Abstract
Background
Guidelines recommend delaying coronary artery bypass grafting (CABG) for 5 days after discontinuing clopidogrel. However, platelet function may recover quicker in certain individuals.
Hypothesis
We hypothesized that perioperative measurement of platelet function with a point‐of‐care P2Y12 inhibitor assay could predict bleeding during CABG in patients exposed to clopidogrel.
Methods
Verify Pre‐Op TIMI 45 was a prospective pilot study of 39 patients on clopidogrel who subsequently underwent CABG. Preoperative on‐treatment platelet reactivity was assessed with VerifyNow P2Y12 Reaction Units (PRU), with higher PRU indicating more reactive platelets. Outcomes were stratified by PRU quartiles, as well as prespecified cutpoints for the lowest quartile (PRU 173), a cutpoint for major bleeding determined by the Youden index using receiver operator curve analysis (PRU 207), and clopidogrel resistance (PRU 230).
Results
Patients in higher PRU quartiles experienced smaller decreases in hemoglobin and hematocrit (P < 0.05 for all comparisons), less major bleeding (P = 0.021), and less major or minor bleeding (P = 0.003). Patients above the PRU 207 and 230 cutpoints had less chest‐tube output (P = 0.041 and P = 0.012, respectively), less major bleeding (P = 0.005 and P = 0.036, respectively), and less major or minor bleeding (P = 0.013 and P < 0.001, respectively). By receiver operator curve analysis, preoperative PRU ≤ 207 discriminated between patients with and without major bleeding during surgery (area under the curve: 0.76, 95% confidence interval: 0.59‐0.94, P = 0.018).
Conclusions
In this pilot study, we found that point‐of‐care platelet function assessment could predict bleeding in patients recently exposed to clopidogrel undergoing CABG.
Introduction
A substantial proportion of patients referred for coronary artery bypass grafting (CABG) are exposed to a P2Y12 inhibitor prior to surgery.1, 2, 3, 4 Given the association between P2Y12 inhibitor use and surgical bleeding, the American College of Cardiology Foundation and American Heart Association advise discontinuation of clopidogrel for ≥5 days prior to elective CABG (class I recommendation, level of evidence [LOE] B). These guidelines reflect data from studies that associate perioperative clopidogrel exposure with increased bleeding, blood‐product transfusion, reoperation, and longer hospitalization,1, 5, 6, 7, 8, 9, 10, 11 particularly if administered within 5 days prior to CABG.1, 5, 8, 9
However, the antiplatelet effect of clopidogrel is quite variable, and studies suggest platelet function may normalize in < 5 days in some individuals.12, 13, 14 Thus, certain patients may be able to undergo CABG prior to the recommended 5‐day waiting period without heightened bleeding risk. In this context, preoperative platelet function testing may confirm platelet function recovery and advise an optimal timing of surgery.
In recognition of this, guidelines from the Society of Thoracic Surgeons (STS) suggest that for patients on dual antiplatelet therapy, platelet function testing may be considered prior to CABG (class IIb, LOE B), and that it is reasonable to make decisions about surgical timing based on tests of platelet inhibition, rather than applying a prespecified period of surgical delay (class IIa, LOE B).15, 16 However, there are limited prospective data correlating any of the available bedside assays with perioperative bleeding. We therefore conducted a proof‐of‐principle study to determine whether preoperative point‐of‐care platelet function testing could predict bleeding in patients undergoing CABG, with an aim to establish a threshold of on‐treatment platelet reactivity (OTR) above which CABG could be performed with an acceptable bleeding risk.
Methods
Study Design
Verify Pre‐Op—TIMI 45 was a prospective pilot study of 39 patients who received clopidogrel, underwent coronary angiography, and were scheduled for on‐pump CABG. Patients were enrolled between April 11, 2006, and December 21, 2007, at 2 centers. Patients not on clopidogrel 75 mg daily for ≥3 days prior were loaded with clopidogrel 300 mg or 600 mg. All patients were also on aspirin 81 to 325 mg for ≥7 days prior to enrollment. Enrollment must have occurred within 12 hours of angiography, and blood samples were obtained daily from time of enrollment to 72 hours after surgery. Platelet function was assessed daily using the VerifyNow P2Y12 assay (Accumetrics, San Diego, CA). Results were reported as P2Y12 Reaction Units (PRU), with higher PRU indicating more reactive platelets. All providers were blinded to the results of the VerifyNow P2Y12 assay during the study. Timing of clopidogrel discontinuation and all aspects of preoperative and postoperative care (including timing of heparin discontinuation) were at the discretion of the surgeon. There were no specific exclusion criteria. Written informed consent was obtained from all patients, and the protocol was approved by the relevant institutional review boards.
Blood Sampling
Blood samples of 2 mL were collected via a 19‐gauge or larger needle in Vacuette partial‐fill blood‐collection tubes (Greiner Bio‐One, Monroe, NC) containing 3.2% sodium citrate following a specified discard (5 mL for indwelling catheters and 2 mL for peripheral draws) at time intervals specified per the study design. The sample immediately prior to CABG was obtained as close to the time of surgery as possible (either the morning of or the night prior to surgery). Blood was allowed to sit at room temperature for a minimum of 10 minutes but no longer than 4 hours after collection, as required by the VerifyNow P2Y12 assay.
The VerifyNow P2Y12 Assay
The VerifyNow P2Y12 assay is a point‐of‐care test that measures adenosine diphosphate (ADP)‐mediated platelet activation rapidly, and it is a sensitive measure of the antiplatelet effect of clopidogrel.17 Whole blood is exposed to 20 μM ADP, which activates the platelets, increasing expression of glycoprotein IIb/IIIa receptors, which crosslink by binding to fibrinogen‐coated microparticles. Light transmittance increases in proportion to the degree of microparticle‐platelet cross‐linking. The channel also contains 22 nM prostaglandin E1, which suppresses activation by P2Y1 receptors, allowing for a truer assessment of P2Y12 receptor response.
Endpoints
Primary endpoints were the absolute decrease and percentage decrease in hemoglobin (Hgb), hematocrit (HCT), and chest‐tube output within 24 hours. Secondary endpoints were major bleeding, minor bleeding, and the composite of any bleeding (major or minor) within 24 hours. Patients were also assessed for surgical outcomes within 24 hours, including number of packed red blood cell (PRBC) and platelet transfusions, periprocedural myocardial infarction (defined as increase of >5× the upper limit of normal creatine kinase–myocardial band), aprotonin use, protamine use, duration of surgery, time on cardiopulmonary bypass, time on mechanical ventilation, need for reoperation for bleeding, and mortality.
Change in Hgb or HCT was calculated by subtracting the first value available ≥24 hours after surgery from the last available value prior to CABG. The average time for the blood draw for this calculation was approximately 24 hours. Values were corrected for perioperative PRBC transfusions by subtracting 1 g/dL from the postoperative Hgb value and 3% from the postoperative HCT value for each unit transfused. Bleeding events were based on the Thrombolysis in Myocardial Infarction (TIMI) definitions of bleeding, with major bleeding defined as a clinically significant drop in Hgb of ≥5 g/dL or HCT ≥15%, or bleeding that resulted in death within 7 days. Minor bleeding was defined as hemorrhage resulting in a drop in Hgb of 3 to < 5 g/dL or HCT 9% to < 15%.
Statistical Analysis
In this pilot study, we planned to enroll approximately 40 patients, for approximately 10 patients per PRU quartile, with a goal to detect whether there was a signal of significance associating PRU with the primary endpoints. Forty‐two patients were enrolled, but 3 were excluded due to unknown laboratory difficulties with the PRU assay. Baseline characteristics and endpoints were analyzed for the 39 remaining patients by PRU quartiles, defined as follows: Q1, PRU 0 to 173 (n = 10); Q2, PRU 174 to 207 (n = 10); Q3, PRU 208 to 276 (n = 10); and Q4, PRU ≥277 (n = 9). One patient in Q1 was missing bleeding laboratory data due to an error filling out the case‐report form and was thus excluded from endpoint analyses. Patients were also stratified by the prespecified cutpoint for the lowest quartile (PRU ≤ 173) and the cutpoint for major bleeding as determined by the Youden index in the context of receiver operating characteristic (ROC) curve analysis (PRU ≤ 207). The cutpoint of PRU ≤ 230 was also investigated, as prior observational studies have correlated PRU ≥230 with adverse events after percutaneous coronary intervention (PCI).18, 19, 20 In addition, the relationship between PRU and bleeding outcome was examined by a restricted cubic spline method21 (see Supporting Figures 1A and 1B in the online version of this article).
Figure 1.
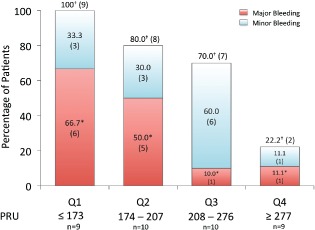
Bleeding outcomes specified by PRU quartiles. Values are depicted as percentage of group total with absolute number of patients in parentheses. * indicates statistical significance at P = 0.021; † indicates statistical significance at P = 0.0003. Abbreviations: PRU, P2Y12 Reaction Units; Q, quartile.
Data are presented as number and percentage for categorical variables and as mean and SD for continuous variables. Categorical variables were compared using Fisher exact tests and continuous variables by Wilcoxon rank‐sum tests. For continuous variables with >3 groups, the Kruskal‐Wallis test was applied. Statistical dependence of the primary endpoints on PRU was determined by Spearman rank correlation. Receiver operating characteristic curve analysis tested the ability of PRU values to discriminate between patients with and without bleeding events. The exact confidence intervals were calculated for sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).22 Optimal cutpoints were calculated by determining the values that provided the greatest Youden index, which is sum of sensitivity and specificity −1 at each observed value of PRU.23 Association between bleeding and PRU was also evaluated by logistic regression. The last PRU prior to CABG and the number of days post withdrawal of clopidogrel were also compared. Endpoints were also analyzed based on the number of days waited off of clopidogrel prior to CABG. A P value of < 0.05 was considered significant. Analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC). The funding agency had no role in data interpretation.
Results
Baseline Characteristics
Baseline characteristics are shown in Table 1. The study population had high rates of medical comorbidities and medication use. In most cases, the indication for catheterization was unstable angina or non–ST‐segment elevation myocardial infarction, and the majority had at least 3‐vessel coronary artery disease.
Table 1.
Baseline Characteristics of the Study Population (N = 39)
| Characteristic | Value |
|---|---|
| Age, y, mean (SD) | 64.6 (10.7) |
| BMI, kg/m2, mean (SD) | 31.6 (7.6) |
| EF, %, mean (SD) | 53 (13) |
| Male sex, n (%) | 31 (80) |
| Hypertension, n (%) | 32 (82) |
| Hyperlipidemia, n (%) | 35 (90) |
| Current smoker, n (%) | 6 (15) |
| DM, n (%) | 17 (44) |
| CKD, n (%) | 5 (13) |
| History of prior MI, n (%) | 17 (44) |
| History of CHF, n (%) | 7 (18) |
| Reason for catheterization, n (%) | |
| Stable angina | 3 (8) |
| UA or NSTEMI | 28 (72) |
| STEMI | 3 (8) |
| Coronary anatomy, n (%) | |
| Left‐main disease | 13 (33) |
| ≥3‐vessel disease | 25 (64) |
| 2‐vessel disease | 10 (26) |
| 1‐vessel disease | 4 (10) |
| Medications in prior 24 hours, n (%) | |
| Unfractionated heparin | 21 (54) |
| Enoxaparin | 5 (13) |
| Glycoprotein IIb/IIIa inhibitor | 6 (15) |
| β‐Blocker | 36 (93) |
| ACEI/ARB | 29 (76) |
| Statin | 20 (51) |
| Aspirin | 37 (95) |
| <324 mg | 6 (15) |
| ≥324 mg | 31 (80) |
| Laboratory parameters, mean (SD) | |
| BUN, mg/dL | 19 (7) |
| Cr, mg/dL | 1.2 (1.1) |
| PT, sec | 14.0 (1.5) |
| aPTT, sec | 44.6 (20.8) |
| Platelet count, thousands, mm3 | 238 (78) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; aPTT, activated partial thromboplastin time; ARB, angiotensin receptor blocker; BMI, body mass index; BUN, blood urea nitrogen; CHF, congestive heart failure; CKD, chronic kidney disease; Cr, creatinine; DM, diabetes mellitus; EF, ejection fraction; MI, myocardial infarction; NSTEMI, non–ST‐segment elevation myocardial infarction; PT, prothrombin time; SD, standard deviation; STEMI, ST‐segment elevation myocardial infarction; UA, unstable angina.
At enrollment, mean and median PRU for the study population were 221 (71) and 207 (173–277), respectively. Forty‐one percent of patients (n = 16) had PRU values ≥230 PRU at time of CABG. There was a small difference in platelet count in the PRU ≤ 207 group compared with the PRU >207 group (216 000 vs 263 000, respectively; P < 0.05). Likewise, there was a small difference in prothrombin time in patients with PRU ≤ 207 compared with PRU >207 (14.0 vs 13.6, P < 0.05). No other baseline characteristics differed among quartiles or by any cutpoint (see Supporting Information, Appendix, Table 1 in the online version of this article). Glycoprotein IIb/IIIa use did not appear to have a significant effect on PRU (P = 0.125 across quartiles).
Primary Endpoints
There was significantly less perioperative bleeding (during the 24 hours post‐surgery) across quartiles of higher OTR. Specifically, as PRU rose from Q1 to Q4, there was a smaller decrease in Hgb (P = 0.006), percentage decrease in Hgb (P = 0.019), decrease in HCT (P = 0.005), and percentage decrease in HCT (P = 0.023; Table 2).
Table 2.
Bleeding Outcomes Stratified by PRU Quartiles
| Outcome | Q1 ≤ 173 PRU (n = 9) | Q2 174–207 PRU (n = 10) | Q3 208–276 PRU (n = 10) | Q4 ≥ 277 PRU (n = 9) | Trend P Value |
|---|---|---|---|---|---|
| Hgb decrease, g/dL | 6.0 (2.2) | 4.8 (2.4) | 3.4 (1.2) | 2.6 (1.5) | 0.006a |
| Hgb percentage decrease | 41.6 (15.3) | 35.1 (18.0) | 26.3 (9.2) | 21.1 (10.9) | 0.019a |
| HCT decrease, % | 18.1 (6.1) | 13.8 (7.4) | 10.1 (4.0) | 7.5 (5.1) | 0.005a |
| HCT percentage decrease | 44.1 (14.8) | 35.7 (19.7) | 27.0 (10.2) | 21.4 (13.0) | 0.023a |
| CT output | 1236 (927) | 964 (489) | 665 (339) | 669 (238) | 0.213 |
Abbreviations: CT, chest tube; HCT, hematocrit; Hgb, hemoglobin; PRU, P2Y12 Reaction Units; Q, quartile; SD, standard deviation.
All values are given as mean (SD).
Indicates statistical significance at α < 0.05.
When split by cutpoints, there were significantly larger declines in Hgb and HCT below (compared with above) each of the PRU 230, 207, and 173 cutpoints (P < 0.05 for all comparisons; Table 3). Patients below the PRU 230 and 207 cutpoints had significantly greater chest‐tube output compared with patients above (P = 0.012 and P = 0.041, respectively; Table 3).
Table 3.
Bleeding Outcomes Stratified by PRU Cutpoint
| Outcome | ≤230 PRU (n = 22) | >230 PRU (n = 16) | ≤207 PRU (n = 19) | >207 PRU (n = 19) | ≤173 PRU (n = 9) | >173 PRU (n = 29) |
|---|---|---|---|---|---|---|
| Hgb decrease, g/dL | 5.1 (2.2)a | 2.8 (1.4)a | 5.3 (2.3)a | 3.0 (1.4)a | 6.0 (2.2)a | 3.6 (1.9)a |
| Hgb percentage decrease | 37.4 (15.7)a | 22.2 (9.9)a | 38.2 (16.7)a | 23.8 (10.1)a | 41.6 (15.3)b | 27.7 (14.1)b |
| HCT decrease, % | 15.2 (6.7)a | 8.5 (4.8)a | 15.9 (6.9)a | 8.9 (4.6)a | 18.1 (6.1)a | 10.6 (6.1)a |
| HCT percentage decrease | 38.6 (16.8)a | 23.0 (11.8)a | 39.7 (24.3)a | 24.3 (11.6)a | 44.1 (14.8)b | 28.3 (15.5)b |
| CT output | 1077 (685)b | 622 (220)b | 1093 (722)b | 678 (288)b | 1236 (927) | 777 (386) |
Abbreviations: CT, chest tube; HCT, hematocrit; Hgb, hemoglobin; PRU, P2Y12 Reaction Units; SD, standard deviation.
All values are given as mean (SD).
Indicates statistical significance at P < 0.01.
Indicates statistical significance at P < 0.05. Significance determined by Student t test within each PRU cutpoint group.
As a continuous variable, higher platelet reactivity (PRU) was inversely correlated with each major endpoint. Specifically, greater PRU was correlated with a smaller decrease in Hgb (Spearman rs = 0.54, P < 0.001), percentage decrease in Hgb (rs = 0.48, P = 0.002), decrease in HCT (rs = 0.54, P < 0.001), percentage decrease in HCT (rs = 0.47, P = 0.003), and chest‐tube output (rs = 0.37, P = 0.024).
Secondary Endpoints
Major and Minor Bleeding
Overall, 34.2% of patients (n = 13) experienced a major bleeding event and 68.4% (n = 26) experienced the composite of any bleeding event following surgery. Both major bleeding and composite major and minor bleeding were significantly lower across Q1 to Q4 in a stepwise fashion (P = 0.021 and P = 0.003, respectively; Figure 1). In total, 100% of patients in Q1 (PRU ≤ 173) had major or minor bleeding within 24 hours of surgery. In contrast, only 22.2% of patients in Q4 (PRU ≥277) experienced a bleeding event.
There were also significant differences in major bleeding observed at each of the PRU cutpoints (Figure 2). Specifically, 50% of patients with PRU ≤ 230 had a major bleed, compared with 12.5% of patients above (P = 0.036; Figure 2). Similarly, major bleeding occurred in 57.9% of patients with PRU ≤ 207, vs 10.5% in patients above (P = 0.005). Furthermore, 100% of patients with PRU ≤ 173 had a major or minor bleeding event, compared with 58.6% of patients with PRU >173 (P = 0.036).
Figure 2.
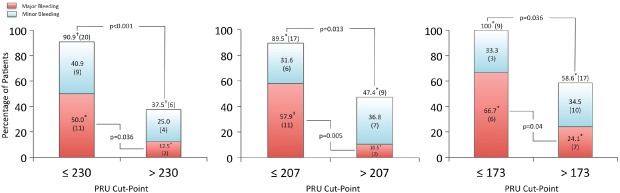
Bleeding outcomes stratified by PRU cutpoints. Values are depicted as percentage of group total with absolute number of patients underneath. * indicates statistical significance at P < 0.05; † indicates statistical significance at P < 0.001. Abbreviations: PRU, P2Y12 Reaction Units.
By ROC curve analysis, preoperative PRU assessment was effective at discriminating between patients who had major bleeding at the time of surgery from those did not (area under the curve [AUC]: 0.76, 95% confidence interval [CI]: 0.59‐0.94, P = 0.018). As above, the optimal cutoff for prediction of major bleeding was PRU ≤ 207. This cutpoint had an 85% sensitivity, 68% specificity, 58% PPV, and 89% NPV for major bleeding, with corresponding positive likelihood ratio of 2.64 (95% CI: 1.43‐4.90) and negative likelihood ratio of 0.23 (95% CI: 0.06‐0.83). The assay also discriminated between patients with any bleeding event (major or minor) from those without (AUC: 0.84, 95% CI: 0.70‐0.97, P = 0.005). The optimal PRU cutoff for the prediction of any bleeding event was ≤ 236, with a sensitivity of 81%, specificity of 83%, PPV of 91%, and NPV of 67%, with a corresponding positive likelihood ratio of 4.85 (95% CI: 1.35‐17.41) and negative likelihood ratio of 0.23 (95% CI: 0.10‐0.53).
By logistic regression, every 1‐unit increase in PRU decreased the odds of a major bleeding event within the first 24 hours by 1.5% (odds ratio [OR]: 0.985, 95% CI: 0.973‐0.997, P = 0.018, C statistic 0.763). Likewise, every 1‐unit increase in PRU decreased the odds of any bleeding event by 2.3% (OR: 0.977, 95% CI: 0.962‐0.993, P = 0.005, C statistic 0.837). For every 10‐point increase in PRU, the risk of a major bleeding was 14% lower and the risk of major or minor bleeding was 20.5% lower.
Excessive Chest‐Tube Output
Excessive chest‐tube output was defined as chest‐tube output within the top tertile, or >935 mL, within 24 hours. Similar to major bleeding, the optimal PRU value for prediction of excessive chest‐tube output was PRU ≤ 207 (AUC: 0.78, 95% CI: 0.63‐0.93, P = 0.0261), with a sensitivity of 77%, specificity of 64%, PPV 53%, and NPV 84%. Among patients with PRU ≤ 207, 58% (n = 11) had excessive chest‐tube output, vs 21% (n = 4) with PRU >207 (P = 0.045). By logistic regression, every 1‐unit increase in PRU decreased the odds of excessive chest‐tube output within the first 24 hours by 1.3% (OR: 0.987, 95% CI: 0.976‐0.998, P = 0.026).
Intraoperative Characteristics, Outcomes, and Blood‐Product Transfusions
The number of vessels bypassed was ≥3 for 33 (74%) patients, and 37 (95%) received a left internal mammary artery graft. Aminocaproic acid was given to 30 (77%) patients and aprotinin to 2 (5%). The average duration of cross‐clamp time was 88 ± 30 minutes, and the time on bypass was 113 ± 38 minutes.
There were few adverse events during surgery. There was 1 (3%) death following CABG, and 2 (5%) patients needed reoperation for bleeding. Only 3 (8%) patients had a periprocedural myocardial infarction, and 2 (5%) had renal failure requiring dialysis. There were no perioperative strokes. Mean time on mechanical ventilation was 12 ± 5 hours.
Regarding blood‐product use, 23 (59%) patients required ≥1 PRBC transfusion, and the mean number of transfusions was 1.7 ± 1.9 units. Ten (28%) patients required a platelet transfusion, and the mean number of platelet transfusions was 0.97 ± 2.5 bags.
Surgical outcomes and blood product transfusions did not significantly differ based on any PRU cutpoint (see Supporting Information, Appendix, Table 2 in the online version of this article).
Days Off Clopidogrel and Outcomes
The number of days off clopidogrel prior to CABG ranged from 1 to 9, with a median of 4 days. The rise in PRU for each day off clopidogrel was 17 ± 33 PRU. The number of days off clopidogrel tended to be associated with greater PRU, and a difference in PRU emerged in patients who held clopidogrel ≥5 days (n = 30) per guideline recommendations, compared with those who held it < 5 days (n = 9; 270 ± 69 vs 206 ± 82 PRU, P = 0.021).
Discussion
In this pilot study, we found that preoperative clopidogrel reactivity testing with the VerifyNow P2Y12 assay can predict bleeding within the first 24 hours after CABG. To the best of our knowledge, this is the first prospective study to use this point‐of‐care assay to determine a specific threshold of preoperative platelet function above which major bleeding may potentially be minimized. This information may allow for individualized determination of platelet recovery and advise optimal surgical timing for clopidogrel‐treated patients awaiting CABG.
By ROC analysis, a PRU threshold of ≤207 was the optimal threshold for discrimination of major bleeding during surgery, with 85% sensitivity and an 89% NPV. Rates of major bleeding were 58% vs 10.5% if PRU were ≤207 or >207, respectively (P = 0.013), and this cutpoint predicted excessive chest‐tube output as well. Additionally, PRU ≤ 236 was the optimal cutpoint for discrimination of any bleeding event (major or minor). This was not a prespecified cutpoint, so we did not include dichotomous analysis based on this PRU. However, this was remarkably close to the prespecified cutpoint for clopidogrel resistance, PRU 230. Furthermore, although PRU ≥277 was not a prespecified cutpoint, this did mark the highest PRU quartile, and only 22.2% of patients had either a major or minor bleeding event above this value (Figure 1). Putting this into practice, if a clinician wished to mitigate the risk of major bleeding and minimize excessive chest‐tube output, a PRU of 207 may suffice. Allowing PRU to rise above 236, or even above 277, would suggest a low risk of even minor bleeding. With this in mind, it may be reasonable to consider preoperative platelet transfusion in patients with an elevated PRU as a means of normalizing platelet function and expediting surgery, though this was not addressed by our study.24
Our findings support the 2012 STS guidelines, which recommend that point‐of‐care platelet function testing may be considered to inform the timing of surgery in patients on clopidogrel prior to CABG, rather than using a uniform waiting period of 5 days.16 In our study, the average increase in PRU was 17 units per day off clopidogrel. Each 10‐unit increase in PRU was associated with 14% lower risk of major bleeding and 20.5% lower risk of any bleeding. Interestingly, in this modest‐sized cohort, the number of days off clopidogrel did not predict bleeding, even though this has been reported in other studies. This is potentially because of individual PRU variability or may be due to the small sample size of our study. The PRU testing did predict outcomes, suggesting that platelet‐reactivity assessment may be a better way to minimize bleeding than simply waiting a fixed number of days.
Our results fit with prior studies that show the VerifyNow P2Y12 assay predicts bleeding in settings other than cardiac surgery. In patients undergoing PCI, one study found that the optimal cutpoint for bleeding complications within 30 days of PCI was PRU ≤ 178,25 and another post‐PCI study found that a PRU ≤ 189 was most predictive of 30‐day bleeding outcomes.26 As CABG constitutes major surgery, it is intuitive that the threshold for OTR needs to be higher to undergo CABG safely.
The VerifyNow P2Y12 assay also accurately assesses the antiplatelet effect of the newer, more potent antiplatelet agents prasugrel and ticagrelor.27, 28 We did not study patients treated with these, and although our data may be useful in guiding decisions on surgical delay in patients on these agents, further study is needed to determine this. Additionally, although our study was conducted in patients undergoing CABG, it seems reasonable that these results may be applicable to individuals undergoing other cardiac, vascular, or major surgeries.
It should be noted that Timing Based on Platelet Function Strategy to Reduce Clopidogrel‐Associated Bleeding Related to CABG (TARGET‐CABG) was the first study to utilize platelet function testing to guide decision‐making with regard to timing of CABG after discontinuation of clopidogrel. Clopidogrel response was assessed with thrombelastography ADP‐induced platelet‐fibrin clot strength (MAADP), and patients with MAADP values >50 mm, 35 to 50 mm, and < 35 mm were assigned to CABG within 1 day, 3 to 5 days, and 5 days after discontinuation of clopidogrel, respectively. Chest‐tube output and blood‐product transfusion did not differ between clopidogrel‐treated patients and clopidogrel‐naïve patients, regardless of days waited prior to CABG, indicating this may be a useful treatment strategy. The mean waiting time in clopidogrel‐treated patients was 2.7 days, ∼46% shorter than the waiting time recommended by the current guidelines. The current study builds on this concept, as we have identified a threshold of platelet reactivity using the VerifyNow P2Y12 assay above which major bleeding appears to be low, setting the stage for a larger, strategy‐based follow‐up study using this assay to guide surgical timing.
Our study does have certain limitations. As a pilot study, our sample size was admittedly small, and thus our results regarding PRU cutpoints should be considered hypothesis‐generating rather than definitive. Given our small sample size, we were underpowered to detect differences in surgical outcomes, including the effect of bleeding on ischemic events and mortality. However, our link of platelet testing to metrics of perioperative bleeding adds to other larger clinical studies that link major bleeding and chest‐tube output to worse outcomes following surgery. The small sample size may have overestimated the magnitude of differences in bleeding outcomes and limited our power to perform a more robust multivariable adjustment. Similarly, the PRU cutpoints for major and minor bleeding may be refined in a larger study with more power. Also, we did not correct for cell‐saver units “auto‐transfused” during surgery, as this is not actual blood “loss.” This definition may be why we did not detect differences in transfusions based on PRU. Additionally, we did not collect data on certain other factors that may have affected bleeding during surgery (such as urgency of case and European System for Cardiac Operative Risk Evaluation [EuroSCORE]), and nonstandardized surgical care may have biased results. Finally, we were limited in our clinical assessment of bleeding, which may overestimate the clinical importance of our results.29
Conclusion
In this study, we found that point‐of‐care platelet function testing could predict perioperative bleeding in patients exposed to clopidogrel. These results support the current STS guidelines, which suggest that the use of point‐of‐care platelet function testing in patients exposed to clopidogrel prior to CABG may advise optimal timing of surgery. As this was a pilot study, our results should be considered hypothesis‐generating rather than definitive. Larger studies are needed to confirm whether a strategy of surgical timing based on preoperative PRU assessment leads to an improvement in surgical outcomes.
Supporting information
Appendix S1. Online Appendix
The relationship of any bleeding event (major or minor) and PRU as a function of probability by restricted cubic spline method. The relationship between PRU and any bleeding event appears to be roughly linear (i.e. the log odds of probability of a major or minor bleeding event decreases as PRU increases.
The relationship between major bleeding and PRU as a function of probability by restricted cubic spline method. The relationship between PRU and major bleeding appears to be linear for most patients (33 subjects < =300 PRU), however, the linearity of the curve appears to change, indicating a possible threshold around 250 PRU. The modest sample size in this pilot study does not give us enough power to arrive any statistical conclusions of this non‐linearity, and thus this will need to be verified by a larger study.
Acknowledgments
The authors acknowledge the contributions of Jacki Buros for statistical assistance.
The study is supported by Accumetrics. Dr. Cannon reports research grants from Accumetrics, Arisaph, AstraZeneca, Boehringer‐Ingelheim, and Janssen; grants and personal fees from GlaxoSmithKline, Merck, and Takeda; and consulting fees from BMS, CSL Behring, Essentialis, Lipimedix, Pfizer, Regeneron, and Sanofi. Arvnid Agnihotri – Consultant (proctor) and Advisory Board, Edwards Lifesciences. Andrew O. Maree – Speakers bureau Daichi Sankyo and Boehringer Ingelheim, and Advisory Board, Abbott Vascular.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Mehta RH, Roe MT, Mulgund J, et al. Acute clopidogrel use and outcomes in patients with non–ST‐segment elevation acute coronary syndromes undergoing coronary artery bypass surgery. J Am Coll Cardiol. 2006;48:281–286. [DOI] [PubMed] [Google Scholar]
- 2. Nijjer SS, Watson G, Athanasiou T, et al. Safety of clopidogrel being continued until the time of coronary artery bypass grafting in patients with acute coronary syndrome: a meta‐analysis of 34 studies. Eur Heart J. 2011;32:2970–2988. [DOI] [PubMed] [Google Scholar]
- 3. Hillis LD, Smith PK, Anderson JL, et al. 2011. ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol . 2011;58:e123–e210. [DOI] [PubMed] [Google Scholar]
- 4. Thukkani AK, Fonarow GC, Cannon CP, et al. Get With the Guidelines Steering Committee and Investigators. Quality of care for patients with acute coronary syndromes as a function of hospital revascularization capability: insights from Get With the Guidelines‐CAD. Clin Cardiol. 2014;37:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berger JS, Frye CB, Harshaw Q, et al. Impact of clopidogrel in patients with acute coronary syndromes requiring coronary artery bypass surgery: a multicenter analysis. J Am Coll Cardiol. 2008;52:1693–1701. [DOI] [PubMed] [Google Scholar]
- 6. Herman CR, Buth KJ, Kent BA, et al. Clopidogrel increases blood transfusion and hemorrhagic complications in patients undergoing cardiac surgery. Ann Thorac Surg. 2010;89:397–402. [DOI] [PubMed] [Google Scholar]
- 7. Kremke M, Tang M, Bak M, et al. Antiplatelet therapy at the time of coronary artery bypass grafting: a multicentre cohort study. Eur J Cardiothorac Surg. 2013;44:e133–e140. [DOI] [PubMed] [Google Scholar]
- 8. Yusuf S, Zhao F, Mehta SR, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation [published corrections appear in N Engl J Med. 2001;345:1506 and 2001;345:1716]. N Engl J Med . 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 9. Ascione R, Ghosh A, Rogers CA, et al. In‐hospital patients exposed to clopidogrel before coronary artery bypass graft surgery: a word of caution. Ann Thorac Surg. 2005;79:1210–1216. [DOI] [PubMed] [Google Scholar]
- 10. Chu MW, Wilson SR, Novick RJ, et al. Does clopidogrel increase blood loss following coronary artery bypass surgery? Ann Thorac Surg. 2004;78:1536–1541. [DOI] [PubMed] [Google Scholar]
- 11. Kapetanakis EI, Medlam DA, Petro KR, et al. Effect of clopidogrel premedication in off‐pump cardiac surgery: are we forfeiting the benefits of reduced hemorrhagic sequelae? Circulation. 2006;113:1667–1674. [DOI] [PubMed] [Google Scholar]
- 12. Price MJ, Coleman JL, Steinhubl SR, et al. Onset and offset of platelet inhibition after high‐dose clopidogrel loading and standard daily therapy measured by a point‐of‐care assay in healthy volunteers. Am J Cardiol. 2006;98:681–684. [DOI] [PubMed] [Google Scholar]
- 13. Gurbel PA, Bliden KP, Butler K, et al. Randomized double‐blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor vs clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–2585. [DOI] [PubMed] [Google Scholar]
- 14. Price MJ, Teirstein PS. Dynamics of platelet functional recovery following a clopidogrel loading dose in healthy volunteers. Am J Cardiol. 2008;102:790–795. [DOI] [PubMed] [Google Scholar]
- 15. Ferraris VA, Brown JR, Despotis GJ, et al. Society of Thoracic Surgeons Blood Conservation Guideline Task Force. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg . 2011;91:944–982. [DOI] [PubMed] [Google Scholar]
- 16. Ferraris VA, Saha SP, Oestreich JH, et al. 2012 update to the Society of Thoracic Surgeons guideline on use of antiplatelet drugs in patients having cardiac and noncardiac operations. Ann Thorac Surg. 2012;94:1761–1781. [DOI] [PubMed] [Google Scholar]
- 17. Price MJ. Bedside evaluation of thienopyridine antiplatelet therapy. Circulation. 2009;119:2625–2632. [DOI] [PubMed] [Google Scholar]
- 18. Price MJ, Berger PB, Teirstein PS, et al. GRAVITAS Investigators. Standard‐ vs high‐dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial [published correction appears in JAMA. 2011;305:2174]. JAMA . 2011;305:1097–1105. [DOI] [PubMed] [Google Scholar]
- 19. Price MJ, Endemann S, Gollapudi RR, et al. Prognostic significance of post‐clopidogrel platelet reactivity assessed by a point‐of‐care assay on thrombotic events after drug‐eluting stent implantation. Eur Heart J. 2008;29:992–1000. [DOI] [PubMed] [Google Scholar]
- 20. Patti G, Nusca A, Mangiacapra F, et al. Point‐of‐care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention: results of the ARMYDA‐PRO (Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty–Platelet Reactivity Predicts Outcome) study. J Am Coll Cardiol. 2008;52:1128–1133. [DOI] [PubMed] [Google Scholar]
- 21. Devlin TF, Weeks BJ. Spline functions for logistic regression modeling In: Proceedings of the 11th Annual SAS Users Group International Conference. Cary, NC: SAS Institute, Inc; 1986:646–651. [Google Scholar]
- 22. Gardner MJ, Altman DG. Statistics with Confidence. London, UK: British Medical Journal Publishing Group; 1989. [Google Scholar]
- 23. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 24. Sharma S, Bhambi B. Use of platelet transfusion to facilitate surgery in patients on clopidogrel and aspirin therapy after drug‐eluting stent percutaneous coronary intervention. Catheter Cardiovasc Interv. 2012;79:498–500. [DOI] [PubMed] [Google Scholar]
- 25. Mangiacapra F, Patti G, Barbato E, et al. A therapeutic window for platelet reactivity for patients undergoing elective percutaneous coronary intervention: results of the ARMYDA‐PROVE (Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty–Platelet Reactivity for Outcome Validation Effort) study. JACC Cardiovasc Interv. 2012;5:281–289. [DOI] [PubMed] [Google Scholar]
- 26. Patti G, Pasceri V, Vizzi V, et al. Usefulness of platelet response to clopidogrel by point‐of‐care testing to predict bleeding outcomes in patients undergoing percutaneous coronary intervention (from the Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty–Bleeding Study). Am J Cardiol. 2011;107:995–1000. [DOI] [PubMed] [Google Scholar]
- 27. Varenhorst C, James S, Erlinge D, et al. Assessment of P2Y(12) inhibition with the point‐of‐care device VerifyNow P2Y12 in patients treated with prasugrel or clopidogrel coadministered with aspirin. Am Heart J . 2009;157:562e1–562e9. [DOI] [PubMed] [Google Scholar]
- 28. Gurbel PA, Bliden KP, Butler K, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation. 2010;121:1188–1199. [DOI] [PubMed] [Google Scholar]
- 29. Cannon CP, Harrington RA, James S, et al. Platelet Inhibition and Patient Outcomes Investigators. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double‐blind study. Lancet . 2010;375:283–293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Online Appendix
The relationship of any bleeding event (major or minor) and PRU as a function of probability by restricted cubic spline method. The relationship between PRU and any bleeding event appears to be roughly linear (i.e. the log odds of probability of a major or minor bleeding event decreases as PRU increases.
The relationship between major bleeding and PRU as a function of probability by restricted cubic spline method. The relationship between PRU and major bleeding appears to be linear for most patients (33 subjects < =300 PRU), however, the linearity of the curve appears to change, indicating a possible threshold around 250 PRU. The modest sample size in this pilot study does not give us enough power to arrive any statistical conclusions of this non‐linearity, and thus this will need to be verified by a larger study.


