the significant predictors with regard to NRF after primary PCI. Variables with a significant association (P < 0.1) with NRF based on univariate analyses were entered into a multivariate model. All the significant variables were then used to establish a clinical score system. The individual score for each variable was calculated based on its regression coefficient in the final step of the multiple logistic regression model. Each individual score was yielded via a linear transformation of the corresponding β regression coefficient, which was divided by 0.439 (the lowest β value, corresponding to collateral circulation grade ≤1), multiplied by a constant (2), and then rounded to the nearest integer. The overall score of a patient was the sum of all individual scores.
Validation of the risk score was performed using the test dataset. The discriminating properties of the NRF prediction model were investigated by calculating the area under a ROC curve. The ROC curve reflects the relationship between the sensitivity and the 1‐specificity. The area under the curve (AUC) shows that to what extent this model could distinguish the patients with NRF from those with normal blood flow. The larger AUC means the better ability of prediction regarding the risk of NRF incidence. Based on the ROC curve, the following values were calculated: the sensitivity, specificity, positive predictive value, and negative predictive value. The selection of the optimal cutpoint was based on the Youden index, the maximum sum of sensitivity and specificity.
Statistical analyses were performed using SPSS 18 (SPSS Inc., Chicago, IL) and/or MedCalc 9 (MedCalc Software, Mariakerke, Belgium). All P values reported are 2‐tailed, and P < 0.05 was considered to be statistically significant.
Results
Between January 2007 and January 2011, 2017 patients with STEMI were admitted and assessed with regard to the eligibility of primary PCI. Among them, 241 were not eligible for the study protocol. After the primary PCI procedure, 367 of 1776 patients (20.7%) suffered from a TIMI flow grade ≤2 (NRF group). The training dataset and the test dataset contained 912 (NRF 22.3%) and 864 (NRF 19.0%) patients, respectively. Admission clinical characteristics and angiographic and PCI characteristics of the 2 sets are detailed in Table 1 and Table 2.
Table 1.
Clinical Characteristics of Patients in the Training and Test Datasets
| Characteristics | Training Dataset, n = 912 | Test Dataset, n = 864 | Total, N = 1776 |
|---|---|---|---|
| Age, y | 62.3 ± 11.8 | 59.6 ± 12.1 | 61.1 ± 12.1 |
| Age ≥55 years | 572 (62.7) | 566 (65.5) | 1138 (64.1) |
| Male sex | 682 (74.8) | 720 (83.3) | 1402 (78.9) |
| Hypertension | 485 (53.2) | 475 (54.9) | 960 (54.1) |
| DM | 221 (24.2) | 231 (26.7) | 452 (25.5) |
| Smoking status | |||
| Current smoker | 378 (41.4) | 191 (22.1) | 569 (32.0) |
| Ex‐smoker | 79 (8.7) | 45 (5.2) | 124 (7.0) |
| Never smoked | 455 (49.9) | 628 (72.7) | 1083 (61.0) |
| Prior PCI | 63 (6.9) | 56 (6.5) | 119 (6.7) |
| Preinfarction angina | 468 (51.3) | 505 (58.4) | 973 (54.8) |
| Medication before MI | |||
| ASA | 158 (17.3) | 70 (8.1) | 228 (12.8) |
| ACEI | 56 (6.1) | 106 (12.3) | 162 (9.1) |
| β‐Blocker | 151 (16.6) | 96 (11.1) | 247 (13.9) |
| CCB | 257 (28.2) | 237 (27.4) | 494 (27.8) |
| Statin | 43 (4.7) | 40 (4.6) | 83 (4.7) |
| Pain‐to‐PCI time, h | 7.4 ± 6.4 | 7.1 ± 6.0 | 7.2 ± 6.3 |
| Pain‐to‐PCI time ≥4 h | 415 (45.5) | 324 (37.5) | 739 (41.6) |
| Physical findings on admission | |||
| SBP, mm Hg | 118 ± 25 | 121 ± 23 | 119 ± 24 |
| DBP, mm Hg | 69 ± 15 | 73 ± 14 | 71 ± 16 |
| Heart rate, bpm | 76 ± 18 | 76 ± 17 | 76 ± 18 |
| Plasma glucose, mmol/L | 10.3 ± 4.8 | 9.4 ± 4.2 | 9.9 ± 4.6 |
| Plasma glucose ≥12.0, mmol/L | 119 (13.0) | 112 (12.9) | 231 (13.0) |
| BNP, mmol/L | 576.2 ± 1.8 | 537.8 ± 1.7 | 567.8 ± 1.7 |
| BNP ≥250, mmol/L | 769 (84.3) | 706 (81.7) | 1475 (83.1) |
| Neutrophil count, 109/counts/L | 9.56 ± 3.18 | 9.45 ± 2.89 | 9.50 ± 3.02 |
| Neutrophil count ≥8.81, 109/counts/L | 328 (36.0) | 291 (33.7) | 619 (34.9) |
| CK‐MB, U/L | 95 ± 113 | 87 ± 107 | 92 ± 109 |
| Killip class | |||
| 1 | 422 (46.3) | 446 (51.6) | 868 (48.9) |
| 2 | 380 (41.7) | 396 (45.8) | 776 (43.7) |
| 3 | 32 (3.5) | 31 (3.6) | 63 (3.5) |
| 4 | 36 (3.9) | 33 (3.8) | 69 (3.9) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ASA, aspirin; BNP, B‐type natriuretic peptide; CCB, calcium channel blocker; CK‐MB, creatine kinase‐MB; DBP, diastolic blood pressure; DM, diabetes mellitus; MI, myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SD, standard deviation.
Data are presented as mean ± SD or n (%).
Table 2.
Angiographic and PCI Characteristics of Patients in the Training and Test Datasets
| Characteristics | Training Dataset, n = 912 | Test Dataset, n = 864 | Total, n = 1776 |
|---|---|---|---|
| NRF | 203 (22.3) | 164 (19.0) | 367 (20.7) |
| IRA | |||
| LM | 9 (1.0) | 6 (0.7) | 15 (0.8) |
| LAD | 410 (45.0) | 407 (47.1) | 817 (46.0) |
| LCX | 124 (13.6) | 153 (17.7) | 277 (15.6) |
| RCA | 362 (39.7) | 305 (35.3) | 667 (37.6) |
| No. of narrowed coronary arteries | |||
| 1 | 186 (20.4) | 282 (32.6) | 468 (26.4) |
| 2 | 236 (25.9) | 327 (37.8) | 563 (31.7) |
| 3 | 489 (53.6) | 256 (29.6) | 745 (41.9) |
| Initial TIMI flow | |||
| 0 | 599 (65.7) | 524 (60.6) | 1123 (63.2) |
| 1 | 23 (2.5) | 42 (4.9) | 65 (3.7) |
| 2 | 57 (6.3) | 55 (6.4) | 112 (6.3) |
| 3 | 231 (25.3) | 245 (28.4) | 476 (26.8) |
| Pre‐PCI thrombus score | |||
| 0–1 | 190 (20.8) | 209 (24.2) | 399 (22.5) |
| 2 | 30 (3.3) | 83 (9.6) | 113 (6.4) |
| 3 | 38 (4.2) | 303 (35.1) | 341 (19.2) |
| 4 | 41 (4.5) | 72 (8.3) | 113 (6.4) |
| 5 | 611 (67.0) | 199 (23.0) | 810 (45.6) |
| Collateral circulation | |||
| 0–1 | 757 (83.0) | 671 (77.7) | 1428 (80.4) |
| 2 | 124 (13.6) | 156 (18.1) | 280 (15.8) |
| 3 | 29 (3.2) | 39 (4.5) | 68 (3.8) |
| Type of intervention | |||
| Stenting | 870 (95.4) | 826 (95.6) | 1696 (95.5) |
| Balloon angioplasty | 42 (4.6) | 38 (4.4) | 80 (4.5) |
| Maximal inflation pressure, atm | 15.7 ± 3.4 | 15.9 ± 3.3 | 15.8 ± 3.3 |
| Stent diameter, mm | 3.02 ± 0.71 | 3.09 ± 0.76 | 3.08 ± 0.72 |
| Total stent length, mm | 24.7 ± 6.3 | 24.8 ± 6.4 | 24.6 ± 6.4 |
| Tirofiban usage (only tirofiban in China) | 301 (33.0) | 252 (29.2) | 553 (31.1) |
| Aspiration thrombectomy | 496 (54.4) | 387 (44.8) | 883 (49.7) |
Abbreviations: IRA, infarct‐related artery; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main artery; NRF, no reflow; PCI, percutaneous coronary intervention; RCA, right coronary artery; SD, standard deviation; TIMI, Thrombolysis In Myocardial Infarction grade.
Data are presented as mean ± SD or n (%).
The results of multiple stepwise logistic regression analysis showed that 7 major predictors highly significantly correlated with the incidence of NRF: age (≥55 years; odds ratio [OR]: 3.15, 95% confidence interval [CI]: 2.23‐4.44, P < 0.001), prolonged pain‐to‐PCI time (≥4 hours; OR: 1.69, 95% CI: 1.25‐2.28, P = 0.001), high neutrophil count (≥8.81 × 109/L; OR: 6.36, 95% CI: 4.66‐8.69, P < 0.001), high admission plasma glucose level (≥12 mmol/L; OR: 2.03, 95% CI: 1.38‐3.02, P < 0.001), high thrombus burden (a pre‐PCI thrombus score of ≥2; OR: 3.23, 95% CI: 2.06‐5.06, P < 0.001), low grading of collateral circulation (≤1; OR: 1.55, 95% CI: 1.03‐2.34, P = 0.037), and high Killip class (4; OR: 1.87, 95% CI: 1.30‐2.69, P = 0.001). These identified predictors were then used to build a clinical score system regarding the prediction of postprocedural NRF. The overall score for a patient ranged from 0 to 29 (Table 3).
Table 3.
Multivariate Analysis for NRF and Scoring Systema
| Variablesb | OR (95% CI) | P Value | β‐Coefficient | Risk Scorec |
|---|---|---|---|---|
| Age ≥55 years | ||||
| Yes | 3.15 (2.23‐4.44) | <0.001 | 1.147 | 5 |
| No | 0 | |||
| Pain‐to‐PCI time ≥4 h | ||||
| Yes | 1.69 (1.25‐2.28) | 0.001 | 0.522 | 2 |
| No | 0 | |||
| Admission plasma glucose ≥12.0 mmol/L | ||||
| Yes | 2.03 (1.38‐3.02) | <0.001 | 0.712 | 4 |
| No | 0 | |||
| Neutrophil count ≥8.81 109/counts/L | ||||
| Yes | 6.36 (4.66‐8.69) | <0.001 | 1.851 | 8 |
| No | 0 | |||
| Killip class 4 | ||||
| Yes | 1.87 (1.30‐2.69) | 0.001 | 0.626 | 3 |
| No | 0 | |||
| Pre‐PCI thrombus score ≥2 | ||||
| Yes | 3.23 (2.06‐5.06) | <0.001 | 1.173 | 5 |
| No | 0 | |||
| Collateral circulation grade ≤1 | ||||
| Yes | 1.55 (1.03‐2.34) | 0.037 | 0.439 | 2 |
| No | 0 |
Abbreviations: BNP, B‐type natriuretic peptide; CI, confidence interval; DBP, diastolic blood pressure; hsCRP, high‐sensitivity C‐reactive protein; IRA, infarct‐related artery; NRF, no reflow; OR, odds ratio; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; TIMI, Thrombolysis In Myocardial Infarction grade.
The backward stepwise multiple logistic regression was used.
Only the variables entering the last step of stepwise logistic regression were shown in the table. Basically, variables entering the multiple logistic regression model included sex, age, smoking status, preinfarction angina, pain‐to‐PCI time, SBP, DBP, hsCRP, BNP, fasting plasma glucose, neutrophil count, IRA, β‐blocker usage, maximal inflation pressure, initial TIMI flow, pre‐PCI thrombus score, collateral circulation grade, tirofiban usage, and aspiration thrombectomy.
Assignment of scores to risk factors was based on a linear transformation of the corresponding β regression coefficient. The coefficient of each variable was divided by 0.439 (the lowest β value, corresponding to collateral circulation grade ≤1), multiplied by a constant (2), and rounded to the nearest integer.
The validity of the NRF score was then tested in the other half of the study population (n = 864). The optimal cutoff score was ≥14 (sensitivity 76.14%, specificity 70.78%; Table 4). The positive predictive value and negative predictive value for the cutoff score of ≥14 were 43.8% and 90.8%, respectively. The ROC curve for the weighted score showed good discriminant power with a c statistic of 0.800 (95% CI: 0.772‐0.826; Figure 1).
Table 4.
Sensitivity and Specificity of Each Cutoff Value for the Risk Score for NRF in the Test Dataset
| Cutoff Value | No. (%)a | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| 10 | 7 (8.1) | 88.32 | 50.3 | 34.6 | 97.5 |
| 11 | 1 (14.3) | 87.31 | 52.11 | 35.3 | 93.2 |
| 12 | 20 (16.0) | 77.16 | 67.92 | 41.8 | 90.9 |
| 13 | 1 (7.7) | 76.65 | 69.73 | 43.1 | 90.9 |
| 14b | 1 (12.5) | 76.14 | 70.78 | 43.8 | 90.8 |
| 15 | 34 (8.9) | 58.88 | 84.19 | 52.7 | 87.3 |
| 16 | 4 (13.3) | 56.85 | 88.1 | 58.8 | 87.3 |
| 17 | 1 (33.3) | 56.85 | 88.55 | 60.5 | 87.4 |
| 18 | 12 (57.1) | 50.76 | 89.91 | 60.3 | 85.9 |
| 19 | 11 (61.1) | 45.18 | 90.96 | 60.0 | 84.8 |
| 20 | 49 (50.0) | 20.81 | 98.34 | 78.5 | 80.6 |
| 21 | 3 (100) | 19.29 | 98.34 | 77.2 | 80.3 |
| 22 | 2 (100) | 19.29 | 98.64 | 80.5 | 80.4 |
| 23 | 14 (82.4) | 12.18 | 99.1 | 80.2 | 79.0 |
| 24 | 15 (75) | 5.08 | 99.85 | 88.4 | 77.8 |
| 25 | 1 (100) | 5.08 | 100 | 100 | 77.9 |
Abbreviations: NPV, negative predictive value; NRF, no reflow; PPV, positive predictive value.
Incidence of NRF for each level of the score.
Optimal cutoff point.
Figure 1.
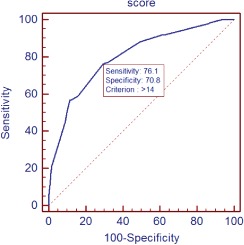
ROC curve for the NRF risk model in the test dataset. Abbreviations: NRF, no reflow; ROC, receiver operating characteristic.
Discussion
No‐reflow phenomenon is by far an important issue in the treatment of AMI patients using PCI techniques. In this study, based on multiple logistic regression, 7 significant indicators were located and were then used to build a clinical score system that could be used to identify patients at high risk of NRF. Based on the score system, clinical staff would be able to readily and rapidly identify those who might require further care, such as using thrombus aspiration techniques and implanting embolic protection devices, all of which are either expensive or unnecessary for low‐risk patients in the clinical practice but would be beneficial to those identified. We also tested the accuracy of this newly built score system in STEMI patients and found that, with clinical and procedural variables, its efficacy is good in predicting postprocedural NRF (AUC = 0.800).
In the present study, in terms of the significance in the predication, the order of the 7 indicators are level of neutrophil at admission, pre‐PCI thrombus score, age, random blood glucose levels, Killip class, pain‐to‐PCI time, and collateral circulation grade. In agreement with Takahashi et al, who reported association of neutrophil counts on admission with microvascular injury in AMI patients treated with PCI,16 we found that neutrophil level had a strong independently predictive effect. Patients in the highest quartile of neutrophil count were nearly 3× more likely to experience post‐PCI NRF than were those in the lowest quartile. Various mechanisms have been described to explain why, in patients with AMI, worse TIMI flow significantly correlates to elevated levels of neutrophils. Advances in basic science have now highlighted that ischemia‐reperfusion injury is essential to the pathophysiology of NRF.17, 18 Neutrophils have been shown to play a causative role in reperfusion injury and are a major source of oxidants in hearts reperfused after prolonged ischemia.19 In addition, neutrophil recruitment and activation at the site of endothelial damage is considered an initial event in thrombus formation. Neutrophil‐derived tissue factors are associated with hypercoagulability and reduce probability of early successful reperfusion.20, 21 The benefits of clopidogrel in improving IRA patency following fibrinolysis is also shown to be attenuated in STEMI patients with an elevated baseline neutrophil count.22 These findings lend further support for therapies targeting both platelets and leukocytes in STEMI.
Both collateral circulation and thrombus burden are important angiographic predisposing factors of the outcome of PCI. Yip et al found that presence of accumulated thrombus is an independent predictor of postprocedural NRF in patients undergoing PCI.8 Results in our study support this finding. The coronary collateral circulation, as an alternative source of blood supply, has been shown to be beneficial with regard to several clinical endpoints in AMI patients.23 Well‐developed collaterals tend to have a protective effect on coronary microcirculation and may lead to less NRF in patients with STEMI. In this study, collateral circulation was also an independent angiographic predictor of the incidence of NRF. Therefore, attentive evaluation of collateral circulation and thrombus burden during PCI procedure, especially before intervention, is highly recommended.
In the present study, proportion of subjects with TIMI grade 3 flow (476, 26.8%) is higher than that in some previous studies, such as Primary Angioplasty in Myocardial Infarction (PAMI) trials, where TIMI‐3 flow was present at initial angiography (spontaneous reperfusion) in 16% of patients.24 No baseline demographic characteristics were confirmed as predictors in terms of spontaneous reperfusion. We note that, however, more patients received preprocedural clopidogrel in addition to aspirin and unfractionated heparin in the emergency department than the participants in PAMI did. This may partly explain the higher initial TIMI‐3 rate.
Selection of an optimal cutoff point largely depends on the purpose of utilizing the risk score and needs to balance its sensitivity and specificity. When it comes to the prediction of post‐PCI NRF, which is associated with mortality and great expense, our purpose is to identify more potentially risky patients and higher sensitivity is preferred. Therefore, a score ≥14 was used as the cutoff point, and the sensitivity and the specificity were 76.1% and 70.8%, respectively.
Study Limitations
Although this study is a carefully designed multicenter study with a large sample, it has some limitations. First, the predictive performance of the score was tested on the same population in which the risk score was developed. Its predictive efficacy needs to be further tested in a well‐designed large‐scale prospective study. Second, tirofiban was the only formulation of glycoprotein IIb/IIIa inhibitor approved by China's State Food and Drug Administration (SFDA). Bivalirudin was not administered in the present study, and this may have influenced the outcomes. Thirdly, intravascular ultrasound could be useful tool in the prediction of postprocedural NRF after primary PCI.25 However, intravascular ultrasound has not been routinely adopted during PCI in patients with STEMI in China. This information would be added in our future studies.
Conclusion
We have developed a simple risk score for the prediction of NRF based on 7 variables: age, pain‐to‐PCI time, admission plasma glucose, neutrophil count, Killip class, pre‐PCI thrombus grade, and collateral circulation grade. Given the high risk of mortality of NRF, a risk score with excellent performance would help clinicians to identify high‐risk subjects requiring intensive treatment.
Acknowledgements
The authors thank Xiaoling Zhu for study management and Zhechun Zeng for statistical oversight.
Jin‐Wen Wang, MD, and Zi‐Qiang Zhou, MD, contributed equally to the study and are considered as co–first authors. The co–first authors made the greatest contribution to the article.
This study was supported by Beijing Municipal Science and Technology Commission (Z121107001012002).
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. [DOI] [PubMed] [Google Scholar]
- 2. Gibson CM, Cannon CP, Murphy SA, et al; TIMI Study Group . Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long‐term outcomes after thrombolytic administration in acute myocardial infarction. Circulation. 2002;105:1909–1913. [DOI] [PubMed] [Google Scholar]
- 3. Rezkalla SH, Kloner RA. Coronary no‐reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2008;72:950–957. [DOI] [PubMed] [Google Scholar]
- 4. Piana RN, Paik GY, Moscucci M, et al. Incidence and treatment of 'no‐reflow' after percutaneous coronary intervention. Circulation. 1994;89:2514–2518. [DOI] [PubMed] [Google Scholar]
- 5. Brosh D, Assali AR, Mager A, et al. Effect of no‐reflow during primary percutaneous coronary intervention for acute myocardial infarction on six‐month mortality. Am J Cardiol. 2007;99:442–445. [DOI] [PubMed] [Google Scholar]
- 6. Bolognese L, Carrabba N, Parodi G, et al. Impact of microvascular dysfunction on left ventricular remodeling and long‐term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109:1121–1126. [DOI] [PubMed] [Google Scholar]
- 7. Cura FA, L'Allier PL, Kapadia SR, et al. Predictors and prognosis of suboptimal coronary blood flow after primary coronary angioplasty in patients with acute myocardial infarction. Am J Cardiol. 2001;88:124–128. [DOI] [PubMed] [Google Scholar]
- 8. Yip HK, Chen MC, Chang HW, et al. Angiographic morphologic features of infarct‐related arteries and timely reperfusion in acute myocardial infarction: predictors of slow‐flow and no‐reflow phenomenon. Chest. 2002;122:1322–1332. [DOI] [PubMed] [Google Scholar]
- 9. Iwakura K, Ito H, Ikushima M, et al. Association between hyperglycemia and the no‐reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol. 2003;41:1–7. [DOI] [PubMed] [Google Scholar]
- 10. Kirma C, Izgi A, Dundar C, et al. Clinical and procedural predictors of no‐reflow phenomenon after primary percutaneous coronary interventions: experience at a single center. Circ J. 2008;72:716–721. [DOI] [PubMed] [Google Scholar]
- 11. Su Q, Li L, Liu Y. Short‐term effect of verapamil on coronary no‐reflow associated with percutaneous coronary intervention in patients with acute coronary syndrome: a systematic review and meta‐analysis of randomized controlled trials. Clin Cardiol. 2013;36:E11–E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. TIMI Study Group . The thrombolysis in myocardial infarction (TIMI) trial. N Engl J Med. 1985;312:932–936. [DOI] [PubMed] [Google Scholar]
- 13. Gibson CM, de Lemos JA, Murphy SA, et al. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 substudy. Circulation. 2001;103:2550–2554. [DOI] [PubMed] [Google Scholar]
- 14. van 't Hof AW, Liem A, Suryapranata H, et al; Zwolle Myocardial Infarction Study Group . Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Circulation. 1998;97:2302–2306. [DOI] [PubMed] [Google Scholar]
- 15. Niccoli G, Burzotta F, Galiuto L, et al. Myocardial no‐reflow in humans. J Am Coll Cardiol. 2009;54:281–292. [DOI] [PubMed] [Google Scholar]
- 16. Takahashi T, Hiasa Y, Ohara Y, et al. Relation between neutrophil counts on admission, microvascular injury, and left ventricular functional recovery in patients with an anterior wall first acute myocardial infarction treated with primary coronary angioplasty. Am J Cardiol. 2007;100:35–40. [DOI] [PubMed] [Google Scholar]
- 17. Turschner O, D'hooge J, Dommke C, et al. The sequential changes in myocardial thickness and thickening which occur during acute transmural infarction, infarct reperfusion and the resultant expression of reperfusion injury. Eur Heart J. 2004;25:794–803. [DOI] [PubMed] [Google Scholar]
- 18. Galiuto L, DeMaria AN, del Balzo U, et al. Ischemia‐reperfusion injury at the microvascular level: treatment by endothelin A‐selective antagonist and evaluation by myocardial contrast echocardiography. Circulation. 2000;102:3111–3116. [DOI] [PubMed] [Google Scholar]
- 19. Duilio C, Ambrosio G, Kuppusamy P, et al. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. Am J Physiol Heart Circ Physiol. 2001;280:H2649–H2657. [DOI] [PubMed] [Google Scholar]
- 20. Madjid M, Awan I, Willerson JT, et al. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44:1945–1956. [DOI] [PubMed] [Google Scholar]
- 21. Turner SJ, Ketch TR, Gandhi SK, et al. Routine hematologic clinical tests as prognostic markers in patients with acute coronary syndromes. Am Heart J. 2008;155:806–816. [DOI] [PubMed] [Google Scholar]
- 22. O'Donoghue M, Morrow DA, Cannon CP, et al. Association between baseline neutrophil count, clopidogrel therapy, and clinical and angiographic outcomes in patients with ST‐elevation myocardial infarction receiving fibrinolytic therapy. Eur Heart J. 2008;29:984–991. [DOI] [PubMed] [Google Scholar]
- 23. Desch S, Eitel I, Schmitt J, et al. Effect of coronary collaterals on microvascular obstruction as assessed by magnetic resonance imaging in patients with acute ST‐elevation myocardial infarction treated by primary coronary intervention. Am J Cardiol. 2009;104:1204–1209. [DOI] [PubMed] [Google Scholar]
- 24. Stone GW, Cox D, Garcia E, et al. Normal flow (TIMI‐3) before mechanical reperfusion therapy is an independent determinant of survival in acute myocardial infarction: analysis from the primary angioplasty in myocardial infarction trials. Circulation. 2001;104:636–641. [DOI] [PubMed] [Google Scholar]
- 25. Nakamura T, Kubo N, Ako J, et al. Angiographic no‐reflow phenomenon and plaque characteristics by virtual histology intravascular ultrasound in patients with acute myocardial infarction. J Interv Cardiol. 2007;20:335–339. [DOI] [PubMed] [Google Scholar]


