ABSTRACT
Emerging evidence suggesting the possibility that interventions able to prevent cardiovascular disease (CVD) may also be effective in the prevention of cancer have recently stimulated great interest in the medical community. In particular, data from both experimental and observational studies have demonstrated that aspirin may play a role in preventing different types of cancer. Although the use of aspirin in the secondary prevention of CVD is well established, aspirin in primary prevention is not systematically recommended because the absolute cardiovascular event reduction is similar to the absolute excess in major bleedings. By adding to its cardiovascular prevention benefits, the potential beneficial effect of aspirin in reducing the incidence of mortality and cancer could tip the balance between risks and benefits of aspirin therapy in primary prevention in favor of the latter and broaden the indication for treatment with aspirin in populations at average risk. Prospective and randomized studies are currently investigating the effect of aspirin in prevention of both cancer and CVD; however, clinical efforts at the individual level to promote the use of aspirin in global (or total) primary prevention already could be made on the basis of a balanced evaluation of the benefit/risk ratio.
Introduction
The prevention of cardiovascular disease (CVD) has profoundly changed over the past 2 decades. It has crossed the boundaries of epidemiology and mere identification and impact of risk factors, as well as interventions based on more or less effective lifestyle modifications, and is steadily moving toward pharmacological interventions aimed at lowering high levels of blood pressure, blood glucose, cholesterol, and atherothrombotic burden.1
Because CVD and cancer are unanimously recognized as the principal causes of mortality and morbidity in most geographic areas and largely share the same cluster of risk factors,2 emerging evidence suggesting the possibility that interventions able to prevent CVD are also effective in the prevention of cancer have recently stimulated growing interest in the medical community.3
In particular, similarly to CVD,4, 5 smoking,6 unhealthy diet,7 harmful use of alcohol,8 physical inactivity,9, 10 low socioeconomic status,11, 12 and air pollution13, 14 are reported as risk factors for many types of cancer. Recently, Rasmussen‐Torvik et al showed that adherence to the 7 ideal health factors defined in the American Heart Association (AHA) 2020 goals—smoking, physical activity, obesity, dietary intake, total cholesterol, blood pressure, and blood sugar—is associated with lower cancer incidence.15 Therefore, it appears reasonable to postulate that targeted interventions to achieve changes of lifestyle can be effective, especially when combined, in reducing cardiovascular (CV) risk as well as the risk of cancer.
In addition to this, numerous recent studies have indicated that some drugs currently used for the prevention and treatment of CVD, such as aspirin, angiotensin‐converting enzyme inhibitors (ACEIs),16 angiotensin receptor blockers (ARBs),17, 18 β‐blockers,19 and statins,20, 21 may have a role in preventing cancer development. Thus, the question arises about whether a possibility does exist about achieving cancer prevention with the same pharmacological strategies used to prevent CVD.
Over the last years, several studies demonstrated the crucial role of inflammation not only in atherogenesis, but also in cancer development. Inflammation is thought to be a common and plausible mechanism through which risk factors may predispose to atherosclerosis and cancer development, and consequently may represent an important therapeutic target.22, 23, 24
To date, reports on ACEIs, ARBs, β‐blockers, and statins in cancer prevention are not uniform and mostly derived from animal studies. In humans, evidence is often derived from post‐hoc retrospective analysis. Prospective randomized clinical trials (RCTs) specifically designed to test cancer chemoprevention as a primary efficacy endpoint with adequate follow‐up would much better define the effects of these drugs on cancer prevention.
Data on the role of aspirin in cancer prevention are very encouraging. Hence, we will focus our discussion on the possible role of aspirin in cancer prevention, analyzing the main evidence in the literature, the mechanisms of action, and the overall balance of benefits and harms.
Aspirin and Cancer Prevention
The suggestion that aspirin could be of benefit against cancer initially arose from a small case‐control study of 700 patients with colorectal cancer (CRC).25 Subsequently, numerous experimental and epidemiological studies have described a role of aspirin and other nonsteroidal anti‐inflammatory drugs (NSAIDs) in preventing CRC, and also other types of cancer.26, 27
Cohort studies in patients with nonmetastatic CRC also reported a reduction in death from any cause and death from cancer in patients who began taking aspirin regularly after the diagnosis.28, 29
Evidence from prospective, placebo‐controlled RCTs is only available in patients with colon adenoma or previous CRC undergoing regular endoscopic follow‐up, in which low‐dose aspirin was demonstrated to significantly reduce the recurrence of adenoma.30, 31, 32
Important evidence for the beneficial role of aspirin in the prevention of cancer came also from the results of the meta‐analyses by Rothwell and colleagues.33, 34, 35, 36 This group of researchers, analyzing patients' data from RCTs of daily aspirin vs control, designed originally to evaluate prevention of vascular events, demonstrated that long‐term use (>5 years) of daily aspirin (75–300 mg/d) reduces incidence and mortality for CRC after a latent period of about 8 to 10 years.33
Subsequently, the same authors investigated the effects of daily aspirin on long‐term risk of death due to all cancers. They found that aspirin reduces 20‐year total cancer mortality by 20% to 30%; benefits were unrelated to aspirin dose (75 mg upward), but increased in relation to duration of treatment.34 In 2012, Rothwell and colleagues also examined the effects of aspirin on all major outcomes with duration of treatment. They showed that the reduced risk of major vascular events in patients on aspirin was initially offset by an increased risk of major bleeding, but effects on both of these outcomes diminished with increasing follow‐up, such that the reduced risk of cancer (absolute reduction: 3.13, 95% confidence interval [CI]: 1.44‐4.82 per 1000 patient‐years) is the only significant effect from 3 years onward.35 Furthermore, aspirin was shown to reduce the risk of metastases.36
Although compelling, the results obtained by Rothwell et al present some limitations that should be mentioned.37 Their meta‐analyses are based on clinical studies originally designed to evaluate CV endpoints; thus, the detection of cancer‐related endpoints may be less accurate than what would be possible in a randomized, controlled prospective study. Moreover, their meta‐analyses do not include data from 2 large RCTs on aspirin in primary prevention, which both failed to demonstrate a protective effect of aspirin on cancer development: the Women's Health Study (WHS), of 39 786 women treated with aspirin 100 mg every other day or placebo; and the Physicians' Health Study (PHS), including 22 017 men treated with alternate‐day 325 mg aspirin or placebo over 5 years.38, 39 More recently, however, analysis of long‐term observational follow‐up data of the WHS revealed a reduction in CRC risk in the aspirin group, emerging after a median follow‐up of 18 years (hazard ratio: 0.80, 95% CI: 0.67‐0.97).40
Aspirin use has also been associated with reduced incidence of other cancers, including prostate,41 breast,42 esophagus,43 head and neck, and mostly larynx cancer.44 However, benefits of aspirin to other cancer sites are less consistent than in the CRC studies.45, 46, 47 A recent meta‐analysis of case‐control and cohort studies showed a reduction in the risk of gastric cancer of about 10% in patients treated with aspirin for ≥4 years and a reduction of 29% in patients treated for >12 years.48
As previously mentioned, the main evidence of the beneficial effect of aspirin in cancer prevention derives from observational studies and post‐hoc analysis of previous RCTs. However, these results may be influenced by the “healthy‐user bias” of patients treated with aspirin, due to a higher attention to self‐care and healthy lifestyles with more frequent access to medical care and a better predisposition to follow medical advice, which can explain timeliness in cancer diagnosis and consequent better outcomes.
For the reason stated above, RCTs are needed to better clarify the role of aspirin in cancer prevention. Currently ongoing are the studies Aspirin in Reducing Events in the Elderly (ASPREE; NCT01038583), a randomized, double‐blind trial that aims to evaluate aspirin effect on the incidence of CV events, cancer, and bleeding in patients age >65 years; and the Study to Assess the Efficacy and Safety of Enteric‐Coated Acetylsalicylic Acid in Patients at Moderate Risk of Cardiovascular Disease (ARRIVE), a randomized, double‐blind trial (NCT00501059) aiming to study the effect of aspirin in a population with moderate CV risk, related to CV endpoints and to the development of malignancy (Table 1). It would be desirable that all future trials in CV diseases include specific data relating to the occurrence of malignancies between endpoints.
Table 1.
Overview of Main RCTs Investigating the Effect of Aspirin in the Primary Prevention of Cancer
| Trial | Study Design, Setting, and Participants | Intervention | Mean Follow‐up, y | Inclusion Criteria | Endpoints | Outcomes |
|---|---|---|---|---|---|---|
| Physicians' Health Study39 | Randomized, double‐blind, placebo‐controlled trial; 22 071 US male physicians | Alternate‐day 325 mg aspirin (n = 11 037) or placebo (n = 11 024) and β‐carotene 50 mg every other day vs placebo | 5 | — | CV mortality; incidence of invasive and noninvasive colorectal tumors | Aspirin reduced the risk for MI but did not reduce total CV mortality; no substantial reduction in the incidence of CRC. |
| Women's Health Study38 | Randomized 2 × 2 factorial trial, conducted from 1992 to 2004 in 39 876 US women | 100 mg of aspirin (n = 19 934) or 600 IU of vitamin E vs placebo (n = 19 942) administered every other day | 10.1 | Women age ≥45 y without previous history of cancer, CVD, or other major chronic illness | Occurrence of cancer or CV events | Alternate‐day use of low‐dose aspirin did not lower risk of total, breast, CRC, or other site‐specific cancers. |
| ASPREE (NCT01038583) | Randomized, double‐blind, parallel assignment, phase 4; 19 000 pts | 100 mg enteric‐coated aspirin daily vs placebo | Age ≥65 y | All‐cause mortality; fatal and nonfatal CV events including CAD death, nonfatal MI, fatal and nonfatal stroke, and hospitalization for HF; fatal and nonfatal cancer, excluding nonmelanoma skin cancer; dementia or persistent physical disability; major hemorrhagic events | Currently underway | |
| ARRIVE (NCT00501059) | Randomized, double‐blind, placebo‐controlled, multicenter, parallel group; 12 551 pts | Aspirin (ASA, BAYE4465) 100 mg, enteric coated, taken daily vs placebo | Males age ≥55 y with 2–4 CV risk factors; females age ≥60 y with ≥3 CV risk factors | Time to first occurrence of MI, stroke, CV death, UA, or TIA, and incidence of all cancers, excluding nonmelanoma skin cancer | Currently underway |
Abbreviations: ARRIVE, Study to Assess the Efficacy and Safety of Enteric‐Coated ASA in Patients at Moderate Risk of Cardiovascular Disease; ASA, acetylsalicylic acid; ASPREE, Aspirin in Reducing Events in the Elderly; CAD, coronary artery disease; CRC, colorectal cancer; CV, cardiovascular; CVD, cardiovascular disease; HF, heart failure; MI, myocardial infarction; pts, patients; RCT, randomized clinical trial; TIA, transient ischemic attack; UA, unstable angina; US, United States.
Postulated Mechanisms of Aspirin Protection Toward Cancer
When administered at low doses (75–100 mg daily), aspirin acts primarily by inhibiting the platelet‐cyclooxygenase‐1 (COX‐1) and the production of thromboxane A2 (TxA2), which is a central mechanism for the prevention of atherothrombosis.49 At higher doses (>325 mg daily), aspirin exerts an efficacious analgesic and anti‐inflammatory action by affecting COX‐2–dependent prostanoids in inflammatory cells.
Because the COX‐2 enzyme is often overexpressed in CRC cells and is involved in cell proliferation and tumor promotion,50 the inhibition of COX‐2 has long been considered the mechanism by which aspirin and NSAIDs may reduce cancer incidence. However, because low‐dose aspirin (75–160 mg) given once daily has demonstrated to reduce the risk of cancer,33 it has been hypothesized that the mechanism underlying the prevention of thrombotic events and cancer may be coincident, being represented by the permanent inactivation of the platelet‐COX‐1 activity and the release of mediators such as TxA2, and also platelet‐derived growth factor, transforming growth factor β, and prostaglandin E2, that are involved in platelet‐dependent induction of cell transformation.51 Therefore, it has been proposed that low‐dose aspirin and high‐dose aspirin may affect different steps of the colon neoplastic process (Figure 1). It is intriguing to note that, acting through different pathways, other CV drugs (such as ACEIs, ARBs, and statins) also may reduce angiogenesis, cell proliferation, and migration.52, 53, 54, 55 This could support the hypothesis of a beneficial effect of current CV therapy in cancer prevention and thus the tight link between pharmacological intervention used for primary CV prevention and cancer prevention. It is obvious that such a hypothesis would need to be proven through specific prospective studies.
Figure 1.
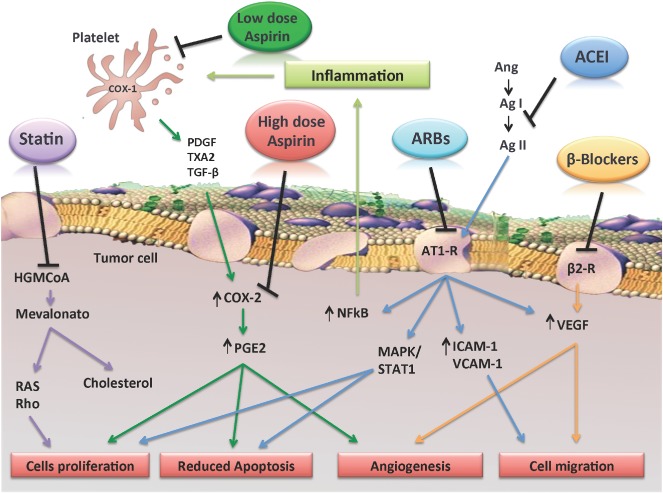
Possible mechanisms underlying antitumor effect of aspirin and other drugs used in the treatment of CVD. Low‐dose aspirin and high‐dose aspirin may affect different steps of colon tumorigenesis. By selectively blocking platelet‐COX‐1 activity and the release of mediators such as TxA2, PDGF, TGF‐β, and PGE2, low‐dose aspirin may inhibit relatively early events in the progression from normal mucosa to adenoma that are involved in platelet‐dependent induction of cell transformation. In particular, low‐dose aspirin might counteract the overexpression of COX‐2 and subsequent increase of PGE2, induced by platelets, in stromal and epithelial intestinal cells, thereby inhibiting cell proliferation and angiogenesis. High‐dose aspirin might directly inhibit COX‐2 activity once it is expressed in intestinal adenomas, reducing the progression of the neoplastic lesion and metastasis. It has also been proposed that statins, ACEIs, ARBs, and β‐blockers, acting through different pathways, may reduce angiogenesis, cell proliferation and migration, and inflammation. Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; Ag I, angiotensin I; Ag II, angiotensin II; Ang, angiotensinogen; ARB, angiotensin receptor blocker; β2‐R, β2 receptor; AT1‐R, angiotensin II type I receptor; COX‐1, cyclooxygenase‐1; CVD, cardiovascular disease; HMG‐CoA, HMG‐CoA reductase; ICAM‐1, intercellular adhesion molecule‐1; MAPK, mitogen‐activated protein kinase; NFκB, nuclear factor κ B; PDGF, platelet‐derived growth factor; PGE2, prostaglandin E2; RAS, RAS protein superfamily of small GTPases; Rho, rho mainfamily of small GTPases; STAT1, signal transducer and activator of transcription‐1; TGF‐β, transforming growth factor β; TXA2, thromboxane A2; VCAM‐1, vascular cell adhesion molecule‐1; VEGF, vascular endothelial growth factor.
Aspirin in Primary Prevention: The Need for a Risk Score
Although the use of aspirin in the secondary prevention of CVD is well established, many aspects of primary prevention are indeed still debated. In fact, it has been shown that the absolute CV event reduction is only slightly greater as compared with the absolute excess in major bleedings. In a large meta‐analysis conducted on 95 000 patients, the use of aspirin in primary prevention resulted in a modest reduction in the absolute risk of CV events (absolute risk reduction: 0.06% per year), compared with a marked increase in the incidence of extracranial bleeds, in particular gastrointestinal (GI).56 Therefore, the most recent European guidelines do not recommend aspirin for primary prevention of CVD; when no previous history of CVD is detected, aspirin may be considered instead in hypertensive patients, those with reduced renal function, or those at high CV risk (IIb).57 Also, the AHA guidelines recommend aspirin for patients with diabetes mellitus and no previous history of vascular disease who are at high risk of CV events (those with a 10‐year risk >10%) and who are not at increased risk for bleeding (Table 2).58
Table 2.
Current Recommendations for Aspirin in Primary Prevention of CVD From Different Guidelines
| Antithrombotic Therapy and Prevention of Thrombosis, 9th ed.: ACCP Guidelines (2012)71 | For persons age ≥50 y without symptomatic CVD, we suggest low‐dose aspirin 75–100 mg daily over no aspirin therapy. |
| European guidelines on CVD prevention in clinical practice (2012)57 | Antiplatelet therapy may be considered in hypertensive patients without a history of CVD but with reduced renal function or at high CV risk (level of evidence IIb). |
| Aspirin for Primary Prevention of Cardiovascular Events in People With Diabetes: A Position Statement of the ADA, a Scientific Statement of the AHA, and an Expert Consensus Document of the ACCF (2010)58 | Low‐dose (75–162 mg/d) aspirin use for prevention: |
| Is reasonable for adults with DM and no previous history of vascular disease who are at increased CVD risk (10‐year risk of CVD events >10%) and who are not at increased risk for bleeding (based on a history of previous GI bleeding or peptic‐ulcer disease or concurrent use of other medications that increase bleeding risk, such as NSAIDs or warfarin). Those adults with DM at increased CVD risk include most men age >50 y and women age >60 y who have ≥1 of the following additional major risk factors: smoking, hypertension, dyslipidemia, family history of premature CVD, and albuminuria. (ACCF/AHA class IIa, level of evidence B; ADA level of evidence C) | |
| Might be considered for those with DM at intermediate CVD risk (younger patients with ≥1 risk factors, or older patients with no risk factors, or patients with 10‐year CVD risk of 5%–10%) until further research is available. (ACCF/AHA class IIb, level of evidence C; ADA level of evidence E) | |
| USPSTF (2009)59 | The USPSTF recommends the use of aspirin for: |
| Men age 45–79 y when the potential benefit due to a reduction in MIs outweighs the potential harm due to an increase in GI hemorrhage. | |
| Women age 55–79 y when the potential benefit of a reduction in ischemic strokes outweighs the potential harm of an increase in GI hemorrhage. | |
| NICE (2008)72 | Offer low‐dose aspirin, 75 mg daily: |
| To patients with DM age ≥50 y if BP is <145/90 mm Hg. | |
| To patients with DM age <50 y with significant other CV risk factors (features of the metabolic syndrome, strong early family history of CVD, smoking, hypertension, extant CVD, microalbuminuria). |
Abbreviations: ACCF, American College of Cardiology Foundation; ACCP, American College of Chest Physicians; ADA, American Diabetes Association; AHA; American Heart Association; BP, blood pressure; CV, cardiovascular; CVD, cardiovascular disease; DM, diabetes mellitus; GI, gastrointestinal; MI, myocardial infarction; NICE, National Institute for Health and Clinical Excellence; NSAIDs, nonsteroidal anti‐inflammatory drugs; USPSTF, US Preventive Services Task Force.
With regard to cancer prevention, in 2007 the US Preventive Services Task Force (USPSTF) recommended against the routine use of aspirin and NSAIDs to prevent colorectal cancer in individuals at average risk (adults age 50–70 years, including those with a family history of colorectal cancer, and not individuals with specific inherited syndromes such as Lynch syndrome or familial adenomatous polyposis, or those with inflammatory bowel disease), and yet currently no guidelines recommend routine use of aspirin for primary prevention of either cancer or CVD,59 although expert documents are supporting an individualized decision.60, 61
In fact, a reduction of the overall incidence and mortality of cancer, by adding benefits to CV prevention, could tip the balance between risks and benefits of aspirin therapy in the primary prevention in favor of the latter, thereby broadening the indication for treatment with aspirin in people at average risk.51
The time elapsed from now to the end of the ongoing prospective studies with aspirin in the prevention of cancer will not be short, and this may turn out to be a disadvantage for patients who could benefit from the treatment. In the meantime, therefore, clinical efforts at the individual level to promote the use of aspirin in global (or total) primary prevention could be made on the basis of a balanced evaluation of the risk/benefit ratio. Although the use of a risk/benefit chart or score would be highly desirable, this is made difficult by the different time course of the potential benefits of aspirin on the risk of bleeding, atherothrombotic events, and cancer. Nonetheless, this clinical strategy may prove useful and contribute to saving lives, provided that it is applied with caution and with a conservative approach to the individual patient.
Accordingly, a key question arises regarding the ideal patient to whom to prescribe aspirin in primary prevention. To answer that question, the CV risk, the risk of cancer, and the risk of bleeding should be considered, possibly at the time of clinical decision.
Concerning the CV risk stratification, different algorithms, mainly based on the presence or the absence of traditional modifiable and nonmodifiable CV risk factors, have been developed. The Systemic Coronary Risk Estimation (SCORE) projects,62 which estimate the 10‐year risk of a fatal atherosclerotic event, and the more recent Pooled Cohort Equations, developed by the ACC/AHA Risk Assessment Work Group to estimate 10‐year atherosclerotic CVD risk ( also including the risk for fatal and nonfatal stroke),63 have been thoroughly validated and largely used in clinical practice. Similarly, various bleeding risk scores have been validated for bleeding risk in anticoagulated patients. As an example, the HAS‐BLED score considers the presence of hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly age, and use of drugs or alcohol. Although this is currently used in patients with atrial fibrillation with the indication for anticoagulant therapy, and it is not appropriate for the primary‐prevention population,64 it might represent a suggestive example of how practical, simple tools can be conceived and specifically designed to stratify hemorrhagic susceptibility in aspirin users.
Regarding the risk of cancer, over the years the understanding of the factors involved in the development of cancer has grown, and subsequently an increase in published risk‐prediction models for various types of cancer, such as esophageal, lung, and breast cancer, has been observed.65, 66, 67 Also, for CRC, several risk‐prediction models have been developed. Freedman et al proposed a model that estimates the probability of developing CRC given a specific age, risk‐factor profile (including sigmoidoscopy and/or colonoscopy and adenoma history in the last 10 years, number of relatives with CRC, current leisure and time of activity, regular use of aspirin/NSAIDs, smoking, vegetable intake, body mass index, and, for women, estrogen status within the last 2 years) and time period (eg, 10 years) in white men and women age ≥50 years.68 More recently, an easy‐to‐use risk calculator was created from Wells et al and validated on >180 000 patients followed up for 11.5 years (Figure 2).69
Figure 2.
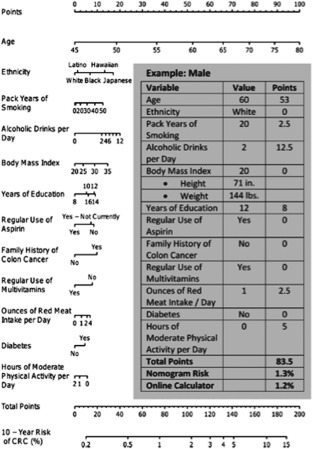
Model for predicting CRC risk in men. Instructions: Draw a perpendicular line from the patient's age to the “points” axis and record the value. Repeat this process for the remaining variables and tally. The 10‐year risk of CRC is identified where a line drawn straight down from the “total points” axis intersects the “10‐year risk of CRC (%).” From Wells et al.69 Abbreviations: CRC, colorectal cancer.
In view of the fact that, to date, benefits of aspirin are more trustworthy for CRC than for other types of cancer, it could be reasonable to assume that the decision to prescribe aspirin in primary prevention may be guided by the estimation, through the appropriate risk‐prediction model, of the risk of CRC and CVD, on one hand, and, on the other hand, of the risk of bleeding. The development of a composite risk‐prediction model may help physicians in making a more objective selection of the primary prevention strategy (aspirin use or not) in the individual patient. Figure 3 schematically represents a simplified approach to use in clinical practice to evaluate the use of aspirin in global primary prevention.
Figure 3.
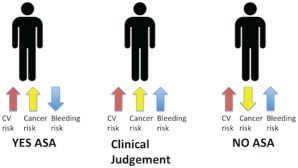
When to prescribe aspirin in primary prevention: 3 different clinical settings. The setting of low‐risk CVD is not shown because in this case aspirin is not indicated, whatever the risk of cancer. Abbreviations: ASA, acetylsalicylic acid (aspirin); CVD, cardiovascular disease.
Recently, data of the WHS were used to develop models for treatment‐effect prediction of alternate‐day aspirin on the combination of CVD, cancer, and major GI bleeding in initially healthy women. Although aspirin was associated with a modestly decreased 15‐year risk of CVD and CRC, aspirin treatment resulted in small benefit or even harm in the majority of women if GI bleeding were also taken into account. However, treating only women age ≥65 years resulted in a higher net benefit with regard to the combined outcomes compared with other treatment strategies, including prediction‐based treatment.70 However, the importance of age in the estimation of “global” risk (including risk of CVD, cancer, and bleeding) must also be confirmed in men.
Conclusion
Cancer and CVD share numerous risk factors, and the interventions aimed to prevent CVD have proven to be effective in the prevention of cancer. Also, many of the drugs used for the prevention and treatment of CVD are thought to prevent the development and progression of certain types of tumors. In particular, several studies demonstrated that long‐term treatment with aspirin can reduce incidence of and mortality from CRC and also other types of tumors. Accordingly, in evaluating the risk/benefit ratio of aspirin in primary prevention of CVD, convincing prospective data in cancer prevention would lead to a wider and safer use. The development of a risk score to evaluate the individual risk‐benefit ratio of the individual patient may help the clinician in making a personalized choice after considering the different components of a complex equation.
Hopefully, ad hoc prospective studies currently underway will clarify in the future the role of aspirin in the prevention of CVD and neoplastic disease.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Jørgensen T, Capewell S, Prescott E, et al. Population‐level changes to promote cardiovascular health. Eur J Prev Cardiol. 2013;20:409–421. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global Status Report on Noncommunicable Diseases 2010. Geneva: World Health Organization; 2011. [Google Scholar]
- 3. Volpe M, Erhardt LR, Williams B. Managing cardiovascular risk: the need for change. J Hum Hypertens. 2008;22:154–157. [DOI] [PubMed] [Google Scholar]
- 4. Wilson K, Gibson N, Willan A, et al. Effect of smoking cessation on mortality after myocardial infarction: meta‐analysis of cohort studies. Arch Intern Med. 2000;160:939–944. [DOI] [PubMed] [Google Scholar]
- 5. Bendinelli B, Masala G, Saieva C, et al. Fruit, vegetables, and olive oil and risk of coronary heart disease in Italian women: the EPICOR Study. Am J Clin Nutr. 2011;93:275–283. [DOI] [PubMed] [Google Scholar]
- 6. Warren TY, Barry V, Hooker SP, et al. Sedentary behaviors increase risk of cardiovascular disease mortality in men. Med Sci Sports Exerc. 2010;42:879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Couto E, Boffetta P, Lagiou P, et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br J Cancer. 2011;104:1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nelson DE, Jarman DW, Rehm J, et al. Alcohol‐attributable cancer deaths and years of potential life lost in the United States. Am J Public Health. 2013;103:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Löllgen H, Böckenhoff A, Knapp G. Physical activity and all‐cause mortality: an updated meta‐analysis with different intensity categories. Int J Sports Med. 2009;30:213–224. [DOI] [PubMed] [Google Scholar]
- 10. Wolin KY, Yan Y, Colditz GA, et al. Physical activity and colon cancer prevention: a meta‐analysis. Br J Cancer. 2009;100:611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stringhini S, Sabia S, Shipley M, et al. Association of socioeconomic position with health behaviors and mortality. JAMA. 2010;303:1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh GK, Miller BA, Hankey BF, et al. Area Socioeconomic Variations in US Cancer Incidence, Mortality, Stage, Treatment, and Survival, 1975–1999. NCI Cancer Surveillance Monograph Series, no. 4. Bethesda, MD: National Cancer Institute; 2003. NIH publication 03‐5417. [Google Scholar]
- 13. Cohen AJ, Ross Anderson H, Ostro B, et al. The global burden of disease due to outdoor air pollution. J Toxicol Environ Health A. 2005;68:1301–1307. [DOI] [PubMed] [Google Scholar]
- 14. Fajersztajn L, Veras M, Barrozo LV, et al. Air pollution: a potentially modifiable risk factor for lung cancer. Nat Rev Cancer. 2013;13:674–678. [DOI] [PubMed] [Google Scholar]
- 15. Rasmussen‐Torvik LJ, Shay CM, Abramson JG, et al. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation. 2013;127:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lever AF, Hole DJ, Gillis CR, et al. Do inhibitors of angiotensin‐I‐converting enzyme protect against risk of cancer? Lancet. 1998;352:179–184. [DOI] [PubMed] [Google Scholar]
- 17. Rao GA, Mann JR, Shoaibi A, et al. Angiotensin receptor blockers: are they related to lung cancer? J Hypertens. 2013;31:1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Volpe M, Azizi M, Danser AH, et al. Twisting arms to angiotensin receptor blockers/antagonists: the turn of cancer. Eur Heart J. 2011;32:19–22. [DOI] [PubMed] [Google Scholar]
- 19. Wang HM, Liao ZX, Komaki R, et al. Improved survival outcomes with the incidental use of β‐blockers among patients with non‐small‐cell lung cancer treated with definitive radiation therapy. Ann Oncol. 2013;24:1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boudreau DM, Yu O, Johnson J. Statin use and cancer risk: a comprehensive review. Expert Opin Drug Saf. 2010;9:603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chrispin J, Martin SS, Hasan RK, et al. Landmark lipid‐lowering trials in the primary prevention of cardiovascular disease. Clin Cardiol. 2013;36:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Libby P. History of discovery: inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elinav E, Nowarski R, Thaiss CA, et al. Inflammation‐induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. [DOI] [PubMed] [Google Scholar]
- 24. Atsumi T, Singh R, Sabharwal L, et al. Inflammation amplifier: a new paradigm in cancer biology. Cancer Res. 2014;74:8–14. [DOI] [PubMed] [Google Scholar]
- 25. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399–4404. [PubMed] [Google Scholar]
- 26. Elwood PC, Gallagher AM, Duthie GG, et al. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–1309. [DOI] [PubMed] [Google Scholar]
- 27. Algra AM, Rothwell PM. Effects of regular aspirin on long‐term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. [DOI] [PubMed] [Google Scholar]
- 28. Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCowan C, Munro AJ, Donnan PT, et al. Use of aspirin post‐diagnosis in a cohort of patients with colorectal cancer and its association with all‐cause and colorectal cancer–specific mortality. Eur J Cancer. 2013;49:1049–1057. [DOI] [PubMed] [Google Scholar]
- 30. Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer [published correction appears in N Engl J Med. 2003;348:1939]. N Engl J Med. 2003;348:883–890. [DOI] [PubMed] [Google Scholar]
- 31. Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. [DOI] [PubMed] [Google Scholar]
- 32. Cole BF, Logan RF, Halabi S, et al. Aspirin for the chemoprevention of colorectal adenomas: meta‐analysis of the randomized trials. J Natl Cancer Inst. 2009;101:256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rothwell PM, Wilson M, Elwin CE, et al. Long‐term effect of aspirin on colorectal cancer incidence and mortality: 20‐year follow‐up of five randomised trials. Lancet. 2010;376:1741–1750. [DOI] [PubMed] [Google Scholar]
- 34. Rothwell PM, Fowkes FG, Belch JF, et al. Effect of daily aspirin on long‐term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. [DOI] [PubMed] [Google Scholar]
- 35. Rothwell PM, Price JF, Fowkes FG, et al. Short‐term effects of daily aspirin on cancer incidence, mortality, and non‐vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. [DOI] [PubMed] [Google Scholar]
- 36. Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. [DOI] [PubMed] [Google Scholar]
- 37. Chan AT, Cook NR. Are we ready to recommend aspirin for cancer prevention? Lancet. 2012;379:1569–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cook NR, Lee IM, Gaziano JM, et al. Low‐dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. [DOI] [PubMed] [Google Scholar]
- 39. Gann PH, Manson JE, Glynn RJ, et al. Low‐dose aspirin and incidence of colorectal tumors in a randomized trial. J Natl Cancer Inst. 1993;85:1220–1224. [DOI] [PubMed] [Google Scholar]
- 40. Stürmer T, Glynn RJ, Lee IM, et al. Aspirin use and colorectal cancer: post‐trial follow‐up data from the Physicians' Health Study. Ann Intern Med. 1998;128:713–720. [DOI] [PubMed] [Google Scholar]
- 41. Mahmud S, Franco E, Aprikian A. Prostate cancer and use of nonsteroidal anti‐inflammatory drugs: systematic review and meta‐analysis. Br J Cancer. 2004;90:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Luo T, Yan HM, He P, et al. Aspirin use and breast cancer risk: a meta‐analysis. Breast Cancer Res Treat. 2012;131:581–587. [DOI] [PubMed] [Google Scholar]
- 43. Corley DA, Kerlikowske K, Verma R, et al. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta‐analysis. Gastroenterology. 2003;124:47–56. [DOI] [PubMed] [Google Scholar]
- 44. Wilson JC, Murray LJ, Hughes CM, et al. Non‐steroidal anti‐inflammatory drug and aspirin use and the risk of head and neck cancer. Br J Cancer. 2013;108:1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bosetti C, Rosato V, Gallus S, et al. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23:1403–1415. [DOI] [PubMed] [Google Scholar]
- 46. Oh SW, Myung SK, Park JY, et al. Aspirin use and risk for lung cancer: a meta‐analysis. Ann Oncol. 2011;22:2456–2465. [DOI] [PubMed] [Google Scholar]
- 47. Ni X, Ma J, Zhao Y, et al. Meta‐analysis on the association between non‐steroidal anti‐inflammatory drug use and ovarian cancer. Br J Clin Pharmacol. 2013;75:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ye X, Fu J, Yang Y, et al. Frequency‐risk and duration‐risk relationships between aspirin use and gastric cancer: a systematic review and meta‐analysis. PLoS One. 2013;8:e71522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Patrono C, Baigent C, Hirsh J, et al; American College of Chest Physicians. Antiplatelet drugs: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):199S–233S. [DOI] [PubMed] [Google Scholar]
- 50. Soumaoro LT, Uetake H, Higuchi T, et al. Cyclooxygenase‐2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10:8465–8471. [DOI] [PubMed] [Google Scholar]
- 51. Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9:259–267. [DOI] [PubMed] [Google Scholar]
- 52. Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10–19. [PubMed] [Google Scholar]
- 53. Zhao Y, Chen X, Cai L, et al. Angiotensin II/angiotensin II type I receptor (AT1R) signaling promotes MCF‐7 breast cancer cells survival via PI3‐kinase/Akt pathway. J Cell Physiol. 2010;225:168–173. [DOI] [PubMed] [Google Scholar]
- 54. Bernabé DG, Tamae AC, Biasoli ÉR, et al. Stress hormones increase cell proliferation and regulates interleukin‐6 secretion in human oral squamous cell carcinoma cells. Brain Behav Immun. 2011;25:574–583. [DOI] [PubMed] [Google Scholar]
- 55. Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. [DOI] [PubMed] [Google Scholar]
- 56. Baigent C, Blackwell L, Collins R, et al; Antithrombotic Trialists' (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 58. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Circulation. 2010;121:2694–2701. [DOI] [PubMed] [Google Scholar]
- 59. Preventive US Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404. [DOI] [PubMed] [Google Scholar]
- 60. Halvorsen S, Andreotti F, ten Berg JM, et al. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology working group on thrombosis. J Am Coll Cardiol. 2014;64:319–327. [DOI] [PubMed] [Google Scholar]
- 61. Volpe M, Abrignani MG, Borghi C, et al. Italian intersocietary consensus document on aspirin therapy in primary cardiovascular prevention [article in Italian]. G Ital Cardiol (Rome). 2014;15:442–451. [DOI] [PubMed] [Google Scholar]
- 62. Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten‐year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 63. Goff DC Jr, Lloyd‐Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014;63(25 part B):3026]. J Am Coll Cardiol. 2013;63(25 part B):2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pisters R, Lane DA, Nieuwlaat R, et al. A novel userfriendly score (HAS‐BLED) to assess one‐year risk of major bleeding in atrial fibrillation patients: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 65. Moghtadaei M, Hashemi Golpayegani MR, Almasganj F, et al. Predicting the risk of squamous dysplasia and esophageal squamous cell carcinoma using minimum classification error method. Comput Biol Med. 2014;45:51–57. [DOI] [PubMed] [Google Scholar]
- 66. Freedman AN, Seminara D, Gail MH, et al. Cancer risk prediction models: a workshop on development, evaluation, and application. J Natl Cancer Inst. 2005;97:715–723. [DOI] [PubMed] [Google Scholar]
- 67. Powell M, Jamshidian F, Cheyne K, et al. Assessing breast cancer risk models in Marin County, a population with high rates of delayed childbirth. Clin Breast Cancer. 2014;14:212.e1–220.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Freedman AN, Slattery ML, Ballard‐Barbash R. Colorectal cancer risk prediction tool for white men and women without known susceptibility. J Clin Oncol. 2009;27:686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wells BJ, Kattan MW, Cooper GS, et al. Colorectal cancer predicted risk online (CRC‐PRO) calculator using data from the multi‐ethnic cohort study. J Am Board Fam Med. 2014;27:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. van Kruijsdijk RC, Visseren FL, Ridker PM, et al. Individualised prediction of alternate‐day aspirin treatment effects on the combined risk of cancer, cardiovascular disease and gastrointestinal bleeding in healthy women. Heart. 2014;doi: 10.1136/heartjnl-2014-306342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines [published correction appears in Chest. 2012;141:1129]. Chest. 2012;141(2 suppl):e637S–e668S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. National Collaborating Centre for Chronic Conditions . Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). http://www.nice.org.uk/guidance/cg87/evidence/cg66‐type‐2‐diabetes‐full‐guideline2. Update published July 2014. [Google Scholar]


