Abstract
Purpose:
To examine the relationship between food cravings and food addiction, and to assess the effects of these variables on weight loss during a 14-week group lifestyle modification program.
Methods:
Data were from 178 participants who were prescribed a 1000-1200 kcal/day portion-controlled diet and provided with weekly group lifestyle modification sessions. Participants completed the Yale Food Addiction Scale and Food Craving Inventory pre- and post-treatment. Weight was measured weekly.
Results:
Participants with food addiction reported more frequent overall food cravings relative to those without food addiction. More frequent food cravings at baseline were associated with less weight loss over the 14 weeks. Analyzed categorically, participants in the highest tertile of baseline food cravings lost 7.6±0.5% of initial weight, which was significantly less compared to those in the lowest tertile who lost 9.1±0.5%. Percent weight loss did not differ significantly between participants with food addiction (6.5±1.2%) and those without food addiction (8.6±0.3%). Food cravings and food addiction symptoms significantly declined from pre- to post-treatment.
Conclusions:
Addictive-like eating behaviors tended to decline during behavioral weight loss. However, participants with frequent food cravings lost less weight than their peers. Targeted interventions could improve weight loss in these individuals.
Keywords: obesity, cravings, weight loss, food addiction, treatment
Introduction
Addictive-like eating, such as food addiction and food cravings, is commonly described by patients as contributors to their obesity and barriers to weight loss (Hammarström et al., 2014; Ruddock & Hardman, 2017). Food addiction is most widely measured with the Yale Food Addiction Scale (YFAS) which conceptualizes food addiction as eating patterns that share behavioral similarities with substance use disorders (Gearhardt et al., 2009a, 2009b). The YFAS was developed by applying the seven criteria for substance dependence from the Diagnostic and Statistical Manual of Mental Disorders (DSM) IV-TR to food and eating behaviors (Gearhardt et al., 2009b). The criteria are: giving up or reducing important social, occupational, or recreational activities due to issues with certain foods or eating; taking the substance in larger amounts and for a longer period than intended; persistent desire or repeated unsuccessful attempts to quit; much time/activity to obtain, use, recover; withdrawal symptoms when cutting down on certain foods; use despite knowledge of adverse consequences; and tolerance. A diagnosis of food addiction is made if individuals endorse at least three dependence symptoms and have clinically significant impairment or distress. In 2013, the American Psychiatric Association updated the criteria for substance use disorders to include cravings, a strong desire or urge to use a substance (American Psychiatric Association, 2013). While craving is a core feature of substance use disorders, the empirical basis for adding this criterion to the food addiction diagnosis has not been well investigated.
A food craving is defined as an intense desire to consume a particular food or food type that is difficult to resist (White et al., 2002b). Food cravings are commonly reported among individuals with obesity and are associated with more frequent consumption of craved foods and with increased body mass index (BMI) and weight gain (Boswell & Kober, 2016; Chao et al., 2014). In studies of community and internet-based samples, more frequent food cravings were related to symptoms of and a diagnosis of food addiction (Joyner et al., 2015; Meule & Kübler, 2012). It is not clear whether individuals with obesity who meet criteria for food addiction have more frequent food cravings relative to those with a similar BMI who do not meet criteria. More studies are needed to assess the relevance of cravings to the food addiction construct.
Additionally, research is needed on the effects of food cravings and food addiction on weight loss. While food cravings tend to decrease with weight loss (Kahathuduwa et al., 2017), findings are inconsistent about whether food cravings are associated with attrition and weight loss during obesity treatment (Batra et al., 2013; Buscemi et al., 2017; Gilhooly et al., 2007; Martin et al., 2006; Moldovan et al., 2016; Smithson & Hill, 2016). In addition, higher food addiction symptoms have been linked with poor weight loss during obesity treatment in some (Burmeister et al., 2013; Tompkins et al., 2017), but not all studies (Lent et al., 2014; Sawamoto et al., 2017). One source of variability in study findings may be the type of dietary prescription used. The present study extends the literature by examining the relationships of food craving and food addiction to weight loss achieved with a structured dietary plan that incorporated meal replacements with behavioral obesity treatment.
Meal replacements help address several challenges related to weight loss and can help improve outcomes relative to isocaloric diets of conventional foods (Heymsfield et al., 2003a; Keogh & Clifton, 2005).They provide 1000-1200 kcal/day in the form of liquid shakes, meal bars, and prepared entrees (Heymsfield et al., 2003a). The food is portion-controlled, so people know precisely how many calories they are consuming and can avoid the calorie underestimation commonly seen with conventional foods (Lichtman et al., 1992). In addition, meal replacements help reduce the number of food choices and the possibility of higher calorie food selections. The rigid structure and restricted nature of meal replacements, however, may be challenging for individuals with frequent food cravings and those who meet criteria for food addiction, as suggested by research on dietary restraint (Polivy & Herman, 1985; Ruderman, 1986). Disruption of dietary restraint may result in overeating or binge eating. Similarly, in the case of substance use disorders, cravings appear to play a central role in drug relapse (Niaura, 2000).
This study had three aims, the first which was to examine whether individuals with food addiction had increased levels of total food cravings and cravings for specific food types (e.g., sweets, high fats). We hypothesized that participants who met criteria for food addiction would have more frequent food cravings relative to individuals without food addiction. The second aim was to assess the effects of baseline food cravings and food addiction on attrition and weight loss during a 14-week group lifestyle modification program that included a 1000-1200 kcal/day meal-replacement diet. We predicted that individuals with more frequent baseline food cravings would have greater attrition and lose less weight at 14 weeks than those with less frequent cravings. We assessed the same hypothesis for food addiction. Third, we explored whether changes in food craving frequency and food addiction symptoms were associated with weight loss. We anticipated that greater reductions in food cravings and food addiction symptoms would be associated with more weight loss.
Methods
Study Design
Details of the parent study have been described previously (Chao et al., 2017; Shaw Tronieri et al., 2017, 2018). The present investigation used data from a prospective, single-arm study of a 14-week group behavioral weight loss program with a structured meal replacement diet (Health Management Resources; HMR800). The program was designed to induce a ≥5% loss of initial body weight (Wadden et al., 2011; Wadden et al., 2004).
Participants
Major inclusion criteria were: being ≥21 and ≤65 years of age; having a body mass index (BMI) ≥33 and ≤55 kg/m2 or (BMI ≥30 kg/m2 with an obesity-related comorbidity); and receiving routine medical care from a primary care provider. The primary exclusion criteria were: clinically significant medical or psychiatric conditions that would contraindicate weight loss; diabetes; pregnant or nursing; current major depressive episode, active suicidal ideation, or history of suicide attempts; antidepressant or antipsychotic use in the past 14 days; ≥10 lb weight loss within the past 3 months; history or plans for bariatric surgery; or inability to walk 5 blocks.
Procedures
Participants were recruited via local advertisements. Eligibility was assessed using a telephone prescreen and in-person screening visit. At the screening visit, informed consent was obtained, and participants completed a behavioral evaluation with a clinical psychologist. Participants then met with a study nurse practitioner or physician to complete a medical history and physical exam. Applicants were enrolled if they met eligibility criteria and confirmed their desire to participate in the study. The study was approved by the University of Pennsylvania Institutional Review Board.
Behavioral Weight Loss Intervention
Participants were provided 14 weekly, 90-minute, in-person group lifestyle modification sessions, as described previously (Wadden et al., 2004). Groups consisted of 10 to 15 participants and were led by registered dietitians or psychologists. At week 1, participants were instructed to record their dietary intake and make modifications to prepare their home for the meal replacement program. From weeks 2 to 12, they were instructed to consume a 1000-1200 kcal/day diet that consisted of four servings daily of chocolate or vanilla liquid shakes (HMR; 160-170 kcal per shake), a prepackaged (or frozen) food entrée (250-300 kcal), 1-2 servings of fruit, and a side salad (Wadden et al., 2011; Wadden et al., 2004). (This diet was selected because it increases weight loss by approximately 3-4 kg as compared to a 1200 kcal/day diet comprised of conventional foods (Heymsfield et al., 2003b; Wadden et al., 2004). Participants were provided with the liquid shakes and frozen food entrees free of charge. From weeks 12-14, participants were prescribed a refeeding diet that gradually replaced the consumption of shakes with conventional foods, so that the use of meal replacements was terminated by week 14. Beginning at week 6, participants were encouraged to gradually increase their physical activity to reach 175 minutes/week by week 14. Group sessions were conducted at the University of Pennsylvania’s Center for Weight and Eating Disorders, following a protocol adapted from prior studies (Wadden et al., 2011; Wadden et al., 2004).
Measures
At each clinic visit, weight was measured on an electronic scale (Detecto, model 6800A) with applicants dressed in light clothing, without shoes. Height was assessed with a wall-mounted stadiometer (Veeder-Root, Elizabethtown, NC). Each measure was performed twice, and the averages were used to calculate each participant’s BMI. Approximately 1 to 2 weeks before starting the weight loss program, participants completed a set of questionnaires. Questionnaires were repeated at an assessment visit at week 14. Demographic data on age, gender, and race/ethnicity were collected using a form designed for this study.
Food cravings were measured with the Food Craving Inventory (FCI) (White et al., 2002a), a validated and reliable measure that asked about cravings for specific foods over the past 28 days. Craving frequency was assessed on a scale of 1 (never) to 5 (always/almost every day). The FCI generates a total score and subscale scores for high fats, sweets, complex carbohydrate/starches, and fast-food fat cravings. The Cronbach’s alpha for the total score was 0.92. The Cronbach’s alpha for the subscale scores were: high fats=0.80; sweets=0.86; complex carbohydrates/starches=0.81; and fast-food fats=0.67.
Food addiction was assessed using the original version of the Yale Food Addiction Scale (YFAS). This is a 25-item instrument that applies the DSM-IV-TR criteria for substance use to food and eating behaviors, and does not include questions on food cravings (Gearhardt et al., 2009b). The measure was scored using a symptom count ranging from 0 to 7, with higher scores indicating more symptoms of dependence (i.e., consumed more than planned; continued use despite physical or psychologic problems; desire or repeated failed attempts to reduce or stop consumption; great deal of time spent in activities necessary to obtain, use, or recover; giving up other important activities; tolerance; withdrawal). A diagnosis of food addiction was given to persons who endorsed three or more symptoms of dependence and reported impairment or distress due to these behaviors. In this sample, the Kuder–Richardson's alpha was 0.71. Participants were also asked to select all foods that they have problems with from a list of 26 items (e.g. pizza, cookies, cakes).
Statistical Analysis
Analyses were performed using SPSS version 24.0 (Armonk, NY). Correlations, Fisher’s exact tests, independent t-tests, and Mann-Whitney U tests were used to examine the relationship among food cravings, food addiction status/symptom count, and attrition. Participants were classified into an attrition group if they did not attend any visits after week 10.
To assess the effects of baseline food cravings and food addiction on percent weight loss over the 14 weeks, we used intention-to-treat linear mixed models with an unstructured variance covariance form. Separate models were used to examine the variables of interest (i.e., food cravings and food addiction). Analyses were conducted with and without adjustments for demographic and clinical variables that may influence weight loss (i.e., race, gender, age, and baseline BMI). Supplementary analyses were also conducted using tertile scores of baseline food craving frequencies to predict percent weight loss.
Paired t-tests were used to examine changes in food cravings and food addiction symptoms over time. To examine whether pre- to post-treatment changes in food cravings and food addiction symptoms were associated with weight loss, we conducted a completers analysis with food cravings and food addiction entered as time-varying variables. Statistical significance was defined as a p<0.05.
Results
Participant Characteristics
Participants had a mean age of 44.2±11.2 yr and baseline BMI of 40.9±5.9 kg/m2 (Table 1). Most of the sample was female (87.6%), and 71.3% of participants were black, 21.9% white, and 6.8% were categorized as other race/ethnicity.
Table 1.
Participant Characteristics (N=178)
| Sex (female) | 156 (87.6%) |
| Race | |
| Black | 127 (71.3) |
| White | 39 (21.9) |
| Other | 12 (6.8) |
| Age, years | 44.22±11.19 |
| Baseline weight (kg) | 114.63±20.99 |
| Baseline BMI (kg/m2) | 40.94±5.92 |
Note. Mean±standard deviation or N(%).
The mean±standard deviation (SD) for the FCI total score was 2.3±0.7. The averages for the FCI subscales are presented in Table 2. Participants had the highest cravings for fast food fats. The most frequently craved foods were chips, pizza, fried chicken, French fries, and chocolate.
Table 2.
Food Craving and Food Addiction Symptoms at Baseline and Changes from Pre- to Post-Treatment
| Baselinea | Pre-Treatmentb | Post-Treatmentb | P-valuec | ||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| Cravingsd | |||||||
| Total | 2.25 | 0.65 | 2.24 | 0.66 | 1.87 | 0.55 | <0.001 |
| Sweets | 2.35 | 0.83 | 2.32 | 0.84 | 1.86 | 0.69 | <0.001 |
| High Fats | 2.09 | 0.72 | 2.08 | 0.73 | 1.81 | 0.63 | <0.001 |
| Complex Carbs/Starches | 2.11 | 0.77 | 2.11 | 0.78 | 1.70 | 0.63 | <0.001 |
| Fast-food Fats | 2.67 | 0.81 | 2.66 | 0.82 | 2.32 | 0.75 | <0.001 |
| Food addiction symptomse | 2.28 | 1.55 | 2.24 | 1.58 | 1.93 | 1.24 | 0.02 |
Includes entire baseline sample (N=178).
Includes individuals who completed the post-treatment assessment (n=138).
The p-value value is from paired-tests for individuals who completed both the pre-treatment and post-treatment assessment (n=138).
Scores range from 1 (never) to 5 (always/almost every day).
Scores range from 0 to 7 with higher scores indicating more dependence symptoms.
We have reported the prevalence of food addiction for this sample previously (Chao et al., 2017). Twelve of the 178 (6.7%) participants met criteria for food addiction. The average number of food addiction symptoms in the total sample was 2.3±1.6 out of a potential 7 symptoms (Table 2). On the YFAS, participants endorsed having the most problems with controlling their intake of chips (52.2% of the total sample), cookies (43.3%), French fries (42.1%), ice cream (40.4%), and cake (37.1%). Participants selected an average of 6.2±4.0 of the 26 foods listed on the YFAS as contributing to problematic eating. Demographic characteristics (i.e., age, sex, race) and BMI were not significantly different between individuals who did and did not meet criteria for food addiction (ps>0.05).
Relationships between Food Cravings and Food Addiction
Compared to participants who did not meet criteria for food addiction, individuals who met criteria had significantly higher total food cravings (p=0.01), and cravings for sweets (p=0.001), complex carbohydrates/starches (p=0.01), and fast-food fats (p=0.01). Cravings for high-fat foods did not differ between groups (p=0.28; Figure 1). Food addiction symptoms were positively correlated with total food cravings (r=0.21, p=0.007), sweet cravings (r=0.25, p=0.001), and complex carbohydrate/starch cravings (r=0.19, p=0.01). Food addiction symptoms were not significantly correlated with high fat cravings (r=0.08, p=0.28) or fast-food fat cravings (r=0.15, p=0.05). Selecting more foods as problematic on the YFAS was correlated with reporting higher total food cravings (r=0.51, p<0.001).
Figure 1:
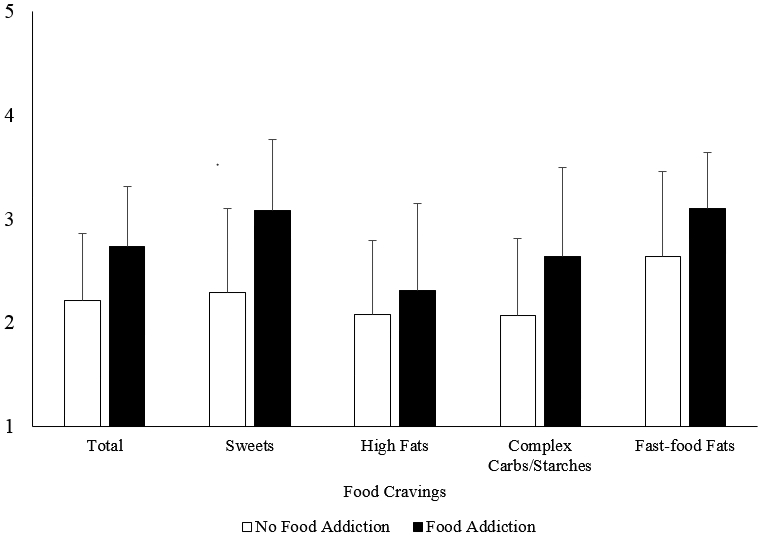
Mean±SD values for total food cravings and cravings for specific food types by food addiction status. Scores range from 1 (never) to 5 (always/almost every day).
Effects on Attrition of Food Cravings and Addiction
Of the 178 participants who began treatment, 150 participants completed the 14-week behavioral weight loss program (84.3%). Total food cravings at baseline did not differ between individuals who did and those who did not complete the program (p=0.63). Specific cravings for high fats, sweets, complex carbohydrates/starches, and fast-food fats did not differ between those who completed and did not complete the program (p=0.86, 0.80, 0.63, and 0.33, respectively). Baseline food addiction status was not significantly associated with attrition (p=0.10); four (33.3%) participants with and 24 (14.5%) participants without food addiction did not complete treatment. Baseline food addiction symptom count did not differ significantly between individuals who did (2.3±1.6) and did not complete the program (2.4±1.5; p=0.78).
Effects on Weight Loss of Food Cravings and Addiction
Overall, participants lost an average of 9.0±0.3% of initial weight over the 14 weeks. Higher frequencies of baseline total food cravings were associated with significantly lower weekly weight losses (B=0.07; SE=0.03; p=0.03; Figure 2). Analyzed categorically, participants in the highest tertile of baseline food cravings lost 7.6±0.5%, which was significantly less than the 9.1±0.5% lost by those in the lowest tertile (p=0.03). Participants in the middle food cravings tertile lost 8.8±0.5% of initial weight, which was not significantly different from those in lowest (p=0.59) or highest tertile (p=0.09).
Figure 2:
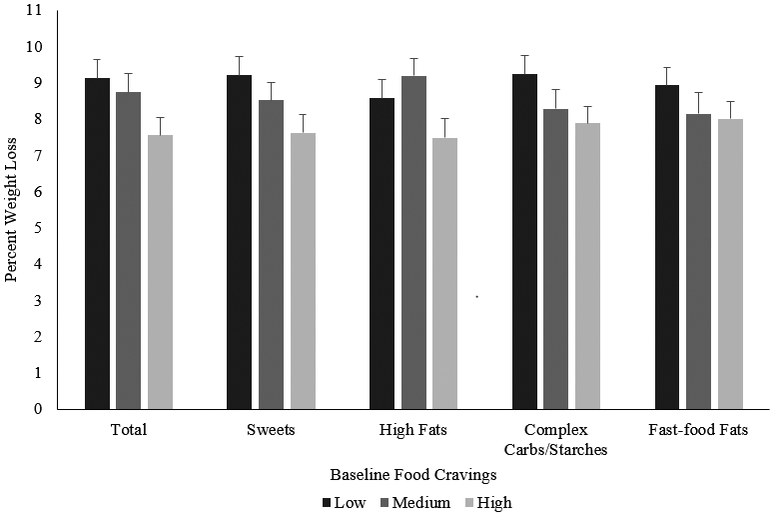
Percent weight loss estimates (mean±SE) at week 14 based on baseline food cravings. Results are from linear mixed models and adjusted for race, gender, age, and baseline BMI.
Exploratory, post-hoc analyses were conducted with the FCI subscale scores. Individuals with higher cravings for sweets had significantly smaller weekly weight losses (B=0.67, SE=0.03, p=0.04). Participants in the highest tertile of sweet food cravings lost 7.6±0.5% of initial weight, which was significantly less than the 9.2±0.5% lost by those in the lowest tertile (p=0.03; Figure 2). Cravings for high fats were significantly associated with smaller weekly weight losses (B=0.07, SE=0.03, p=0.03). Those in the highest tertile of high-fat cravings lost 7.5±0.5% of initial weight, which was significantly less than the 9.2±0.5% for participants in the middle tertile (p=0.02). For high-fat cravings, those in the highest tertile did not differ in percent weight loss from those in the lowest tertile (8.6±0.5%; p=0.14). More frequent baseline cravings for complex carbohydrates/starches and fast-food fats were not significantly associated with weekly weight losses (p=0.12, 0.30, respectively).
Participants who met criteria for food addiction lost 6.5±1.2% of initial body weight. This did not differ significantly from the 8.6±0.3% lost by those without food addiction (p=0.09). Results are from linear mixed models and adjusted for race, gender, age, and baseline BMI.
Changes in Food Cravings, Food Addiction, and Weight Loss
Among the 138 participants who completed the pre- and post-treatment assessment visit, total food cravings and cravings for high fats, sweets, complex carbohydrates/starches, and fast-food fats declined significantly from pre- to post-treatment (ps<0.001; Table 2). YFAS symptoms also declined significantly from before (2.2±1.6) to after treatment (1.9±1.2; p=0.02). Two people met criteria for food addiction post-treatment. Changes in total food cravings and YFAS symptoms were not associated with weight loss (ps=0.10 and 0.80, respectively).
Discussion
Relative to other clinical samples, few participants (6.7%) in this sample of persons who sought weight loss treatment met criteria for food addiction (Pursey et al., 2014). Consistent with an addiction framework, those with food addiction had significantly higher total food cravings than those who did not meet criteria. Food addiction was not significantly related to weight loss during this lifestyle intervention that incorporated meal replacements, though the limited number of participants who met criteria for food addiction limited power to detect differences. More frequent total food cravings at baseline were associated with attenuated weight loss. Participants with the lowest frequencies of food cravings lost 1.6% more of initial body weight than those with the highest frequencies of food cravings. Overall, participants who completed the follow-up assessment reported significant declines in food cravings and food addiction symptoms from pre- to post-treatment.
A growing body of literature has demonstrated behavioral and physiologic similarities (as well as differences) between substance use disorders and problematic consumption of highly palatable foods (Avena, Rada, et al., 2008; Davis et al., 2011; Long et al., 2015; Rogers, 2017; Volkow et al., 2011). In line with models of addiction (Tiffany & Wray, 2012), as well as previous studies (Joyner et al., 2015; Meule & Kübler, 2012), we found that craving appeared to be a component of food addiction. However, the majority of this sample endorsed some level of craving, and food cravings are commonly reported in the general population (Chao et al., 2014). Conversely, cravings for substances (e.g., nicotine, alcohol) are generally thought to be uniquely associated with substance use disorders. People without substance use disorders generally do not report that they have experienced cravings for specific types of drugs (Agrawal et al., 2011). Thus, a primary differentiating factor between cravings for foods versus cravings for substances is the commonality of food cravings and the finding that food cravings may occur outside of food addiction due to factors such as chronic stress or menstrual cycle hormonal changes.(Chao et al., 2015; Krishnan et al., 2016). Food cravings appear to play a role in food addiction, and the YFAS has also been recently modified to reflect the new DSM-5 criteria for substance use disorders, including the addition of food cravings (Gearhardt et al., 2016). Further research is needed to determine thresholds for pathological food cravings, and how such cravings relate to the development and maintenance of addictive-like eating.
All FCI subscales were associated with food addiction except the high fats foods subscale. One explanation for this is that there are differences in the addictive nature of various foods. In animal models, it has been suggested that sugar and fat may have distinct roles and mechanisms underlying addictive-like eating behaviors (Avena et al., 2009). For example, rats with addictive-like eating of sugar experience signs of anxiety and distress (e.g., teeth chattering, aggression) if given an opiate antagonist (Avena, Bocarsly, et al., 2008). Yet, these opiate-like withdrawal symptoms are not seen among rats maintained on fat-rich diets (Avena et al., 2009). The FCI subscales were not created based on a macronutrient analysis, and items that load onto the high fat food construct include items high in protein (e.g., fried chicken, bacon, steak), as well as those that are high in carbohydrates (i.e., cornbread). No definitive evidence has implicated particular foods or nutrients as addictive in humans.
An alternative explanation for the finding is that the foods on the high-fat food subscale are more meal-type foods compared to the snack and fast-food items listed on other subscales (e.g., cookies, candy, chips). Differences in frequencies may be the result of the availability and ease with which the food can be obtained and consumed. Additional research is needed to assess whether nutrient composition influences addictive-like eating or whether behavioral factors may promote addictive-like eating behaviors. Since no addictive components in food have yet been conclusively identified, we agree with others about conceptualizing food addiction as a behavioral disorder, rather than a substance-based disorder, and recommend the term addictive-like eating behaviors to characterize this phenotype (Hebebrand et al., 2014; Ruddock et al., 2017).
Contrary to our hypothesis, food addiction was not associated with attrition or weight-loss during a behavioral obesity treatment that included meal replacements. This finding is congruent with other studies in which individuals with and without food addiction did not differ in weight loss (Lent et al., 2014; Sawamoto et al., 2017). Our study expands these results by demonstrating that with a more rigid lifestyle program that used meal replacements, food addiction did not negatively affect weight loss. The use of meal replacements may have decreased contact with problem foods and subsequent addictive-like eating of these foods. However, results should be interpreted cautiously given the small number of people who met criteria for food addiction in this sample.
More frequent food cravings at baseline were associated with attenuated weight loss. This suggests that individuals with more frequent cravings may have more difficulty decreasing consumption of craved foods during a behavioral weight loss program. Individuals with frequent food cravings may benefit from specific interventions that target cravings, such as stimulus control or acceptance-based behavioral techniques (Forman et al., 2007). Acceptance-based strategies focus on normalizing cravings, accepting thoughts about cravings rather than attempting to change them, redefining and stepping back from cravings, and acting in ways that are in accordance with patient’s goals and values (Forman et al., 2007). These strategies may improve weight loss outcomes among patients with high levels of baseline food cravings.
Patients and providers are often concerned that food cravings may increase during weight loss and dieting, especially with dietary monotony. It has been theorized that causes of food cravings include dietary restriction and energy deficiency (Hill, 2007). However, like other studies, we did not find support for this notion (Harvey et al., 1993; Kahathuduwa et al., 2017; Martin et al., 2006). The current study of a lifestyle intervention that incorporated meal replacements showed that on average, participants reported significant declines in general cravings and cravings for specific foods over the 14 weeks. Taken together, this supports the view that, for most people, food cravings are likely the result of conditioned stimuli or cognitive processes. Weight loss interventions that disengage the pairing between stimuli and food intake appear to be effective in reducing food cravings, at least in the short-term. Further research is needed to assess the longer-term stability of these results, and to examine which components of the intervention (e.g., behavioral weight loss counseling, meal replacements, or the combination) are most effective for reducing food cravings.
This study had several limitations. The sample was predominantly female and black, and all participants were treatment-seeking, which may limit generalizability of results. The findings apply to short-term weight loss, and future studies are necessary to examine the effects of food cravings and food addiction on weight loss maintenance. The fast-food fats craving measure had low reliability in this sample, and results should be interpreted cautiously. Follow-up data on food cravings were only collected on individuals at their 14-week assessment; thus, only baseline scores were available for individuals who did not complete the treatment. While baseline food cravings and food addiction symptoms did not differ between individuals who did and did not complete the program, it is possible that participants who had increases on these measures were not as likely to complete the program. This may have contributed to our non-significant findings for the relationship between change in these measures and weight loss.
In conclusion, a lifestyle intervention program that used meal replacements produced significant reductions in food cravings and food addiction symptoms. However, frequent food cravings at baseline appear to be a risk factor for attenuated weight loss during behavioral obesity treatment. Patients with high levels of baseline food cravings who seek weight loss may benefit from targeted interventions focused on cravings including stimuli control and acceptance-based behavioral therapy. This study supports the clinical and diagnostic utility of considering food cravings in obesity treatment.
Acknowledgments
Funding: AMC was supported by a Ruth L. Kirschstein National Research Service Award postdoctoral fellowship from the National Institute of Nursing Research/National Institutes of Health #T32NR007100. Support was also provided by an investigator-initiated grant from Eisai Pharmaceuticals and from HMR Weight Management Services Corp, Boston, MA (TAW). These funders had no role in the current study design or the collection, analysis, or interpretation of data, writing the manuscript or the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: TAW reports receiving personal fees for serving on advisory boards for Novo Nordisk, and Weight Watchers and has received grant support on behalf of the University of Pennsylvania from Eisai Pharmaceutical and Novo Nordisk. TAW and AMC have received grant support on behalf of the University of Pennsylvania from Shire Pharmaceuticals. JST discloses serving as a consultant for Novo Nordisk. RLP discloses serving as a consultant for Weight Watchers. The other authors declare no conflicts of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- Agrawal A, Heath AC, & Lynskey MT (2011). DSM-IV to DSM-5: The impact of proposed revisions on diagnosis of alcohol use disorders. Addiction, 106(11), 1935–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5): American Psychiatric Pub. [Google Scholar]
- Avena NM, Bocarsly ME, Rada P, Kim A, & Hoebel BG (2008). After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiology and Behavior, 94(3), 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, & Hoebel BG (2008). Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience and Biobehavioral Reviews, 32(1), 20–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, & Hoebel BG (2009). Sugar and fat bingeing have notable differences in addictive-like behavior. The Journal of Nutrition, 139(3), 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra P, Das SK, Salinardi T, Robinson L, Saltzman E, Scott T, … Roberts SB (2013). Relationship of cravings with weight loss and hunger. Results from a 6month worksite weight loss intervention. Appetite, 69, 1–7. [DOI] [PubMed] [Google Scholar]
- Boswell RG, & Kober H (2016). Food cue reactivity and craving predict eating and weight gain: a meta-analytic review. Obesity Reviews, 17(2), 159–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister JM, Hinman N, Koball A, Hoffmann DA, & Carels RA (2013). Food addiction in adults seeking weight loss treatment. Implications for psychosocial health and weight loss. Appetite, 60, 103–110. [DOI] [PubMed] [Google Scholar]
- Buscemi J, Rybak TM, Berlin KS, Murphy JG, & Raynor HA (2017). Impact of food craving and calorie intake on body mass index (BMI) changes during an 18-month behavioral weight loss trial. Journal of Behavioral Medicine, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A, Grilo CM, White MA, & Sinha R (2014). Food cravings, food intake, and weight status in a community-based sample. Eating Behaviors, 15(3), 478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A, Grilo CM, White MA, & Sinha R (2015). Food cravings mediate the relationship between chronic stress and body mass index. Journal of Health Psychology, 20(6), 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A, Shaw JA, Pearl RL, Alamuddin N, Hopkins CM, Bakizada ZM, … Wadden TA (2017). Prevalence and psychosocial correlates of food addiction in persons with obesity seeking weight reduction. Comprehensive Psychiatry, 73, 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS, & Kennedy JL (2011). Evidence that ‘food addiction’is a valid phenotype of obesity. Appetite, 57(3), 711–717. [DOI] [PubMed] [Google Scholar]
- Forman EM, Hoffman KL, McGrath KB, Herbert JD, Brandsma LL, & Lowe MR (2007). A comparison of acceptance-and control-based strategies for coping with food cravings: An analog study. Behaviour Research and Therapy, 45(10), 2372–2386. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Corbin WR, & Brownell KD (2009a). Food addiction: an examination of the diagnostic criteria for dependence. Journal of Addiction Medicine, 3(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Corbin WR, & Brownell KD (2009b). Preliminary validation of the Yale Food Addiction Scale. Appetite, 52(2), 430–436. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Corbin WR, & Brownell KD (2016). Development of the Yale Food Addiction Scale Version 2.0. Psychology of Addictive Behaviors, 30(1), 113–121. [DOI] [PubMed] [Google Scholar]
- Gilhooly C, Das S, Golden J, McCrory M, Dallal G, Saltzman E, … Roberts S (2007). Food cravings and energy regulation: the characteristics of craved foods and their relationship with eating behaviors and weight change during 6 months of dietary energy restriction. International Journal of Obesity, 31(12), 1849–1858. [DOI] [PubMed] [Google Scholar]
- Hammarström A, Wiklund AF, Lindahl B, Larsson C, & Ahlgren C (2014). Experiences of barriers and facilitators to weight-loss in a diet intervention-a qualitative study of women in Northern Sweden. BMC Women’s Health, 14(1), 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Wing RR, & Mullen M (1993). Effects on food cravings of a very low calorie diet or a balanced, low calorie diet. Appetite, 21(2), 105–115. [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Albayrak Ö, Adan R, Antel J, Dieguez C, de Jong J, … Murphy M (2014). “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neuroscience and Biobehavioral Reviews, 47, 295–306. [DOI] [PubMed] [Google Scholar]
- Heymsfield S, Van Mierlo C, Van der Knaap H, Heo M, & Frier H (2003a). Weight management using a meal replacement strategy: meta and pooling analysis from six studies. International Journal of Obesity, 27(5), 537–549. [DOI] [PubMed] [Google Scholar]
- Heymsfield S, Van Mierlo C, Van der Knaap H, Heo M, & Frier H (2003b). Weight management using a meal replacement strategy: meta and pooling analysis from six studies. International Journal of Obesity, 27(5), 537–549. [DOI] [PubMed] [Google Scholar]
- Hill AJ (2007). The psychology of food craving. Proceedings of the Nutrition Society, 66(2), 277–285. [DOI] [PubMed] [Google Scholar]
- Joyner MA, Gearhardt AN, & White MA (2015). Food craving as a mediator between addictive-like eating and problematic eating outcomes. Eating Behaviors, 19, 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahathuduwa C, Binks M, Martin C, & Dawson J (2017). Extended calorie restriction suppresses overall and specific food cravings: a systematic review and a meta-analysis. Obesity Reviews, 18(10), 1122–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh J, & Clifton P (2005). The role of meal replacements in obesity treatment. Obesity Reviews, 6(3), 229–234. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Tryon RR, Horn WF, Welch L, & Keim NL (2016). Estradiol, SHBG and leptin interplay with food craving and intake across the menstrual cycle. Physiology and Behavior, 165, 304–312. [DOI] [PubMed] [Google Scholar]
- Lent MR, Eichen DM, Goldbacher E, Wadden TA, & Foster GD (2014). Relationship of food addiction to weight loss and attrition during obesity treatment. Obesity, 22(1), 52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman SW, Pisarska K, Berman ER, Pestone M, Dowling H, Offenbacher E, … Heymsfield SB (1992). Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. New England Journal of Medicine, 327(27), 1893–1898. [DOI] [PubMed] [Google Scholar]
- Long CG, Blundell JE, & Finlayson G (2015). A systematic review of the application and correlates of YFAS-diagnosed ‘food addiction’in humans: are eating-related ‘addictions’a cause for concern or empty concepts? Obesity Facts, 8(6), 386–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, O’Neil PM, & Pawlow L (2006). Changes in food cravings during low-calorie and very-low-calorie diets. Obesity, 14(1), 115–121. [DOI] [PubMed] [Google Scholar]
- Meule A, & Kübler A (2012). Food cravings in food addiction: The distinct role of positive reinforcement. Eating Behaviors, 13(3), 252–255. [DOI] [PubMed] [Google Scholar]
- Moldovan CP, Weldon AJ, Daher NS, Schneider LE, Bellinger DL, Berk LS, … Peters WR (2016). Effects of a meal replacement system alone or in combination with phentermine on weight loss and food cravings. Obesity, 24(11), 2344–2350. [DOI] [PubMed] [Google Scholar]
- Niaura R (2000). Cognitive social learning and related perspectives on drug craving. Addiction, 95(8s2), 155–163. [DOI] [PubMed] [Google Scholar]
- Polivy J, & Herman CP (1985). Dieting and binging: A causal analysis. American Psychologist, 40(2), 193–201. [DOI] [PubMed] [Google Scholar]
- Pursey KM, Stanwell P, Gearhardt AN, Collins CE, & Burrows TL (2014). The prevalence of food addiction as assessed by the Yale Food Addiction Scale: a systematic review. Nutrients, 6(10), 4552–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers PJ (2017). Food and drug addictions: Similarities and differences. Pharmacology Biochemistry and Behavior, 153, 182–190. [DOI] [PubMed] [Google Scholar]
- Ruddock HK, Christiansen P, Halford JC, & Hardman CA (2017). The development and validation of the Addiction-like Eating Behaviour Scale. International Journal of Obesity, 41(11), 1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddock HK, & Hardman CA (2017). Food addiction beliefs amongst the lay public: What are the consequences for eating behaviour? Current Addiction Reports, 4(2), 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman AJ (1986). Dietary restraint: A theoretical and empirical review. Psychological Bulletin, 99(2), 247. [PubMed] [Google Scholar]
- Sawamoto R, Nozaki T, Nishihara T, Furukawa T, Hata T, Komaki G, & Sudo N (2017). Predictors of successful long-term weight loss maintenance: a two-year follow-up. Biopsychosocial Medicine, 11(1), 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw Tronieri J, Alfaris N, Chao AM, Pearl RL, Alamuddin N, Bakizada ZM, … Wadden TA (2017). Lorcaserin plus lifestyle modification for weight loss maintenance: Rationale and design for a randomized controlled trial. Contemporary Clinical Trials, 59, 105–112. [DOI] [PubMed] [Google Scholar]
- Shaw Tronieri J, Wadden TA, Berkowitz RI, Chao AM, Pearl RL, Alamuddin N, … Alfaris N (2018). A randomized controlled trial of lorcaserin and lifestyle counseling for maintaining weight loss achieved with a low-calorie diet. Obesity, 26(2), 299–309. [DOI] [PubMed] [Google Scholar]
- Smithson E, & Hill A (2016). It is not how much you crave but what you do with it that counts: behavioural responses to food craving during weight management. European Journal of Clinical Nutrition, 71, 625–630. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, & Wray JM (2012). The clinical significance of drug craving. Annals of the New York Academy of Sciences, 1248(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins CL, Laurent J, & Brock DW (2017). Food addiction: a barrier for effective weight management for obese adolescents. Childhood Obesity, 13(6), 462–469. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Tomasi D, & Baler R (2011). Food and drug reward: overlapping circuits in human obesity and addiction Brain Imaging in Behavioral Neuroscience (pp. 1–24): Springer. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Faulconbridge LF, Jones-Corneille LR, Sarwer DB, Fabricatore AN, Thomas JG, … Webb VL (2011). Binge eating disorder and the outcome of bariatric surgery at one year: a prospective, observational study. Obesity, 19(6), 1220–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Foster GD, Sarwer DB, Anderson DA, Gladis M, Sanderson RS, … Phelan S (2004). Dieting and the development of eating disorders in obese women: results of a randomized controlled trial. The American Journal of Clinical Nutrition, 80(3), 560–568. [DOI] [PubMed] [Google Scholar]
- White MA, Whisenhunt BL, Williamson DA, Greenway FL, & Netemeyer RG (2002a). Development and validation of the Food-Craving Inventory. Obesity Research, 10(2), 107–114. [DOI] [PubMed] [Google Scholar]
- White MA, Whisenhunt BL, Williamson DA, Greenway FL, & Netemeyer RG (2002b). Development and validation of the Food-Craving Inventory. Obesity, 10(2), 107–114. [DOI] [PubMed] [Google Scholar]


