ABSTRACT
Pulmonary arterial hypertension (PAH) is a rare disease, but it boasts significant morbidity and mortality. Although remarkable achievements have been made in the medical treatment of PAH, there is a role for invasive or surgical procedures in patients with progressive disease despite optimal medical therapy or with no access to such therapy. Atrial septostomy creates a right‐to‐left intracardiac shunt to decompress the overloaded right ventricle. Despite significant advances to validate and improve this palliative procedure, as well as recent reports of improved outcomes, it is only slowly being adopted. This article aims to detail the history, indications, contraindications, procedural techniques, and outcomes of atrial septostomy. We will also shed light on some of the newer interventions, inspired by the same physiological concept, that are being evaluated as potential palliative modalities in patients with PAH.
Overview
Background
Infants that are born with cyanotic heart disease such as transposition of the great arteries usually survive without surgery for a few days because the foramen ovale (FO) allows mixing of oxygenated and nonoxygenated blood and, hence, delivery of some oxygen to the body. When the FO closes a few days after birth, the cyanosis usually worsens, and unless corrective surgery takes place, the infant dies.
In 1966, William Rashkind, an American pediatric cardiologist at the Children's Hospital of Philadelphia, invented the lifesaving procedure that bears his name.1 Atrial septostomy (AS), or Rashkind septostomy, is an endovascular intervention that maintains this vitally important opening between the right and left atria until definitive surgery is performed.
Pediatric and adult patients with pulmonary arterial hypertension (PAH) have certain similarities that can be exploited, as this article will show. It is known that patients with idiopathic PAH who have a patent FO survive longer than those who do not.2 Furthermore, patients who have Eisenmenger syndrome live longer and develop heart failure less frequently than patients with idiopathic PAH or secondary pulmonary hypertension.3 Moreover, Austen et al showed that in animals, interatrial flow decompressed the right ventricle (RV) and augmented systemic blood flow, especially with exercise.4 Right ventricular function and cardiac index (CI) are very important in the prognosis of PAH.5
Collectively, these observations allow us to consider AS a potential therapeutic intervention in patients with PAH. One reason is that the atrial defect decompresses the RV, thereby delaying the catastrophic event of RV failure. Another is that the defect shunts more blood to the left side and increases cardiac output, which, despite decreased oxygen concentration from right‐to‐left shunting, improves overall oxygen delivery to the peripheral tissues. Decompressing the right atrium dramatically improves signs and symptoms of systemic congestion, which include ascites and lower‐extremity edema.6
In 1983, Rich and Lam first described AS for the purpose of alleviating PAH in humans.7 Several subsequent published case reports and case series, despite showing encouraging results, have not increased utilization of this procedure for the reasons that will be discussed below.
Indications
In addition to infants with cyanotic heart disease, specifically transposition of the great arteries, AS is currently recommended for patients with specific criteria that can be considered palliative or bridging.6, 8, 9
Severe PAH and intractable right heart failure despite maximal medical therapy, including optimized PAH‐specific agents and inotropes.
Palliation with restoration and maintenance of clinical stability until lung transplantation can be performed.
When no other option exists, for example in regions where modern PAH medical therapy is not available due to technical difficulty or cost.
It should be emphasized that one should consider the procedure before hemodynamic compromise and end‐organ dysfunction are too advanced.
Contraindications
Retrospective analyses examining the outcomes of patients who underwent AS for PAH identified several predictors of higher morbidity and mortality with the procedure. It is recommended not to perform AS in patients with (1) severe RV failure on cardiorespiratory support, (2) mean right atrial pressure (mRAP) >20 mm Hg, (3) room‐air resting O2 saturation <90%, (4) left ventricular end diastolic pressure (LVEDP) >18 mm Hg.6, 8, 10 The most common cause of death was resistant hypoxemia. Of note, the higher the right‐sided pressure, the more likely the shunt will be significant enough to cause uncontrollable hypoxemia. In cases where LVEDP is already elevated, increased left ventricular (LV) preload from the shunt would lead to pulmonary edema and respiratory failure.8
Technical Considerations
Atrial septostomy is an invasive procedure that shares the inherent risks of all intravascular procedures. Universal antiseptic precautions and preprocedural preparation, including a “time out” protocol to confirm patient identity and type and location of procedure, are mandatory.
Currently there are no specific training programs; however, comfort with use of cardiac catheters, and specifically with transseptal puncture, is mandatory.
Best Practices
The basic principle behind AS requires that an opening is formed and maintained in the atrial septum to relieve right‐heart strain and improve left‐sided filling. The increase in cardiac output offsets the decrease in arterial oxygen concentration. Ideally, the appropriately sized shunt should decrease the arterial oxygen concentration by no more than 5% to 10%. If the interatrial defect is too large, the increased cardiac output may not be able to compensate for the degree of hypoxemia, leading to tissue hypoxia.8
Procedure Planning
Due to increased right‐sided pressures, the anatomy of the right atrium and atrial septum may not be elucidated as clearly under fluoroscopy.11 Because both techniques of AS involve initial puncturing of the atrial septum, it is not surprising that, even in the most experienced hands, pericardial tamponade occurs in about 1.2% of cases, mostly due to left atrial wall puncture.12
Transthoracic echocardiography does not represent the optimal imaging modality.13, 14, 15 Direct visualization of the atrial septum with transesophageal echocardiography or intracardiac echocardiography may decrease procedural complications.16, 17, 18
Radiofrequency perforation of the target area in the septum allows for precise puncturing and potentially a decreased incidence of free wall perforations and unnecessary cardiac muscle activation leading to arrhythmias.19
Cardiac magnetic resonance imaging is considered by many authorities to be the gold standard for the evaluation of RV function, and therefore it would certainly be helpful in the evaluation of septostomy patients pre‐ and postprocedure. Animal data suggest that cardiac magnetic resonance imaging can be used to guide AS, but no such data exist in humans.20
Complication Prevention
The major factor prohibiting the widespread use of AS despite its benefits is the associated high direct mortality rate. One analysis showed a 24‐hour mortality rate of 7.1%, as well as a 14.8% 1‐month mortality rate.8 Nevertheless, recommendations to minimize complications have been in practice and are thought to decrease procedure‐related mortality and morbidity.6 A recent review emphasized the following recommendations to decrease mortality and complications: (1) avoidance of AS in patients with ≥1 of the contraindications described above, (2) experienced operator in an experienced center with surgical backup, and (3) preprocedural, intraprocedural, and postprocedural measures to help avoid complications and improve outcome.
Preprocedural
Optimization of cardiac function with adequate right‐heart filling pressures and additional inotropic support as needed.
Intraprocedural
Supplemental oxygen, sedation, continuous monitoring of oxygen saturation, and monitoring of mean right atrial and left atrial pressures (mRAP and mLAP) with attempt to tailor the size of the defect to cause a decrease in left atrial O2 saturation of <10%.
Postprocedural
Optimization of O2 delivery using red blood cell transfusions or hematopoietic medications as needed to maintain appropriate oxygen‐carrying capacity, per PAH guidelines.9 Long‐term anticoagulation is mandatory.
Outcomes
Immediate Clinical Outcomes
Several case series have reported a procedure‐related mortality rate of about 15%, which has been confirmed in a review of 223 cases of AS in PAH.8 Moreover, the 24‐hour mortality rate was close to 7%. The most common etiology was refractory hypoxemia from an oversized shunt. Progressive RV failure was the second most common culprit. Procedural complications such as cardiac tamponade, arrhythmias, and hemoptysis were rarer causes of death.8
Univariate analysis of periprocedural death with selected variables revealed that patients who had the poorest outcomes were those with RAP >20 mm Hg (HR [hazard ratio]: 30) and those with higher World Health Organization (WHO) class (HR: 8.5). The higher the SaO2% postprocedure, the better the chance of survival (HR: 0.90). Performing AS in patients with PAH who presented with syncope was associated with significantly better outcome (HR: 0.14).8
In a more recent report, the 1‐month mortality rate was reported as 2%. The authors attributed this improvement in outcome to improved selection of patients, adherence to safety recommendations mentioned above, and using balloon‐dilation AS with a graded step‐by‐step protocol. In this particular series, 28% of the patients had a RV end‐diastolic pressure (RVEDP) <10 mm Hg, reflecting better baseline RV function and arguing for earlier utilization of this technique to decrease its associated mortality.21
Patients who survive AS usually live to experience significant improvement of their symptoms at 2 to 4 weeks, as evidenced by both their WHO class (improved from 3.57 ± 0.6 to 2.07 ± 0.3) and performance on the 6‐minute walk test (6MWT; improved from 107 ± 127 m to 217 ± 108 m).19 Other symptoms such as syncope and signs of RV failure such as edema and ascites improve in close to 90% of patients.8 Brain natriuretic peptide (BNP) levels were also reported to improve post‐AS (Table 1).22
Table 1.
Clinical Effects of Atrial Septostomy
Immediate Hemodynamic Response
The most notable hemodynamic changes immediately after AS are a drop in RVEDP (14.5 ± 6 mm Hg to 10.7 ± 5.9 mm Hg) and a concomitant increase in LVEDP (6.4 ± 3.4 mm Hg to 8.8 ± 2.7 mm Hg).21 The mRAP drops an average of 3 mm Hg, accompanied by a similar increase in mLAP.6 The SaO2% drops from 93.3 ± 4.1% to 83 ± 8.5%. This is offset by increased CI of 0.6 to 0.7 L/min/m2, leading to improved systemic oxygen delivery.6, 8, 21, 23 Although post‐AS exercise hemodynamics have not been studied in humans, animal studies suggest that in cases of RV hypertension, the presence of atrial septal defect allows for a dramatic increase in LV output during exercise without concomitant increase in RVEDP.4, 23 Improvement in the 6MWT is an indirect indicator of a more marked improvement of hemodynamics during exercise.
Interestingly, pulmonary and systemic arterial pressures do not change significantly; therefore, the clinical improvement is thought to be mostly due to RV decompression and improved oxygen delivery, especially during exercise.24, 25 Another interesting point is that the hemodynamic response was inversely related to the baseline mRAP. Patients with mRAP <10 mm Hg experienced almost no hemodynamic changes. In contrast, those with mRAP >20 mm Hg had the most significant postprocedural changes, including improvement of CI; however, as noted earlier, they also had the highest complications and mortality rates. The group with the best risk/benefit ratio seemed to be those with mRAP between 10 and 20 mm Hg8 (Table 2).
Table 2.
Hemodynamic Effects of Atrial Septostomy Related to Baseline Resting mRAP
| Variable | mRAP <10 mm Hg (n = 42) | mRAP 11–20 mm Hg (n = 49) | mRAP >20 mm Hg (n = 26) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | P Value | Before | After | P Value | Before | After | P Value | |
| mRAP | 6.6 ± 2.4 | 5.9 ± 3.2 | 0.214 | 14.8 ± 2.8 | 11.9 ± 3.5 | 0.0001 | 26.6 ± 4.4 | 19.9 ± 3.8 | 0.0001 |
| mPAP | 62 ± 16 | 64 ± 19 | 0.329 | 66.4 ± 17 | 66.4 ± 16 | 1.000 | 63.4 ± 20 | 67.5 ± 20 | 0.102 |
| mLAP | 5.0 ± 2.7 | 6.8 ± 2.4 | 0.005 | 5.3 ± 3.6 | 7.8 ± 4.5 | 0.0001 | 7.9 ± 3.0 | 10.9 ± 4.0 | 0.029 |
| CI | 2.36 ± 0.6 | 2.89 ± 0.7 | 0.0001 | 2.04 ± 0.7 | 2.65 ± 1.0 | 0.0001 | 1.55 ± 0.5 | 2.14 ± 0.6 | 0.0001 |
| mSAP | 83 ± 15 | 83 ± 13 | 0.931 | 84.5 ± 14 | 88.8 ± 15 | 0.065 | 78 ± 20 | 81 ± 18 | 0.254 |
Abbreviations: CI, cardiac index; mLAP, mean left atrial pressure; mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; mSAP, mean systemic arterial pressure; NYHA, New York Heart Association.
Modified from Keogh et al8 with permission from Elsevier.
Late Clinical Outcomes
On average, most survivors live slightly more than 60 months. Mortality is best predicted by a worsening functional class postprocedure. Eventually the typical cause of death is progression of the pulmonary vascular disease, with reappearance of dyspnea and cyanosis.21, 26 This has been reported in association with a reduction in pulmonary perfusion on lung scans, indicating worsening right‐to‐left shunt.23
The combination of medical treatment and AS has been recently shown to be superior to AS alone. The median survival was 83 months for patients who underwent AS in addition to medical therapy, compared with 53 months in those who underwent AS alone.21 Higher exercise endurance and SaO2 were predictors of better outcomes. Unlike prior studies in which AS was used as a salvage therapy after failure of medical treatment, in this study AS was performed first, followed by the addition of medical therapy. The lesser stage of disease state likely explains the much lower mortality rate reported in this series.21
Late Hemodynamic Response
There is some evidence that improved hemodynamics (eg, CI and RAP) are sustained for 2 or more years.6, 27 Echocardiographic evidence of decreased RV and RA pressures was demonstrated 5.5 months after AS in 1 study.28
Sandoval et al reported sustained long‐term functional improvement of patients who underwent AS and remained alive in their series. The WHO class improved from 2.1 ± 0.3 to 1.5 ± 0.5 (n = 10, P < 0.05), and the 6MWT improved from 191 ± 101 m to 284 ± 73 m (n = 10, P < 0.05).23
Spontaneous closure of the septal defect can occur; it is usually heralded by worsening symptoms and signs of RV failure, such as reappearance of ascites, or by a spontaneous “improvement” in the SaO2%. Occasionally, the closure is detected on follow‐up echocardiography. Repeat AS has been reported and seems to be safe.21, 23 Some investigators have used fenestrated Amplatzer, stents, and most recently cryoplasty at the time of the initial septostomy to prevent closure of the defect. The usefulness of these approaches remains to be proved.29, 30, 31, 32
Periprocedural Care
Patient Education and Consent
The patient and family should be informed about the risks and benefits of the procedure. The operator should fully discuss and disclose any potential and predictable bad outcomes, including death. It is mandatory that an experienced operator either performs or supervises for the duration of the procedure.
Preprocedure Planning
Careful planning involves a thorough and accurate identification of the anatomy. As stated earlier, echocardiographic imaging can provide essential information before and during the procedure. Both transesophageal echocardiography and intracardiac echocardiography have been shown to provide accurate imaging of the atrial septum.16, 17, 18 Hemodynamic evaluation is performed to diagnose PAH. Measurement of CI, RVEDP, LVEDP, and venous and arterial saturation is essential prior to creating the septal defect. The procedure is performed in a cardiac catheterization laboratory with fluoroscopic capability. The following equipment is required:
Venous access.
Venous sheath (usually 8 F–10 F).
Either a Brockenbrough needle to puncture the atrial septum or a catheter with a retractable blade to create the initial incision in the atrial septum.
A guidewire to advance the balloon(s).
Balloon catheters of different diameters.
Patient Preparation
General anesthesia is usually not required. Conscious sedation that is routinely performed in the cardiac catheterization laboratory is generally used. Nevertheless, the presence of intubation and mechanical ventilation capability is mandatory because hypoxemia is a major complication. Likewise, the patient needs to be able to lie completely supine for the duration of the procedure. Regarding preprocedural and postprocedural anticoagulation, we suggest that low‐molecular‐weight heparin can be started days before and continued days after the procedure, assuming there is no bleeding, and the patient can eventually revert to oral anticoagulation. During the procedure, the interventional cardiologist may use standard heparin, per normal heart‐catheterization procedure.
Technique
The Rashkind procedure was originally performed in children while the atrial septal defect was still present. A venous catheter is advanced from the right to the left atrium, when a balloon at the tip of the catheter is inflated. The catheter is then retracted to the right atrium, “tearing” the atrial septum on its way back.1
Because this technique is not generally feasible in adults, or in infants in whom the FO is closed, one of the following techniques should be used:
Graded balloon dilation atrial septostomy (BDAS). Here an initial atrial septal puncture is performed with a Brockenbrough needle. The puncture is subsequently dilated using gradually larger balloons that are advanced into the atrial septum through the Mullins sheath. Hemodynamic variables like mRAP, mLAP, SaO2%, and LVEDP are measured before each dilation with a larger balloon. This technique offers the advantage of a better control over the degree of changes in the hemodynamics and O2 saturation, thus minimizing the risks of an oversized shunt leading to severe hypoxemia. BDAS is associated with a lower postprocedural mortality rate, thus is considered the procedure of choice for AS in PAH (Figure 1).8, 23
Blade balloon atrial septostomy (BBAS). Park et al performed this technique initially on animals and in vitro human hearts in 1975.33 The procedure involves repeated (3–6) atrial septal incisions using a blade within the catheter. This may or may not be followed by balloon dilation. Unlike the graded balloon technique, it does not allow for repeated measurements of hemodynamics. However, in BBAS a repeat septostomy for spontaneous closure is needed in only 3% of patients, whereas as many as 15% of patients who undergo BDAS will require a repeat procedure.34, 35
Figure 1.
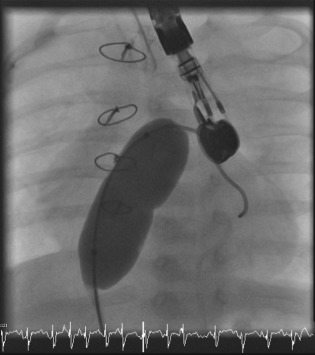
An example of balloon AS, under TEE guidance, in a child with congenital heart disease complicated by pulmonary hypertension. Notice the “waist” on the balloon, which indicates the site of the atrial septum. Image courtesy of Dr. Alexander Davidson from the Children's Hospital of Philadelphia. Abbreviations: AS, atrial septostomy; TEE, transesophageal echocardiography.
Alternative Methods to Decompress the Right Ventricle
The surgical creation of a direct anastomosis between the descending aorta and the left pulmonary artery is called Potts shunt. The shunt can be pressure‐restrictive to control the degree of hypoxemia. Another potential advantage is through shunting hypoxemic blood beyond cerebral and coronary circulations. Potts shunt was evaluated in 8 children with idiopathic PAH and severe symptoms such as syncope and right‐heart failure. Of these 8 children, 6 experienced symptomatic improvement, had improved RV dimensions on echocardiography, and had lower BNP levels. At a mean follow‐up of 63 months, those 6 patients continued to do well.36 More recently, 4 adults received percutaneous aortopulmonary shunt creation via a puncture between the descending aorta and left pulmonary artery with subsequent stent placement. Two of the 4 patients died within 8 days of the postoperative period.37 Both surgical and percutaneous creation of aortopulmonary shunts share the physiological principle behind AS; however, more data are needed before any recommendation can formally be made about them.
Monitoring and Follow‐up
For the first 48 hours after an uncomplicated procedure, all patients need to be observed in an intensive care unit setting where hemodynamic monitoring can be performed on a continuous basis.19 Supplemental oxygen availability is mandatory, as the most feared complication is systemic hypoxia, which can be catastrophic if uncontrolled. Fortunately, devastating post‐AS hypoxia has been salvaged by using inhaled iloprost until the patient was stabilized and weaned off of the medication, and this approach may be used if needed.38
To assure an appropriate oxygen delivery after septostomy, hemoglobin levels may need to be maintained at near normal levels. Patients with Eisenmenger syndrome tend to have good tissue oxygenation, and this has been attributed, at least partially, to appropriate hemoglobin levels.39
Special attention should be given to oxygen saturation. Survivors can be monitored clinically for recurrence of symptoms of right‐sided heart failure: edema, ascites, or a spontaneous “improvement” in SaO2%. Development of any should be investigated with echocardiography to look for spontaneous closure of the shunt.21 Routine serial echocardiography at preset intervals may be performed. Functional assessments, including 6MWT, have been used in case series to evaluate the efficacy of AS.6, 8
Of note, data from case series suggest that to redo septostomy is no more or less risky than the original procedure. In one series, the rate of repeat septostomy was 29.4%. In that series, it was noted that using PAH‐specific treatment after AS led to elimination of the need to repeat septostomies.21
Conclusion
The most updated algorithm on the management of PAH recommended that balloon AS should be considered (class IIa–c recommendation) in patients who either do not respond to maximal medical therapy or as a bridge to lung transplantation. It was also recommended for palliation when medical therapy is not available.40 On the other hand, the United Kingdom's National Institute for Health and Clinical Excellence (NICE) only reviewed the evidence behind medical treatment and failed to mention AS as a potential therapeutic modality.41 Although there are several areas of further study, such as AS plus medical therapy vs medical therapy alone, our current thought is that this procedure is underutilized, which is probably related to overestimation of its risks and underestimation of its benefits. Hopefully we were able to reintroduce this potentially effective therapeutic modality in patients with PAH.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Rashkind WJ, Miller WW. Creation of an atrial septal defect without thoracotomy: a palliative approach to complete transposition of the great arteries. JAMA. 1966;196:991–992. [PubMed] [Google Scholar]
- 2. Rozkovec A, Montanes P, Oakley CM. Factors that influence the outcome of primary pulmonary hypertension. Br Heart J. 1986;55:449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hopkins WE, Ochoa LL, Richardson GW, et al. Comparison of the hemodynamics and survival of adults with severe primary pulmonary hypertension or Eisenmenger syndrome. J Heart Lung Transplant. 1996;15(1 part 1):100–105. [PubMed] [Google Scholar]
- 4. Austen WG, Morrow AG, Berry WB. Experimental studies of the surgical treatment of primary pulmonary hypertension. J Thorac Cardiovasc Surg. 1964;48:448–455. [PubMed] [Google Scholar]
- 5. Collins TJ, Moore JW, Kirby WC. Atrial septostomy for pulmonary hypertension. Am Heart J. 1988;116:873–874. [DOI] [PubMed] [Google Scholar]
- 6. Sandoval J, Rothman A, Pulido T. Atrial septostomy for pulmonary hypertension. Clin Chest Med. 2001;22:547–560. [DOI] [PubMed] [Google Scholar]
- 7. Rich S, Lam W. Atrial septostomy as palliative therapy for refractory primary pulmonary hypertension. Am J Cardiol. 1983;51:1560–1561. [DOI] [PubMed] [Google Scholar]
- 8. Keogh AM, Mayer E, Benza RL, et al. Interventional and surgical modalities of treatment in pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 suppl):S67–S77. [DOI] [PubMed] [Google Scholar]
- 9. McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. [DOI] [PubMed] [Google Scholar]
- 10. Bhamra‐Ariza P, Keogh AM, Muller DW. Percutaneous interventional therapies for the treatment of patients with severe pulmonary hypertension. J Am Coll Cardiol. 2014;63:611–618. [DOI] [PubMed] [Google Scholar]
- 11. Sager JS, Ahya VN. Surgical therapies for pulmonary arterial hypertension. Clin Chest Med. 2007;28:187–202. [DOI] [PubMed] [Google Scholar]
- 12. Roelke M, Smith AJ, Palacios IF. The technique and safety of transseptal left heart catheterization: the Massachusetts General Hospital experience with 1279 procedures. Cathet Cardiovasc Diagn. 1994;32:332–339. [DOI] [PubMed] [Google Scholar]
- 13. Cafri C, de la Guardia B, Barasch E, et al. Transseptal puncture guided by intracardiac echocardiography during percutaneous transvenous mitral commissurotomy in patients with distorted anatomy of the fossa ovalis. Catheter Cardiovasc Interv. 2000;50:463–467. [DOI] [PubMed] [Google Scholar]
- 14. Zanchetta M, Rigatelli G, Pedon L, et al. Role of intracardiac echocardiography in atrial septal abnormalities. J Interv Cardiol. 2003;16:63–77. [DOI] [PubMed] [Google Scholar]
- 15. Zanchetta M, Rigatelli G, Pedon L, et al. Intracardiac echocardiography: gross anatomy and magnetic resonance correlations and validations. Int J Cardiovasc Imaging. 2005;21:391–401. [DOI] [PubMed] [Google Scholar]
- 16. Unger P, Stoupel E, Vachiery JL, et al. Atrial septostomy under transesophageal guidance in a patient with primary pulmonary hypertension and absent right superior vena cava. Intensive Care Med. 1996;22:1410–1411. [DOI] [PubMed] [Google Scholar]
- 17. Bidoggia H, Maciel JP, Alvarez JA. Transseptal left heart catheterization: usefulness of the intracavitary electrocardiogram in the localization of the fossa ovalis. Cathet Cardiovasc Diagn. 1991;24:221–225. [DOI] [PubMed] [Google Scholar]
- 18. Moscucci M, Dairywala IT, Chetcuti S, et al. Balloon atrial septostomy in end‐stage pulmonary hypertension guided by a novel intracardiac echocardiographic transducer. Catheter Cardiovasc Interv. 2001;52:530–534. [DOI] [PubMed] [Google Scholar]
- 19. Sakata Y, Feldman T. Transcatheter creation of atrial septal perforation using a radiofrequency transseptal system: novel approach as an alternative to transseptal needle puncture. Catheter Cardiovasc Interv. 2005;64:327–332. [DOI] [PubMed] [Google Scholar]
- 20. Raval AN, Karmarker PV, Guttman MA. Real‐time MRI‐guided atrial septal puncture and balloon septostomy in swine. Catheter Cardiovasc Interv. 2006;67:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sandoval J, Gaspar J, Peña H, et al. Effect of atrial septostomy on the survival of patients with severe pulmonary arterial hypertension. Eur Respir J. 2011;38:1343–1348. [DOI] [PubMed] [Google Scholar]
- 22. O'Byrne ML, Rosenzweig ES, Barst RJ. The effect of atrial septostomy on the concentration of brain‐type natriuretic peptide in patients with idiopathic pulmonary arterial hypertension. Cardiol Young. 2007;17:557–559. [DOI] [PubMed] [Google Scholar]
- 23. Sandoval J, Gaspar J, Pulido T, et al. Graded balloon dilation atrial septostomy in severe primary pulmonary hypertension: a therapeutic alternative for patients nonresponsive to vasodilator treatment. J Am Coll Cardiol. 1998;32:297–304. [DOI] [PubMed] [Google Scholar]
- 24. Sandoval J, Gaspar J. Atrial septostomy In: Peacock AJ, Rubin LJ, eds. Pulmonary Circulation. 2nd ed London: Edward Arnold; 2004:319–333. [Google Scholar]
- 25. Bristow MR, Zisman LS, Lowes BD, et al. The pressure‐overloaded right ventricle in pulmonary hypertension. Chest. 1998;114(1 suppl):101S–106S. [DOI] [PubMed] [Google Scholar]
- 26. Barst RJ. Role of atrial septostomy in the treatment of pulmonary vascular disease [published correction appears in Thorax. 2000;55:254]. Thorax. 2000;55:95–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hopkins WE. The remarkable right ventricle of patients with Eisenmenger syndrome. Coron Artery Dis. 2005;16:19–25. [DOI] [PubMed] [Google Scholar]
- 28. Espinola‐Zavaleta N, Vargas‐Barrón J, Tazar JI, et al. Echocardiographic evaluation of patients with primary pulmonary hypertension before and after atrial septostomy. Echocardiography. 1999;16(7 part 1):625–634. [DOI] [PubMed] [Google Scholar]
- 29. Althoff TF, Knebel F, Panda A, et al. Long‐term follow‐up of a fenestrated Amplatzer atrial septal occluder in pulmonary arterial hypertension. Chest. 2008;133:283–285. [DOI] [PubMed] [Google Scholar]
- 30. Troost E, Delcroix M, Gewillig M, et al. A modified technique of stent fenestration of the interatrial septum improves patients with pulmonary hypertension. Catheter Cardiovasc Interv. 2009;73:173–179. [DOI] [PubMed] [Google Scholar]
- 31. Roy AK, Gaine SP, Walsh KP. Percutaneous atrial septostomy with modified butterfly stent and intracardiac echocardiographic guidance in a patient with syncope and refractory pulmonary arterial hypertension. Heart Lung Circ. 2013;22:668–671. [DOI] [PubMed] [Google Scholar]
- 32. Guerrero M, Cajigas H, Awdish R, et al. First‐in‐man experience with cryoplasty during graded balloon atrial septostomy to reduce spontaneous closure in a patient with severe pulmonary arterial hypertension. EuroIntervention. 2014;9:1235–1236. [DOI] [PubMed] [Google Scholar]
- 33. Park SC, Zuberbuhler JR, Neches WH, et al. A new atrial septostomy technique. Cathet Cardiovasc Diagn. 1975;1:195–201. [DOI] [PubMed] [Google Scholar]
- 34. Reichenberger F, Pepke‐Zaba J, McNeil K, et al. Atrial septostomy in the treatment of severe pulmonary arterial hypertension. Thorax. 2003;58:797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ali Khan MA, Bricker JT, Mullins CE, et al. Blade atrial septostomy: experience with the first 50 procedures. Cathet Cardiovasc Diagn. 1991;23:257–262. [DOI] [PubMed] [Google Scholar]
- 36. Baruteau AE, Serraf A, Lévy M, et al. Potts shunt in children with idiopathic pulmonary arterial hypertension: long‐term results. Ann Thorac Surg. 2012;94:817–824. [DOI] [PubMed] [Google Scholar]
- 37. Esch JJ, Shah PB, Cockrill BA, et al. Transcatheter Potts shunt creation in patients with severe pulmonary arterial hypertension: initial clinical experience. J Heart Lung Transplant. 2013;32:381–387. [DOI] [PubMed] [Google Scholar]
- 38. Kurzyna M, Dabrowski M, Bielecki D, et al. Atrial septostomy in treatment of end‐stage right heart failure in patients with pulmonary hypertension. Chest. 2007;131:977–983. [DOI] [PubMed] [Google Scholar]
- 39. Sandoval J, Torbicki A. Atrial septostomy In: Voelkel NF, Schranz D, eds. The Right Ventricle in Health and Diseases. New York, NY: Humana Press/Springer Science + Business Media; 2015:419–437. [Google Scholar]
- 40. Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D60–D72. [DOI] [PubMed] [Google Scholar]
- 41. National Institute for Health and Clinical Excellence . Appraisal of the drug treatments used in pulmonary arterial hypertension. http://www.nice.org.uk/guidance/gid‐tag382/documents/pulmonary‐arterial‐hypertension‐association‐uk4. Published May 2007. Accessed December 31, 2014.


