ABSTRACT
Background
There are limited data on the comparative effectiveness of medical therapy, supervised exercise, and revascularization to improve walking and quality of life in patients with intermittent claudication (IC).
Hypothesis
Supervised exercise and revascularization was superior to medical therapy in IC.
Methods
We studied the comparative effectiveness of exercise training, medications, endovascular intervention, and surgical revascularization on outcomes including functional capacity (walking distance and timing), quality of life, and mortality. We searched PubMed, EMBASE, and the Cochrane Database of Systematic Reviews from January 1995 to August 2012 for relevant English‐language studies. Two investigators independently collected data. Meta‐analyses with random‐effects models of direct comparisons were supplemented by mixed‐treatment analyses to incorporate data from placebo comparisons, head‐to‐head comparisons, and multiple treatment arms.
Results
Thirty‐five unique studies evaluated treatment modalities in 7475 patients with IC. Compared with usual care, only exercise training improved both maximal walking distance (150 meters; 95% confidence interval: 35–266 meters, P = 0.01) and initial claudication distance (39 meters; 95% confidence interval: 9–65 meters, P = 0.003). All modalities were associated with improved quality of life (Short Form‐36 physical functioning score) compared with usual care, but there were no differences between treatments. There were insufficient safety data to assess treatment‐related complications. All‐cause mortality was not significantly different between modalities.
Conclusions
Evidence is insufficient to determine treatment superiority for improving quality of life and walking parameters in IC patients. Further studies with attention to study design, standardized efficacy and safety endpoints, and appropriate subgroup reporting are necessary to determine comparative effectiveness.
Introduction
Intermittent claudication (IC) affects an estimated 8 million people worldwide and is the most common symptomatic manifestation of peripheral artery disease (PAD).1 Intermittent claudication is associated with significant medical costs,2 decreased quality of life,3 and decreased walking endurance.4 The current American College of Cardiology/American Heart Association (ACC/AHA) guidelines give class I indications to cilostazol, exercise rehabilitation, and lower‐extremity revascularization as therapies in selected patients with IC.5 However, considerable controversy remains as to the comparative effectiveness of these treatments. A recent meta‐analysis addressing the comparative effectiveness of treatment strategies for IC6 did not assess quality of life or the effect of cilostazol, and it did not include the landmark Claudication Exercise Versus Endoluminal Revascularization (CLEVER) trial,7 which evaluated optimal medical care vs supervised exercise vs endovascular revascularization for IC.
Given the gaps in comparative effectiveness data describing quality of life and functional capacity outcomes among multiple guideline‐recommended treatments in patients with IC, we conducted a systematic review and meta‐analysis to evaluate (1) the comparative effectiveness of medical therapy, supervised exercise, and endovascular or surgical revascularization in patients with IC; (2) the variation in effectiveness of these treatments by subgroup; and (3) the safety of these treatments for IC.
Methods
Data Sources and Searches
We searched PubMed, EMBASE, and the Cochrane Database of Systematic Reviews using strategies similar to the National Library of Medicine's medical subject headings. Searches were limited to articles published from January 1995 to August 2012 to capture contemporary studies on medical therapy, supervised exercise, and endovascular and surgical revascularization for IC. Exact search strings are listed in the full Agency for Healthcare Research and Quality (AHRQ) report.8 We studied the comparative effectiveness of exercise training, medications, endovascular intervention, and surgical revascularization on outcomes including functional capacity (walking distance and timing), quality of life, and mortality.
Study Selection
Studies were limited to populations age ≥18 years with lower‐extremity PAD (eg, ankle‐brachial index <0.9) who were symptomatic with intermittent claudication. We included English‐language randomized trials or observational studies with relevant treatment comparisons (eg, medical therapy, supervised exercise, endovascular revascularization, surgical revascularization, usual care) and outcomes. Outcomes assessed for our review were walking distance, claudication distance, all‐cause mortality, and quality of life (eg, Short Form‐36 [SF‐36], walking impairment questionnaire, peripheral artery questionnaire). Safety concerns associated with each treatment strategy included adverse drug reactions, bleeding, contrast nephropathy, radiation exposure, infection, and periprocedural complications causing acute limb ischemia. Inclusion and exclusion criteria are described in the full report.8
Two reviewers (SV and WSJ) independently examined titles and abstracts for potential relevance. Articles included by either reviewer underwent full‐text screening where 2 independent reviewers read each article to determine eligibility for data abstraction. A third‐party arbitrator reconciled disagreements.
Data Extraction and Quality Assessment
Abstracted data included study design, patient characteristics overall and by study group (age, sex, race), and intervention‐specific factors (antiplatelet therapy, exercise training, type of endovascular revascularization, type of surgical revascularization).
Two reviewers evaluated the quality of individual studies independently as described in AHRQ's Methods Guide for Effectiveness and Comparative Effectiveness Reviews,9 assigning summary ratings of good, fair, or poor. Disagreements were resolved via consensus.
Data Synthesis and Analysis
We summarized the primary literature by abstracting relevant continuous data (eg. age, event rates) and categorical data (eg, race, presence of coronary disease risk factors). Continuous variable outcomes were summarized by how the studies reported them. These included means, medians, SDs, interquartile ranges, ranges, and associated P values. Dichotomous variables were summarized by proportions and associated P values.
Two reviewers evaluated the strength of evidence using the 4 required domains described in AHRQ's Methods Guide9: risk of bias, consistency, directness, and precision. We assigned an overall grade for the strength of evidence as high (evidence reflects the true effect), moderate (further research may change the estimate of effect), low (further research is likely to change the estimate), or insufficient (an estimate of effect is not possible with the available data).
Meta‐analysis was considered for comparisons where ≥3 studies reported the same outcome. Random‐effects models were used for all outcomes because of the heterogeneity of the studies. Continuous outcome measures comparing 2 treatments that used a similar scale were combined without transformation using a random‐effects model as implemented in Comprehensive Meta‐Analysis Version 2 (Biostat, Englewood, NJ). Dichotomous outcome measures comparing 2 treatments were combined, and odds ratio (ORs) were computed using a random‐effects model as implemented in Comprehensive Meta‐Analysis.
Studies reporting continuous outcome measures on different scales (eg, functional capacity, quality‐of‐life measures) were combined using a random‐effects meta‐regression model on the effect sizes as implemented in the SAS procedure NLMIXED (SAS Institute Inc., Cary, NC). Effect size interpretation was based on Cohen's d, whereby 0 equates to no effect, 0.2 equates to a small effect, 0.5 equates to a medium effect, 0.8 equates to a large effect, and effects >1.0 equate to very large effects.10
Studies reporting dichotomous outcome measures were combined using a random‐effects, multiple logistic model as implemented in EGRET (Cytel Software Corp., Cambridge, MA). To minimize the impact that study populations and disease severity may have on clinical outcomes, we reviewed the definition of PAD for study inclusion and baseline population characteristics and found similar eligibility criteria and mean ankle‐brachial indices at study enrollment (within 1 SD of each other); therefore, we did not perform statistical adjustment for baseline severity of PAD.
We tested for statistical heterogeneity between studies (Q and I 2 statistics) while recognizing that the power to detect such heterogeneity may be limited. Summary estimates, standard errors, and confidence intervals (CIs) are presented.
Results
Our initial search yielded 5908 citations and, after applying inclusion and exclusion criteria, resulted in 35 unique studies (see Supporting Information, Appendix, in the online version of this article) assessing the comparative effectiveness of medications, exercise training, endovascular interventions, or surgical revascularization in 7475 PAD patients with IC (Figure 1). Of these studies, 10 randomized controlled trials (RCTs) compared cilostazol or pentoxifylline (ie, medical therapy) with usual care, 10 RCTs compared exercise training with usual care, 5 RCTs compared endovascular intervention with usual care, and 9 RCTs compared endovascular intervention with exercise training. For the current analysis, observational studies were excluded and only RCTs were included.
Figure 1.
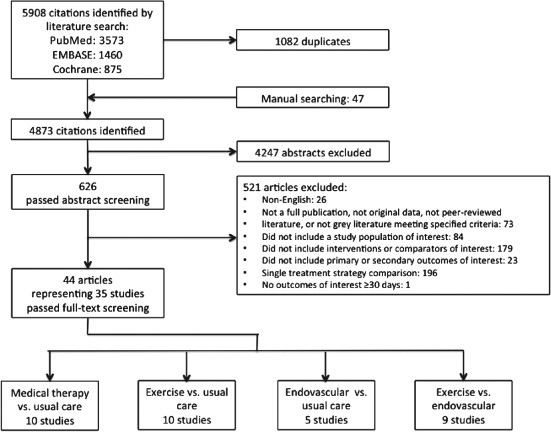
Literature flow diagram.
Study Characteristics
The Table 1 lists the studies by therapeutic comparison. There were 27 RCTs included in the current analysis: 12 (44%) were good quality, 13 (48%) were fair quality, and 2 (7%) were poor quality. Many studies used different measures for the same outcome. For example, peak performance or walking ability was measured by maximal walking distance (MWD), maximal walking time (MWT), absolute claudication distance (ACD), or peak walking time (PWT). Likewise, claudication onset was measured by pain‐free walking distance (PFWD), pain‐free walking time (PFWT), initial claudication distance (ICD), or claudication onset time (COT).
Table 1.
Study Characteristics
| Study | Study Design (N) | Quality | Outcomes |
|---|---|---|---|
| Cilostazol vs usual care | |||
| Beebe, 1999 | RCT (418) | Good | MWD, PFWD, quality of life, safety, mortality, amputation |
| Hobbs, 2007 | RCT (38) | Good | ACD, ICD, safety |
| Strandness, 2002 | RCT (393) | Fair | MWD, safety, mortality |
| Cilostazol vs placebo | |||
| Dawson, 1998 | RCT (77) | Good | ACD, ICD, safety |
| Money, 1998 | RCT (212) | Fair | ACD, ICD, quality of life, safety, mortality |
| Hiatt, 2008; Stone, 2008 | RCT (1435) | Good | Mortality, safety |
| Soga, 2009 | RCT (78) | Good | Mortality, safety, amputation |
| Cilostazol vs pentoxifylline vs placebo | |||
| Dawson, 2000 | RCT (699) | Fair | MWD, PFWD, safety |
| Exercise vs usual care | |||
| Bronas, 2011 | RCT (41) | Good | MWD, PFWD |
| Crowther, 2008 | RCT (21) | Fair | PFWT |
| Gardner, 2002 | RCT (61) | Fair | ACD, ICD, mortality, quality of life, safety, amputation |
| Gardner, 2011 | RCT (119) | Good | PWT, COT, quality of life |
| Gelin, 2001 | RCT (264) | Fair | MWD, mortality, amputation, quality of life |
| Gibellini, 2000 | RCT (40) | Fair | ACD, ICD |
| Hobbs, 2006 | RCT (23) | Fair | ACD, ICD, safety |
| Hobbs, 2007 | RCT (38) | Good | ACD, ICD, safety |
| Tsai, 2002 | RCT (53) | Poor | PWT, COT, quality of life |
| Exercise vs endovascular | |||
| Greenhalgh, 2008 | RCT (127) | Fair | MWD, ICD, mortality, safety, quality of life |
| Kruidenier, 2011 | RCT (70) | Good | ACD, quality of life |
| Mazari, 2012 | RCT (178) | Good | MWD, ICD, quality of life, safety |
| Perkins, 1996 | RCT (56) | Fair | MWD, mortality, safety |
| Gelin, 2001 | RCT (264) | Fair | MWD, mortality, amputation, quality of life |
| Spronk, 2009 | RCT (150) | Fair | MWD, PFWD, mortality, quality of life, safety |
| Hobbs, 2006 | RCT (23) | Fair | ACD, ICD, safety |
| Nordanstig, 2011 | RCT (200) | Good | MWD, mortality, quality of life, amputation |
| Endovascular vs usual care | |||
| Gelin, 2001 | RCT (264) | Fair | MWD, mortality, quality of life, amputation |
| Hobbs, 2006 | RCT (23) | Fair | ACD, ICD, safety |
| Nylaende, 2007 | RCT (56) | Good | MWD, PFWD, mortality, quality of life |
| Whyman, 1997 | RCT (62) | Fair | ICD, MWD |
| Endovascular vs usual care vs exercise | |||
| Murphy, 2012 | RCT (111) | Good | PWT, COT, quality of life, safety |
| Endovascular vs exercise | |||
| Greenhalgh, 2008 | RCT (127) | Fair | ICD, MWD, quality of life, mortality, safety |
| Hobbs, 2006 | RCT (23) | Fair | ACD, ICD, safety |
| Nordanstig, 2011 | RCT (200) | Good | MWD, mortality, quality of life, amputation |
| Kruidenier, 2011 | RCT (70) | Good | ACD, quality of life |
| Mazari, 2012 | RCT (178) | Good | MWD, ICD, quality of life, safety |
| Gelin, 2001 | RCT (264) | Fair | MWD, mortality, amputation, quality of life |
| Perkins, 1996 | RCT (56) | Fair | MWD, mortality, safety |
| Spronk, 2009 | RCT (150) | Fair | MWD, PFWD, mortality, quality of life, safety |
Abbreviations: ACD, absolute claudication distance; COT, claudication onset time; ICD, initial claudication distance; MWD, maximal walking distance; MWT, maximal walking time; N, number of patients; PFWD, pain‐free walking distance; PWT, peak walking time; RCT, randomized controlled trial; TWD, total walking distance.
Effects on Maximal Walking Measures
Twenty‐five studies reported the maximal walking measures MWD, ACD, or PWT. There was significant heterogeneity in the study protocols and data reporting. Of these studies, 16 reported the distance measures MWD or ACD and met criteria for inclusion in the random‐effects meta‐analysis (Figure 2A). Due to inconsistent and imprecise results and low strength of evidence, pentoxifylline studies were removed from this analysis. When compared with usual care, large effects were seen with exercise training (9 studies, moderate strength of evidence) and with endovascular intervention plus exercise (2 studies, low strength of evidence). Moderate effects were seen with cilostazol (6 studies, low strength of evidence) and with endovascular intervention (5 studies, moderate strength of evidence). Clinically, these equate to improvements in MWD or ACD of 150 meters for exercise training (95% CI: 35 to 266 meters, P = 0.01), 93 meters for cilostazol (95% CI: −30 to 216 meters, P = 0.14), 78 meters for endovascular intervention (95% CI: −54 to 210 meters, P = 0.25), and 184 meters for endovascular intervention plus exercise (95% CI: −17 to 383 meters, P = 0.7).
Figure 2.
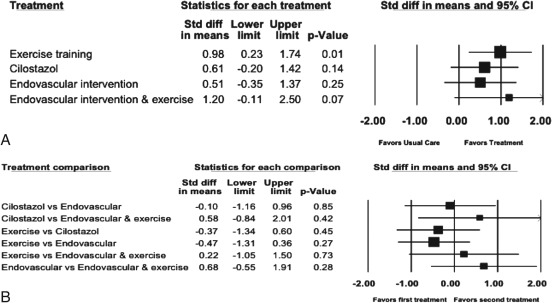
Meta‐analysis of treatment effects on MWD showing (A) treatment vs usual care and (B) treatments vs each other. Data marker size is relative to study weight. Large standardized mean difference = 0.8, medium standardized mean difference = 0.5, small standardized mean difference = 0.2. Abbreviations: CI, confidence interval; MWD, maximal walking distance; std diff, standardized difference.
When indirectly compared with each other, none of the treatment arms were found to be statistically significantly different (Figure 2B). There was a minimal effect between cilostazol and endovascular intervention, favoring cilostazol (MWD or ACD of 15 meters; 95% CI: 176 to −146 meters). There was a small effect between exercise and the combination of endovascular intervention plus exercise, favoring the combination (MWD or ACD of 33 meters; 95% CI: 228 to −159 meters). There were medium effects seen between exercise and endovascular intervention, favoring exercise (MWD or ACD of 71 meters; 95% CI: 199 to −55 meters). Medium effects also were seen between cilostazol and exercise, favoring exercise (MWD or ACD of 56 meters; 95% CI: 201 to −90 meters); between cilostazol and the combination of endovascular intervention plus exercise, favoring the combination (MWD or ACD of 88 meters; 95% CI: 305 to −127 meters); and between endovascular intervention and the combination of endovascular intervention plus exercise, favoring the combination (MWD or ACD of 103 meters; 95% CI: 290 to −83 meters).
Effects on Claudication Onset Measures
Twenty‐one studies reported the claudication onset measures PFWD, ICD, or COT. There was significant heterogeneity in the study protocols and data reporting. Of these studies, 12 met criteria for inclusion in the random‐effects meta‐analysis comparing the multiple treatment arms on continuous measures (PROC NLMIXED; Figure 3). Compared with usual care, the summary effect sizes were 0.63 (95% CI: −0.02 to 1.29, P = 0.059) favoring cilostazol; 0.69 (95% CI: 0.23 to 1.15, P = 0.003) favoring exercise training; and 0.79 (95% CI: 0.29 to 1.29, P = 0.002) favoring endovascular intervention. Clinically, these equate to improvements in ICD or PFWD of 35 meters for cilostazol (95% CI: −1.3 to 45 meters, P = 0.059), 39 meters for exercise training (95% CI: 9 to 65 meters, P = 0.003), and 44 meters for endovascular intervention compared with usual care (95% CI: 16.3 to 71.7 meters, P = 0.002).
Figure 3.
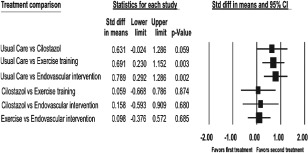
Meta‐analysis of treatment effects on ICD. Data marker size is relative to study weight. Large standardized mean difference = 0.8, medium standardized mean difference = 0.5, small standardized mean difference = 0.2. Abbreviations: CI, confidence interval; ICD, initial claudication distance; std diff, standardized difference.
There was no effect between exercise training and cilostazol (effect size, 0.06). Clinically, this equated to an improvement of 3 meters (95% CI: −37 to 43.6 meters, P = 0.874). There were small effects between endovascular intervention and cilostazol (effect size, 0.16) and between endovascular intervention and exercise (effect size, 0.10), both favoring endovascular intervention. Clinically, these equated to improvements of 9 meters (95% CI: −33 to 50 meters, P = 0.680) and 6 meters (95% CI: −21 to 31.5 meters, P = 0685), respectively. The overall strength of evidence was rated low for all comparisons.
Effects on Quality‐of‐Life Measures
Thirteen studies reported measures of quality of life, such as the SF‐36, Walking Impairment Questionnaire (WIQ), European Quality of Life questionnaire (EuroQOL), Vascular Quality of Life questionnaire (VascuQOL), or Peripheral Artery Questionnaire (PAQ). However, only SF‐36 was used frequently enough to allow for meta‐analysis. There was significant heterogeneity in the study protocols and data reporting. Of these studies, 10 met criteria for inclusion in the random‐effects meta‐analysis comparing the multiple treatment arms on continuous measures (PROC NLMIXED; Figure 4A). Compared with usual care, the summary effect sizes were statistically significant for cilostazol (2 studies; P = 0.03), exercise training (7 studies; P = 0.0003), and endovascular intervention (6 studies; P = 0.0001). Thus, cilostazol and exercise training had moderate effects on physical functioning, whereas endovascular intervention had large effects. Clinically, the range of the SF‐36 physical functioning domain is 0 to 100, and the effect sizes above equate to an improvement in SF‐36 physical functioning domain scores of 4.4 for cilostazol (95% CI: 0.5 to 8.3, P = 0.0278), 5.6 for exercise training (95% CI: 2.5 to 8.6, P < 0.001), and 6.1 for endovascular intervention (95% CI: 3.0 to 9.2, P < 0.001). Figure 4B shows that the effect sizes comparing cilostazol, exercise training, and endovascular intervention were negligible or small, ranging from 0.05 to 0.38. This corresponded to improvements in the SF‐36 physical functioning domain scores of 0.5 to 3.8. The overall strength of evidence was rated low for all comparisons on the basis of consistent results of an indirect analysis with a wide CI.
Figure 4.
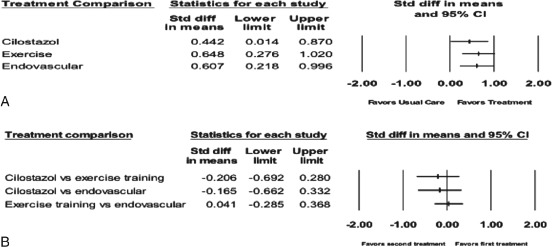
Meta‐analysis of treatment effects on quality of life showing (A) treatment vs usual care and (B) treatments vs. each other. Data marker size is relative to study weight. Large standardized mean difference = 0.8, medium standardized mean difference = 0.5, small standardized mean difference = 0.2. Abbreviations: CI, confidence interval; std diff, standardized difference.
Effects on Safety
Seventeen RCTs reported safety concerns. Harms were measured in 10 studies comparing medical therapy and usual care, 3 comparing exercise training and usual care, 3 comparing endovascular revascularization and usual care, and 5 comparing endovascular revascularization and exercise training. Five studies reported both headache and diarrhea, 5 reported serious adverse events, and 3 reported bleeding.
A random‐effects meta‐analysis of the 5 studies comparing cilostazol with placebo and reporting headache showed an estimated OR of 3.00 (95% CI: 2.29 to 3.95) favoring placebo. There was no evidence of heterogeneity (Q = 2.46 for 4 degrees of freedom; P = 0.65, I 2 = 0.00). A random‐effects meta‐analysis of the 5 studies comparing cilostazol with placebo and reporting diarrhea showed an estimated OR of 2.51 (95% CI: 1.58 to 3.97) favoring placebo. There was no evidence of heterogeneity (Q = 5.85 for 4 degrees of freedom; P = 0.21, I 2 = 31.61). A meta‐analysis of the 3 studies comparing cilostazol with placebo and reporting palpitations showed an estimated OR of 18.11 (95% CI: 5.95 to 55.13) favoring placebo. There was no evidence of heterogeneity (Q = 0.78 for 2 degrees of freedom; P = 0.68, I 2 = 0.00).
We identified no studies that measured contrast nephropathy, radiation, infection, or exercise‐related harms. No studies reported on whether any harms varied by subgroup such as age, sex, race, risk factors, comorbidities, or anatomic location of disease.
Discussion
We conducted a systematic review and meta‐analysis of the medical literature to evaluate the comparative effectiveness of medical therapy, supervised exercise, and endovascular revascularization in patients with IC. Our review has 4 major findings. First, a meta‐analysis of 16 studies suggests that, compared with usual care, maximal walking measures were improved to a greater extent with supervised exercise than with medical therapy or endovascular intervention (low strength of evidence). However, when compared indirectly, there were no significant differences between medical therapy (with cilostazol), supervised exercise, and endovascular intervention. Second, meta‐analysis of 12 studies demonstrates that exercise training and endovascular intervention, but not cilostazol, improved initial claudication measures compared with usual care (low strength of evidence). Third, with respect to quality of life, a meta‐analysis of 13 studies suggests that although all treatment modalities were superior to usual care, there was no significant difference between modalities (low strength of evidence). Fourth, heterogeneity in functional endpoints, single‐arm observational study design, and poor subgroup reporting significantly limit comparative effectiveness analysis in PAD.
Role of Optimal Medical Therapy
Our findings on the benefits of supervised exercise are consistent with existing systematic reviews of exercise therapy in patients with IC.11, 12 Although several different outcome measures for walking distance and time were reported, the existing data consistently demonstrate improved walking measures with exercise training when indirectly compared with usual care or medical therapy. In contrast to a 2008 Cochrane review,13 however, our analysis found an insignificant benefit for cilostazol compared with usual care for improving maximal walking parameters and only a trend toward benefit in claudication distance. This discrepancy may be because the previous review included unpublished studies and studies performed prior to 1995 and assessed only ICDs. Given that medical therapy for PAD and PAD risk factors has improved since 1995, our analysis has the benefit of a more contemporary and larger total patient sample, including the CLEVER trial, which is the first multicenter comparative effectiveness trial in IC to contain and optimal medical‐therapy arm.7 Although the CLEVER trial was not powered to assess the relative efficacy of cilostazol in the medical‐therapy arm, our results in a larger set of patients are consistent with the findings in CLEVER, where medical therapy did not result in significant improvement in maximal walking measures. Taken together, these findings raise questions about the relative therapeutic usefulness of cilostazol in treating contemporary patients with IC.
Comparison With National Institute for Health and Clinical Excellence Guidelines
Furthermore, our findings contradict the recent systematic review for the National Institute for Health and Clinical Excellence (NICE) guidelines,14 where, from a limited number of studies, the NICE investigators found improvements in maximum walking distance from supervised exercise alone or in combination with angioplasty when compared with usual care. The discrepancy is likely due to the differing methodologies and sample sizes between the NICE review and the present review. The NICE systematic review focused on direct comparisons of specific therapies, so the number of studies identified for each comparison was low. In contrast, by using a network meta‐analytic approach to allow for indirect comparisons, we increased study sample size and subsequently assessed comparative effectiveness across all treatment strategies—medical therapy, exercise training, endovascular intervention, and surgical revascularization—using an effect size analysis on continuous measures (eg, walking distance, claudication onset, and quality of life) and a random‐effects meta‐regression model for dichotomous outcomes (eg, mortality, amputation, periprocedural complications). Our methodology also allowed for meta‐analysis of quality‐of‐life data, which was seen in only a small number of studies per comparison in the NICE review, thereby preventing meta‐analysis.
Research Gaps
Given that the clinical presentation and comorbidities of patients with IC are highly variable, it is likely that no single treatment strategy will be appropriate for all patients. Knowing the relative efficacies of multiple treatment modalities in various clinical subgroups is important for clinical decision‐making. Thus, further studies of comparative effectiveness in IC should target relevant anatomic and disease subgroups. Our review found the literature insufficient to allow for the evaluation of the impact of anatomic locations, comorbidities, and disease severity on the relative efficacy of treatment modalities. Although CLEVER was designed to address comparative effectiveness of treatment modalities in a subgroup (aortoiliac disease) of IC patients, other populations in which endovascular or surgical revascularization may be less durable and associated with greater rates of adverse outcomes (such as patients with chronic renal disease and diabetes mellitus) may represent attractive targets for future comparative effectiveness studies in IC.
To effectively carry out such studies, standardization of both functional and quality‐of‐life outcomes will be necessary. The present study found that the currently available evidence demonstrated significant heterogeneity in the use of outcome measures to assess functional capacity in the IC population. Functional endpoints included MWD, MWT, ACD, and PWT. Likewise, claudication onset was measured by ICD, PFWD, COT, PFWT, and others. As a result, effect‐size analysis had to be performed across treatment strategies for our report. Quality‐of‐life measures also varied among 5 instruments (SF‐36, EuroQOL, WIQ, PAQ, and VascuQOL). We focused on the results of the SF‐36 physical functioning score because it was most commonly reported. However, generic health‐related quality‐of‐life measures, such as the SF‐36, are theoretically less responsive to change than disease‐specific measures. From the limited studies we analyzed, there was a large effect of various therapies on improving quality of life. Validation of this finding in future research using both general and disease‐specific quality‐of‐life measures is encouraged, and treatment studies that evaluate changes in quality of life with medical therapy, exercise, and invasive approaches are needed.
Study Limitations
Our review was limited to English‐language studies and focused on those that compared ≥2 treatment modalities. This excluded the single‐arm studies examining endovascular or surgical therapy, which encompass most of the current literature on IC. We further limited studies to those completed after 1995 because of significant subsequent changes in the quality of adjunctive medical care, including antiplatelet agents, antihypertensive agents, oral hypoglycemic agents, and lipid‐lowering agents such as statins. In doing so, we may have excluded studies that assessed the efficacy of surgical revascularization, the most mature treatment modality for IC. We were also unable to ascertain either baseline or follow‐up ankle‐brachial index testing results, mainly due to underreporting. Although a network meta‐analysis allows indirect evidence for comparative effectiveness studies, it also relies on similar patient populations, study designs, outcome measures, and follow‐up, a fact that may introduce potential bias in the current report.15
Furthermore, we excluded pentoxifylline from our meta‐analysis due to insufficient strength of evidence. Most pentoxifylline studies were conducted prior to 1995, and those conducted since 1995 were marked by very low sample size and inconsistency, leading to imprecise effect‐size estimates. This is consistent with the ACC/AHA recommendation of pentoxifylline as an alternative to cilostazol (IIb) because its clinical effectiveness in IC is marginal and not well established.16
Despite our stringent inclusion criteria, the strength of evidence for the outcomes assessed was low, thus highlighting one of the major limitations of the existing literature: substandard study designs lacking appropriate medical therapy or comparison groups.
Conclusion
This systematic review and meta‐analysis found that several therapies were effective at improving walking measures and quality of life. However, comparisons between treatment modalities were limited by low strength of evidence, reflecting multiple gaps in the existing literature. These gaps include few direct treatment strategy trials, heterogeneity of endpoints, and poor subgroup reporting.
Despite the National Heart, Lung, and Blood Institute's PAD awareness campaign and the explosion of endovascular and surgical techniques for lower‐extremity revascularization, our analysis indicates that the evidence base has not kept pace. With an ever‐increasing array of treatment modalities available, rigorous treatment strategy trials and real‐world multicenter registries with appropriate endpoints and inclusion of key subgroups will be needed to guide clinical practice.
Supporting information
AppendixS1. Study References
Acknowledgments
The authors thank Megan von Isenburg, MSLS, for help with the literature search and retrieval; Rachael Posey, MSLS, and Megan Chobot, MSLS, for project coordination; and Liz Wing, MA, for editorial help.
This project was funded under contract no. 290‐2007‐10066‐I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. MRP has received research grants from Johnson & Johnson, Pluristem, and AstraZeneca and has served as a consultant for Baxter, Genzyme, Bayer, and Ortho‐McNeil‐Janssen. WSJ has received research grants from AstraZeneca, Boston Scientific Corporation, Bristol‐Myers Squibb, and the American Heart Association and has served as a consultant/received honoraria from the American College of Physicians and American Physician Institute SV has received research grants from Boston Scientific Corporation.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–1264. [DOI] [PubMed] [Google Scholar]
- 2. Mahoney EM, Wang K, Keo HH, et al; Reduction of Atherothrombosis for Continued Health (REACH) Registry Investigators . Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes. 2010;3:642–651. [DOI] [PubMed] [Google Scholar]
- 3. Feinglass J, McCarthy WJ, Slavensky R, et al; The Chicago Claudication Outcomes Research Group . Effect of lower extremity blood pressure on physical functioning in patients who have intermittent claudication. J Vasc Surg. 1996;24:503–512. [DOI] [PubMed] [Google Scholar]
- 4. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. [DOI] [PubMed] [Google Scholar]
- 5. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery. J Am Coll Cardiol. 2011;58:2020–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahimastos AA, Pappas EP, Buttner PG, et al. A meta‐analysis of the outcome of endovascular and noninvasive therapies in the treatment of intermittent claudication [provisional abstract]. J Vasc Surg. 2011;54:1511–1521. [DOI] [PubMed] [Google Scholar]
- 7. Murphy TP, Cutlip DE, Regensteiner JG, et al; CLEVER Study Investigators . Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six‐month outcomes from the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) study. Circulation. 2012;125:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones WS, Schmit KM, Vemulapalli S, et al. Treatment Strategies for Patients With Peripheral Artery Disease Comparative Effectiveness Review No. 118. AHRQ publication 13‐EHC090‐EF (prepared by the Duke Evidence‐based Practice Center under contract no. 290‐2007‐10066‐I). http://www.effectivehealthcare.ahrq.gov/search‐for‐guides‐reviews‐and‐reports/?pageaction=displayproduct&productid=1518. Published May 2013. Accessed November 1, 2014.
- 9. Agency for Healthcare Research and Quality . Methods Guide for Effectiveness and Comparative Effectiveness Reviews Rockville, MD: Agency for Healthcare Research and Quality; January 2014 (update). http://www.effectivehealthcare.ahrq.gov/ehc/products/60/318/CER‐Methods‐Guide‐140109.pdf. Accessed March 16, 2012.
- 10. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- 11. Leng GC, Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2000;2:CD000990. [DOI] [PubMed] [Google Scholar]
- 12. Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2008;4:CD000990. [DOI] [PubMed] [Google Scholar]
- 13. Robless P, Mikhailidis DP, Stansby GP. Cilostazol for peripheral arterial disease. Cochrane Database Syst Rev. 2008;1:CD003748. [DOI] [PubMed] [Google Scholar]
- 14. National Institute for Health and Clinical Excellence . Lower Limb Peripheral Arterial Disease: Diagnosis and Management. NICE Clinical Guideline. http://www.nice.org.uk/guidance/cg147. Published August 2012. Accessed February 13, 2015. [Google Scholar]
- 15. Cipriani A, Higgins JP, Geddes JR, et al. Conceptual and technical challenges in network meta‐analysis. Ann Intern Med. 2013;159:130–137. [DOI] [PubMed] [Google Scholar]
- 16. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AppendixS1. Study References


