ABSTRACT
Introduction: The appraisal of medicines is often a complex and iterative process. We compared the health technology assessment (HTA) process in England and France taking as a case study the example of ixazomib for multiple myeloma.
Methods: We undertook an analysis of eight relevant published documents identifed from the websites of the French and English HTA bodies (HAS and NICE, respectively). We analyse patients’ availability of ixazomib resulting in the different stages of the appraisal process.
Results: We identified differences in the assessment, one of these being the use of an appraisal scope in England allowing the differentiation of populations and comparators according to previously approved treatments. Ixazomib became available earlier in France as part of an early access programme, but the availability was soon discontinued for newly eligible patients following an HAS determination that Ixazomib yielded no additional benefit. This opinion resulted in long pricing discussions. In England, despite the absence of an early access programme and following a process that included cost-effectiveness evaluation combined with pricing discussions, the medicine was fairly rapidly recommended for use.
Conclusions: Differences in the HTA process may result in appreciable differences in time from marketing authorisation to health service adoption of newly licensed drugs.
KEYWORDS: NICE, HAS, health technology assessment, France, England, Ixazomib, multiple myeloma
Introduction
There has been considerable public concern expressed about perceived delays in making new medicines available for sick patients. In the European union, the granting of marketing authorisation denotes that the benefit/risk balance for a new medicine is deemed favourable by the European Medicines Agency (EMA).
However, decisions about costs and reimbursement by Health Insurance Systems are taken by each individual State member based on advice from country-specific Health Technology Assessment (HTA) bodies.
Ixazomib is an orally administered proteasome inhibitor which was approved by the EMA in November 2016 for treating patients with relapsed or refractory multiple myeloma (RRMM) [1]. It is used in combination with lenalidomide-dexamethasone (LEN-DEX).
Since the marketing authorisation of ixazomib in RRMM, a number of EU countries (e.g., Germany, Austria, and Denmark) have provided a positive opinion on its reimbursement [2].
As of July 2019, ixazomib is available for patients with RRMM via national reimbursement schemes in both France and England, albeit there are more restrictions in England according to the number of prior lines of treatment. This current end result in the two countries does not fully reflect if there have been potential differences in the HTA processes involved.
The National Authority for Health (HAS) in France and the National Institute for Health and Care Excellence (NICE) in England have a central functional role in the assessment of health technologies and in ensuring rational price settings.
Our objective was to compare health technology assessment in France and England and to assess their differences and limitations using the example of ixazomib as a case study. We define the health technology assessment process in terms of the main steps between the start of appraisal until the final decision on reimbursement.
Our choice of ixazomib derived from the involvement of several authors in the appraisal of ixazomib by NICE.
Methods
First, we provide an overview of the national drug assessment processes in France and England, and then we present the methods for our case-study to compare HTA and the decision-making processes for ixazomib in both countries.
Overview of national drug assessment in France and England
The English Health Technology Assessment (HTA) process applies to new medicines which have been screened and assessed as likely to incur significant increased expenditures for the National Health Service (NHS) [3].
Conversely, in France, the process of HTA is systematic for all newly available medicines, whether marketing authorisation is granted nationally or at a European level essentially through a centralized procedure [4].
The major difference between the two processes lies in the paradigm of assessment. In England, NICE is asked to determine first whether the new drug is clinically effective compared to standard of care, and second whether the drug represents good value for money [5].
For this purpose, the cost-effectiveness of the drug is examined against a threshold between £20,000 and £30,000 per Quality Adjusted Life Years (QALY) gained compared to appropriate comparator(s) [6]. As part of this appraisal, companies are invited to propose commercial access agreements to NHS England, which are taken into account in assessing the cost-effectiveness of the drug [7].
In France, the process follows several stages. First, the Transparency Committee of the French HAS assesses the level of actual medical benefit (SMR for ‘Service Médical Rendu’) on the basis of the clinical effectiveness and safety profile of the drug together with considerations of disease severity, and the drug’s impact in terms of the quality of public health [8].
Next, should the SMR be judged sufficient, the same committee appraises the level of improvement of medical benefit (ASMR for ‘Amélioration du Service Médical Rendu’) which accounts for the added clinical benefit of the new drug relative to existing treatment(s). ASMR ratings are given on a scale from I (major benefit) to V (absence of/no additional benefit) [8]. Based on the SMR/ASMR ratings provided by HAS, the next step is the pricing discussion between the National Economic Committee (CEPS for ‘Comité Economique des Produits de Santé’) and the drug company [9]. Pricing discussion encompasses the costs of the drug and the price of comparators together with the anticipated number of eligible patients [9].
Unlike the NICE process, HAS does not systematically examine the cost-effectiveness of new drugs. An assessment of cost-effectiveness in France is undertaken by the Committee of Economic Evaluation and Public Health (CEESP) of the French HAS, which is distinct from the Transparency committee. Cost-effectiveness evaluation is restricted to a] drugs deemed innovative (defined as drugs for which Companies claim an ASMR rating between I to III) and b] the likelihood the health system will incur significant expenditures (defined as those for which the anticipated yearly budget is at least 20 million euros nationally) [10]. Opinions by CEESP on the drug cost-effectiveness are taken into account by the CEPS at the stage of pricing. There is no French threshold to define whether a new technology is cost-effective [11]. In this particular case Ixazomib did not meet the two French criteria (a] and b]) requiring a cost-effectiveness evaluation.
Methods of our case-study
The HTA process related to ixazomib was examined and compared: all relevant documents within the internet websites of NICE and HAS were identified and analysed. For NICE, we identified the following relevant documents [12]: draft and final scope, committee papers, appraisal consultation document (ACD), final appraisal document (FAD), guidance. For HAS, we identified the transparency committee opinion and the minutes of transparency committee meetings [2]. A narrative account is presented.
Results
Figure 1 depicts the main milestones of the technology assessment of ixazomib in England and France and shows the resulting decisions regarding the availability of the drug for patients.
Figure 1.
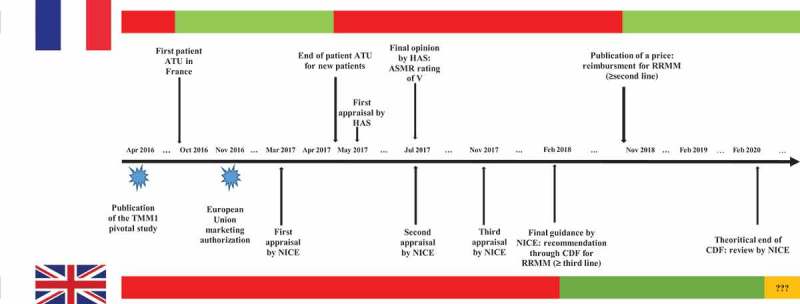
Comparative availability over time of ixazomib for new patients with relapsed or refractory multiple myeloma between France and England and main milestones of the appraisal. The red bars denote periods of time during which ixazomib was not reimbursed in each country's health-care system, the green bars denote periods of time during which ixazomib was reimbursed in each country’s health-care system, the orange bar denotes potential uncertainty in the reimbursement of ixazomib.TMM-1 = Tourmaline MM1 trial; NICE = National Institute for Health and Care Excellence; HAS = French High Health Authority; CDF = Cancer Drugs Fund; ASMR = improvement of actual medical benefit; RRMM = relapsed refractory multiple Myeloma; ATU = Early Access Program.
Just 6 months following the publication of the pivotal phase III trial [13], and 1 month before ixazomib was granted a marketing authorisation [1], France was the first country allowing access to ixazomib to patients with RRMM. This was possible through the patient early access program also called ‘ATU’ for ‘Autorisation Temporaire d’Utilisation’ which is one of the mainstays of access to innovative medicines in France [14]. ATUs can be issued by the French regulatory agency of medicines (ANSM) for individuals who cannot participate in a clinical trial and where the product is expected to be of benefit for the patient [15] and where no other therapeutic option is available. As part of the access program, patients are fully covered for the expenses by the National Health Insurance system.
Over the period October 2016-May 2017, 35 individual patients benefited from ixazomib within this scope [2]. However, from May, the 2nd 2017 onwards, ixazomib was only made available for those who were initiated prior to this date, which means the availability of the drug to potentially further newly eligible patients was no longer possible. The reason for discontinuation for newly eligible patients at this time is not clearly reported and may be related to the concomitant appraisal of ixazomib by HAS [2].
The first appraisal committee meetings at NICE and HAS took place in March 2017 and May 2017, respectively.
Both NICE and HAS have assessed the clinical effectiveness of ixazomib according to its label indication, namely patients with multiple myeloma who failed at least one prior regimen.
The evidence submitted by the manufacturer of ixazomib (Takeda) to support the clinical effectiveness was similar between jurisdictions and relied on the findings from the Tourmaline MM1 (TMM-1) trial. TMM-1 is a randomised controlled trial (RCT) that enrolled 771 RRMM patients who received either ixazomib and LEN-DEX (IXA-LEN-DEN) or placebo-LEN-DEX as 2nd, 3rd or 4th line treatment [13]. The primary endpoint was progression-free survival (PFS) assessed by an independent review committee (IRC) while overall survival (OS) was a secondary endpoint.
Neither NICE nor HAS have identified major methodological issues with the design and the conduct of the TMM-1 trial which was deemed of good quality. However, a common source of concern pertained to the presence of a treatment effect with ixazomib compared with placebo. The PFS Hazard Ratio (HR) based on the first interim analysis (15 months follow-up) suggested a 26% reduction in the risk of progression or death (HR 0.74, 95% CI 0.5, 0.94; p = 0.012). However, further trial analyses which were not part of the pre-specified statistical analysis plan, conducted upon request by the US Food and Drug Administration (FDA) using slightly more mature follow-up data (23 months median follow up), found a smaller treatment effect of 18% risk reduction which was not statistically significant (HR 0.82, 95% CI 0.67, 1.0; p = 0.054).
Given that the analyses of more mature data were non-inferential, NICE and HAS acknowledged that, from a formal statistical standpoint, PFS benefit had been demonstrated, but both agencies commented that the results based on PFS overall lacked robustness [2,12].
NICE and HAS highlighted the immaturity of TMM-1 trial data for analysis OS in both first and second interim analyses; no benefit from adding ixazomib to LEN DEX was observed (HR for death of 0.90, 95% CI 0.62, 1.32 and 0.87, 95% CI 0.64, 1.18, respectively). Longer-term follow-up would be needed to draw conclusions on OS outcomes.
A notable difference between French and English assessments was the consideration of subgroup analyses from the TMM-1 trial.
In France, the transparency committee in effect assessed ixazomib strictly according to its label indication (patients with RRMM who received at least one prior therapy and therefore examined evidence for the entire TMM-1 population; no assessment was made differentiating patients according to the number of prior therapies received although TMM-1 trial subgroup analyses according to number of prior therapies were reported in the transparency committee document these were not further commented upon and apparently were not taken into consideration for decision-making [16].
Conversely, the appraisal by NICE relied on a pre-appraisal scoping document which indicated that the assessment should be differentiated according to the number of prior regimens received by patients [17]. This was because not all comparator regimens were equally recommended within the NHS with regard to the number of therapies previously received.
Interestingly, while bortezomib (with or without dexamethasone) was mentioned as the appropriate standard in RRMM patients at the second line (one prior therapy) in the NICE scope, lenalidomide-dexamethasone (LEN-DEX) was deemed the most relevant comparator in patients at the third or fourth line (two or three prior therapies). Within this scope, IXA-LEN-DEX was to be assessed against bortezomib-based regimens for the second line, while it was to be assessed against LEN-DEX for third or fourth line treatment.
In a first (non-final) appraisal, NICE concluded that IXA-LEN-DEX did not represent good value for money compared with bortezomib-based regimens; this conclusion was based on results from indirect treatment comparisons of benefit and on cost-effectiveness modelling. Similarly, at first appraisal, IXA-LEN-DEX was not recommended for third or fourth line therapy versus LEN-DEX; this was after consideration of direct subgroup evidence from the TMM-1 trial indicating a HR 0.62 (95% 0.45, 0.86) for PFS and a trend for improved OS.
In subsequent submissions to NICE by the Company, IXA-LEN-DEX was repositioned exclusively as a 3rd or 4th line therapy, with LEN-DEX as the relevant comparator. The resulting clinical effectiveness data coupled with new price discounts, in the judgement of the appraisal committee, rendered ixazomib potentialy cost-effective for this sub-population.
Ixazomib was eventually recommended as a third/fourth line option, not through routine commissioning, but within the Cancer Drugs Fund (CDF) [18]. The principle of the CDF is to allow further data collection with the aim of diminishing clinical uncertainty [19]. On the basis of these appraisal stages, it appears very unlikely that NICE could recommend ixazomib in the absence of differentiation of patients according to the number of prior therapies and referral to the CDF.
In France, the Transparency Committee published an opinion in July 2017 concluding that there was an absence of medical benefit improvement for IXA-LEN-DEX compared with LEN-DEX alone (ASMR rating of V) [2]. In its submission to HAS, the Company Takeda claimed an ASMR rating of IV for ixazomib [20]. Given the submitted evidence exhibiting a lack of robustness for effectiveness in PFS and immaturity in OS data, an ASMR rating of V for the label indication (i.e., no discrimination according to the number of previous therapies) is unsurprising.
Since the additional cost of ixazomib had to be considered against no treatment (LEN-DEX alone), no pricing arrangements could be found at the time between the French CEPS and the Company.
Eventually, in October 2018 a final decision was made on the list price of ixazomib in France [21]. This means that access to ixazomib for potential newly eligible patients was discontinued for 18 months (Figure 1).
Had the transparency committee considered clinical effectiveness data differentiated according to the number of prior therapies, it appears likely that the same ASMR rating of V would have pertained for second-line treatment.
However, the ASMR rating could have been more favourable if results observed in patients with more prior treatments had been included. In such a situation, presumably, a much earlier price arrangement might have been arrived at allowing the reimbursement of ixazomib in such a subgroup of patients.
Discussion
We have emphasised that access to ixazomib occurred much earlier in France compared to England thanks to the ATU programme, but that it subsequently became unavailable to newly eligible patients for a long period as pricing negotiations were ongoing between the Company and the French CEPS.
It may seem paradoxical that a new drug is judged so promising that it is made available under the ATU programme, but it is eventually granted an ASMR rating of V even though the same set of data on clinical effectiveness was used. However, based on the submitted evidence, we would agree that the ASMR of V granted to ixazomib appeared appropriate.
Although recently the criteria used to judge ASMR have been more clearly explained [22], this paradoxical situation may suggest the adoption of a more consistent policy of access to innovation in France involving ANSM and HAS and an appraisal process differentiating the populations most likely to benefit from a drug.
The uncertainty around clinical effectiveness estimates, especially for OS because of the immaturity of data, was highlighted in appraisals. This likely influenced the final opinion provided by HAS (ASMR rating of V). In such situations, temporary mechanisms of funding such as the one in place with the CDF in England are worthy of consideration, i.e., allowing patients to benefit from innovative medicines whilst collecting data with the aim of reducing uncertainty. This sort of funding source might usefully be implemented in France; however, analyses of data should be mandatory within a specified timeline.
The two appraisals conducted in France and England for ixazomib can be compared according to pricing. The basic NHS list price of ixazomib is £6,336 (excluding VAT) for a pack of three capsules irrespective of the strength [12]. The discounted price included in the commercial access agreement is confidential but presumably much lower. The final ICER produced under the proposed commercial access agreement has been publicly disclosed (£31,691 per QALY gained compared to LEN-DEX alone) which is useful to help understand the decision-making.
In France, the officially published price in October 2018 is €3,875 (excluding VAT) for three capsules [21]. It is highly likely that this price is a discounted one obtained after pricing negotiation, which denotes an apparently higher degree of transparency in France. In the absence of formal cost-effectiveness evaluation, it is unclear what factors were accounted for in the pricing of ixazomib in France. This seems particularly important given the poor ASMR rating (ASMR of V) the drug received compared to LEN-DEX alone. Indeed, while ASMR ratings of V can be provided for new medicines compared to other active agents, here the ASMR rating of V for ixazomib was given compared to LEN-DEX alone, which denotes no additional benefit compared to placebo.
It is important to bear in mind that in France, as in other countries, the published price following negotiations is usually not the real one, since prices are determined based on a price/volume agreement, which involves confidential national rebates.
Although we have pointed to a more direct and simplified availability of ixazomib in England, it is important to highlight the more restricted circumstance of use in England since the CDF only allows funding of the drug for RRMM patients at the third or fourth line. More specifically, there are issues associated with patients treated at the second line. Indeed, LEN-DEX is currently no longer funded at this stage through the CDF and this drug regimen is only used in patients at the third or fourth line. Similarly, retreatment with bortezomib at the second line is no longer funded through the CDF. There is, therefore, a lack of recommended therapies for patients within the NHS at second line who were previously treated with bortezomib-based regimens at first line [12].
Our work has some limitations. The main one is that our method of analysis did not include interviews with stakeholders, it is possible that we have not fully captured the full complexity of the decision-making process. This includes pricing discussions on commercial access agreements which remain confidential to public observers. We have only focussed on a single example so that our observations may not as widely generalizable as we would wish.
Based on our comparison of England and France, our view is that cost-effectiveness evaluation could become more systematically used in France allowing a more transparent process of pricing based on a defined willingness to pay threshold.
Further research is required to comprehensively assess the usefulness of the early access program for innovative drugs in France.
Since English funding through the CDF is said to be provisional, it will be interesting to observe the next review of ixazomib by NICE, based on more mature clinical effectiveness data. As any other drug, it is also expected that ixazomib will be re-reviewed by the French HAS in the near future.
Although we have indicated a more rapid final decision taken in England compared to France, the decision of reimbursement was taken even more rapidly in other European countries since ixazomib was reimbursed in Germany, Austria, and Denmark less than 3 months following EMA approval.
In conclusion, based on our specific observation of France and England, it appears that differences in the HTA process such as the differentiation of populations and/or comparators according to previous treatments can result in appreciable differences in time from marketing authorisation to health service adoption of newly licensed drugs.
Acknowledgments
The authors would like to thank Dominique Polton for providing comments on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Ninlaro | European Medicines Agency Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ninlaro
- [2].Haute Autorité de Santé - NINLARO (ixazomib), antinéoplasique Available from: https://www.has-sante.fr/portail/jcms/c_2779830/fr/ninlaro-ixazomib-antineoplasique
- [3].National Institute for Health and Care Excellence (NICE) , Topic selection | Our programmes | What we do | About. Available from: https://www.nice.org.uk/about/what-we-do/our-programmes/topic-selection
- [4].Haute Autorité de Santé - Commission de la transparence Available from: https://www.has-sante.fr/portail/jcms/c_412210/fr/commission-de-la-transparence
- [5].National institute for health and care excellence: overview of technology appraisals for patient and carer organisations, Public Involvement Programme. April 2014. Available from: https://www.nice.org.uk/Media/Default/About/NICE-Communities/Public-involvement/Developing-NICE-guidance/Overview-of-technology-appraisals-patient-carer-groups.pdf [Google Scholar]
- [6].McCabe C, Claxton K, Culyer AJ.. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26:733–6. [DOI] [PubMed] [Google Scholar]
- [7].National Institute for Health and Care Excellence (NICE) , Patient access schemes, commercial access agreements and flexible pricing | Guide to the processes of technology appraisal | Guidance | NICE. Available from: https://www.nice.org.uk/process/pmg19/chapter/patient-access-schemes-commercial-access-agreements-and-flexible-pricing [PubMed]
- [8].Haute Autorité de Santé - Le service médical rendu (SMR) et l’amélioration du service médical rendu (ASMR) Available from: https://www.has-sante.fr/portail/jcms/r_1506267/fr/le-service-medical-rendu-smr-et-l-amelioration-du-service-medical-rendu-asmr
- [9].Giorgi D. [The French medecine pricing committee]. Ann Pharm Fr. 2017;75:373–384. [DOI] [PubMed] [Google Scholar]
- [10].Toumi M, Motrunich A, Millier A, et al. Analysis of health economics assessment reports for pharmaceuticals in France - understanding the underlying philosophy of CEESP assessment. J Mark Access Health Policy. 2017;5:1344088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Haute Autorité de Santé: L’évaluation médico-économique des Médicaments et Dispositifs Médicaux. Dossier de presse: Conférence de presse; 2014. décembre 18. [Google Scholar]
- [12].National Institute for Health and Care Excellence (NICE) , Ixazomib with lenalidomide and dexamethasone for treating relapsed or refractory multiple myeloma | Guidance and guidelines | NICE. Available from: https://www.nice.org.uk/guidance/ta505
- [13].Moreau P, Masszi T, Grzasko N, et al. TOURMALINE-MM1 study group: oral Ixazomib, Lenalidomide, and Dexamethasone for multiple Myeloma. N Engl J Med. 2016;374:1621–1634. [DOI] [PubMed] [Google Scholar]
- [14].Direction Générale de l'Offre de Soins (DGOS): Le forfait innovation Available from: https://solidarites-sante.gouv.fr/systeme-de-sante-et-medico-social/recherche-et-innovation/forfait-innovation
- [15].Agence Nationale de Sécurité du Médicament et des produits de santé à la référence , Qu’est ce qu’une autorisation temporaire d’utilisation? - ANSM: Agence nationale de sécurité du médicament et des produits de santé. Available from: https://www.ansm.sante.fr/Activites/Autorisations-temporaires-d-utilisation-ATU/Qu-est-ce-qu-une-autorisation-temporaire-d-utilisation/(offset)/0
- [16].Haute Autorité de Santé, NINLARO 17052017 CT15988 TRANSCRIPTION Available from: https://www.has-sante.fr/portail/plugins/ModuleXitiKLEE/types/FileDocument/doXiti.jsp?id=c_2803717
- [17].National Institute for Health and Care Excellence (NICE) , Ixazomib with lenalidomide and dexamethasone for treating relapsed or refractory multiple myeloma | Final Scope | NICE. Available from: https://www.nice.org.uk/guidance/ta505/history
- [18].Armoiry X, Connock M, Tsertsvadze A, et al. Ixazomib for relapsed or refractory multiple Myeloma: review from an evidence review group on a NICE single technology appraisal. Pharmacoeconomics. 2018;36:1073–1081. [DOI] [PubMed] [Google Scholar]
- [19].Dillon A, Landells LJ. NICE, the NHS, and cancer drugs. JAMA. 2018;319:767–768. [DOI] [PubMed] [Google Scholar]
- [20].Haute Autorité de Santé,COMPTE RENDU CT 17052017 Available from: https://www.has-sante.fr/portail/plugins/ModuleXitiKLEE/types/FileDocument/doXiti.jsp?id=c_2775511
- [21].Avis relatif aux prix de spécialités pharmaceutiques , JORF n°0252 du 31 octobre 2018 texte n° 142, https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000037543195&categorieLien=id.
- [22].Haute Autorité de Santé - La commission de la transparence précise et adapte ses principes d’évaluation des médicaments Available from: https://www.has-sante.fr/portail/jcms/c_2877573/fr/la-commission-de-la-transparence-precise-et-adapte-ses-principes-d-evaluation-des-medicaments
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ninlaro | European Medicines Agency Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ninlaro
- Haute Autorité de Santé - NINLARO (ixazomib), antinéoplasique Available from: https://www.has-sante.fr/portail/jcms/c_2779830/fr/ninlaro-ixazomib-antineoplasique


