Abstract
The influence of type 2 diabetes mellitus on cardiac remodeling has been evaluated for decades; however, the majority of investigations were focused only on the left ventricle. The impact of diabetes on the left atrial (LA) function is less researched. LA enlargement has been shown as an independent predictor of cardiovascular morbidity and mortality in the general and diabetic population; however, LA dysfunction has been proven to be an independent predictor only in the general population. There are not much follow‐up data about the influence of diabetes on LA function. New echocardiographic techniques, such as 2‐dimensional speckle tracking imaging, provide more accurate, sensitive, and reliable information about LA function than traditional, volumetric methods. The aim of this review was to summarize the most recent reports about the influence of diabetes on LA function, as well as to discuss the possible mechanisms and potential clinical implications of the relationship between diabetes and LA remodeling.
Introduction
Type 2 diabetes mellitus represents a frequent cardiovascular risk factors whose prevalence constantly increases over the years, predominantly due to epidemic obesity, sedentary lifestyle, and long‐term exposure to stress. The effect of diabetes on cardiac remodeling has been extensively investigated in the last several decades. However, the research has been mainly focused on the left ventricle1, 2, 3, 4 and more recently on the right ventricle.5, 6, 7 The impact of diabetes on left atrial (LA) function is significantly less studied. The importance of LA function on left ventricular diastolic dysfunction8, 9, 10 and overall morbidity and mortality in the general population11 , 12 and in patients with diabetes,13 as well as the significance of left atrial–ventricular coupling,14 increase the interest in LA remodeling in different conditions such as ischemic heart disease, cardiac valve disease, hypertension, and diabetes.15
The aim of this review article is to summarize current knowledge about the influence of diabetes on LA remodeling using echocardiographic, computed tomography (CT), and cardiac magnetic resonance (CMR) studies that evaluated LA volume, function, and mechanics in diabetic patients.
Left Atrial Structure and Function in Diabetes
Left Atrial Size
Determination of LA size represents a challenging echocardiography task. Namely, the enlargement of the LA is often asymmetrical, which is why LA volume reflects LA size more accurately than LA anteroposterior diameter, which is still widely used in clinical practice.16 Therefore, LA volume represents a better predictor of cardiovascular outcome than LA diameter.17
The recently published CARDIA (Coronary Artery Risk Development in Young Adults) study investigated the influence of modifiable cardiovascular risk factors on LA size in 2903 young adults (age 23–35 years) over a 20‐year follow‐up period.18 After a 5‐year period, investigators showed that diabetes was not associated with unindexed LA diameter and LA diameter indexed for body surface area or height. Whereas, after a 20‐year follow‐up period, diabetes was associated with the increased unindexed and indexed LA diameters.18 The TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) trial, which included 455 adolescents with type 2 diabetes (average age 18 years), showed that LA diameter, even after indexing for height, did not correlate with hemoglobin (Hb) A1c level or inability to maintain normal glycemic control.19 These results raise the question of the usefulness of LA diameters in diabetic population.
On the other hand, the usage of LA volumes in detection of subclinical cardiac damage in diabetic patients is more convincing. Namely, the majority of studies published in the last 10 years that investigated LA remodeling in diabetes confirmed enlargement of LA size assessed by LA volumes and corresponding indexes.9, 20, 21 Not all of the investigations confirmed an enlarged LA in the diabetic patients22; however, the main reason for these results is a small sample size that was the main obstacle in reaching statistical significance.
The studies that investigated LA volume in the diabetic population with other imaging techniques such as CT and CMR are scarce.23 Mahabadi et al showed that diabetes correlated with LA enlargement23; however, in multivariable regression, only body mass index, blood pressure, antihypertensive medication, and smoking remained associated with LA size.23 The same group of authors recently reported that CT‐derived LA size is associated with major cardiovascular events (coronary event, stroke, cardiovascular death) independently of cardiovascular risk factors and coronary artery calcium in a large population of subjects, age 45 to 75 years old, without prevalent cardiovascular disease.24
Graca et al showed that CMR‐derived LA volumes were similar between the controls and the diabetic subjects. However, they found significant difference in LA function between these 2 groups.25
The number of studies that compare different cardiovascular imaging modalities for determination of LA volumes is constantly increasing. The investigations showed a good correlation between 2‐dimensional echocardiography (2DE)‐derived and CT‐obtained LA volumes.26 Kataoka et al reported that CT‐derived LA volumes correlated well with 2DE‐ and 3‐dimensional echocardiography (3DE)‐derived LA volumes27; whereas Rohner et al obtained similar findings comparing 3DE and CT LA volumes.28 However, both techniques—2DE and 3DE—significantly and equally underestimated LA volumes in comparison with CT. On the other hand, studies that used CMR showed that 3DE‐derived LA volumes are more accurate than 2DE‐based analysis, compared with CMR‐obtained LA volumes, even if 3DE measurements still underestimate real LA volumes.29, 30
The advantages of CMR and CT over the echocardiographic technique are independence of quality of acoustic window, accurate delineation of endocardial border, and precise determination of all parts of the LA, including the LA appendage.31 This could partly explain the difference between 3DE‐ and CT/CMR‐calculated LA volumes. However, only 3DE and CT could obtain a true 3‐dimensional dataset, whereas CMR could provide only 1 in selected sequences. Additionally, CT is related with usage of iodine contrast and radiation, whereas CMR could be used in patients with pacemakers or defibrillators only with caution.
Studies showed that reproducibility of 3DE, CT, and CMR is very high. Intraclass correlation for 3DE‐ and CT‐derived LA volumes is 0.99, and interclass correlation for 3DE and CT LA volumes is 0.97–0.99.28 Interobserver and intraobserver variability for LA volume were 7% ± 4% and 6% ± 4%, for 2DE, 6% ± 4% and 5% ± 3% for 3DE, and 6% ± 4% and 4% ± 3% for CT, respectively.32 Artang et al reported that intraobserver and interobserver variability for CMR‐derived LA maximum volume were 1.8% and 6.4 %, respectively, and 2% and 5.1% for 3DE‐derived LA volume.33
Left Atrial Phasic Function
Traditional determination of LA phasic function means evaluation of LA volumes in a different phase of the cardiac cycle (maximum, minimum, and pre‐atrial contraction volumes), as well as the assessment of various emptying fractions (total, passive, and active emptying fraction).15 Traditional 2DE volumetric methods of the LA assessment are presented in Figure 1.
Figure 1.
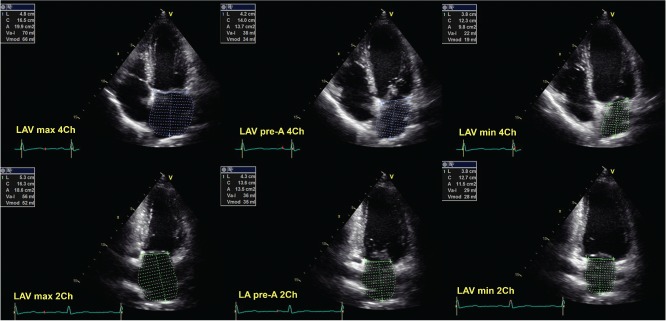
Traditional 2‐dimensional echocardiographic volumetric methods of evaluation of the left atrium in 2‐chamber (2Ch) and 4‐chamber (4Ch) apical views. Abbreviations: LAV, left atrial volume; LV, left ventricle; max, maximum; min, minimum.
Total emptying volume corresponds with the LA reservoir function, and it is calculated as the difference between maximum and minimum LA volume. Passive emptying volume resembles LA conduit function, and it is computed as the difference between LA maximum and pre‐atrial contraction volume; whereas active emptying volume describes LA booster function and is calculated as the difference between pre‐atrial contraction and minimum LA volume.
Recent studies have shown that LA minimal volume is associated with the worsening diastolic dysfunction, even more than LA maximal volume.34 Furthermore, minimal LA volume increases even in mild left ventricular diastolic dysfunction, whereas maximal LA volume increases in later stages of diastolic dysfunction. These findings are very interesting considering the fact that a majority of investigations are focused on maximal LA volume as a surrogate marker of left ventricular diastolic dysfunction and a predictor of cardiovascular outcomes. This suggests that minimal LA volume could be a more sensitive marker of left ventricular dysfunction than maximal LA volume, and also underlines the necessity of comprehensive evaluation of LA phasic function, and not simplification to only 1 parameter—maximal LA volume. The same analysis showed that LA reservoir function correlated well with left ventricular longitudinal function and ejection fraction.34 Additionally, Murata et al demonstrated that LA reservoir function is associated with worsening of left ventricular diastolic function.8 These findings could be very important for diabetic patients, because early detection of an impaired LA reservoir function in diabetic patients could prevent the development of left ventricular systolic and diastolic dysfunction and consequent heart failure. Mirza et al also showed that LA reservoir function represents an independent predictor of atrial fibrillation occurrence,35 which might explain the predisposition to this arrhythmia in diabetic patients.
According to some authors, LA reservoir function assessed by volumetric method, is not different between diabetics and healthy controls.20, 36 Difficulties exist in evaluation of LA conduit and pump function. Huang et al showed that LA passive emptying volume and fraction are lower in diabetic patients, whereas LA active volume and emptying fraction are higher in this group.20 We obtained similar results in diabetic subjects, whereas Mondillo et al did not find any difference in conduit and booster pump function between the diabetic subjects and the controls.36 However, Mondillo et al investigated only diabetic patients with normal LA size and included a small number of subjects, which could explain the results of this investigation. Table 1 shows all characteristics of 2DE and 3DE assessment of LA function, and Table 2 summarizes the most important studies that investigated LA remodeling in diabetic patients.
Table 1.
The Strengths and Limitation in Left Atrial Assessment Using 2DE and 3DE Volumetric Methods and 2DE Speckle Tracking Imaging
| Volumetric Assessment | Speckle Tracking Assessment | ||
|---|---|---|---|
| 2DE | 3DE | LA Strain/Strain Rates | |
| Technical consideration | |||
| Availability | High | Moderate | Moderate/high |
| Cost | Low | Moderate | Low |
| Typical scan duration (min)* | 3–5 | 1 | 3–5 |
| Typical time for analysis (min) | 4–6 | 5–7 | 2–3 |
| Problem with imaging window | Present | Present | Present |
| Temporal resolution | +++ | + | ++/+++ |
| Spatial resolution | +++ | ++ | +++ |
| Authentic 3‐dimensional imaging | No | Yes | No |
| Assessment of LA structure | + | ++ | No |
| Assessment of LA volumes | |||
| Static | + | ++ | No |
| Phasic | + | ++ | No |
| Assessment of LA function | + | ++ | ++ |
| Major advantages | Low cost | No geometric assumptions | Short time of analysis |
| High availability | High reproducibility | High reproducibility | |
| LA appendage could be evaluated | |||
| Major limitations | Measurements are made only in 2‐chamber, 4‐chamber, and apical long‐axis views | Cost | |
| Geometric assumptions | Stable cardiac rhythm | Relatively low availability | |
| Long time for analysis | Acquisition of LA full‐volume | Higher cost than for volumetric method | |
| LA appendage is not measured | Visualization of LA endocardial border | Stable cardiac rhythm is preferred | |
Abbreviations: 2DE, 2‐dimensional echocardiography; 3DE, 3‐dimensional echocardiography; LA, left atrium.
= low, ++ = moderate, +++ = high, ++++ = very high major limitation of the modality.
Time only for scanning.
Table 2.
The Studies That Investigated LA Volumes and Mechanics in Diabetic Population
| Reference | Imaging Techniques | Patients/ Controls, No. | Age, y | Main Findings |
|---|---|---|---|---|
| CARDIA study18 | 2DE | 2903 | 23–35 | After a 5‐year period diabetes was not associated with LA diameter, and LA diameter indexed for body surface area or height. After a 20‐year follow‐up period, diabetes was associated with the increased unindexed and indexed LA diameters. |
| TODAY study19 | 2DE | 455 | ∼18 | LA diameter, even after indexation for height, did not correlate with hemoglobin A1c level. |
| Kadappu et al9 | 2DE | 73/73 | 43 ± 10 | LA enlargement in diabetes is independent of hypertension and diastolic function, and it is associated with LA dysfunction evaluated by 2DE strain. |
| Poulsen et al13 | 2DE | 305 | 58.6 ± 11.3 | Increased LA volume index was an independent predictor of cardiovascular morbidity and mortality in diabetic patients free of cardiovascular disease. |
| Huang et al20 | 2DE | 58/40 | 32–78 | Maximal, minimal, and pre‐atrial contraction LA volumes were higher in diabetic patients than in controls. LA reservoir and conduit function were reduced, whereas LA pump function was increased in diabetic patients. |
| Muranaka et al22 | 2DE strain | 39/16 | 62 ± 9 | 2DE strain imaging detected impairment of LA reservoir and conduit functions in diabetic patients, even in the absence of left ventricular hypertrophy and LA dilatation. |
| Graca et al25 | CMR | 45/24 | 45–75 | CMR detection of LA dysfunction in asymptomatic diabetic patients: reduced LA reservoir and conduit functions. LA pump function did not differ. |
| Mondillo et al36 | 2DE strain | 155/36 | 65 ± 11 | LA deformation is impaired in diabetic patients, even if LA volumes were similar between groups. |
| Jarnert et al43 | 2DE VVI strain | 87 | 60 ± 7 | LA strain by VVI is impaired in patients with type 2 diabetes mellitus. LA strain distinguished normal from abnormal diastolic function. |
| Liu et al44 | 2DE strain | 164/26 | 51 ± 11 | Hypertension leads to abnormal LA reservoir and conduit functions, and coexisting diabetes can further impair conduit function. |
Abbreviations: 2DE, 2‐dimensional echocardiography; CMR, cardiac magnetic resonance; LA, left atrium; LV, left ventricle; VVI, vector velocity imaging.
CT and CMR studies that researched LA phasic function in the diabetic population are not common. Graca et al have recently shown that CMR successfully detects subtle LA dysfunction in asymptomatic DM patients: reduced LA reservoir and conduit functions.25 The authors demonstrated that diabetes was independently associated with LA reservoir function, but not with LA conduit function.25
Buechel et al found high correlations between 3DE and CMR for total LA emptying fraction (r = 0.92, P < 0.001), and active LA emptying fraction (r = 0.87, P < 0.001). Similarly, Bland‐Altman analysis revealed narrow limits of agreement for total LA emptying fraction (−11.2%–14.9 %), and active LA emptying fraction (−10.6%–6.8 %).37 There was no difference in LA reservoir function assessed by 3DE and CMR. However, LA active function obtained by CMR was slightly higher than calculated by 3DE.37
Kataoka et al revealed that CT‐derived LA reservoir function was similar to 3DE‐obtained, but significantly lower than 2DE‐derived LA total emptying fraction (25.3% ± 13.1%, 30.2% ± 6.8%, and 33.9% ± 8.9%, respectively). The correlation coefficients for LA total emptying fraction of interobserver variation were 0.64, 0.77, and 0.34, respectively for CT, 3DE, and 2DE.27
Left Atrial Mechanics in Diabetes
Determination of LA volumes during the whole cardiac cycle has long been the only method of LA mechanics assessment. Development of new echocardiographic tools, initially speckle tracking imaging, enables the usage of strain and strain rates as a feasible, sensitive, rather simple, and reliable method for evaluation of LA deformation.38 More important is that investigations revealed that global LA strain is a strong and independent predictor of cardiovascular events, even superior to LA conventional parameters (indexed LA volume, LA total emptying fraction, LA area, and LA diameter).39 Cameli et al showed that overall predictive value of cardiovascular events was the highest for global longitudinal LA strain (area under receiver operator characteristic curve: global LA strain 0.83, indexed LA volume 0.71, LA total emptying fraction 0.69, LA area 0.64, and LA diameter 0.59).39 Additionally, Hirose et al demonstrated the relationship between LA strain and new onset of atrial fibrillation40 and stroke.41 It was also reported that LA strain and strain rates correlate with the level of left ventricular diastolic dysfunction.42 All of these findings suggest that LA dysfunction has significant prognostic implications, and that LA strain could detect LA dysfunction earlier than volumetric measurements, and emphasize the need for inclusion of LA strain in a routine echocardiographic examination and report.
LA strain/strain rate during systole correspond with LA reservoir function, LA strain/strain rate during early diastole describe LA conduit function, and LA strain/strain rate during late diastole represent the measure of LA booster pump function. The evaluation of LA mechanics by 2DE speckle tracking is presented in Figure 2.
Figure 2.
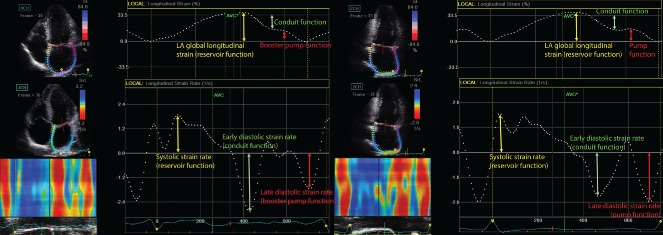
Determination of left atrial (LA) mechanics using 2‐dimensional speckle tracking imaging (strain and strain rates) in 2‐chamber (2Ch) and 4‐chamber (4Ch) apical views. Abbreviation: AVC, aortic valve closure.
Mondillo et al showed that LA deformation is impaired in patients with hypertension or diabetes with normal LA size.36 They also reported that coexistence of both conditions further impairs LA performance in an additive fashion, which is very important for clinical practice where these conditions are usually met together. The authors demonstrated a good correlation between LA global longitudinal strain and left ventricular diastolic function assessed by pulsed and tissue Doppler.
Kadappu et al revealed that longitudinal strain in all 6 segments of the LA is lower in the diabetic patients compared with the controls.9 However, LA global strain was similar between the patients with diabetes and hypertension. LA reservoir, conduit, and pump function, evaluated by systolic, early, and late diastolic strain rates, were reduced in the diabetic subjects.9 Considering the fact that transmitral velocities during late diastole obtained by pulsed and tissue Doppler were increased in the subjects with diabetes, one would expect that pump function, assessed by late diastolic strain rate, is also increased due to a compensatory increase in late diastolic blood flow as a consequence of left ventricular diastolic dysfunction. However, this was not observed, implying changed intrinsic LA function with a reduction in atrial deformation even during its contractile phase.
Velocity vector imaging of the LA showed that LA strain is reduced in the diabetic patients with mild and moderate left ventricular diastolic dysfunction.43 The authors reported the difference in systolic LA strain and early diastolic strain rate (reservoir and conduit LA function, respectively) between the diabetic patients with no and mild diastolic dysfunction, the difference in systolic LA strain and late diastolic strain rate (reservoir and pump LA function, respectively) among the participants with no and moderate diastolic dysfunction, whereas the difference between the subjects with mild and moderate diastolic dysfunction existed only in late diastolic strain rate (LA pump function).43
Liu et al, in the subjects with diabetes and hypertension, found lower strain and strain rates during systole and early diastole, whereas no difference was found during late diastole.44 This corresponds with impaired‐reduced LA reservoir and conduit function, and preserved pump function. The authors reported that patients with hypertension and diabetes had decreased strain/strain rate only during early diastole, which corresponds with LA conduit function. Similar results were obtained by Muranaka et al, who studied the difference between patients with coexisting diabetes and hypertension, and diabetic subjects.33
Mechanical function of the LA is best assessed by 2DE strain. However, the most recent studies even use other modalities like 3DE or CMR in determination of 3‐dimensional LA strain,45, 46 but not yet in diabetic patients.
The mean difference for intraobserver agreement for 2DE LA global longitudinal strain was −1.1% (95% confidence interval [CI]: −3.1% to 0.9%).47 The mean difference for interobserver agreement for 2DE LA global longitudinal strain was 2.8% (95% CI: 0.3% to 5.3%).47 Mondillo et al reported that variability coefficients are <6% for 2DE LA longitudinal strain measurements in both interobserver and intraobserver analyses.36
Mechanisms of Left Atrial Remodeling in Diabetes
Atrial remodeling could be considered in 3 different ways: structural, functional, and electrical. The precise mechanisms that lead to LA remodeling in diabetes are not quite clear; however, there are several mechanisms that could explain the relation between altered atrial phasic function and impaired glucose regulation (Figure 3). First, left ventricular diastolic dysfunction has been proven as an independent predictor of atrial phasic function,48 and diabetic patients usually have diastolic dysfunction.42 Second, subendocardial fibrosis, which occurs in the diabetic atrium, causes decreased wall elasticity, an important factor for regulation of atrial reservoir function.49 Third, atrial enlargement seen in diabetes might be the reason of impaired LA atrial function. Fourth, increased blood pressure induced by insulin resistance in diabetic patients could also impact LA mechanics.36, 44 Fifth, electrical remodeling of the LA, reflected in conduction and refractory disorders,50 could make additional problems in atrial phasic function in diabetic patients. Furthermore, autonomic nervous system disbalance with enhanced sympathetic activation in diabetic hearts could be suitable atrial arrhythmogenic substrate, which could result with LA dysfunction and atrial fibrillation.51 Oxidative stress and inflammation, frequently associated with diabetes, might also cause LA deformation changes. Studies already demonstrated that markers of oxidative stress and inflammation correlated with left ventricular deformation in diabetes,52 thus it is reasonable to hypothesize that the same relationship exists between these markers and parameters of LA mechanics.
Figure 3.
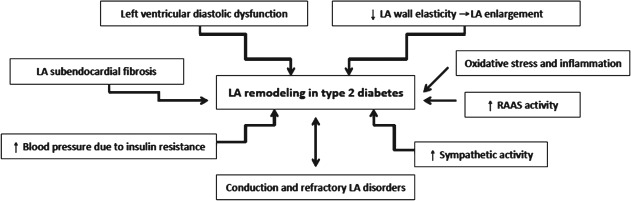
Mechanisms that induce left atrial (LA) remodeling in type 2 diabetes. Abbreviation: RAAS, renin‐angiotensin‐aldosterone system.
Impact of LA Remodeling Morbidity and Mortality in Type 2 Diabetes
There are very limited data about the impact of LA volumes and mechanics on the prognosis in diabetic patients. Poulsen et al reported that LA volume index is an independent and incremental predictor of cardiovascular morbidity and mortality in diabetic patients without history of cardiovascular disease.13 In this study, increased LA volume index was a predictor of death and major adverse cardiac events, even after adjustment for age and hypertension. Additionally, the authors demonstrated that LA volume index predicted cardiovascular morbidity and mortality independently of myocardial ischemia.13
The Strong Heart Study, which included patients without overt cardiovascular disease but with high prevalence of hypertension and diabetes, demonstrated that high LA systolic force, a measure of LA mechanical function, was a predictor of cardiovascular events, including heart failure, independent of the traditional risk factors.53
Cameli et al, in a population of patients with prevalent hypertension (63%) and diabetes (25%), found that global longitudinal LA strain is a strong and independent predictor of cardiovascular events, even better than conventional parameters of LA analysis.39
Investigations demonstrated that pulsed Doppler measurements of LA appendage flow velocity are excellent predictors of thrombus formation in patients with atrial fibrillation, as well as predictors of successful cardioversion, maintenance of sinus rhythm after both electrical cardioversion and pulmonary vein isolation, and risk of thromboembolism.54, 55 A recent study showed that left ventricular diastolic dysfunction is associated with reduced LA appendage function in patients with atrial fibrillation, which represents a potential risk factor for formation of thrombus and stroke.56 This is especially important for diabetic patients who frequently have left ventricular diastolic dysfunction.1, 2, 3, 4, 8, 9, 10, 11
Potential Clinical Implications
There are many clinical implications of LA dysfunction in diabetic patients. LA dysfunction is highly associated with left ventricular diastolic dysfunction, which is closely associated with abnormal myocardial perfusion on myocardial perfusion scintigraphy, as well as with vascular function in diabetic subjects.4 Studies also show that LA dysfunction is an important predictor of cardiovascular events (myocardial infarction, stroke, new onset of atrial fibrillation) and mortality in diabetic population. LA dysfunction is associated with development of atrial fibrillation and significantly contributes to the worsening of systolic and diastolic left ventricular function, which leads to heart failure, an important causes of death in the modern world. It was reported that HbA1c correlates with LA function, which provides us an effective, indirect follow‐up method of LA function improvement in diabetic patients.
These significant clinical implications of LA dysfunction demonstrate the importance of comprehensive assessment of LA function in everyday clinical life and emphasize that determination of only LA maximal volume, or even worse, only LA diameter, is not enough to estimate the cardiovascular risk of our patients with diabetes. In light of recent studies that show that normal LA size in diabetic and hypertensive patients does not necessarily mean normal LA function,36 it is even more important to introduce a more sophisticated and sensitive LA echocardiographic analysis—2‐dimensional speckle tracking—into daily practice to reliably assess baseline LA function and, more importantly, the changes induced by pharmacologic and lifestyle interventions.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Fonarow GC, Srikanthan P. Diabetic cardiomyopathy. Endocrinol Metab Clin North Am. 2006;35:575–599. [DOI] [PubMed] [Google Scholar]
- 2. Ernande L, Bergerot C, Rietzschel ER, et al. Diastolic dysfunction in patients with type 2 diabetes mellitus: is it really the first marker of diabetic cardiomyopathy? J Am Soc Echocardiogr. 2011;24:1268–1275. [DOI] [PubMed] [Google Scholar]
- 3. Roos CJ, Scholte AJ, Kharagjitsingh AV, et al. Changes in multidirectional LV strain in asymptomatic patients with type 2 diabetes mellitus: a 2‐year follow‐up study. Eur Heart J Cardiovasc Imaging. 2014;15:41–47. [DOI] [PubMed] [Google Scholar]
- 4. Poulsen MK, Henriksen JE, Dahl J, et al. Left ventricular diastolic function in type 2 diabetes mellitus: prevalence and association with myocardial and vascular disease. Circ Cardiovasc Imaging. 2010;3:24–31. [DOI] [PubMed] [Google Scholar]
- 5. Kosmala W, Colonna P, Przewlocka‐Kosmala M, et al. Right ventricular dysfunction in asymptomatic diabetic patients. Diabetes Care. 2004;27:2736–2738. [DOI] [PubMed] [Google Scholar]
- 6. Kosmala W, Przewlocka‐Kosmala M, Mazurek W. Subclinical right ventricular dysfunction in diabetes mellitus—an ultrasonic strain/strain rate study. Diabet Med. 2007;24:656–663. [DOI] [PubMed] [Google Scholar]
- 7. Tadic M, Ivanovic B, Celic V, et al. The impact of metabolic syndrome, recently diagnosed diabetes and hypertension on right ventricular remodeling. Is there difference between risk factors? Clin Exp Hypertens. 2014;36:295–301. [DOI] [PubMed] [Google Scholar]
- 8. Murata M, Iwanaga S, Tamura Y, et al. A real‐time three‐dimensional echocardiographic quantitative analysis of left atrial function in left ventricular diastolic dysfunction. Am J Cardiol. 2008;102:1097–1102. [DOI] [PubMed] [Google Scholar]
- 9. Kadappu KK, Boyd A, Eshoo S, et al. Changes in left atrial volume in diabetes mellitus: more than diastolic dysfunction? Eur Heart J Cardiovasc Imaging. 2012;13:1016–1023. [DOI] [PubMed] [Google Scholar]
- 10. Hsiao SH, Lin KL, Chiou KR. Comparison of left atrial volume parameters in detecting left ventricular diastolic dysfunction versus tissue Doppler recordings. Am J Cardiol. 2012;109:748–755. [DOI] [PubMed] [Google Scholar]
- 11. Gupta S, Matulevicius SA, Ayers CR, et al. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J. 2013;34:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leung DY, Chi C, Allman C, et al. Prognostic implications of left atrial volume index in patients in sinus rhythm. Am J Cardiol. 2010;105:1635–1639. [DOI] [PubMed] [Google Scholar]
- 13. Poulsen MK, Dahl JS, Henriksen JE, et al. Left atrial volume index: relation to long‐term clinical outcome in type 2 diabetes. J Am Coll Cardiol. 2013;62:2416–2421. [DOI] [PubMed] [Google Scholar]
- 14. Miyoshi H, Mizuguchi Y, Oishi Y, et al. Early detection of abnormal left atrial‐left ventricular‐arterial coupling in preclinical patients with cardiovascular risk factors: evaluation by two‐dimensional speckle‐tracking echocardiography. Eur J Echocardiogr. 2011;12:431–439. [DOI] [PubMed] [Google Scholar]
- 15. Leung DY, Boyd A, Ng AA, et al. Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J. 2008;156:1056–1064. [DOI] [PubMed] [Google Scholar]
- 16. Lester SJ, Ryan EW, Schiller NB, et al. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–832. [DOI] [PubMed] [Google Scholar]
- 17. Tsang TS, Abhayaratna WP, Barnes ME, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018–1023. [DOI] [PubMed] [Google Scholar]
- 18. Armstrong AC, Gidding SS, Colangelo LA, et al. Association of early adult modifiable cardiovascular risk factors with left atrial size over a 20‐year follow‐up period: the CARDIA study. BMJ Open. 2014;4:e004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. TODAY Study Group . Alterations in left ventricular, left atrial, and right ventricular structure and function to cardiovascular risk factors in adolescents with type 2 diabetes participating in the TODAY clinical trial [published online ahead of print January 22, 2014]. Pediatr Diabetes. doi: 10.1111/pedi.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang G, Zhang L, Xie M, et al. Assessment of left atrial function in diabetes mellitus by left atrial volume tracking method. J Huazhong Univ Sci Technolog Med Sci. 2010;30:819–823. [DOI] [PubMed] [Google Scholar]
- 21. Zapolski T, Wysokiński A. Left atrium volume index is influenced by aortic stiffness and central pulse pressure in type 2 diabetes mellitus patients: a hemodynamic and echocardiographic study. Med Sci Monit. 2013;19:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Muranaka A, Yuda S, Tsuchihashi K, et al. Quantitative assessment of left ventricular and left atrial functions by strain rate imaging in diabetic patients with and without hypertension. Echocardiography. 2009;26:262–271. [DOI] [PubMed] [Google Scholar]
- 23. Mahabadi AA, Lehmann N, Sonneck NC, et al; on behalf of the Heinz Nixdorf Recall Study Investigative Group . Left atrial size quantification using non‐contrast‐enhanced cardiac computed tomography—association with cardiovascular risk factors and gender‐specific distribution in the general population: the Heinz Nixdorf Recall study [published online ahead of print October 10, 2013]. Acta Radiol. doi: 10.1177/0284185113507446. [DOI] [PubMed] [Google Scholar]
- 24. Mahabadi AA, Geisel MH, Lehmann N, et al. Association of computed tomography‐derived left atrial size with major cardiovascular events in the general population: the Heinz Nixdorf Recall Study. Int J Cardiol. 2014;174:318–323. [DOI] [PubMed] [Google Scholar]
- 25. Graca B, Ferreira MJ, Donato P, et al. Left atrial dysfunction in type 2 diabetes mellitus: insights from cardiac MRI [published online ahead of print July 17, 2014]. Eur Radiol. doi: 10.1007/s00330-014-3299-2 [DOI] [PubMed] [Google Scholar]
- 26. Gweon HM, Kim SJ, Kim TH, et al. Evaluation of left atrial volumes using multidetector computed tomography: comparison with echocardiography. Korean J Radiol. 2010;11:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kataoka A, Funabashi N, Takahashi A, et al. Quantitative evaluation of left atrial volumes and ejection fraction by 320‐slice computed‐tomography in comparison with three‐ and two‐dimensional echocardiography: a single‐center retrospective‐study in 22 subjects. Int J Cardiol. 2011;153:47–54. [DOI] [PubMed] [Google Scholar]
- 28. Rohner A, Brinkert M, Kawel N, et al. Functional assessment of the left atrium by real‐time three‐dimensional echocardiography using a novel dedicated analysis tool: initial validation studies in comparison with computed tomography. Eur J Echocardiogr. 2011;12:497–505. [DOI] [PubMed] [Google Scholar]
- 29. Mor‐Avi V, Yodwut C, Jenkins C, et al. Real‐time 3D echocardiographic quantification of left atrial volume: multicenter study for validation with CMR. JACC Cardiovasc Imaging. 2012;5:769–777. [DOI] [PubMed] [Google Scholar]
- 30. Shimada YJ, Shiota T. Underestimation of left atrial volume by three‐dimensional echocardiography validated by magnetic resonance imaging: a meta‐analysis and investigation of the source of bias. Echocardiography. 2012;29:385–390. [DOI] [PubMed] [Google Scholar]
- 31. To AC, Flamm SD, Marwick TH, et al. Clinical utility of multimodality LA imaging: assessment of size, function, and structure. JACC Cardiovasc Imaging. 2011;4:788–798. [DOI] [PubMed] [Google Scholar]
- 32. Miyasaka Y, Tsujimoto S, Maeba H, et al. Left atrial volume by real‐time three‐dimensional echocardiography: validation by 64‐slice multidetector computed tomography. J Am Soc Echocardiogr. 2011;24:680–686. [DOI] [PubMed] [Google Scholar]
- 33. Artang R, Migrino RQ, Harmann L, et al. Left atrial volume measurement with automated border detection by 3‐dimensional echocardiography: comparison with Magnetic Resonance Imaging. Cardiovasc Ultrasound. 2009;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Russo C, Jin Z, Homma S, et al. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. 2012;98:813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mirza M, Caracciolo G, Khan U, et al. Left atrial reservoir function predicts atrial fibrillation recurrence after catheter ablation: a two‐dimensional speckle strain study. J Interv Card Electrophysiol. 2011;31:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mondillo S, Cameli M, Caputo ML, et al. Early detection of left atrial strain abnormalities by speckle‐tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr. 2011;24:898–908. [DOI] [PubMed] [Google Scholar]
- 37. Buechel RR, Sommer G, Leibundgut G, et al. Assessment of left atrial functional parameters using a novel dedicated analysis tool for real‐time three‐dimensional echocardiography: validation in comparison to magnetic resonance imaging. Int J Cardiovasc Imaging. 2013;29:601–608. [DOI] [PubMed] [Google Scholar]
- 38. Vianna‐Pinton R, Moreno CA, Baxter CM, et al. Two‐dimensional speckle‐tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr. 2009;22:299–305. [DOI] [PubMed] [Google Scholar]
- 39. Cameli M, Lisi M, Focardi M, et al. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am J Cardiol. 2012;110:264–269. [DOI] [PubMed] [Google Scholar]
- 40. Hirose T, Kawasaki M, Tanaka R, et al. Left atrial function assessed by speckle tracking echocardiography as a predictor of new onset non‐valvular atrial fibrillation: results from a prospective study in 580 adults. Eur J Echocardiogr. 2012;13:243–250. [DOI] [PubMed] [Google Scholar]
- 41. Shih JY, Tsai WC, Huang YY, et al. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J Am Soc Echocardiogr. 2011;24:513–519. [DOI] [PubMed] [Google Scholar]
- 42. Otani K, Takeuchi M, Kaku K, et al. Impact of diastolic dysfunction grade on left atrial mechanics assessed by two‐dimensional speckle tracking echocardiography. J Am Soc Echocardiogr. 2010;23:961–967. [DOI] [PubMed] [Google Scholar]
- 43. Jarnert C, Melcher A, Caidahl K, et al. Left atrial velocity vector imaging for the detection and quantification of left ventricular diastolic function in type 2 diabetes. Eur J Heart Fail. 2008;10:1080–1087. [DOI] [PubMed] [Google Scholar]
- 44. Liu Y, Wang K, Su D, et al. Noninvasive assessment of left atrial phasic function in patients with hypertension and diabetes using two‐dimensional speckle tracking and volumetric parameters. Echocardiography. 2014;31:727–735 [DOI] [PubMed] [Google Scholar]
- 45. Mochizuki A, Yuda S, Oi Y, et al. Assessment of left atrial deformation and synchrony by three‐dimensional speckle‐tracking echocardiography: comparative studies in healthy subjects and patients with atrial fibrillation. J Am Soc Echocardiogr. 2013;26:165–174. [DOI] [PubMed] [Google Scholar]
- 46. Imai M, Venkatesh BA, Samiei S, et al. Multi‐Ethnic Study of Atherosclerosis: association between left atrial function using tissue tracking from cine MR imaging and myocardial fibrosis. Radiology. 131971 Accessed July 14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saraiva RM, Demirkol S, Buakhamsri A, et al. Left atrial strain measured by two‐dimensional speckle tracking represents a new tool to evaluate left atrial function. J Am Soc Echocardiogr. 2010;23:172–180. [DOI] [PubMed] [Google Scholar]
- 48. Sun JP, Yang Y, Guo R, et al. Left atrial regional phasic strain, strain rate and velocity by speckle‐tracking echocardiography: normal values and effects of aging in a large group of normal subjects. Int J Cardiol. 2013;168:3473–3479. [DOI] [PubMed] [Google Scholar]
- 49. Kato T, Yamashita T, Sekiguchi A, et al. What are arrhythmogenic substrates in diabetic rat atria? J Cardiovasc Electrophysiol. 2006;17:890–894. [DOI] [PubMed] [Google Scholar]
- 50. Watanabe M, Yokoshiki H, Mitsuyama H, et al. Conduction and refractory disorders in the diabetic atrium. Am J Physiol Heart Circ Physiol. 2012;303:H86–H95. [DOI] [PubMed] [Google Scholar]
- 51. Otake H, Suzuki H, Honda T, et al. Influences of autonomic nervous system on atrial arrhythmogenic substrates and the incidence of atrial fibrillation in diabetic heart. Int Heart J. 2009;50:627–641. [DOI] [PubMed] [Google Scholar]
- 52. Zhao CT, Wang M, Siu CW, et al. Myocardial dysfunction in patients with type 2 diabetes mellitus: role of endothelial progenitor cells and oxidative stress. Cardiovasc Diabetol. 2012;11:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Simone G, Devereux RB, Roman MJ, et al. Does cardiovascular phenotype explain the association between diabetes and incident heart failure? The Strong Heart Study. Nutr Metab Cardiovasc Dis. 2013;23:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Palinkas A, Antonielli E, Picano E, et al. Clinical value of left atrial appendage flow velocity for predicting of cardioversion success in patients with non‐valvular atrial fibrillation. Eur Heart J. 2001;22:2201–2208. [DOI] [PubMed] [Google Scholar]
- 55. Zabalgoitia M, Halperin JL, Pearce LA, et al. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J Am Coll Cardiol. 1998;31:1622–1626. [DOI] [PubMed] [Google Scholar]
- 56. Demircelik MB, Cetin M, CiCekcioglu H, et al. Effect of left ventricular diastolic dysfunction on left atrial appendage function and thrombotic potential in nonvalvular atrial fibrillation. Anadolu Kardiyol Derg. 2014;14:256–260. [DOI] [PubMed] [Google Scholar]


