Abstract
Recent public health emergencies have highlighted the unique vulnerabilities of pregnant women and infants to emerging health threats and the critical role of public health surveillance. Surveillance systems can collect critical data to measure the impact of a disease or disaster and can be used to inform clinical guidance and prevention strategies. These systems can also be tailored to collect data on vulnerable populations, such as pregnant women and their infants. Novel surveillance systems to assess risks and outcomes of pregnant women and infants have been established during public health emergencies but typically cease data collection once the public health response has ended, limiting our ability to collect data to understand longer-term outcomes. State-based birth defects surveillance systems are not available in all states, and no national surveillance system linking pregnancy exposure data to longitudinal outcomes for infants and children exists. In this report, we describe ongoing surveillance efforts to monitor congenital syphilis, Zika virus infection during pregnancy, and neonatal abstinence syndrome. We describe the need and rationale for an ongoing integrated surveillance system to monitor pregnant women and their infants and to detect emerging threats. We also discuss how data collected through this type of system can better position federal, state, and local health departments to more rapidly and comprehensively respond to the next public health emergency.
Keywords: infectious diseases, emerging threats, pregnant women, infants, preparedness
Introduction
Recent public health emergencies have highlighted unique vulnerabilities of pregnant women and infants to emerging health threats and the critical role of public health surveillance. Surveillance systems can collect data to describe the impact of a disease or disaster that can be used to inform clinical guidance and prevention strategies.1
Pregnant women might be more susceptible to infection or experience more severe illness, mortality, pregnancy complications, or adverse birth outcomes.2 For example, during the 2009–2010 H1N1 influenza pandemic, infected pregnant women had more severe illness and higher rates of hospitalization than the general population.3 Similarly, fetuses and infants may be at increased risk for adverse outcomes during public health emergencies, including stillbirth, preterm birth, low birthweight, birth defects, infant disorders, and developmental disabilities. For example, maternal Zika virus infection can cause serious brain and eye abnormalities in fetuses and infants and has been linked to neurodevelopmental disabilities in early childhood.4,5
Emerging threats to the health of pregnant women and infants are not limited to infectious diseases. Environmental exposures and natural disasters can introduce toxins (e.g., lead in the water supply), limit the supply of clean water and safe food, and may limit access to health care.6 Pregnant women and infants are also uniquely vulnerable populations impacted by the current opioid crisis. The rate of diagnosis of opioid use disorder at hospital delivery more than quadrupled between 1999 and 2014 in the United States.7 In parallel with increasing rates of maternal opioid use disorder, the rate of postnatal drug withdrawal among infants prenatally exposed to opioids, commonly known as neonatal abstinence syndrome (NAS), continues to increase. In 2014, a newborn with signs of opioid withdrawal was diagnosed every 15 minutes in the United States.8
Public health surveillance and the timely identification of known and emerging threats and evolving risks that affect pregnant women and infants enables federal, state, tribal, territorial, and local health departments to effectively respond to threats and mitigate adverse pregnancy, infant, and childhood outcomes. Rapid and complete characterization of the impact of health threats on pregnant women and their infants may benefit from an integrated surveillance system that (1) collects data on adverse maternal, pregnancy, and birth outcomes, such as birth defects or other infant disorders; (2) links the mother/infant dyad and collects data on pregnancy exposures; and (3) includes longitudinal monitoring to assess possible longer-term outcomes, such as cognitive impairment or developmental delay.
Rapid and real-time surveillance can quickly provide data to inform the public health response during an acute emergency, clinical practice guidelines for known and emerging threats, and the distribution of critical resources, including specialized health care. In addition, real-time data can monitor the effectiveness of prevention efforts and response strategies, medical countermeasures, and other public health interventions in a timely manner.
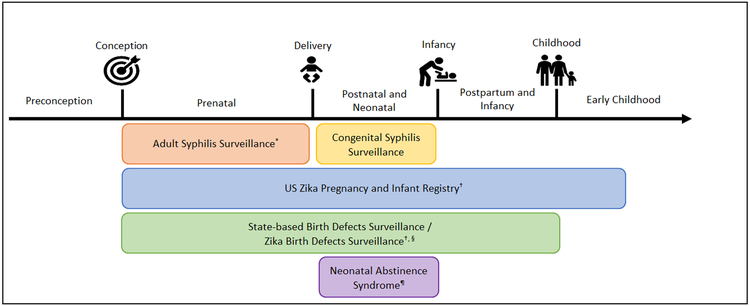
In this report, we describe ongoing surveillance efforts to monitor congenital syphilis, Zika virus infection during pregnancy, and NAS and challenges within existing data systems (Fig. 1).
Figure 1. Non-survey-based National Surveillance Systems for Maternal, Infant, and Child Health Outcomes.
* This system collects case reports of syphilis infections in males and females; pregnancy status is reportable to national surveillance but is not collected in a timely manner.
† Established in 2016 as part of the Centers for Disease Control and Prevention Zika response.
§ Additional birth defects were added to existing state-based birth defects surveillance systems for Zika Birth Defects Surveillance.
¶ The Council of State and Territorial Epidemiologists established a position statement for a standardized surveillance case definition for neonatal abstinence syndrome surveillance that was approved in June.
We also discuss how an ongoing integrated surveillance system to monitor pregnant women and their infants can better position health agencies across the United States to more rapidly and comprehensively respond to the next public health emergency. As new health threats emerge and others resurge, this type of integrated surveillance system provides an innovative, efficient, and modifiable method to collect and exchange information and to inform public health strategies to protect pregnant women and infants. An overview of these topics was presented at CDC’s Public Health Grand Rounds on September 18, 2018.9
Congenital Syphilis
Syphilis is caused by the bacteria Treponema pallidum and is most often spread through sexual contact. Congenital syphilis occurs when an infected woman transmits syphilis to her fetus during pregnancy. Congenital syphilis can result in miscarriage, stillbirth, preterm birth, low birthweight, and infant death. Up to 40% of infants born to women with untreated syphilis may be stillborn or die from the infection as newborns.10 Infants born with congenital syphilis may experience anemia, hepatosplenomegaly, neurological impairment, including deafness, and birth defects of the long bones and teeth.11 When maternal syphilis is detected and appropriate treatment is initiated at least 30 days before delivery, congenital syphilis can be prevented.12
The Centers for Disease Control and Prevention (CDC) recommends universal syphilis screening at the first prenatal visit and additional screening for women at high risk, including those living in areas of high morbidity, between 28–32 weeks gestational age, and again at delivery to treat maternal syphilis and prevent congenital syphilis and its complications.13
Congenital syphilis is a nationally notifiable disease. Public health departments in all 50 states, the District of Columbia, and all U.S. territories report cases of syphilis, including congenital syphilis, to CDC through the National Notifiable Diseases Surveillance System (NNDSS). CDC uses data reported to NNDSS to monitor syphilis trends, identify populations or geographic areas at high risk, plan prevention and control policies and interventions, and allocate resources.14 Despite the availability of effective treatment options that are safe for use during pregnancy, during 2012–2017, the number of infants born with congenital syphilis increased 176%.15,16
In 2017, CDC published a “Syphilis Call to Action” outlining critical gaps in syphilis surveillance and activities needed to reduce rates of syphilis, including the rate among women of reproductive age, which mirrors the rate of congenital syphilis. Female syphilis data collected and shared between health care providers, laboratories, and public health departments are essential for both treating women and preventing congenital syphilis.17 The current surveillance system does not capture pregnancy status for women with syphilis in a timely manner. This limits state and local health departments’ ability to ensure prompt treatment at least 30 days before delivery and slows the triage of women into other preventive services, including health department-led case management programs.
The current surveillance system also makes identifying risk factors for congenital syphilis challenging because it does not link maternal and congenital syphilis cases and does not collect data on infants prenatally exposed to syphilis unless the infant is classified as a congenital syphilis case. Comparing infants born with congenital syphilis to infants who were prenatally exposed to syphilis but did not meet the case definition could identify protective factors to improve congenital syphilis prevention efforts. In addition, current surveillance methods do not collect data on children with prenatal syphilis exposure to assess possible longer-term effects.
A longitudinal surveillance system that enrolls pregnant women at the time of syphilis diagnosis could improve our understanding of developmental outcomes of children with prenatal syphilis exposure, ensure more complete congenital syphilis case ascertainment, allow us to examine maternal and fetal predictors of adverse outcomes, and link pregnant women to perinatal case management.
Zika Infection
Zika is a mosquito-borneflavivirus spread primarily through the bite of an infected Aedes species mosquito. Zika can also be spread through sexual transmission, blood transfusion, and from a woman to a fetus during pregnancy or at birth.18 Zika virus infection during pregnancy can cause severe brain and eye defects as well as other adverse outcomes secondary to neurological damage.4,5,19 In January 2016, CDC began an emergency response to outbreaks of Zika among immunologically naive populations in the Americas.20
In collaboration with health departments, CDC established the US Zika Pregnancy and Infant Registry and the Zika Birth Defects Surveillance system. These complementary surveillance systems provided more complete ascertainment of Zika-associated pregnancy and birth outcomes and improved our understanding of longer-term outcomes that could benefit from clinical follow-up during early childhood. The US Zika Pregnancy and Infant Registry prospectively monitors pregnant women and infants with laboratory evidence of possible Zika infection.21 The Zika Birth Defects Surveillance system retrospectively collects data on fetuses and infants born with Zika-associated birth defects with or without laboratory evidence of maternal Zika infection.22 Some jurisdictions were able to rapidly adapt their methods to report Zika-associated birth defects to CDC’s Zika Birth Defects Surveillance system, but this was only feasible on a short time frame among states with existing birth defects surveillance systems. Using 2013–2014 data from three state-based birth defects sur-veillance programs, CDC was able to estimate the baseline prevalence of birth defects potentially related to congenital Zika infection before the introduction of Zika in the Americas.23 This provided an important comparison to estimate the approximate 30-fold increase in these specific birth defects among pregnancies with laboratory evidence of possible Zika virus infection.
Data collected through the US Zika Pregnancy and Infant Registry and the Zika Birth Defects Surveillance system improved our understanding about the timing, risk, and spectrum of outcomes associated with Zika infection during pregnancy and enabled CDC to rapidly and continuously update travel, testing, and clinical care recommendations based on emerging data. In addition, data collected through these systems informed planning for clinical, public health, and other services needed to support pregnant women and families affected by Zika. The US Zika Pregnancy and Infant Registry continues to monitor infants and young children from >7400 completed pregnancies with laboratory evidence of possible Zika infection during pregnancy delivered between December 1, 2015 and March 31, 2018.
Collaboration between the US Zika Pregnancy and Infant Registry and the Zika Birth Defects Surveillance system resulted in a more robust understanding of the effects of Zika infection during pregnancy and informed clinical and public health efforts.1 However, there were also challenges. An early challenge to Zika surveillance was the absence of standard case definitions for Zika virus disease and congenital Zika infection.
At the start of the outbreak, states with existing birth defects surveillance programs were not collecting data on all birth defects associated with Zika infection in pregnancy. Therefore, states with existing birth defects surveillance programs needed to revise their existing methods. In addition, before the Zika response, relationships between some organizational units within state and local health departments were limited and required more intentional collaboration to share data across surveillance systems and with CDC.
In June 2016, the Council of State and Territorial Epidemiologists (CSTE), with input from CDC, established initial case definitions for congenital and noncongenital Zika virus disease and standardized data collection methods for cases of Zika virus infection across the United States.24 CDC also established a data use working group for the US Zika Pregnancy and Infant Registry to discuss issues around data reporting, analysis, and case definitions with state, local, and territorial partners.
States that successfully established remote access to hospital medical records for case abstraction reported a significant decrease in the amount of time and resources needed to collect and report data to the US Zika Pregnancy and Infant Registry and the Zika Birth Defects Surveillance system. Some states also reported that the multidisciplinary nature of the Zika response improved communication within and between health departments, as well as with health care providers, and led to more comprehensive case reports and improved communication of rapidly changing testing and treatment guidelines.
Neonatal Abstinence Syndrome
Prenatal opioid exposure is associated with poor fetal growth, preterm birth, stillbirth, and NAS, and some studies suggest a possible association with specific birth defects (e.g., orofacial clefts, gastroschisis).25-27 However, the potential longer-term impacts of prenatal opioid exposure are not well understood.
There is a strong body of evidence demonstrating that the teratogenic effects of alcohol use during pregnancy may not manifest until childhood or adolescence.28 Recent evidence suggests that children born with NAS may be more likely to have a developmental delay or speech or language impairment in early childhood compared with children born without NAS.29 Longitudinal surveillance of children with prenatal opioid exposure could improve our understanding of possible teratogenic effects of opioid use during pregnancy and the confounding effects of exposure to other substances during pregnancy, including alcohol, as well as environmental and psychosocial factors.30
Currently, there is no national surveillance of NAS, but the recent establishment of a standardized surveillance case definition by CSTE is an opportunity to improve consistency and comparability of reported cases.31 As of January 3, 2018, six states had laws requiring reporting of NAS cases for public health surveillance.32 On January 10, 2018, Pennsylvania’s governor declared a statewide disaster emergency for the state’s heroin and opioid crisis. The declaration added NAS as a reportable condition for public health surveillance.33 In response, the Pennsylvania Department of Health rapidly established a statewide surveillance system to collect data on NAS cases.
Before the Zika emergency response, Pennsylvania was the largest state in the United States without a state-based birth defects surveillance system.34 However, in response to Zika, the state established a surveillance system to report cases to the US Zika Pregnancy and Infant Registry and the Zika Birth Defects Surveillance system. Establishing pregnancy and birth defects surveillance during the Zika response equipped the state to successfully implement data collection for NAS surveillance within a few weeks of the emergency declaration.
Specifically, the Pennsylvania Department of Health applied their familiarity of data elements captured in birth records to create an NAS case report form and quickly produced a list of birthing facilities and corresponding annual live birth counts to prioritize outreach and education on NAS case reporting. In addition, the Pennsylvania Department of Health rapidly adapted its web-based REDCap system used to report data to the US Zika Pregnancy and Infant Registry and the Zika Birth Defects Surveillance system for NAS data collection.
Building upon the Zika response’s investment and infrastructure enabled the Pennsylvania Department of Health to quickly distribute case reporting guidance to 95 birthing facilities and initiate rapid case ascertainment. Within 9 months, 89% (n = 85) of birthing facilities in Pennsylvania were reporting NAS cases to the Pennsylvania Department of Health.
NAS surveillance not only enabled the Pennsylvania Department of Health to estimate the incidence of NAS in their state but also provided information on maternal and infant characteristics, including maternal demographics, receipt of prenatal care, specific opioid exposures among NAS cases (e.g., medication-assisted treatment, synthetic opioids), symptoms of withdrawal (e.g., irritability, hypertonia), comorbidities among infants born with NAS (e.g., low birthweight, shorter length of gestation), and postnatal care administered to infants, including administration of pharmacological and non-pharmacological treatment. The Pennsylvania Department of Health’s experience implementing a system for rapid NAS surveillance may offer strategies that other states could adopt to rapidly monitor NAS or other emerging health threats to pregnant women and infants.
Summary and Future Directions
Surveillance is a core function of public health practice.35 The timely and systematic collection, analysis, and interpretation of data improves our ability to detect and respond to known and emerging health threats and can inform prevention practices and clinical guidance at both the patient and population level. Previous emergency responses as well as ongoing notifiable disease surveillance have demonstrated the benefits of public health surveillance data to inform prevention strategies and clinical care for pregnant women and infants.
However, we have too often ceased data collection on pregnant women and infants when an emergency response ends, limiting our ability to identify possible longer-term outcomes associated with public health threats to pregnant women and infants. Although NNDSS allows CDC to monitor the incidence and spread of specific infectious and noninfectious conditions, NNDSS does not collect pregnancy status for women who test positive for infection in a timely manner, does not link maternal cases to perinatal, neonatal, and pediatric outcomes, and does not collect longitudinal data.
Adverse outcomes associated with congenital syphilis, Zika infection during pregnancy, and NAS demonstrate vulnerabilities of pregnant women and infants and highlight the critical function of public health surveillance to protect these populations from known and emerging health threats. However, we do not have a comprehensive national surveillance system that (1) monitors adverse maternal and infant outcomes, including birth defects and other infant disorders; (2) links pregnancy exposure or infection data to longitudinal outcomes for mothers and infants; and (3) includes a longitudinal component to assess developmental outcomes among prenatally exposed children.
Existing public health surveillance efforts can be strengthened by consistent documentation and timely reporting of pregnancy status as part of laboratory testing and case reporting and may improve the treatment and management of maternal conditions during the prenatal period. In addition, as demonstrated during the Zika response, linking pregnancy data with infant outcomes can improve our understanding of outcomes associated with harmful exposures during pregnancy. This information can inform better prevention and clinical management and can help to standardize treatment protocols for pregnant women and infants.
This public health surveillance may also improve infant and child health. Longitudinal monitoring of exposed infants can help characterize risk factors for specific adverse outcomes, assess medical needs and possible developmental outcomes among children with specific prenatal exposures, and may facilitate screening for possible functional impairments that may benefit from treatments or interventions.
Building upon our experience with ongoing surveillance of notifiable diseases and lessons learned from surveillance of pregnant women and infants during emergency responses, CDC is working with partners to define core variables for future surveillance of known and emerging health threats to pregnant women and their infants that can be adapted using supplemental modules that might be needed for specific threats. A surveillance system with these core variables could be poised to more rapidly respond to the next public health emergency and collect critical data to protect pregnant women and infants.
Acknowledgments
The authors thank Gail Bolan, Catherine Brown, Julie Dunn, Katherine Flaherty, Michael Fraser, Jennifer Fuld, Kim Hauser, Cathleen Higgins, Callie Howells, Sarah Kidd, Susan Laird, Allison Longenberger, Alicia May, Kara Polen, Jennita Reefhuis, Angie Robertson, Kayleigh Sandhu, Sarah Scotland, and Fan Tait for their important contributions to this study. We also thank the many public health and clinical partners who participated in the September 2018 Public Health Grand Rounds and shared their valuable insights.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Authors Disclosure Statement
No competing financial interests exist.
References
- 1.Gilboa SM, Mai CT, Shapiro-Mendoza CK, et al. Population-based pregnancy and birth defects surveillance in the era of Zika virus. Birth Defects Res 2017;109:372–378. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen SA, Hayes EB. Public health approach to emerging infections among pregnant women. Am J Public Health 2005;95:1942–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in the United States. JAMA 2010;303:1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—Reviewing the evidence for causality. N Engl J Med 2016;374:1981–1987. [DOI] [PubMed] [Google Scholar]
- 5.Rice ME, Galang RR, Roth NM, et al. Vital signs: Zika-associated birth defects and neurodevelopmental abnormalities possibly associated with congenital Zika virus infection—US territories and freely associated states, 2018. Morb Mortal Wkly Rep 2018;67:858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaghan WM, Rasmussen SA, Jamieson DJ, et al. Health concerns of women and infants in times of natural disasters: Lessons learned from Hurricane Katrina. Matern Child Health J 2007;11:307–311. [DOI] [PubMed] [Google Scholar]
- 7.Haight SC, Ko JY, Tong VT, Bohm MK, Callaghan WM. Opioid use disorder documented at delivery hospitalization—United States, 1999–2014. Morb Mortal Wkly Rep 2018;67:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkelman TN, Villapiano N, Kozhimannil KB, Davis MM, Patrick SW. Incidence and costs of neonatal abstinence syndrome among infants with medicaid: 2004–2014. Pediatrics 2018;141:e20173520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Public health grand rounds: surveillance for emerging threats to pregnant women and infants: data for action. Atlanta, GA: US Department of Health and Human Services, CDC; 2018. https://www.cdc.gov/grand-rounds/pp/2018/20180918-pregnancy-threats.html Accessed August 1, 2019. [Google Scholar]
- 10.Ingraham N Jr The value of penicillin alone in the prevention and treatment of congenital syphilis. Acta Derm Venereol 1950;31:60–87. [PubMed] [Google Scholar]
- 11.Holmes K, ed. Sexually transmitted diseases. 4th ed. China: McGraw Hill, 2008. [Google Scholar]
- 12.Alexander JM, Sheffield JS, Sanchez PJ, Mayfield J, Wendel G Jr. Efficacy of treatment for syphilis in pregnancy. Obstet Gynecol 1999;93:5–8. [DOI] [PubMed] [Google Scholar]
- 13.Workowski KA, Bolan GAJMR. Sexually transmitted diseases treatment guidelines, 2015. Morb Mortal Wkly Rep 2015;64:1. [Google Scholar]
- 14.Adams DA, Thomas KR, Jajosky RA, et al. Summary of notifiable infectious diseases and conditions—United States, 2015. Morb Mortal Wkly Rep 2017;64. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017, 2018 https://www.cdc.gov/std/stats17/default.htm Accessed August 1, 2019.
- 16.Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med 2009;163:978–985. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. CDC call to action: Let’s work together to stem the tide of rising syphilis in the United States, 2017. https://www.cdc.gov/std/syphilis/SyphilisCalltoActionApril2017.pdf Accessed August 1, 2019.
- 18.Gregory CJ, Oduyebo T, Brault AC, et al. Modes of transmission of Zika virus. J Infect Dis 2017;216:S875–S883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 2017;171:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. CDC Emergency Operations Center moves to highest level of activation for Zika response, 2016. https://www.cdc.gov/media/releases/2016/s0208-zika-eoca-activation.html Accessed August 1, 2019.
- 21.Reynolds MR, Jones AM, Petersen EE, et al. Vital signs: Update on Zika virus—Associated birth defects and evaluation of all US infants with congenital Zika virus exposure—US Zika Pregnancy Registry, 2016. Morb Mortal Wkly Rep 2017;66:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delaney A, Mai C, Smoots A, et al. Population-based surveillance of birth defects potentially related to Zika virus infection—15 states and US territories, 2016. Morb Mortal Wkly Rep 2018;67:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cragan JD, Mai CT, Petersen EE, et al. Baseline prevalence of birth defects associated with congenital Zika virus infection-Massachusetts, North Carolina, and Atlanta, Georgia, 2013–2014. Morb Mortal Wkly Rep 2017;66:219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Zika virus disease and Zika virus infection 2016 case definition, 2016. https://wwwn.cdc.gov/nndss/conditions/zika/case-definition/2016/06/ Accessed August 1, 2019.
- 25.Broussard CS, Rasmussen SA, Reefhuis J, et al. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol 2011;204:314.e311. [DOI] [PubMed] [Google Scholar]
- 26.Lind JN, Interrante JD, Ailes EC, et al. Maternal use of opioids during pregnancy and congenital malformations: A systematic review. Pediatrics 2017;139:e20164131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yazdy MM, Mitchell AA, Tinker SC, Parker SE, Werler MM. Periconceptional use of opioids and the risk of neural tube defects. Obstet Gynecol 2013;122:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senturias Y, Asamoah A. Fetal alcohol spectrum disorders: Guidance for recognition, diagnosis, differential diagnosis and referral. Curr Probl Pediatr Adolesc Health Care 2014; 44:88–95. [DOI] [PubMed] [Google Scholar]
- 29.Fill M-MA, Miller AM, Wilkinson RH, et al. Educational disabilities among children born with neonatal abstinence syndrome. Pediatrics 2018;142:e20180562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honein MA, Boyle C, Redfield RR. Public health surveillance of prenatal opioid exposure in mothers and infants. Pediatrics 2019;143:e20183801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Council of State and Territorial Epidemiologists (CSTE). Neonatal Abstinence Syndrome Standardized Case Definition. CSTE position statement, 2019. https://cdn.ymaws.com/www.cste.org/resource/resmgr/2019ps/final/19-MCH-01_NAS_final_7.31.19.pdf Accessed August 1, 2019.
- 32.Jilani SM, Frey MT, Pepin D, et al. Evaluation of state-mandated reporting of neonatal abstinence syndrome—Six states, 2013–2017. Morb Mortal Wkly Rep 2019;68:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The Office of the Pennsylvania Governor. Governor Wolf’s disaster declaration for the heroin and opioid epidemic, 2018. https://www.governor.pa.gov/newsroom/governor-wolf-declares-heroin-and-opioid-epidemic-a-statewide-disaster-emergency/ Accessed August 1, 2019. [Google Scholar]
- 34.Lupo PJ, Isenburg JL, Salemi JL, et al. Population-based birth defects data in the United States, 2010–2014: A focus on gastrointestinal defects. Birth Defects Res 2017;109: 1504–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Institute of Medicine. Committee for the Study of the Future of Public Health The future of public health, Vol. 88 Washington (DC): National Academy Press, 1988. [Google Scholar]