Abstract
Myocardial perfusion imaging (MPI), using radiotracers, has been in routine clinical use for over 40 years. This modality is used for the detection of coronary artery disease (CAD), risk stratification, optimizing therapy, and follow‐up of patients with CAD. Molecular cardiovascular imaging using targeted radiotracers provides a unique opportunity for imaging biochemical and metabolic processes, and cell membrane transporter and receptor functions at a cellular and molecular level in experimental animal models as well as in humans. Cardiac imaging using radiolabeled free fatty acid analogues and glucose analogues enable us to image myocardial ischemia directly as an alternative to stress‐rest MPI. Direct ischemia imaging techniques can avoid and overcome some of the limitations of standard stress‐rest MPI. This article describes recent studies using 18F‐fluorodeoxyglucose (18FDG) for myocardial ischemia imaging.
Introduction
The diagnostic evaluation and management of patients with coronary artery disease (CAD) has undergone major new advances in the last few decades. These new developments have resulted in a dramatic decrease in the morbidity and mortality from CAD. Routine availability of safe, simple and reliable noninvasive modalities for the diagnosis, risk stratification, and follow‐up of a wide range of patient populations with established or suspected CAD has greatly facilitated the development and appropriate use of a very wide array of therapeutic options. Myocardial perfusion imaging (MPI) using radiotracers is a notable development in this field.1, 2, 3 Introduction of a new generation of radiotracers for MPI, agents for pharmacological stress test, and advances in gamma imaging cameras and image processing have resulted in its wide clinical acceptance.4, 5, 6, 7, 8 MPI has played an important role in large clinical trials, which resulted in the evolution of the current therapy of CAD.1, 8 Despite all of these strengths, MPI does suffer from several drawbacks. The sensitivity and specificity of MPI for the diagnosis of CAD are relatively suboptimal.4 Furthermore, the sensitivity of MPI for detecting individual coronary vessels with significant obstructive disease is quite low. Abnormalities on MPI can sometimes be unimpressive or even absent despite the presence of significant underlying multivessel disease. Attenuation artifacts, artifacts due to extracardiac radiotracer activity, and technical issues are relatively common. These artifacts are sometimes indistinguishable from true perfusion abnormalities. These artifacts cannot be adequately corrected or prevented using current technology. Some of these drawbacks are as a consequence of the “cold‐spot” nature of this imaging, where the target abnormality (myocardial ischemia) appears as an area of deficient or reduced radiotracer uptake. A wide variety of artifacts can also mimic cold‐spot abnormality. These drawbacks of MPI warrant consideration of alternative imaging modalities for detecting CAD.4, 9, 10
Molecular Cardiovascular Imaging
The repertoire of radionuclide imaging includes tools for noninvasive imaging of biochemical and metabolic phenomena, cell membrane receptors, and transporters in humans. This permits the development of highly innovative, targeted molecular imaging techniques for clinical use. Molecular imaging of the myocardium with ischemia can overcome the drawbacks of MPI.10, 11 The Table 1 provides a list of the established nuclear imaging modalities as well as the investigational modalities in various stages of development. Molecular imaging techniques are under development for studying myocardial metabolism and imaging atheroma, apoptosis, and angiogenesis. This review is focused on molecular imaging techniques for imaging myocardial ischemia.
Table 1.
Established and Experimental/Investigational Scintigraphic Cardiovascular Imaging Techniques
| Indication | Technique | Radiotracers | Current Status |
|---|---|---|---|
| Established nuclear imaging techniques | |||
| Myocardial perfusion imaging | SPECT | Tl‐201 | Less commonly used |
| 99mTc‐sestamibi (Cardiolite) | In extensive clinical use | ||
| 99mTc‐tetrofosmin (Myoview) | In extensive clinical use | ||
| PET | 82Rb | In frequent clinical use | |
| 13N ammonia, 15O‐water | Rarely used | ||
| 18F‐flupiridaz | Investigational, not FDA approved | ||
| Cardiac adrenergic neuronal imaging | SPECT | 123I‐MIBG | Only recently FDA approved |
| Myocardial necrosis imaging | SPECT | 111In‐antimyosin | Not in clinical use anymore |
| Experimental/investigational nuclear imaging techniques | |||
| Myocardial apoptosis imaging | SPECT | 99mTc‐Annexin | Investigational, not FDA approved |
| Myocardial necrosis imaging | SPECT | 99mTc‐glucarate | Investigational, not FDA approved |
| Fatty acid metabolism imaging | SPECT | 123I‐BMIPP | Investigational, not FDA approved |
| Glucose metabolism/imaging, myocardial ischemia imaging | PET | 18FDG | Investigational |
| Vascular calcification imaging | PET | 18F‐NaF | Investigational, not FDA approved |
| Hypoxia imaging | PET | 18F‐fluoromisonidazole | Investigational, not FDA approved |
Abbreviations: 18FDG, 18F‐fluorodeoxyglucose; 99mTc, technetium‐99 m; 123I‐BMIPP, 123I‐labeled β‐methyl iodophenyl pentadecanoic acid; FDA, Food and Drug Administration; PET, positron‐emission tomography; SPECT, single‐photon emission computed tomography.
Myocardial Ischemia Imaging
Direct imaging of myocardial ischemia can be accomplished by targeting regional myocardial hypoxia, which parallels regional myocardial ischemia, or by targeting metabolic accompaniments of myocardial ischemia, which can act as signature of myocardial ischemia.
Hypoxia Imaging
The partial pressure of oxygen, or oxygen tension (pO2), inside the cells in normally oxygenated and adequately perfused tissues ranges from 30 to 50 mm Hg. The pO2 inside the hypoxic cells is substantially lower. pO2 in the hypoxic tumors can be as low as 3 to 10 mm Hg.12, 13 Nitroimidazoles are compounds that have a core nitroimidazole ring with an NO2 moiety and varying side chains.12, 13 Nitroimdazoles are freely diffusible across the cell membranes. Inside the cells with normal pO2, nitroimdazoles undergo a reversible reduction to a reduced moiety (NO2 −). The reduced and nonreduced moieties exist in equilibrium. Under hypoxia, reduced nitroimidazole undergoes further reduction to NO2 −− moiety. This reaction is irreversible. NO2 −− is a highly reactive species that reacts indiscriminately with intracellular macromolecules and binds with them. Thus, NO2 −− is trapped inside the hypoxic cells. Several radiolabeled nitroimidazole derivatives have been used for imaging tissues or organs with hypoxia.12, 13, 18 F‐fluoromisonidazole is a commonly used positron‐emission tomography (PET) imaging agent for imaging tumors that are hypoxic.12, 13 These compounds can be used for imaging tumor hypoxia, but they are not suitable for imaging stress‐induced myocardial ischemia because of the evanescent nature of the later phenomenon.
Metabolic Imaging
An understanding of myocardial energetics and metabolism can provide imaging tools for targeting myocardial ischemia. Cardiac metabolism and its alteration in the ischemic milieu provides possible targets for imaging exercise‐induced myocardial ischemia.13, 14, 15, 16, 17, 18, 19, 20, 21, 22 Myocardium has an extremely high metabolic demand to meet the energy needs of its contractile function. Approximately 90% of the cardiac energy requirement is for its pumping function. Myocardial oxygen consumption is in the range of 8 to 15 mL/min/100 g at rest. This increases to 60 to 70 mL/min/100 g of tissue at a high workload.22 The heart requires a steady supply of energy substrates and oxygen for supporting its metabolism. Normal myocardium extracts and metabolizes a variety of energy substrates such as glucose, pyruvate, lactate, free fatty acids, ketone bodies, and amino acids. Of these substrates, free fatty acids and glucose are the largest substrates for energy production (Figure 1). The relative uptake and proportional contribution of these substrates to cardiac metabolism varies widely and depends on a multitude of factors such as a fed or a fasting state, plasma insulin and catecholamine levels, oxygen availability, and the circulating levels of these substrates in the plasma. While fasting, the insulin and glucose levels are low, and free fatty acid levels are high. Therefore, under conditions of fasting, free fatty acids are the main contributors to energy production. The contribution of glucose metabolism is relatively low. In contrast, blood glucose and insulin levels rise following a carbohydrate‐rich meal. Therefore, glucose contributes to a greater fraction of energy production in the postprandial state. Exercise increases catecholamines and free fatty acid levels, and free fatty acids contribute to a higher proportion of metabolism during exercise. However, free fatty acid metabolism is an obligatory aerobic and is highly sensitive to reduced oxygen tension. Myocardial ischemia profoundly alters myocardial metabolism and substrate utilization (Figure 1). The ischemia‐induced suppression of free fatty acid metabolism persists significantly longer than the duration of ischemia. This provides a signature of myocardial ischemia. Glucose metabolism is a 2‐step process: glycolysis and oxidative phosphorylation. The first step, glycolysis, is an anaerobic process that converts 1 molecule of glucose to 2 molecules of pyruvate in the cytosol. Pyruvate is reduced to lactate under hypoxia. Under normal oxygen tension, pyruvate enters the Krebs' cycle, an obligatory aerobic process. This process occurs in the mitochondria and through a long sequence of oxidative reactions, pyruvate is converted to carbon dioxide and water. Glycolysis becomes the predominant source of energy production, which sustains the myocardium under hypoxia or ischemia. However, glycolysis is a relatively inefficient source of energy production. One mole of glucose generates only 2 net moles of adenosine triphosphate (ATP) through glycolysis. In comparison, oxidative phosphorylation yields approximately 38 moles of ATP. Ischemic myocardium, therefore, requires a large supply of glucose for surviving through glycolysis. An increased glucose uptake by the ischemic myocardium is facilitated by an immediate translocation of highly specialized and efficient glucose transporters GLUT‐4 and GLUT‐1 (GLUT) from cytosol to the sarcolemma with the onset of ischemia.19 An interesting feature of this process is that once GLUT transports are translocated to the sarcolemma, they persist in the sarcolemma significantly longer than the duration of myocardial ischemia.19 Thus, the glycolysis as a signature of myocardial ischemia persists longer than the duration of actual ischemia, providing us with an opportunity to image myocardial ischemia.
Figure 1.
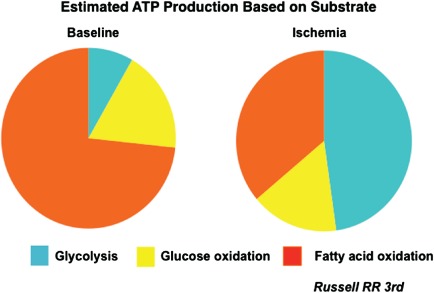
A diagrammatic representation of the relative proportion of myocardial substrate utilization. Under resting conditions, fatty acid metabolism contributes to nearly 70% to 75% energy generation, whereas the remaining comes from glucose metabolism (approximately 8% from glycolysis and 18% from oxidative metabolism). Myocardial substrate utilization changes dramatically with the onset of myocardial ischemia, with glycolysis contributing to nearly 50% of the energy production and a significant reduction in fatty acid uptake and metabolism. Courtesy of Dr. Raymond R. Russell, III, Yale University School of Medicine. Abbreviations: ATP, adenosine triphosphate.
The analogues of free fatty acids and glucose can be used for imaging myocardial ischemia after suitably radiolabeling them. The radiolabeled free fatty acid analogues show a reduced uptake (cold spot), whereas radiolabeled glucose analogues show an enhanced uptake (hot spot) in myocardial regions following an episode of myocardial ischemia. These metabolic changes are instantaneous with the onset of myocardial ischemia, but persist for a significantly longer than the duration of myocardial ischemia. This article reviews the concept and basic principles of scintigraphic imaging of myocardial ischemia imaging.
18F‐Fluorodeoxyglucose for Imaging Myocardial Ischemia
Myocardial ischemia is associated with a significant and persistent upregulation of regional glucose uptake in comparison to the normally perfused myocardium.15, 16, 17, 18, 19 Normal myocardium has a lower glucose uptake on exercising under fasting milieu and a higher uptake of free fatty acids. Therefore, a significant metabolic differential separates the normal from the ischemic myocardium. Deoxyglucose is a glucose analogue, and tracks cellular glucose uptake and initial metabolic steps in the tissues. 18 F‐fluorodeoxyglucose (18FDG) is a commercially available radiotracer that is used for imaging glucose uptake and metabolism. 18FDG is used for imaging a wide variety of malignant tumors that show chronic hypoxia and increased glucose uptake.23 18FDG is also useful for detecting viable or hibernating myocardium.24, 25 18FDG can also be used for imaging exercise‐induced myocardial ischemia. The profound metabolic differential between the normal and the ischemic myocardium on exercise permits the use of 18FDG for myocardial ischemia imaging.26, 27, 28, 29, 30, 31, 32, 33, 34, 35 18FDG is imaged using dedicated PET imaging cameras. However, specially designed single‐photon emission computed tomography (SPECT) cameras with modified sodium iodide detectors and thicker collimators suitable for high‐energy radioisotopes can also be used for 18FDG imaging.29, 32, 36
The potential of 18FDG as a myocardial ischemia imaging agent has been evaluated in several small studies by Abramson et al,26 Camici et al,27 and Araujo et al.28 These investigators observed increased 18FDG uptake in myocardial segments corresponding to the reversible perfusion abnormalities on MPI. Abbott et al observed increased 18FDG in less than one‐half of the vascular territories showing reversible perfusion abnormalities when 18FDG was injected 1 to 1.5 hours following completion of treadmill exercise in CAD patients.30 He et al performed simultaneous perfusion and ischemia imaging of the heart by injecting 18FDG and technetium‐99 m (99mTc)‐sestamibi at peak exercise in 26 patients with angina symptoms and no previous myocardial infarction.29 They used a SPECT camera capable of PET imaging to compare simultaneous perfusion and ischemia imaging. Coronary angiography was used to compare MPI and ischemia imaging. Twenty‐two patients had ≥50% narrowing of ≥1 coronary arteries, of which 18 had abnormalities on MPI (sensitivity 82%), and 20 had abnormally increased cardiac 18FDG uptake (sensitivity 91%, P = not significant). Myocardial perfusion abnormalities were seen in 25 vascular territories out of a total of 51 vessels with ≥50% luminal narrowing (sensitivity 49%). Abnormally increased 18FDG uptake was noticed in 34 vascular territories (sensitivity 67%, P < 0.01). These data provided definitive proof that exercise 18FDG can be used for imaging exercise‐induced myocardial ischemia (Figures 2 and 3). Similar results were obtained by Sasikumar et al, who performed exercise‐rest SPECT MPI and exercise 18FDG PET imaging on separate days in 45 patients with suspected CAD.37, 38 They observed a significantly higher sensitivity (96% vs 56%, P < 0.001), but lower specificity (44% vs 72%) of exercise 18FDG compared to exercise‐rest SPECT MPI.
Figure 2.
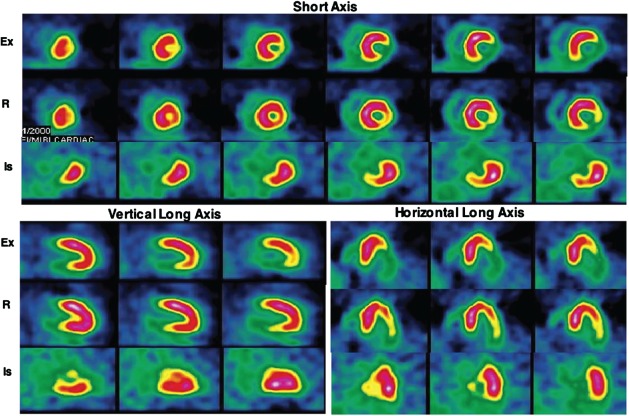
Representative exercise (Ex) and rest (R) technetium‐99 m‐sestamibi and exercise 18 F‐fluorodeoxyglucose (18FDG) images of a patient with angina in the short axis, and vertical and horizontal long axes. This patient had no history of myocardial infarction. A large area of partially reversible perfusion abnormality involving the inferior and lateral walls is seen on the perfusion images. Intense 18FDG uptake is seen in the areas corresponding to the perfusion abnormalities. On coronary angiography, 100% occlusion of the right coronary artery and a 50% narrowing of the left anterior descending coronary artery were observed. Reproduced with permission from Jain and He.34
Figure 3.
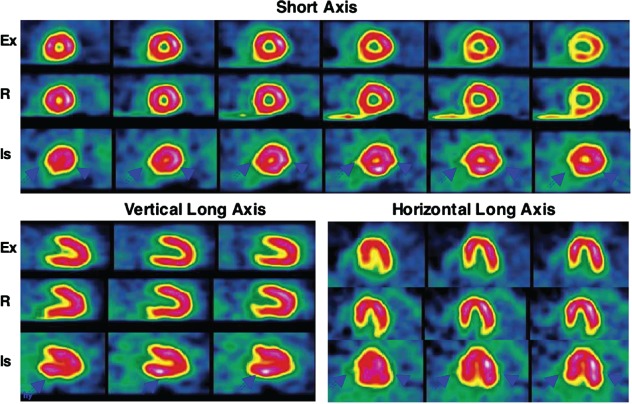
Representative exercise (Ex) and rest (R) technetium‐99 m‐sestamibi and exercise 18 F‐fluorodeoxyglucose (18FDG) images of a patient with angina in the short axis, and vertical and horizontal long axes. This patient had no prior myocardial infarction. No perfusion abnormality is observed on the stress and rest perfusion images. An intense global 18FDG uptake is observed in all 3 vascular territories (solid arrowheads). On coronary angiography, 3‐vessel disease was observed (70% narrowing of the left anterior descending, 60% narrowing of the left circumflex, and 60% narrowing of the right coronary arteries). Reproduced with permission from He et al.30
Dou and colleagues performed exercise and rest cardiac 18FDG and perfusion imaging 24 hours apart in 18 patients with significant CAD.39 Of these 18 patients, 15 showed increased regional 18FDG uptake on exercise images, of which 8 (53%) had no 18FDG uptake, 5 (33%) had reduced 18FDG uptake, and 2 (13%) had persistence of 18FDG uptake on rest 18FDG imaging 24 hours later (Figure 4). Patients with persistence of 18FDG uptake on rest imaging had more extensive 18FDG uptake and developed myocardial ischemia at a lower workload in comparison to the patients with no 18FDG uptake at rest. These data confirm that increased regional myocardial 18FDG uptake on exercise is specific for myocardial ischemia. Increased regional myocardial 18FDG uptake is not observed in a majority of patients if 18FDG is injected 24 hours after an episode of myocardial ischemia. Persistence of increased myocardial 18FDG uptake 24 hours after exercise is a marker for severe CAD and development of high‐risk myocardial ischemia at a low workload. This finding has obvious prognostic and therapeutic implications.
Figure 4.
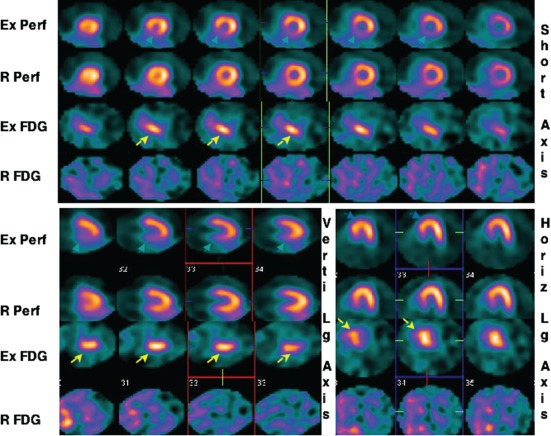
This 49‐year‐old male with exertional angina underwent exercise (Ex) and rest 99mTc‐sestamibi (Perf) and 18 F‐fluorodeoxyglucose (FDG) imaging 24 hours apart. Representative short axis, and vertical (Verti) and horizontal (Horiz) long (Lg) axes slices of the heart are shown. Reversible perfusion abnormality in the posterior septum and inferior walls is observed on this study. Intense FDG uptake on the exercise images (yellow arrows) is seen in the corresponding segments. No FDG uptake is seen in the heart on rest images. This patient was found to have 85% narrowing of the right coronary artery. Reproduced with permission from Dou et al.40
The strengths of myocardial ischemia imaging are evident from these results. The sensitivity of myocardial ischemia imaging is higher compared to stress‐rest MPI for diagnosing CAD. Furthermore, its sensitivity for detecting individual coronary vascular territories with significant luminal obstruction is substantially greater compared to stress‐rest MPI. This is particularly useful in the presence of multivessel CAD, where increased 18FDG may be observed in all diseased vascular territories (Figure 3). It is relatively uncommon to observe reversible perfusion abnormalities in all 3 vascular territories in the presence of severe 3‐vessel CAD. Inferior wall perfusion abnormalities are often difficult to differentiate from artifacts due to diaphragmatic attenuation. Ischemia‐induced increased 18FDG uptake in the inferior wall is not affected by diaphragmatic attenuation artifacts. Exercise 18FDG imaging can also reduce the imaging time and radiation dose to the patients by eliminating the need for rest MPI. These early promising results warrant further large multicenter clinical studies to evaluate the role of stress 18FDG imaging in routine clinical practice.
Fatty Acid Metabolic Imaging for Myocardial Ischemia
The free fatty acid metabolism and its uptake by the heart decrease with the onset of myocardial ischemia. This decreased free fatty acid uptake persists for many hours. Therefore, radiolabeled analogues of free fatty acid can also be used for imaging myocardial ischemia. These agents have also been used for detecting an episode of myocardial ischemia in patients who present several hours after experiencing an episode of chest pain.40, 41, 42, 43, 44 Because this is a cold‐spot imaging agent, where myocardial segments with ischemia show reduced radiotracer uptake, its advantages over MPI are unclear. This technique may be useful for confirming an episode of myocardial ischemia that occurred hours earlier. Several radiolabeled free fatty acid derivatives have been evaluated for imaging cardiac fatty acid metabolism. Of these, 123I‐labeled β‐methyl iodophenyl pentadecanoic acid (123I‐BMIPP) is the most widely studied agent. After its myocardial uptake, it has prolonged myocardial retention. This agent has been in wide use in Japan for the last 20 years. However, in the United States, this is still an investigational agent. Dilsizian and colleagues injected 123I‐BMIPP within a 30‐hour window of exercise‐induced myocardial ischemia in 32 CAD patients.41 They observed decreased regional 123I‐BMIPP uptake in 94% of the myocardial segments with ischemia on stress‐rest MPI. In a meta‐analysis by Inaba and Bergman involving 7 studies and 528 patients, the sensitivity and specificity of resting 123I‐BMIPP imaging for the identifying significant CAD were 78% and 84%, respectively.43 These results support the potential utility of 123I‐BMIPP for imaging metabolic signature of myocardial ischemia or ischemic memory hours following an episode of myocardial ischemia.
Conclusion
Cardiovascular imaging has advanced significantly in the recent years. Recent advances permit ultrafast noninvasive imaging of coronary arterial lumen, atheromatous plaques, and calcium contents of coronary arteries. Nuclear imaging, though lacking the ultrafast speed and high resolution of these techniques, has an unparalleled ability to noninvasively image molecular, biochemical, and metabolic phenomena, and cell membrane receptor and transporter functions under a multitude of physiological and metabolic milieu. Nuclear molecular imaging techniques can be used as clinical imaging tools. Molecular imaging can also provide experimental tools for studying cardiac pathophysiology under various disease conditions and their modulation with therapeutic interventions. Myocardial metabolic imaging using 18FDG and 123I‐BMIPP provides novel diagnostic tools for CAD with different but somewhat complimentary strengths. 18FDG is more suitable for imaging of exercise‐induced myocardial ischemia and diagnosing CAD, whereas 123I‐BMIPP is more suitable for identifying patients presenting with a recent episode of chest pain that resolved spontaneously, leaving no obvious abnormality at presentation, and who warrant closer observation and coronary angiography. Further studies are warranted to establish their role in routine clinical practice.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Iskandrian AE, Hage FG, Shaw LJ, et al. Serial myocardial perfusion imaging: defining a significant change and targeting management decisions. JACC Cardiovasc Imaging. 2014;7:79–96. [DOI] [PubMed] [Google Scholar]
- 2. Zaret BL, Strauss HW, Martin ND, et al. Noninvasive evaluation of regional myocardial perfusion with radioactive potassium: study of patients at rest, exercise, and during anginal pectoris. N Engl J Med. 1973;288:809–812. [DOI] [PubMed] [Google Scholar]
- 3. Cremer P, Hachamovitch R, Tamarappoo B. Clinical decision making with myocardial perfusion imaging in patients with known or suspected coronary artery disease. Semin Nucl Med. 2014;44:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jain D, Zaret BL. Nuclear imaging in cardiovascular medicine In: Rosendorf C, ed. Essential Cardiology 3rd ed New York, NY: Springer; 2013:195–220. [Google Scholar]
- 5. Jain D, Wackers FJ, Mattera J, et al. Biokinetics of technetium‐99 m‐tetrofosmin: myocardial perfusion imaging agent: implications for a one‐day imaging protocol. J Nucl Med. 1993;34:1254–1259. [PubMed] [Google Scholar]
- 6. Travin MI. Cardiac cameras. Semin Nucl Med. 2011;41:182–201. [DOI] [PubMed] [Google Scholar]
- 7. Ghimire G, Hage FG, Heo J, et al. Regadenoson: a focused update. J Nucl Cardiol. 2013;20:284–288. [DOI] [PubMed] [Google Scholar]
- 8. Boden W. Management of chronic coronary disease: is the pendulum returning to equipoise? Am J Cardiol. 2008;101:S69–S74. [DOI] [PubMed] [Google Scholar]
- 9. Zaret BL. Pursuit of the ideal perfusion agent. J Nucl Cardiol. 2002;9:149–150. [DOI] [PubMed] [Google Scholar]
- 10. Beller GA. Will cardiac positron emission tomography ultimately replace SPECT for myocardial perfusion imaging? J Nucl Cardiol. 2009;16:841–843. [DOI] [PubMed] [Google Scholar]
- 11. Jain D, McNulty PH. Exercise‐induced myocardial ischemia: can this be imaged with F‐18‐fluorodeoxyglucose? J Nucl Cardiol. 2000;7:286–288. [DOI] [PubMed] [Google Scholar]
- 12. Lapi SE, Voller TF, Welch MJ. Positron emission tomography imaging of hypoxia. PET Clin. 2009;4:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. J Nucl Med . 2008;49(suppl 2):129S–148S. [DOI] [PubMed] [Google Scholar]
- 14. Neely JR, Rovetto MJ, Oram JF. Myocardial utilization of carbohydrates and lipids. Prog Cardiovasc Dis. 1972;15:289–329. [DOI] [PubMed] [Google Scholar]
- 15. Liedtke AJ. Alterations of carbohydrate and lipid metabolism in the acutely ischemic heart. Prog Cardiovasc Dis. 1981;23:321–336. [DOI] [PubMed] [Google Scholar]
- 16. Schwaiger M, Schelbert HR, Ellison D, et al. Sustained regional abnormalities in cardiac metabolism after transient ischemia in the chronic dog model. J Am Coll Cardiol. 1985;6:336–347. [DOI] [PubMed] [Google Scholar]
- 17. Schwaiger M, Neese RA, Araujo L, et al. Sustained nonoxidative glucose utilization and depletion of glycogen in reperfused canine myocardium. J Am Coll Cardiol. 1989;13:745–754. [DOI] [PubMed] [Google Scholar]
- 18. McNulty PH, Sinusas AJ, Shi CQ, et al. Glucose metabolism distal to a critical coronary stenosis in a canine model of low‐flow myocardial ischemia. J Clin Invest. 1996;98:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McNulty PH, Jagasia D, Cline GW, et al. Persistent changes in myocardial glucose metabolism in vivo during reperfusion of a limited‐duration coronary occlusion. Circulation. 2000;101:917–922. [DOI] [PubMed] [Google Scholar]
- 20. Herraro P, Weinhemer CJ, Dence C, et al. Quantification of myocardial glucose utilization by PET and 1‐carbon‐11‐glucose. J Nucl Cardiol. 2002;9:5–14. [DOI] [PubMed] [Google Scholar]
- 21. Sun D, Nguyen N, Degrado T, et al. Ischemia induces translocation of the insulin‐responsive glucose transporter GLUT 4 to the plasma membrane of cardiac myocytes. Circulation. 1994:89;793–798. [DOI] [PubMed] [Google Scholar]
- 22. Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ciliberto M, Maggi F, Treglia G, et al. Comparison between whole‐body MRI and Fluorine‐18‐Fluorodeoxyglucose PET or PET/CT in oncology: a systematic review. Radiol Oncol. 2013;47:206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Di Carli MF. Assessment of myocardial viability after myocardial infarction. J Nucl Cardiol. 2002;9:229–235. [DOI] [PubMed] [Google Scholar]
- 25. Dilsizian V, Arrighi JA, Diodati JG, et al. Myocardial viability in patients with chronic coronary artery disease. Comparison of 99mTc‐sestamibi with thallium reinjection and 18 F‐fluorodeoxyglucose. Circulation. 1994;89:578–587. [DOI] [PubMed] [Google Scholar]
- 26. Abramson BL, Ruddy TD, de Kemp RA, et al. Stress perfusion/metabolic imaging: a pilot study for a potential new approach to the diagnosis of coronary artery disease in women. J Nucl Cardiol. 2000;7:205–212. [DOI] [PubMed] [Google Scholar]
- 27. Camici P, Araujo LI, Spinks T, et al. Increased uptake of 18 F‐fluorodeoxyglucose in postischemic myocardium of patients with exercise‐induced angina. Circulation. 1986;74:81–88. [DOI] [PubMed] [Google Scholar]
- 28. Araujo LI, McFalls EO, Lammertsma AA, et al. Dipyridamole‐induced increased glucose uptake in patients with single‐vessel coronary artery disease assessed with PET. J Nucl Cardiol. 2001;8:339–346. [DOI] [PubMed] [Google Scholar]
- 29. He ZX, Shi RF, Wu YJ, et al. Direct imaging of exercise‐induced myocardial ischemia with fluorine‐18‐labeled deoxyglucose and Tc‐99 m‐sestamibi in coronary artery disease. Circulation. 2003;108:1208–1213. [DOI] [PubMed] [Google Scholar]
- 30. Abbott BG, Liu YH, Arrighi JA. [18 F]Fluorodeoxyglucose as a memory marker of transient myocardial ischaemia. Nucl Med Commun. 2007;28:89–94. [DOI] [PubMed] [Google Scholar]
- 31. Arrighi JA. F‐18 fluorodeoxyglucose imaging in myocardial ischemia: beyond myocardial viability. J Nucl Cardiol. 2001;8:417–420. [DOI] [PubMed] [Google Scholar]
- 32. He ZX, Shi RF, Wu YJ, et al. Myocardial ischemia, fluorodeoxyglucose, and severity of coronary artery stenosis: The complexities of metabolic remodeling in hibernating myocardium. Circulation. 2004;109:e167–e170. [DOI] [PubMed] [Google Scholar]
- 33. Jain D, He ZX. Direct imaging of myocardial ischemia: a potential new paradigm in nuclear cardiovascular imaging. J Nucl Cardiol. 2008;15:617–630. [DOI] [PubMed] [Google Scholar]
- 34. Jain D, He ZX, Ghanbarinia A. Exercise 18FDG imaging for the detection of CAD: what are the clinical hurdles? Curr Cardiol Rep. 2010;12:170–178. [DOI] [PubMed] [Google Scholar]
- 35. Jain D, He ZX, Ghanbarinia A, et al. Direct imaging of myocardial ischemia with 18FDG: a new potentially paradigm shifting molecular cardiovascular imaging technique. Curr Cardiovasc Imaging Rep. 2010;3:134–150. [Google Scholar]
- 36. Sandler MP, Patton JA. Fluorine‐18 labeled fluorodeoxyglucose myocardial single‐photon emission computed tomography: an alternative for determining myocardial viability. J Nucl Cardiol. 1996;3:342–349. [DOI] [PubMed] [Google Scholar]
- 37. Sasikumar A, Mittal BR, Bhattacharya A, et al. Comparison of Tc‐99 m‐tetrofosmin myocardial perfusion scintigraphy and exercise F‐18‐FDG imaging in detection of myocardial ischemia in patients with coronary artery disease [published online ahead of print August 15, 2014]. J Nucl Cardiol . doi: 10.1007/s12350-014-9954-9. [DOI] [PubMed] [Google Scholar]
- 38. Jain D, He ZX. Direct myocardial ischemia imaging with exercise 18FDG. J Nucl Cardiol. In press. [DOI] [PubMed] [Google Scholar]
- 39. Dou KF, Yang MF, Yang YJ, et al. Direct myocardial ischemia imaging: 18FDG uptake during exercise and at rest in patients with coronary artery disease. J Nucl Med. 2008;49:1986–1991. [DOI] [PubMed] [Google Scholar]
- 40. Herrero P, Gropler RJ. Imaging of myocardial metabolism. J Nucl Cardiol. 2005;12:345–358. [DOI] [PubMed] [Google Scholar]
- 41. Dilsizian V, Bateman TM, Bergmann SR, et al. Metabolic imaging with beta‐methyl‐p‐[(123)I]‐iodophenyl‐pentadecanoic acid identifies ischemic memory after demand ischemia. Circulation. 2005;112:2169–2174. [DOI] [PubMed] [Google Scholar]
- 42. Messina SA, Aras O, Dilsizian V. Delayed recovery of fatty acid metabolism after transient myocardial ischemia: a potential imaging target for "ischemic memory". Curr Cardiol Rep. 2007;9:159–165. [DOI] [PubMed] [Google Scholar]
- 43. Inaba Y, Bergman SR. Diagnostic accuracy of β‐methyl‐p (123I)‐iodophenylpentadecanoic acid (BMIPP) imaging: a metaanalysis. J Nucl Cardiol. 2008;15:345–352. [DOI] [PubMed] [Google Scholar]
- 44. Yoshinaga K, Naya M, Shiga T, et al. Ischaemic memory imaging using metabolic radiopharmaceuticals: overview of clinical settings and ongoing investigations. Eur J Nucl Med Mol Imaging. 2014;41:384–393. [DOI] [PubMed] [Google Scholar]


