ABSTRACT
Hepatitis E virus (HEV) can lead to high mortality during pregnancy. This study was to investigate the adverse pregnancy outcomes caused by different HEV genotypes and their prevention by HEV 239 vaccine in rabbits. Forty-two female rabbits were randomly and equally divided into 7 groups (A-G). HEV 239 vaccine and a placebo were administered to groups E (10 μg×2), F (5 μg×2) and G (1 mL of PBS×2) before copulation. After pregnancy, 1 mL of 1.5×106 copies/mL rabbit HEV3 was inoculated to groups A, E, F and G, swine HEV4/human HEV3 to groups B/C, and group D was a negative control. Anti-HEV antibody, HEV RNA, and alanine aminotransferase (ALT)/aspartate aminotransferase (AST) levels were monitored. Pregnant rabbits infected by HEV manifested HEV infection symptoms including fecal virus shedding, ALT/AST elevation, and histopathological changes, and adverse pregnancy outcomes. Immunized pregnant rabbits in groups E and F showed no HEV infection symptoms and adverse outcomes. The newborn rabbits delivered by pregnant rabbits with/without immunization showed without/with HEV infection symptoms. This study demonstrated that multiple genotypes of HEV infection can cause adverse outcomes and HEV 239 vaccine can prevent HEV-related adverse outcomes in pregnant rabbits.
KEYWORDS: Hepatitis E, pregnancy, HEV vaccine, adverse pregnancy outcomes, vertical transmission
Introduction
Hepatitis E virus (HEV) is the main cause of acute viral hepatitis in developing countries, which has aroused wide public concern [1–3]. Each year, there are approximately 20 million people infected with HEV worldwide, causing 70,000 deaths [4]. HEV, a single-stranded positive RNA virus, spreads through mainly the fecal-oral route and has been recognized as a zoonotic pathogen [5]. HEV can be divided into 8 genotypes (HEV1-8), among which HEV1-4 are closely related to human infection. HEV1 and HEV2 can only infect humans, while HEV3 and HEV4 are zoonotic [1–3].
HEV often causes acute self-limited disease, with a mortality of approximately 0.2–1% [3]. However, in pregnant women, the mortality rate of HEV infection can reach as high as 25% [6–9]. HEV infection in pregnant women can also lead to adverse pregnancy outcomes including miscarriage, stillbirths, fulminant hepatic failure and membrane rupture [10]. However, to date, the mechanism of the high mortality rate of HEV infection in pregnant women remains unclear. HEV-related adverse pregnancy outcomes are caused by mainly HEV1 [10], and whether other genotypes of HEV can lead to adverse outcomes during pregnancy is still unclear. In addition to HEV genotypes, the pathogenesis of HEV-related adverse pregnancy outcomes may be related to changes in cellular immunity and hormones during pregnancy [10], but that possibility warrants further investigation. Currently, there is no recommended drug for HEV-infected pregnant women therefore, an effective prevention strategy is of high priority for this population. Although the HEV 239 vaccine (Hecolin®), which is a bacterially expressed recombinant peptide corresponding to amino acid residues 368–606 of ORF2 of HEV1, has been commercialized in China and can successfully prevent HEV infection for years [11], it still lacks studies on its efficacy for adverse pregnancy outcomes. Previously, we successfully established a HEV-infected pregnant rabbit model that can simulate the HEV-related high mortality rate observed in pregnant women [12]. Therefore, this study aimed to investigate the pathogenicity of different HEV genotypes in pregnant rabbits and the efficacy of the HEV 239 vaccine in preventing adverse outcomes and vertical transmission of HEV infection in pregnant rabbits.
Materials and methods
Ethics statement
The animal experiments were approved by the Committee of Laboratory Animal Welfare and Ethics, Peking University Health Science Center, and were in strict accordance with the protocol for the review of the Laboratory Animal Welfare and Ethics, Peking University Health Science Center (IACUC number: LA2017047).
Animals
Forty-two 7-month-old female Japanese white rabbits weighing between 5.0 and 6.0 kg were obtained from the Department of Laboratory Animal Science of Peking University Health Science Center. Serum and feces specimens from selected rabbits were collected once a week for 2 weeks to test ALT and AST levels and to establish a baseline. Enzyme-linked immunosorbent assay (ELISA) and reverse transcription-nested PCR (RT-nPCR) were performed to ensure that all specimens were negative for HEV RNA and antibodies against HEV.
Vaccines
The HEV 239 vaccine (Hecolin; Xiamen Innovax Biotech, Xiamen, China) is a 26 kDa recombinant polypeptide expressed by the Escherichia coli system derived from the 368–606 aa segment of the HEV1 (GenBank D11092) ORF2 protein. It is currently the only commercially available HEV vaccine globally. To date, the HEV 239 vaccine is only available in the Chinese market and has been approved for use in people older than 16 years, which is indicated for vaccinating individuals at high risk of HEV infection [11].
Viruses
The rabbit HEV strain (CHN-BJ-R14, genotype 3, GenBank JQ768461), swine HEV strain (CHN-SD-SW2, genotype 4, GenBank KP284140) and human HEV strain (CHN-SH-W, genotype 3, GenBank MF996356) used in this study were all isolated from feces. Inocula were prepared as described previously [12]. The viral load of each HEV strain was adjusted to 1.5×106 copies/mL by using a one-step real-time quantitative PCR assay [12]
Experimental design
All selected female rabbits (n = 42) were randomly divided into 7 groups (A-G), with 6 rabbits per group (Supplementary Fig. 1). Before copulation, rabbits in groups E and F were inoculated intramuscularly with a 10 and 5 μg dose of HEV 239 vaccine on weeks 0 and 4 respectively, while rabbits in group G as a vaccine control group were inoculated with a sterile PBS solution. On week 7, all female rabbits (n = 42) copulated with healthy male rabbits. After their pregnancy was confirmed [12], rabbits in groups A, E, F, and G were inoculated intravenously with 1 mL of 1.5×106 copies/mL rabbit HEV strain (rHEV3). Rabbits in groups B and C were inoculated intravenously with 1 mL of 1.5×106 copies/mL swine HEV4 strain (sHEV4) and human HEV3 strain (hHEV3), respectively, and rabbits in group D were inoculated intravenously with a rabbit feces suspension without HEV infection as a negative control.
Sample collection and processing
After virus inoculation, serum and fecal specimens from pregnant rabbits were collected weekly for serum ALT and AST level, fecal HEV RNA load, and serum estrogen and progesterone level testing. Three weeks after newborn rabbits were born, their serum and fecal samples were collected weekly for serum ALT and AST level and fecal HEV RNA load testing. Livers, ovaries, or placentas were collected immediately from dead HEV-infected pregnant rabbits, euthanized HEV-infected pregnant rabbits, dead newborn rabbits and euthanized HEV-infected young rabbits. Each tissue was stored at −80°C for detection of HEV RNA positive and negative strands and HEV viral load. In addition, the tissue specimens were also processed for histopathology, immunofluorescence and immunohistochemistry (IHC) by being immediately fixed in 10% neutral buffered formalin [12].
Detection of pregnancy-related hormones
To confirm the female rabbits’ pregnancy, their serum (day 0, day 10, day 17 and day 24 after pregnancy) and postpartum serum were all tested for progesterone and estrogen by the chemiluminescent enzyme immunoassay method on a Siemens Immulite 2000 Immunoassay System according to the manufacturer’s instructions [12].
Detection of ALT and AST concentrations
All serum samples were tested immediately for ALT and AST concentrations using standard methods on a Hitachi Automatic Clinical Analyzer 7180. When the ALT or AST level became more than 2 times the preinfection baseline value, the rabbit was considered to have liver inflammation [13].
Evaluation of anti-HEV antibodies by ELISA and quantitative detection
All rabbit serum samples were detected by ELISA for total anti-HEV antibodies according to the manufacturer’s instructions (Wantai, Biopharmaceutical, Beijing, China). When the S/CO value > 1, it was considered to be positive [14]. The geometric mean antibody titre (GMT) and quantitation of anti-HEV antibodies were performed as previously described [15].
Extraction and detection of HEV RNA
Total RNA in serum and fecal suspensions was extracted according to the RNAgents Total RNA Isolation System Kit (Promega, Madison, USA) instructions, and HEV RNA fragments were amplified by RT-nPCR [16]. RT-nPCR was carried out to detect HEV RNA as previously described [16]. Tissues with detectable positive-stranded HEV RNA were then assayed for negative-sense HEV RNA by RT-nPCR with the same 2 sets of universal primers, which used the external forward primer for cDNA synthesis and the amplification conditions were the same as those used in the detection of positive-sense HEV RNA [16].
Real-time fluorescence quantitative PCR
According to the literature [17], real-time fluorescence quantitative PCR (RT-qPCR) in this study was performed by using the Taqman probe detection method and QuantiTect® Probe RT–PCR Kit (Qiagen, Hilden, Germany).
Histopathology, immunofluorescence and immunohistochemistry staining
For histopathology, tissue samples were prepared for hematoxylin and eosin (HE) staining, IHC (microscope equipped with a digital camera, Olympus CX31, Japan) and immunofluorescence (Nikon DS-U3, Japan) by first being fixed in 10% neutral buffered formalin immediately following sampling according to the literature [18,19]. HEV proteins were visualized by using HEV ORF2- and ORF3-specific antibodies (1:1600, Bioss, Woburn, USA). Nuclear staining was achieved with DAPI (Beyotime, Shanghai, China). CD4+ and CD8+ cells were visualized with anti-CD4+ (1:1000, Bio-Rad, California, USA) and -CD8+ polyclonal antibodies (1:1500, Goodbio Technology, Wuhan, China).
Statistical analysis
Statistical analysis was performed using the SPSS PASW Statistics v18.0 statistical software package (SPSS, Inc., USA, http://www.ibm.com/cn/). Data were compared using Student’s t-test or chi-squared test. A P-value of <0.05 was considered significant.
Results
Pathogenicity of HEVs infection in pregnant rabbits
To analyze and compare the results of different genotypes of HEV (HEVs) infection in pregnant rabbits, we inoculated pregnant rabbits in groups A, B and C with rabbit HEV3 (rHEV3), swine HEV4 (sHEV4), and human HEV3 (hHEV3) respectively after the pregnancy was confirmed. Group D was set up as a negative control group without HEV infection.
Different incidences of adverse pregnancy outcomes were observed among groups A-D. The overall incidence of adverse pregnancy outcomes in group A (rHEV3) was 83.3% (5/6), with 16.7% (1/6) maternal death, 50% (3/6) stillbirth, and 16.7% (1/6) miscarriage; in group B (sHEV4) was 83.3% (5/6), with 66.7% (4/6) stillbirth and 16.7% (1/6) miscarriage; and in group C (hHEV3) was 66.7% (4/6), with 16.7% (1/6) maternal death and 50% (3/6) stillbirth. All rabbits in group D had normal delivery and no adverse pregnancy outcomes occurred. While maternal death only occurred in groups A (16.7%) and C (16.7%) and miscarriage was only observed in groups A (16.7%) and B (16.7%), stillbirth was observed in groups A (50%, 32.3 ± 1.5 days post pregnancy), B (66.7%, 31.5 ± 1.3 days post pregnancy) and C (50%, 30.5 ± 1.3 days post pregnancy). All HEV genotypes could induce adverse pregnancy outcomes in pregnant rabbits, and there was no statistically significant difference in the incidence of adverse outcomes among groups A, B, and C (P > 0.05) (Table 1).
Table 1. Adverse pregnancy outcomes of pregnant rabbits infected by different genotypes of HEV.
| Groups (NO., HEV genotypes) | Adverse pregnancy outcomesa (%) | Normal deliveryb (nc, %) | P valued | ||
|---|---|---|---|---|---|
| Death | Stillbirth | Miscarriage | |||
| A (6, rHEV3) | 1/6e(16.7) | 3/6(50) | 1/6(16.7) | 1/6(4, 16.7) | 0.015* |
| B (6, sHEV4) | 0/6(0) | 4/6(66.7) | 1/6(16.7) | 1/6(5, 16.7) | 0.015* |
| C (6, hHEV3) | 1/6e(16.7) | 3/6(50) | 0/6(0) | 2/6(7, 33.3) | 0.060 |
| D (6, placebo) | 0/6(0) | 0/6(0) | 0/6(0) | 6/6(38, 100) | – |
| E (6, rHEV3) | 0/6(0) | 0/6(0) | 0/6(0) | 6/6(40, 100) | 1.000 |
| F (6, rHEV3) | 0/6(0) | 1/6(16.7) | 0/6(0) | 5/6(35, 83.3) | 1.000 |
| G (6, rHEV3) | 1/6(16.7) | 3/6(50) | 2/6(33.3) | 0/6(0, 0) | 0.002* |
aNumber of rabbits with adverse pregnancy outcomes/total number of rabbits in the group.
bNumber of rabbits had normal delivery/total number of rabbits in the group.
cNumber of newborn rabbits in the group.
dCompared with group D.
eThe rabbits in group A and C died 34th day and 31st day post pregnancy.
*Significantly different, P < 0.05.
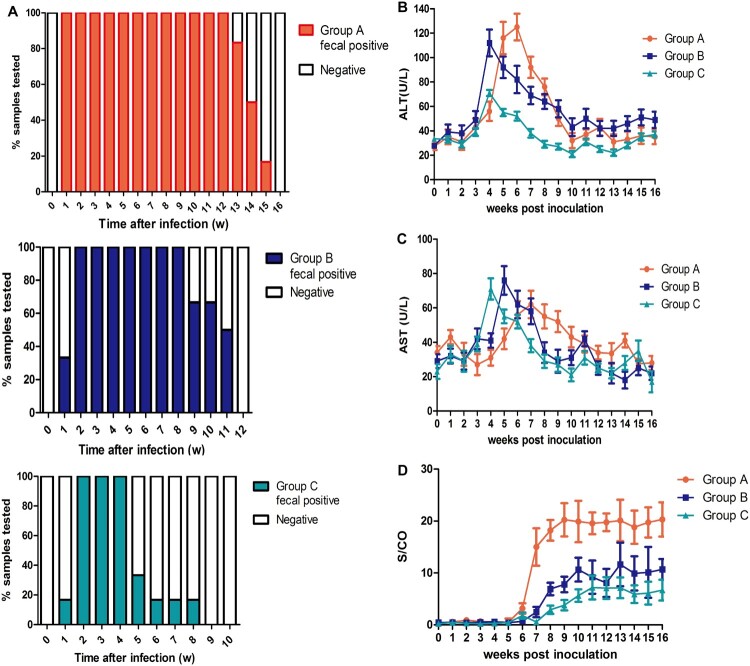
Different durations of fecal virus shedding were observed in groups A-C. Rabbits in group A (rHEV3) began fecal virus shedding at 1 week post inoculation (wpi) that lasted for an average of 13.5 ± 0.4 weeks; rabbits in group B (sHEV4) began shedding at 2wpi that lasted for 10.2 ± 0.5 weeks on average; and rabbits in group C (hHEV3) began shedding at 2wpi that lasted for 4.8 ± 0.7 weeks on average. The durations of fecal virus shedding were statistically different among the three groups, with group A shedding (13.5 ± 0.4 w) being significantly longer than that of group B (10.2 ± 0.5 w) and group C (4.8 ± 0.7 w) (P < 0.05) (Table 2 and Figure 1). The sequences of HEV detected in group A, B and C feces were all in accordance with the inoculation viruses.
Table 2. Markers of HEV infection in pregnant rabbits.
| Groups (NO., HEV genotypes) | Mean duration of fecal virus shedding ± SD (week) | No. of positive rabbits/total no. of rabbits tested post-inoculation | ||||||
|---|---|---|---|---|---|---|---|---|
| Shedding virus | Viremia | +/- stranded HEV RNA in livera |
+/- stranded HEV RNA in placentab |
Anti- HEV (+) |
ALT level (peak/pre ≥2) | AST level (peak/pre ≥2) | ||
| A (6, rHEV3) | 13.5 ± 0.4* | 6/6 | 1/6 | 6/6 | 3/4d | 6/6 | 4/6 | 3/6 |
| B (6, sHEV4) | 10.2 ± 0.5 | 6/6 | 0/6 | 6/6 | 0/5d | 6/6 | 3/6 | 1/6 |
| C (6, hHEV3) | 4.8 ± 0.7 | 6/6 | 0/6 | 3/6d | 0/5d | 4/6 | 1/6 | 0/6 |
| D (6, placebo) | – | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 |
| E (6, rHEV3) | – | 0/6 | 0/6 | 0/6 | 0/6 | 6/6c | 0/6 | 0/6 |
| F (6, rHEV3) | – | 0/6 | 0/6 | 0/6 | 0/6 | 6/6c | 0/6 | 0/6 |
| G (6, rHEV3) | 12.0 ± 1.4 | 6/6 | 1/6 | 6/6 | 1/3 | 6/6 | 3/6 | 3/6 |
aPositive and negative stranded HEV RNA in liver were both detected.
bPositive and negative stranded HEV RNA in placenta were both detected.
cAfter HEV 239 vaccination and before virus inoculation.
dOnly positive stranded HEV RNA were detected in 3/6 of livers in group C, 1/4 of placentas in group A, 2/5 of placentas in group B, 2/5 of placentas in group C.
*Group A was significantly higher than that of group B and group C.
Figure 1.
Duration of fecal virus shedding and dynamic seroconversion of anti-HEV antibodies, ALT and AST of pregnant rabbits. Duration of fecal HEV RNA shedding among group A, B and C. (B) Dynamic ALT changes among three groups. (C) Dynamic AST changes among three groups. (D) Dynamic seroconversion of anti-HEV antibodies among three groups.
In group A, 16.7% (1/6) of pregnant rabbits had viremia, while the rabbits in the other groups did not. Positive and negative HEV RNA strands in the livers and placentas were detected in 100% (6/6) and 50% (3/6) of group A’s rabbits respectively; positive and negative RNA strands were detected in the livers of 100% (6/6) and 50% (3/6) of the rabbits in groups B and C respectively. In groups A, B and C, 66.7% (4/6), 50% (3/6) and 16.7% (1/6) of rabbits showed a significant increase in ALT levels that was more than 2 times the baseline value respectively, while AST levels of 50% (3/6), 16.7% (1/6) and 0% (0/6) of rabbits in groups A, B and C significantly increased more than 2 times the baseline value respectively. In addition, seroconversion of anti-HEV antibodies in groups A (6/6), B (6/6), and C (4/6) was observed, and S/CO values in group A (20.3 ± 3.3) were significantly higher than those in groups B (10.7 ± 2.1) and C (6.1 ± 1.9) (P < 0.05) (Table 2 and Figure 1).
Characteristics of histopathology and immunofluorescence of HEVs infection
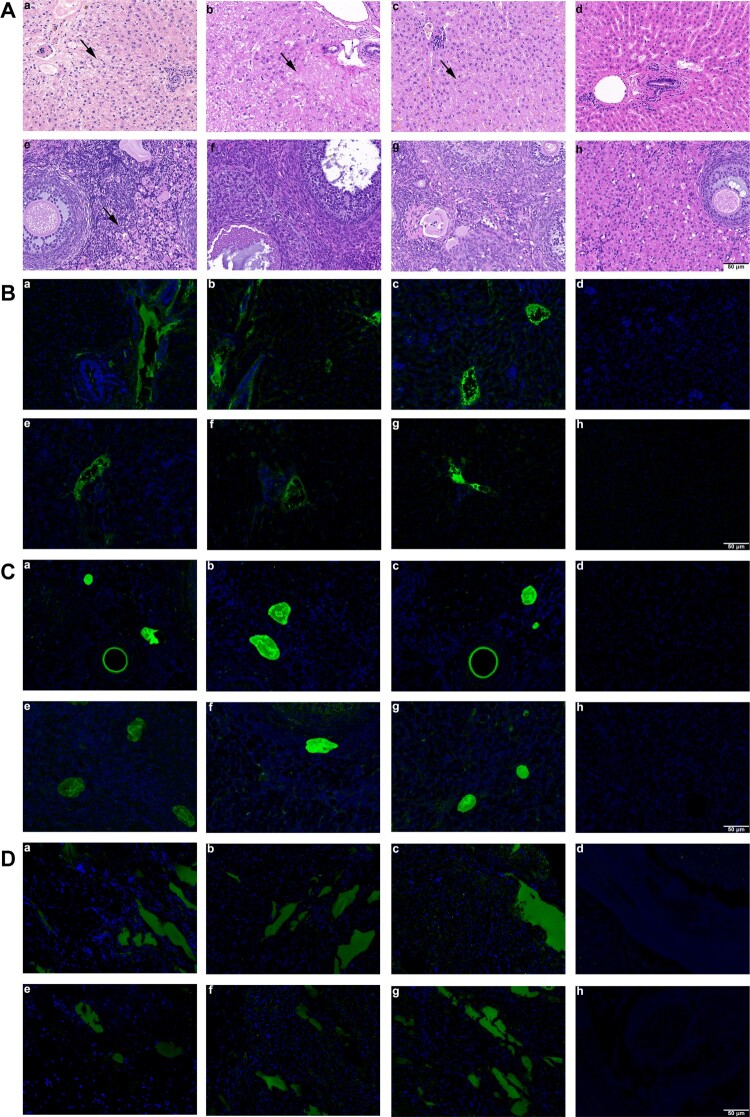
To clarify the histopathological changes in pregnant rabbits’ tissues, we performed HE staining on rabbit livers and other organs (Figure 2). In group A, hepatic hemorrhage was found in livers of pregnant rabbits, with a large number of red blood cells in hepatic sinusoids, extensive edema, degeneration and necrosis of hepatocytes and inflammatory cell infiltration; necrosis and degeneration of hepatocytes with inflammatory cell infiltration in liver tissue of pregnant rabbits of groups B and C were also observed, but the degree of inflammation was milder than that of group A; and no obvious pathological change in the liver tissue was observed in group D. In addition, the ovary tissue of group A showed slight inflammatory cell infiltration, but groups B, C and D showed no obvious pathological changes in ovary tissues.
Figure 2.
Histopathology and immunofluorescence of pregnant rabbits in group A, B, C and D. Liver and ovary histopathology. (a-c) HE staining showed hepatocyte degeneration and necrosis, collagen fibre hyperplasia and fibrosis, and infiltration of inflammatory cells in liver sections of group D, E and F rabbits; (d) liver section of group G showed no visible pathological changes; (e) mild inflammatory cells’ inflitration in ovary section of group A; (f-h) no visible pathological signs of HEV infection in ovary sections of group B-D rabbits. (B) Immunofluorescence staining of HEV ORF2 and ORF3 in liver. (a-c, e-g) Positive signals of HEV ORF2 and ORF3 respectively in liver sections of rabbits in group A-C; (d, h) no positive signals of HEV ORF2 and ORF3 were observed in liver section of rabbits in group D. (C) Immunofluorescence staining of HEV ORF2 and ORF3 in ovary. (a-c, e-g) Positive signals of HEV ORF2 and ORF3 respectively in ovary sections of rabbits in group A-C; (d, h) no positive signals of HEV ORF2 and ORF3 were observed in ovary section of rabbits in group D. (D) Immunofluorescence staining of HEV ORF2 and ORF3 in placenta. (a-c, e-g) Positive signals of HEV ORF2 and ORF3 respectively in placenta sections of rabbits in group A-C; (d, h) no positive signals of HEV ORF2 and ORF3 were observed in placenta section of rabbits in group D. Original magnification,×200.
To assess the replication efficiency of different genotypes of HEV in the liver, ovary and placenta, the expression of HEV ORF2 and ORF3 was detected by immunofluorescence staining. HEV ORF2 and ORF3 antigens were positive in the liver, ovary, and placenta of rabbits in groups A, B, and C but not in the organs of group D (Figure 2).
The estrogen levels and cellular immunity in HEV-infected pregnant rabbits
By regression analysis of serum estrogen levels and fecal viral load in group A after virus inoculation, a positive correlation between serum estradiol levels and fecal viral load in pregnant rabbits (r2 = 0.4339) was observed, which indicated that estrogen levels were correlated with HEV infection and the higher the estrogen level, the higher the fecal viral load in pregnant rabbits was (Supplementary Fig. 2A).
To investigate the relationship between HEV infection and cellular immunity in pregnant rabbits, we investigated the cellular immune response associated with HEV infection in the liver, ovary, and placenta through IHC. IHC experiments on CD4+ and CD8+ T cells showed that the signals of CD4+ T cells and CD8+ T cells in the liver, ovary and placenta of pregnant rabbits infected with rHEV3 were all positive, and the expression of CD8+ T cells was higher than that of CD4+T cells, but pregnant rabbits of group D showed low but similar levels of CD4+ T cells and CD8+ T cells in liver, ovary and placenta (Supplementary Fig. 2B).
HEV infection in newborn rabbits and vertical transmission
By analyzing HEV infection in surviving newborn rabbits delivered by pregnant rabbits infected with different genotypes of HEV, 50% (2/4) of surviving newborn rabbits of group A had fecal virus shedding after birth, 25% (1/4) were positive for anti-HEV antibody without HEV infection and 25% (1/4) did not have HEV infection; in surviving newborn rabbits of group B, 20% (1/5) had viremia after birth and 80% (4/5) did not have HEV infection; and in group C’s surviving newborn rabbits, 100% (7/7) were without HEV infection. The HEV infection rate of surviving newborn rabbits in group A was significantly higher than that of surviving newborn rabbits in group C (P < 0.05) (Supplementary Table 1).
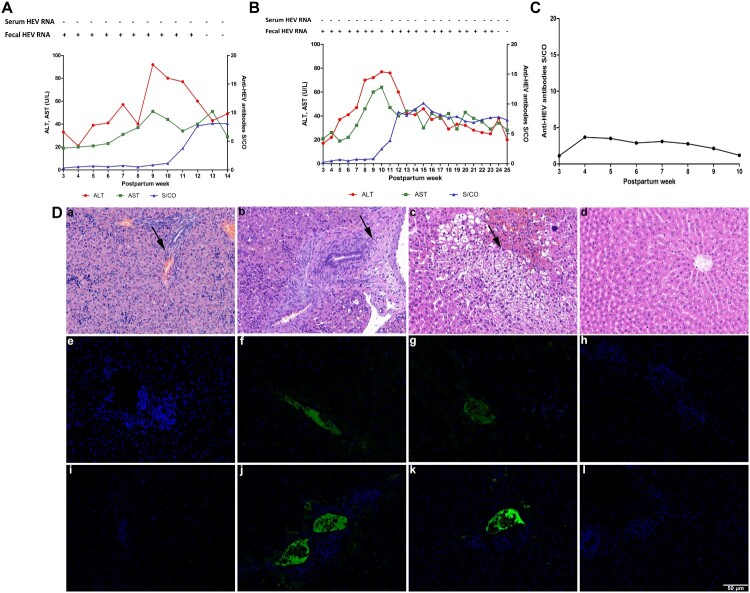
One newborn rabbit of group A (Aa) shed virus in its feces for 12 weeks, and the other (Ab) shed for 23 weeks. Both ALT and AST levels of these two rabbits significantly increased more than 2 times the baseline value and seroconversion of anti-HEV antibodies was also observed (Figure 3A and 3B). The third newborn rabbit (Ac) maintained a low level of anti-HEV antibodies that decreased over time (Figure 3C).
Figure 3.
Dynamic seroconversion of anti-HEV, HEV RNA, ALT, AST, histopathology and immunofluorescence of newborn rabbits. and (B) Dynamic seroconversion of antiHEV, HEV RNA, ALT and AST of group A newborn rabbits Aa and Ab with fecal HEV RNA shedding. (C) Dynamic seroconversion of anti-HEV of group A newborn rabbits Ac with anti-HEV antibodies. (D) Liver histopathology and Immunofluorescence staining of HEV ORF2 and ORF3 in liver of newborn rabbits. (a, d) Liver section of group A newborn dead rabbit and group D newborn rabbit showed no visible pathological signs of HEV infection; (b, c) various pathological changes in liver sections of group A newborn rabbits Aa and Ab respectively; (e, i, h, l) negative signals for HEV ORF2 and ORF3 antigen in liver of group A newborn dead rabbit and group D newborn rabbit; (f, g, j, k) positive signals for HEV ORF2 and ORF3 antigen in livers from group A newborn rabbits Aa and Ab. Original magnification,×200.
The liver tissues of euthanized newborn rabbits were analyzed by histopathology and immunofluorescence. Structural disorder, hepatocyte necrosis and inflammatory cell infiltration were observed in the liver tissue of Aa and extensive hepatocyte edema, balloon-like degeneration and hepatocyte necrosis were observed in the liver tissue of Ab. Immunofluorescence analysis showed positive signals for HEV ORF2 and ORF3 in the liver tissues of Aa and Ab (Figure 3D).
Antibody responses after vaccination
Different doses of the HEV 239 vaccine were administered to pregnant rabbits to evaluate the protective efficacy. Four weeks after the first immunization in groups E and F, the serum anti-HEV antibody-positive rates of rabbits inoculated with the 10 and 5 μg dose HEV 239 vaccine were 83.3% and 66.7%, respectively. After the whole immunization process, all vaccinated rabbits were positive for anti-HEV antibodies, whereas the rabbits of the placebo group, G, were all negative for anti-HEV antibodies (Table 3).
Table 3. The seroconversion rates of anti-HEV antibody after vaccination.
| Groups (No.) | HEV 239 (μg) | The seroconversion rates of anti-HEV antibody (%) | ||
|---|---|---|---|---|
| 1 weeka | 4 weekb | 5 weekc | ||
| E (6) | 2×10 | 2/6(33.3) | 5/6(83.3) | 6/6(100) |
| F (6) | 2×5 | 1/6(16.7) | 4/6(66.7) | 6/6(100) |
| G (6) | 2×0 | 0/6(0) | 0/6(0) | 0/6(0) |
aA week after the first immunization.
bBefore the second immunization.
cA week after the second immunization.
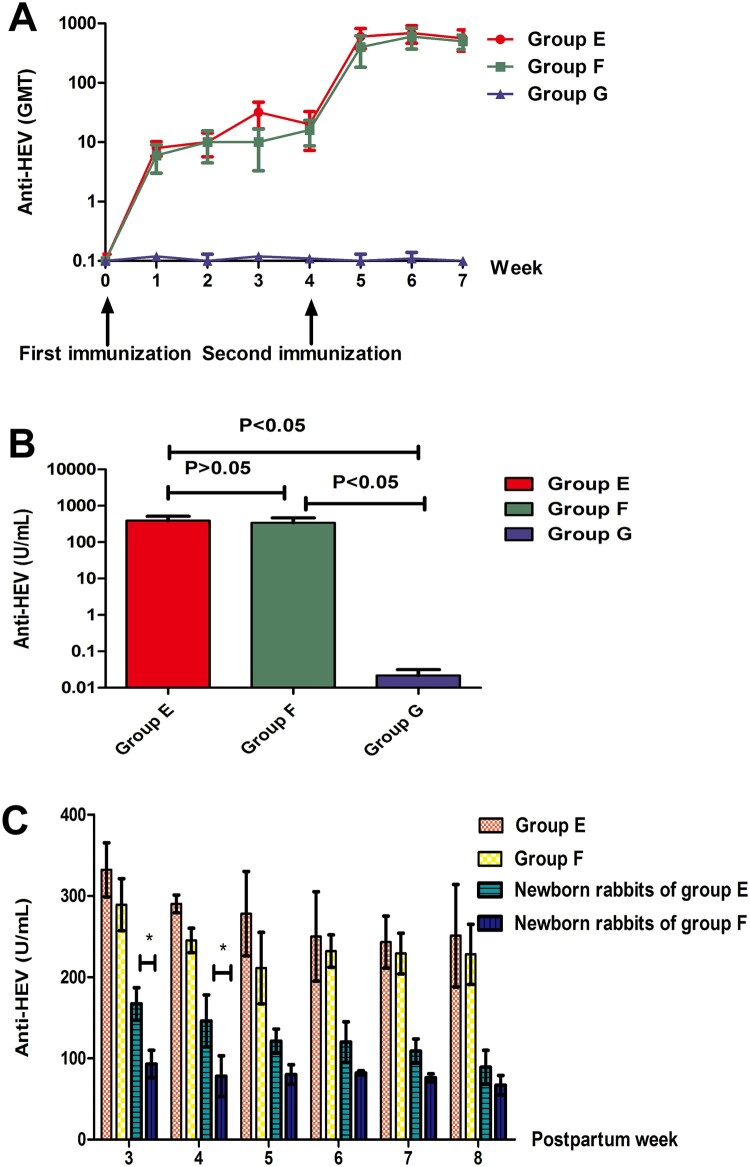
To evaluate the dynamics of anti-HEV antibodies induced by different doses of the HEV 239 vaccine throughout the whole immunization process, the GMT was measured weekly. The GMT induced by the 10 and 5 μg dose vaccines gradually increased after the first immunization and increased rapidly after the second immunization (Figure 4A).
Figure 4.
Antibody responses of rabbits after vaccination. (A) Dynamics of GMT induced by various types of vaccination. (B) The comparison of anti-HEV levels in each group. Y axis represents anti-HEV levels on week 7 expressed in WHO unit/ml. (C) Postpartum protection of rabbits and newborn rabbits. Y axis represents anti-HEV levels on postpartum week 3 to week 8 expressed in WHO unit/ml (* represents P < 0.05).
Three weeks after the whole immunization process, the anti-HEV antibody concentrations of rabbits’ serum were quantified and the average anti-HEV antibody concentrations were compared among the groups. The results showed that the average anti-HEV antibody concentrations between group E and group F were not significantly different (P > 0.05) and that the average anti-HEV antibody concentrations in groups E and F were higher than that in group G (P < 0.05) (Figure 4B).
Protective efficacy of the HEV 239 vaccine in pregnant rabbits
After pregnancy was confirmed, rabbits in groups E, F and G were inoculated with the rHEV3 virus on the 10th day post copulation. None of the rabbits in groups E and F developed HEV infection symptoms, such as viremia, fecal virus shedding and elevation of ALT and AST levels, with only one rabbit in group F having a stillbirth. In contrast, all rabbits in group G began seroconversion to anti-HEV antibodies and fecal virus shedding at 2 wpi. Adverse pregnancy outcomes, including miscarriage, stillbirth, and death, occurred at 4 wpi, indicating that HEV can successfully infect rabbits without HEV 239 vaccination.
Statistical analysis showed that compared with placebo administration, two doses of the 10 μg HEV 239 vaccine not only protected pregnant rabbits from rabbit HEV infection but also prevented the occurrence of adverse pregnancy outcomes (P < 0.05). Two doses of the 5 μg HEV 239 vaccines could protect pregnant rabbits from rabbit HEV infection, with only one case of stillbirth occurring, which also had a significant difference compared with placebo administration (P < 0.05) (Table 4).
Table 4. Protection of rabbits against challenge with rHEV-3.
| Groups (No.) | HEV 239 (μg) | Pre-challenge | Post challenge | |||
|---|---|---|---|---|---|---|
| Mean anti-HEV level ± SD(U/mL) | Number of rabbits shedding virus in fecesa | Ab resp.b | Number of rabbits with adverse pregnancy outcomesc | P valuesf | ||
| E (6) | 2×10 | 389.2 ± 112.3 | 0/6 | − | 0/6 | 0.002* |
| F (6) | 2×5 | 339.5 ± 165.2 | 0/6 | − | 1/6d | 0.015* |
| G (6) | 2×0 | <1 | 6/6 | + (>4fold) | 6/6e | − |
aNumber of rabbits shedding virus in feces/total number of rabbits in the group.
bAb resp. (+) represents the mean anti-HEV level in every group post challenge is four times higher than that pre-challenge.
cNumber of rabbits with adverse pregnancy outcomes/total number of rabbits in the group.
dOne rabbit had stillbirth.
eOne rabbit died, three rabbits had stillbirth and two rabbits had miscarriage.
fThe vaccine efficacy compared to group G.
*Significantly different, P < 0.05.
Serum anti-HEV antibody levels of newborn rabbits in groups E and F were followed. From the 3rd to 8th week after birth, the levels of anti-HEV antibodies continued to decrease and then remained at the same level, while the anti-HEV antibody levels in the pregnant rabbits of groups E and F decreased slightly and remained at the same level. The initial level of anti-HEV antibody in newborn rabbits in group E was higher than that in group F but remained at the same level eventually (Figure 4C).
Discussion
Although studies have suggested that mainly HEV1 infection is associated with high mortality in pregnant women [10], there is limited research to investigate the influence of other HEV genotypes. In this study, the incidences of adverse pregnancy outcomes were not significantly different among the three groups of rabbits infected with rHEV3, sHEV4 and hHEV3, indicating that different genotype HEVs with the same viral load can all cause adverse pregnancy outcomes. This study is the first time that rabbits have been able to be infected by hHEV3, which may be due to the use of different subgenotypes compared with other studies [20] or because that pregnant rabbits are more prone than nonpregnant rabbits to hHEV3 infection. However, the manifestations of different HEV genotypes infection, including fecal virus shedding duration, the anti-HEV S/CO levels and pathological changes, were different among the groups, and rabbits infected with rHEV3 showed more obvious symptoms than the other groups.
The vertical transmission of HEV has always attracted much attention [21–23], and several studies have shown that HEV can replicate in the human placenta [11,24]. In this study, the positive and negative strands of HEV RNA were detected in placentas and immunofluorescence results showed that both HEV ORF2 and ORF3 signals were positive in the placenta, suggesting that HEV may replicate in the placenta, possibly leading to vertical transmission. We also found that the ovaries of HEV-infected pregnant rabbits were positive for HEV ORF2 and ORF3, indicating that HEV may also replicate in rabbit ovaries. Two of the surviving newborn rabbits (Aa and Ab) delivered by HEV-infected pregnant rabbits showed symptoms of HEV infection, suggesting possible vertical transmission.
The alteration in hormone secretion during pregnancy may be associated with hepatitis E-related poor pregnancy outcomes, and estrogen has been proven to be able to promote HEV replication in vitro [25]. Our results showed that the estrogen level was positively correlated with fecal viral load, suggesting that estrogen may be able to promote HEV replication in pregnant rabbits. The decrease in the CD4/CD8 ratio in blood may be related to high mortality and adverse outcomes during pregnancy caused by HEV infection [26,27]. In this study, in accordance with the lowered CD4/CD8 cell ratio in blood samples [26,27], the IHC results of tissues showed that compared with the negative control rabbits, pregnant rabbits infected with HEV were positive for both CD4+ and CD8+ T cells in the liver, ovary, and placenta and that the proportion of CD8+ T cells was higher than that of CD4+ T cells.
In this study, similar to previous studies [28,29], our results showed that the HEV 239 vaccine can effectively prevent rabbit HEV infection in pregnant rabbits. For the first time, our results showed that the HEV 239 vaccine can also prevent the occurrence of adverse pregnancy outcomes in pregnant rabbits. Different doses of vaccination in pregnant rabbits could produce anti-HEV antibodies without significant differences in immunization efficacy. We also noted that anti-HEV antibodies could be detected in newborn rabbits delivered by immunized pregnant rabbits, which may be related to breastfeeding of female rabbits. The anti-HEV antibody persisted in newborn rabbits, and different doses of the HEV 239 vaccine had the same efficacy on newborn rabbits.
In summary, our results showed that different genotypes of HEV can infect pregnant rabbits and lead to adverse pregnancy outcomes and vertical transmission. The HEV 239 vaccine can effectively protect pregnant rabbits from rabbit HEV infection and adverse pregnancy outcomes. Although the animal numbers in the present study are limited, our results still suggest that to avoid hepatitis E-related adverse pregnancy outcomes and vertical transmission, childbearing-age women should be immunized with the HEV vaccine before pregnancy.
Supplementary Material
Acknowledgements
We would like to thank Xiamen Innovax Biotech, Xiamen, China, for supplying the HEV 239 vaccine. We thank Prof. Patrick CY Woo (Department of Microbiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China) for proofreading the manuscript.
Conception and design: Manyu Li and Ling Wang. Manyu Li and Ling Wang performed the experiments and wrote the manuscript. Manyu Li, Shuangshuang Li, Qiyu He, Zhaochao Liang, Lin Wang and Qianhui Wang collected and assembled the data. Manyu Li analyzed the data.
Funding Statement
This work was supported by the National Natural Science Foundation of China [grant number 81772175].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Kamar N, Izopet J, Pavio N, et al. Hepatitis E virus infection. Nat Rev Dis Primers. 2017;3:17086. doi: 10.1038/nrdp.2017.86 [DOI] [PubMed] [Google Scholar]
- 2.Himmelsbach K, Bender D, Hildt E.. Life cycle and morphogenesis of the hepatitis E virus. Emerg Microbes Infect. 2018;7:196. doi: 10.1038/s41426-018-0198-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nimgaonkar I, Ding Q, Schwartz RE, et al. Hepatitis E virus: advances and challenges. Nat Rev Gastroenterol Hepatol. 2018;15:96. doi: 10.1038/nrgastro.2017.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Hepatitis E vaccine: WHO position paper, May 2015. Wkly Epidemiol Rec. 2015;18:185–200. [PubMed] [Google Scholar]
- 5.Ruggeri F M, Di Bartolo I, Ponterio E, et al. Zoonotic transmission of hepatitis E virus in industrialized countries. New Microbiol. 2013;36:331–344. [PubMed] [Google Scholar]
- 6.Khuroo M S, Kamili S, Khuroo M S.. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J Viral Hepat. 2009;16:519–523. doi: 10.1111/j.1365-2893.2009.01101.x [DOI] [PubMed] [Google Scholar]
- 7.Patra S, Kumar A, Trivedi S S, et al. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med. 2007;147:28–33. doi: 10.7326/0003-4819-147-1-200707030-00005 [DOI] [PubMed] [Google Scholar]
- 8.Oncu S, Oncu S, Okyay P, et al. Prevalence and risk factors for HEV infection in pregnant women. Med Sci Monit. 2005;12:CR36–CR39. [PubMed] [Google Scholar]
- 9.Kumar A, Beniwal M, Kar P, et al. Hepatitis E in pregnancy. Int J Gynaecol Obstet. 2004;85:240–244. doi: 10.1016/j.ijgo.2003.11.018 [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Gracia MT, Suay-García B, Mateos-Lindemann ML.. Hepatitis E and pregnancy: current state. Rev Med Virol. 2017;27:e1929. doi: 10.1002/rmv.1929 [DOI] [PubMed] [Google Scholar]
- 11.Zhu F C, Zhang J, Zhang X F, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6 [DOI] [PubMed] [Google Scholar]
- 12.Xia J, Liu L, Wang L, et al. Experimental infection of pregnant rabbits with hepatitis E virus demonstrating high mortality and vertical transmission. J Viral Hepat. 2015;22:850–857. doi: 10.1111/jvh.12406 [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Ge S X, Huang G Y, et al. Evaluation of antibody-based and nucleic acid-based assays for diagnosis of hepatitis E virus infection in a rhesus monkey model. J Med Virol. 2003;71:518–526. doi: 10.1002/jmv.10523 [DOI] [PubMed] [Google Scholar]
- 14.Wang Y C, Zhang H, Xia N, et al. Prevalence, isolation, and partial sequence analysis of hepatitis E virus from domestic animals in China. J Med Virol. 2002;67:516–521. doi: 10.1002/jmv.10131 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zeng H, Liu P, et al. Hepatitis E vaccine immunization for rabbits to prevent animal HEV infection and zoonotic transmission. Vaccine. 2015;33:4922–4928. doi: 10.1016/j.vaccine.2015.07.040 [DOI] [PubMed] [Google Scholar]
- 16.Liu P, Bu Q N, Wang L, et al. Transmission of hepatitis E virus from rabbits to cynomolgus macaques. Emerg Infect Dis. 2013;19:559. doi: 10.3201/eid1904.120827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jothikumar N, Cromeans T L, Robertson B H, et al. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods. 2006;131:65–71. doi: 10.1016/j.jviromet.2005.07.004 [DOI] [PubMed] [Google Scholar]
- 18.Han J, Zeng H, Wang L, et al. Hepatitis E virus infection in farmed rabbits and swine in the Eastern Chinese city Lianyungang: showing no potential interspecies transmission. J Med Virol. 2014;86:1898–1904. doi: 10.1002/jmv.24003 [DOI] [PubMed] [Google Scholar]
- 19.Allweiss L, Gass S, Giersch K, et al. Human liver chimeric mice as a new model of chronic hepatitis E virus infection and preclinical drug evaluation. J Hepatol. 2016;64:1033–1040. doi: 10.1016/j.jhep.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Gong W, Song WT, et al. Different susceptibility and pathogenesis of rabbit genotype 3 hepatitis E virus (HEV-3) and human HEV-3 (JRC-HE3) in SPF rabbits. Vet Microbiol. 2017;207:1–6. doi: 10.1016/j.vetmic.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 21.Kumar R M, Uduman S, Rana S, et al. Sero-prevalence and mother-to-infant transmission of hepatitis E virus among pregnant women in the United Arab Emirates. Eur J Obstet Gynecol Reprod Biol. 2001;100:9–15. doi: 10.1016/S0301-2115(01)00448-1 [DOI] [PubMed] [Google Scholar]
- 22.Morozov V A, Morozov A V, Rotem A, et al. Extended microbiological characterization of göttingen minipigs in the context of xenotransplantation: detection and vertical transmission of hepatitis E virus. PLoS One. 2015;10:e0139893. doi: 10.1371/journal.pone.0139893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma S, Kumar A, Kar P, et al. Risk factors for vertical transmission of hepatitis E virus infection. J Viral Hepat. 2017;24:1067–1075. doi: 10.1111/jvh.12730 [DOI] [PubMed] [Google Scholar]
- 24.Bose P D, Das B C, Hazam R K, et al. Evidence of extrahepatic replication of hepatitis E virus in human placenta. J Gen Virol. 2014;95:1266–1271. doi: 10.1099/vir.0.063602-0 [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Yu W, Bi Y, et al. Increased oestradiol in hepatitis E virus-infected pregnant women promotes viral replication. J Viral Hepat. 2018;25:742–751. doi: 10.1111/jvh.12865 [DOI] [PubMed] [Google Scholar]
- 26.Jilani N, Das B C, Husain S A, et al. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J Gastroenterol Hepatol. 2007;22:676–682. doi: 10.1111/j.1440-1746.2007.04913.x [DOI] [PubMed] [Google Scholar]
- 27.Khaskheli M N, Baloch S, Sheeba A, et al. Acute hepatitis E viral infection in pregnancy and maternal morbidity. J Coll Physicians Surg Pak. 2015;25:734–737. [DOI] [PubMed] [Google Scholar]
- 28.Liu P, Du R J, Wang L, et al. Management of hepatitis E virus (HEV) zoonotic transmission: protection of rabbits against HEV challenge following immunization with HEV 239 vaccine. PLoS One. 2014;9:e87600. doi: 10.1371/journal.pone.0087600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Zeng H, Liu P, et al. Hepatitis E vaccine immunization for rabbits to prevent animal HEV infection and zoonotic transmission. Vaccine. 2015;33:4922–4928. doi: 10.1016/j.vaccine.2015.07.040 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.