Abstract
Semisynthetic proteins engineered to contain non-coded elements such as post-translational modifications (PTMs) represent a powerful class of tools for interrogating biological processes. Here, we introduce a one-pot, chemoenzymatic method that allows broad access to chemically modified proteins. The approach involves a tandem transamidation reaction cascade that integrates intein-mediated protein splicing with enzyme-mediated peptide ligation. We show that this approach can be used to introduce PTMs and biochemical probes into a range of proteins including Cas9 nuclease and the transcriptional regulator MeCP2, which causes Rett Syndrome when mutated. The versatility of the approach is further illustrated through the chemical tailoring of histone proteins within a native chromatin setting. We expect our approach will extend the scope of semisynthesis in protein engineering.
The complexity of biological processes demands the development of precision tools that can shed light on underlying molecular mechanisms. This is exemplified by the generation of designer proteins containing non-coded moieties such as PTMs, unnatural amino acids and biophysical probes1–5. These modified proteins often play pivotal roles in understanding the molecular mechanisms underlying biological function6–8. Generating chemically tailored proteins frequently relies on the amalgamation of chemical synthesis and recombinant DNA technologies9, allowing access to molecular space beyond the reach of either method in isolation. In particular, ligation-based semisynthesis technologies, where targets are assembled from building blocks comprising synthetic peptides and recombinant proteins, are widely used for the generation of proteins bearing non-genetically encoded elements10. Ideally, a ligation-based methodology should have the following capabilities; (i) it should be chemically traceless, leaving behind a minimal (or no) ligation ‘scar’ in the protein product, (ii) it should involve the use of reactive handles or auxiliaries that are straightforward to install, and (iii) it should be high yielding and compatible with low concentrations of polypeptide reactants. While existing methods in this area fulfill one or more of these criteria, arguably no single approach is able to achieve them all, at least in a general fashion (Supplementary Fig. 1). For instance, the widely used Native Chemical Ligation (NCL) and Expressed Protein Ligation (EPL) strategies allow the assembly of semisynthetic proteins bearing a minimal (or often no) ligation scar11,12. However, these techniques require significant expertise in order to install mutually reactive chemical functionalities into peptides and proteins. Moreover, the second order reaction kinetics associated with the chemical ligation step often demand reactant concentrations beyond the tolerance of many proteins, thereby limiting the scope of substrates that may be accessed for downstream applications. Analogously, enzyme-mediated ligation approaches that employ engineered transpeptidases and proteases13–18 each have associated strengths and weaknesses (Supplementary Fig. 1). For example, the commonly used sortase-based ligation method allows the use of building blocks that are accessible to the non-specialist and that can be ligated at relatively low protein concentrations16. However, the process is kinetically reversible and may leave a significant scar of non-native residues in the reaction product that can preclude the traceless installation of modified fragments within a protein sequence (Supplementary Fig. 1). Thus, despite the impressive progress made in the protein ligation field as a whole19–21, a more accessible method to efficiently and tracelessly generate semisynthetic proteins would greatly help in addressing the many remaining problems in protein science.
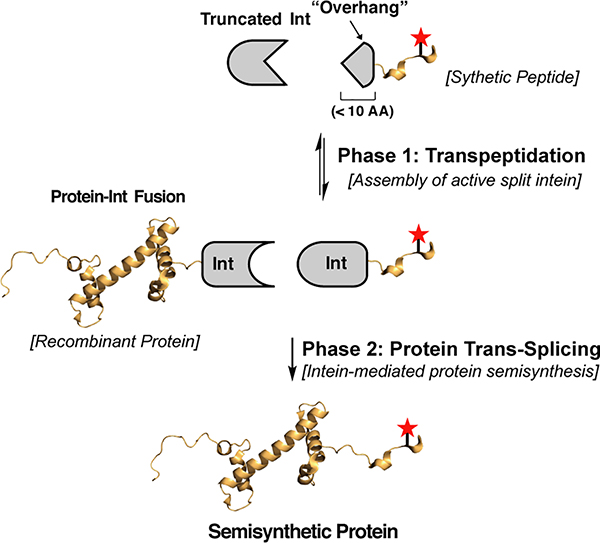
We envisioned a chemoenzymatic ligation method that harnesses the potential of split inteins for semisynthesis22–24. Split inteins can be considered “Nature’s protein ligases” in that they mediate the assembly of appended polypeptide cargoes in a process known as protein trans-splicing (PTS)24. We were especially interested in integrating a recently described consensus-fast split intein (Cfa) into our process25. Importantly, Cfa supports extremely efficient PTS over a broad range of reaction conditions and, because of the intrinsically high affinity of the N- and C-intein fragments (IntN and IntC), PTS can be performed using very low concentrations of reactants24. Despite these favorable characteristics, protein semisynthesis using Cfa is greatly constrained by the large size of the IntN and IntC fragments (~101 and 35 residues, respectively) - one or other of these reactive handles needs to be included in the synthetic building block. We imagined a solution to this problem in which a semisynthetic protein is assembled in two distinct reaction phases (Fig. 1). In the first phase, transpeptidase-mediated ligation is used to assemble an active split-intein fusion from a truncated version of the split intein and a synthetic cargo peptide fused to a short ‘overhang’ comprising the remaining residues of the intein. A second reaction phase then follows whereby the newly generated intein-cargo reacts via PTS with the recombinant protein to be modified, fused to the complementary Cfa intein. This process, which we term Transpeptidase-Assisted Intein Ligation (TAIL), has two important attributes: (i) the synthetic peptide building block is rendered much more accessible due to the short reactive handle employed (the simple, unmodified peptide ‘overhang’), and (ii) the traceless PTS step is irreversible and is expected to drive the overall process to completion, even at low concentrations of reactants, thereby affording the desired semisynthetic product in high overall yield.
Fig. 1. Protein semisynthesis by Transpeptidase-Assisted Intein Ligation (TAIL).
Schematic showing the TAIL workflow. During Phase 1, a functional split-intein fragment is generated through enzymatic transpeptidation of a truncated intein (Truncated Int) and a synthetic peptide bearing a short polypeptide sequence (‘overhang’) that completes the intein sequence. In phase 2, the assembled split-intein fragment undergoes PTS with a recombinant protein fused to the cognate split intein fragment to afford a semisynthetic protein. Note, for clarity the scheme depicts ligation of a synthetic cargo to the C-terminus of a recombinant protein, however, the approach can be used in the inverse configuration.
Results and Discussion
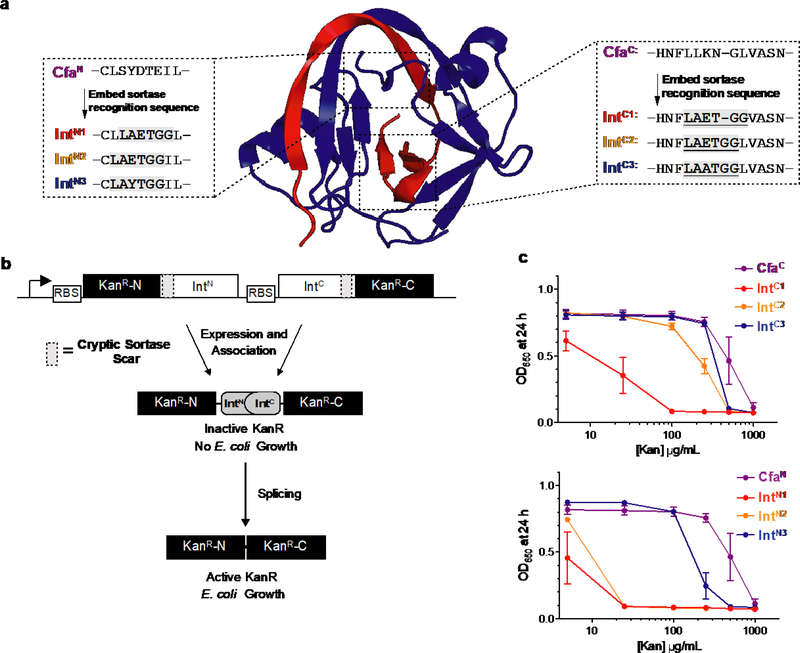
Our investigations began with the identification of functional IntN and IntC sequences that can be assembled through transpeptidation, the first step in the TAIL process (Fig. 1). Ideally, these transpeptidation sites would be placed adjacent to the end of the intein fragment that is fused to the cargo, thereby permitting the use of minimal intein-derived ‘overhangs’. With this stipulation in mind, we identified candidate locations in Cfa IntN and Cfa IntC where we could embed a cryptic transpeptidation motif (-LAxTG-) for an evolved version of a bacterial sortase (eSrt2A)26 (Fig. 2a). In order to rapidly screen the activity of the mutant inteins, we employed an in vivo survival assay that couples PTS activity with resistance of E. coli to the antibiotic kanamycin (Fig. 2b). By varying both the position of the -LAxTG- insertion, as well as the nature of the variable residue within the motif itself, split intein mutants were identified which retained much of the splicing activity of the parent Cfa intein (Fig. 2c). Subsequent in vitro PTS assays confirmed the mutant IntC and IntN sequences were highly active, exhibiting splicing half-lives of roughly 2 and 5 min, respectively (Supplementary Fig. 3–4). More generally, the ability of the Cfa intein fragments to accommodate these embedded transpeptidase motifs speaks to the malleability of the system for protein engineering27.
Fig. 2. Identification of mutant inteins for use in TAIL.
a, Crystal structure of a DnaE split intein (Npu, PDB ID 4l×3) indicating regions of the IntN and IntC fragments (rendered blue and red, respectively) proximal to the splice junction in which to insert transpeptidase recognition sequences. Primary sequences of the CfaN (left) and CfaC (right) split intein fragments, and related mutant inteins (IntC/N) containing the eSrt2A recognition sequences are shown. b, Schematic of an in vivo kanamycin resistance assay for screening ability of split inteins containing embedded sortase recognition sequences to undergo PTS, affording resistance to increasing concentrations of kanamycin. c, Dose-response growth curves of in vivo kanamycin resistance assays for IntN = CfaN and IntC = CfaC, IntC1, IntC2 or IntC3 (upper), and for IntC = CfaC and IntN = CfaN, IntN1, IntN2 or IntN3 (lower). Error bars represent SD (n=3).
The necessary split intein mutants in hand, we next asked whether they could be integrated into the overall TAIL process. We began these studies by exploring the ligation of a synthetic cargo to the C-terminus of a recombinant protein (hereafter referred to as C-TAIL). The ~41 kDa Maltose Binding Protein (MBP) was employed as a model system, representing the approximate median size of proteins in the human proteome28. A synthetic peptide (IntCov-cargo) was prepared using solid-phase peptide synthesis, containing a fluorescein moiety as a representative synthetic ‘cargo’, as well as the requisite 7-amino acid overhang that completes the functional intein sequence (IntCov) and is compliant with eSrt2A-mediated transpeptidation. The other two reactants, namely a genetic fusion of MBP and Cfa IntN (MBP-IntN) and the truncated Cfa IntC fragment containing the transpeptidase recognition element (IntCtr), were readily accessed through recombinant expression in E. coli (see Supplementary Methods). Incubation of these constructs in the presence of a catalytic amount of eSrt2A led to the generation of the modified protein as judged by SDS-PAGE and mass spectrometry (Fig. 3a,b). Control experiments demonstrated that in the absence of an active version of eSrt2A, product formation through background PTS was slow and inefficient (Supplementary Fig. 4), accompanied by significant quantities of cleaved MBP-IntN starting material (to give MBP and IntN). Thus, as per our design, efficient product formation requires the covalent assembly of the truncated split intein fragments. The C-TAIL reaction was efficient over a range of temperatures, typically reaching 75–85% conversion, and accommodated a variety of residues (including glycine, glutamate, arginine and valine) at the splice junction (Fig. 2c, Supplementary Fig. 5–6). Moreover, the process could be easily scaled, allowing the labeled protein to be isolated in multi-milligram amounts (Supplementary Fig. 7). Of note, the transpeptidase-mediated labeling of MBP by eSrt2A alone under similar conditions afforded a maximum of ~60 % conversion to product, which diminished over time due to unfavorable hydrolysis competing with the reversible labeling reaction (Supplementary Fig. 8). This demonstrates the value of coupling transpeptidation with protein splicing to drive the reaction to a favorable thermodynamic endpoint.
Fig. 3. C-terminal protein engineering using TAIL (C-TAIL).
a, Schematic depicting C-TAIL reaction on the model protein MBP. A fluorescent cargo peptide bearing the N-terminal overhang GGLVASN (IntCov-cargo) was reacted with MBP-IntN, a truncated IntC fragment (IntCtr, containing the transpeptidase compliant sequence, LAATGG) and the transpeptidase eSrt2A. (MBP structure: PDB ID 3PGF). b, Analysis of the model C-TAIL reaction at 37 °C. Top: The C-TAIL reaction progress was monitored over time by SDS-PAGE (the gel was visualized by coomassie staining and in-gel fluorescence imaging); Bottom: ESI-TOF MS analysis of the semisynthetic product, MBP-cargo. c, Time courses of the model C-TAIL reaction at different temperatures. Reactions were analyzed by SDS-PAGE and reaction progress determined by densitometry analysis of coomassie stained bands. Lines connect mean values and error bars represent s.d (n=3, individual data points are shown). d, Semisynthesis of linker histone H1 phosphorylated at serine 182 (H1-S182ph). Top: Schematic of the semisynthesis route employed. C-TAIL was used to link recombinant (residues 1–179) and synthetic (180–214) fragments of H1. The ligation was then rendered traceless by converting Cys180 at the ligation junction back to the native alanine using an in situ radical desulfurization step. Bottom: RP-HPLC (left) and ESI-TOF MS (right) of purified semisynthetic H1-S182ph. e, Semisynthesis of the transcriptional regulator MeCP2. Top: C-TAIL was used to assemble the ~50 kDa protein from two fragments in a traceless fashion. This allowed the incorporation of phospho-serine at position 423 and a diazirine-containing analog of leucine (photo-Leu) at position 424. Bottom: RP-HPLC (left) and ESI-TOF MS (right) of purified semisynthetic MeCP2.
A benchmark of current methods for protein semisynthesis is the traceless assembly of modified protein sequences from precursor fragments. With this in mind, we asked whether TAIL could be used to install one or more modifications into an otherwise native protein sequence. As a first example, we chose the 214-residue linker histone, H1, an architectural protein critical in the compaction and organization of chromatin29. Linker histones are extensively decorated with PTMs, which are presumed to regulate the function of the protein, albeit through poorly understood mechanisms29. The semisynthesis of a modified linker histone is yet to be reported, making this an excellent target for our technology. We established a traceless semisynthesis of H1 that combined the C-TAIL approach with a subsequent radical desulfurization step30 (Fig. 3d, and S9). This route permitted the site-specific installation of non-coded elements into the unstructured C-terminal region of the protein, as illustrated by the incorporation of a phosphor-serine PTM at position 182. The semisynthetic protein was isolated in high chemical purity and in sufficient quantities for biochemical studies (Fig. 3c). In a second example, we focused on Methyl-CpG Protein Binding 2 (MeCP2), a 486 residue transcriptional regulator abundant in nerve cells31. Mutation of MeCP2 is associated with multiple human disorders including Rett syndrome32 and, like many chromatin-associated factors, the protein is elaborated with multiple PTMs33. Among the latter, phosphorylation of serine 423 has received considerable attention due to its association with neuronal activation34. Access to this phosphorylated protein would help ongoing efforts to understand its precise role in neuronal function. We successfully used traceless C-TAIL to generate a semisynthetic version of MeCP2 containing this PTM, as well as an adjacent diazirine-containing photocrosslinker (Fig. 3e and S10). This represents the first semisynthesis of this protein and illustrates a powerful feature of the TAIL approach, namely the ability to incorporate multiple different non-coded elements into a single protein.
We next turned to the ligation of synthetic peptides to the N-terminus of recombinant proteins (i.e. N-TAIL). Mirroring the C-TAIL manifold, we initially attempted to ligate a fluorescent cargo peptide to the N-terminus of MBP by reacting the following components: MBP fused to the Cfa IntC fragment (IntC-MBP), a synthetic peptide corresponding to the cargo fused a 7-amino acid overhang containing the transpeptidase recognition element (cargo-IntNov) and a truncated Cfa IntN fragment as a transpeptidase acceptor (IntNtr) (Fig. 4a, scheme (i)). Analysis of the reaction mixture revealed that no ligation product (cargo-MBP) had been generated in the presence of eSrt2A (Supplementary Fig. 11). Rather, we exclusively observed by-products corresponding to premature cleavage of the IntC-MBP building block (to give IntC and MBP), a side-reaction that apparently outcompeted transpeptidase-mediated assembly of the split intein fragment. Increasing the concentration of eSrt2A to stoichiometric levels did not solve this problem, suggesting that transpeptidation of IntNtr is inhibited by the presence of the cognate C-intein binding partner. As a solution to this, we drew inspiration from cellular amide-forming processes such as protein ubiquitination, where isopeptide bond formation between acyl-enzyme intermediates and suitable acyl-acceptors is driven by induced proximity via adaptor proteins35. Thus, we imagined that genetically fusing the eSrt2A transpeptidase to the truncated intein (IntNtr-eSrt2A) would promote a rapid intramolecular relay of a substrate peptide from the transpeptidase to the N-terminus of the truncated intein (Fig. 4a, scheme (ii)). Gratifyingly, this adjustment to the protocol promoted the efficient assembly of semisynthetic cargo-MBP (Fig. 4b,c). Moreover, consumption of the starting material was complete in a few hours, affording the semisynthetic product in high yield (>90%), as assessed by SDS-PAGE analysis (Fig. 4b).
Fig. 4. N-terminal protein engineering using TAIL (N-TAIL).
a, Schematic depicting optimization of the N-TAIL process. (i) A fluorescent cargo peptide bearing the C-terminal overhang CLAYTGG (Cargo-IntNov) was reacted with IntC-MBP, a truncated IntN fragment (IntNtr, containing the transpeptidase acceptor sequence, GG) and the transpeptidase eSrt2A. (ii) Fusion of the transpeptidase to the IntNtr fragment (IntNtr-eSrt2A) was used to overcome premature cleavage of the IntC-protein. MBP structure: PDB ID 3PGF. b, Coomassie stained SDS-PAGE analysis and corresponding in-gel fluorescence of N-TAIL model reaction monitored over time. c, ESI-TOF MS analysis of the semisynthetic product from N-TAIL reaction on the model protein, MBP. d, Schematic depicting labeling of dCas9 using N-TAIL. A synthetic biotinylated peptide bearing the C-terminal overhang (cargo-IntNov) was reacted with IntNtr-eSrt2A and IntC-dCas9 (dCas9 structure: PDB ID 4ZT9). e, Coomassie stained SDS-PAGE analysis of N-TAIL reaction on dCas9 monitored over time, and corresponding western blot using streptavidin as a detection reagent. “Splicing intermediate” refers to the branched-thioester intermediate in the protein trans-splicing cascade.
Encouraged by results of the pilot study, we asked whether the optimized N-TAIL process could be used to modify a more challenging protein target. For this we chose a 158 kDa nuclease-deficient form of Cas9 (dCas9) from Streptococcus pyogenes. We have previously shown that this protein can be used to target synthetic epigenetic effector molecules to specific genomic loci36. However, the preparation of the necessary dCas9-cargo conjugates involved a multistep process that included the chemical synthesis of long (> 40 residue) intein-fusion peptides, thereby constraining the types of applications available to the strategy. Use of the N-TAIL protocol provided a much more convenient and expeditious route to semisynthetic dCas9 conjugates. As an example, we were able to efficiently ligate a biotin-labeled cargo peptide to the protein through treatment with IntNtr-eSrt2A (Fig. 4d,e). The ligation reaction proceeded smoothly using IntC-dCas9 concentrations as low as 1 μM, which illustrates an inherent strength of the chemoenzymatic ligation approach, namely the ability to use low concentrations of reactants.
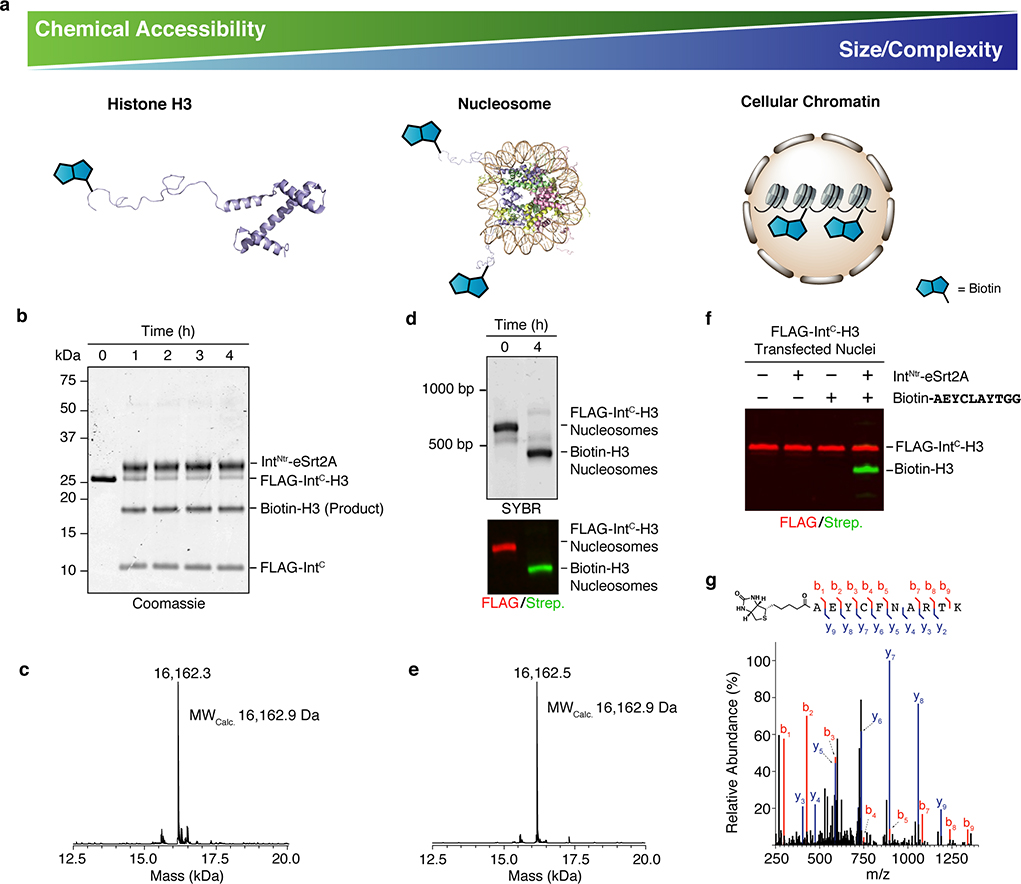
Finally, we asked whether the specificity of TAIL could enable protein ligation in systems of increasing physiological complexity (Fig. 5a). We focused on the N-terminal functionalization of histone H3, a component of eukaryotic chromatin. We posited that N-TAIL, by virtue of its reaction kinetics and chemoselectivity, would allow modification of H3 in the context of the isolated protein, the nucleosome complex and native cellular chromatin (Fig. 5a). To this end, we incubated a recombinant IntC-H3 fusion protein with IntNtr-eSrt2A and a biotinylated peptide, which afforded efficient conversion to a biotin-labeled H3 as judged by SDS-PAGE and ESI-MS analysis (Fig. 5b,c). A similar outcome was observed when the reaction was carried out on preformed nucleosomes containing the IntC-H3 fusion (Fig. 5d,e and S12). Finally, we attempted the labeling reaction on cellular chromatin. Nuclei harvested from 293T cells expressing the IntC-H3 construct were subjected to N-TAIL with IntNtr-eSrt2A and biotinylated peptide. After a 4h incubation at 37 °C, labeled protein was observed in chromatin by western blot, in a manner dependent on treatment with both peptide and IntNtr-eSrt2A (Fig. 5f). Isolation and analysis of the product by mass spectrometry verified the identity of the N-TAIL reaction product (Fig. 5g and S13).
Fig. 5. Application of TAIL to protein modification in increasingly complex environments.
a, The N-TAIL process was used to attach a synthetic biotinylated peptide to histone H3 in different contexts; the isolated protein (left), the protein in a nucleosome complex (middle), and the protein incorporated into native cellular chromatin (right). These three contexts vary in their accessibility to classical semisynthetic methods and their molecular complexity. b, Purified IntC-H3 was reacted with a biotinylated peptide (Cargo-IntNov – see legend to Fig. 4D) in the presence of IntNtr-eSrt2A. The reaction progress was monitored by SDS-PAGE with coomassie staining. c, ESI-TOF MS analysis of the semisynthetic product from N-TAIL on isolated histone H3. d, Mononucleosomes containing IntC-H3 were reacted with cargo-IntNov in the presence of IntNtr-eSrt2A. Top, the N-TAIL reaction was monitored by SYBR gold stained native PAGE and, bottom, subsequent western blot analysis of nucleosomal histone H3 using anti-FLAG antibodies (red; starting material) and streptavidin (green; product). e, ESI-TOF MS analysis of the product from N-TAIL on nucleosomal histone H3. f, N-TAIL reaction in nuclei isolated from 293T cells transfected with Flag-IntC-H3. Nuclei were incubated in the presence or absence of IntNtr-eSrt2A and cargo-IntNov for 4 h, and the chromatin fraction then analyzed by western blot, as per panel D. g, Characterization of the reaction product from the in nucleo N-TAIL reaction by MS/MS. Top, annotated peptide sequence indicating the detected bn and yn ions in the MS/MS spectrum shown below. Importantly, this peptide spans the N-TAIL ligation junction confirming the region-specificity of the reaction in nucleo.
Chemoenzymatic methods for protein engineering hold great promise for the precise generation of complex modified proteins. By combining the amide-making and -breaking activities of both transpeptidase enzymes and split inteins into a single manifold, we have been able to generate modified semisynthetic proteins from short, easily accessible peptides, and recombinant fusion proteins at low concentrations. This transformation avoids challenges associated with the synthesis and manipulation of peptide thioesters used in NCL/EPL and even proceeds in the complex environment of a cell nucleus, demonstrating excellent orthogonality to cellular conditions. Like NCL/EPL, the current version of TAIL leaves a Cys residue at the ligation junction, which may be incompatible with certain proteins. Furthermore, the kinetics of the reaction are influenced by the residues surrounding the splice junction. However, we anticipate that ongoing advances in the discovery and engineering of both inteins27,37,38 and transpeptidases26,39–41 will permit further expansion and refinement of the TAIL method, in the process alleviating the constraints associated with the current version of the method. Specifically, ultrafast split inteins utilizing serine nucleophilies38 could also be adapted to enable cysteine free N- and C- TAIL ligations. Additionally, mutating sequences that are known to affect protein splicing offers a means to engineer more promiscuous inteins for TAIL27. Taken together, the N- and C-TAIL ligation strategies should serve as a simple and productive route to generate synthetically modified proteins that have previously been out of reach.
Methods
Detailed experimental methods, synthetic procedures and characterization of the compounds are described in the Supplementary Information.
Supplementary Material
Acknowledgments
We thank current members of the Muir laboratory (in particular G. Liszczak, M. Haugbro, A. Burton and J. Gramespacher) for discussions and comments. We also thank Tharan Srikumar of the Princeton University Mass Spectrometry Facility. This work was supported by National Institutes of Health (NIH) Grants R37 GM086868 and P01 CA196539. R.E.T. was supported by a Charles H. Revson Foundation postdoctoral fellowship and A.J.S. by a National Science Foundation graduate research fellowship (DGE-1148900).
Footnotes
Competing interests: Authors declare no competing interests.
Materials & Correspondence: Plasmids for the following vectors have been deposited, along with maps and sequences, in Addgene: pET30-His6-Sumo-IntNtr-eSrt2A, Addgene plasmid #126015; pET30-His6-Sumo-MBP-CfaN, Addgene plasmid #126016; pET30-MBP-CfaN-His6, Addgene plasmid #126017; pET30-His6-Sumo-CfaC-MBP, Addgene plasmid #126018; pTXB1-His6-IntCtr-GyrA-His6, Addgene plasmid #126019. All other plasmids reported in this manuscript will be available upon request. All correspondence and material requests should be addressed to T.W.M.
References
- 1.Boutureira O & Bernardes GJL Advances in chemical protein modification. Chem Rev 115, 2174–2195 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Debelouchina GT & Muir TW A molecular engineering toolbox for the structural biologist. Q. Rev. Biophys. 50, 1–41 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang K & Chin JW Cellular Incorporation of Unnatural Amino Acids and Bioorthogonal Labeling of Proteins. Chem. Rev. 114, 4764–4806, (2014). [DOI] [PubMed] [Google Scholar]
- 4.Wang L & Schultz PG Expanding the genetic code. Angew. Chem.-Int. Edit. 44, 34–66, (2005). [DOI] [PubMed] [Google Scholar]
- 5.Spicer CD & Davis BG Selective chemical protein modification. Nat. Commun. 5, 14, (2014). [DOI] [PubMed] [Google Scholar]
- 6.Tarrant MK & Cole PA The chemical biology of protein phosphorylation. Annu. Rev. Biochem. 78, 797–825 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis L & Chin JW Designer proteins: applications of genetic code expansion in cell biology. Nat. Rev. Mol. Cell Biol. 13, 168–182 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Muller MM & Muir TW Histones: At the Crossroads of Peptide and Protein Chemistry. Chem. Rev. 115, 2296–2349 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muir TW Semisynthesis of proteins by expressed protein ligation. Annu. Rev. Biochem. 72, 249–289 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Hackenberger CPR & Schwarzer D Chemoselective Ligation and Modification Strategies for Peptides and Proteins. Angew. Chem.-Int. Edit. 47, 10030–10074 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Dawson PE, Muir TW, Clark-Lewis I & Kent SBH Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Muir TW, Sondhi D & Cole PA Expressed protein ligation: A general method for protein engineering. Proc. Natl. Acad. Sci. U. S. A. 95, 6705–6710 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henager SH et al. Enzyme-catalyzed expressed protein ligation. Nat. Methods 13, 925–927 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson DY et al. A designed peptide ligase for total synthesis of ribonuclease A with unnatural catalytic residues. Science 266, 243–247 (1994). [DOI] [PubMed] [Google Scholar]
- 15.Nguyen GKT et al. Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synthesis. Nat. Chem. Biol. 10, 732–738 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Popp MW, Antos JM, Grotenbreg GM, Spooner E & Ploegh HL Sortagging: a versatile method for protein labeling. Nat. Chem. Biol. 3, 707–708 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Weeks AM & Wells JA Engineering peptide ligase specificity by proteomic identification of ligation sites. Nat. Chem. Biol. 14, 50–57 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang RL et al. Engineering a Catalytically Efficient Recombinant Protein Ligase. J. Am. Chem. Soc. 139, 5351–5358 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M, Toplak A, Quaedflieg P & Nuijens T Enzyme-mediated ligation technologies for peptides and proteins. Curr. Opin. Chem. Biol. 38, 1–7 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Kent S Chemical protein synthesis: Inventing synthetic methods to decipher how proteins work. Bioorg. Med. Chem. 25, 4926–4937 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Bondalapati S, Jbara M & Brik A Expanding the chemical toolbox for the synthesis of large and uniquely modified proteins. Nat. Chem. 8, 407–418 (2016). [DOI] [PubMed] [Google Scholar]
- 22.David Y, Vila-Perello M, Verma S & Muir TW Chemical tagging and customizing of cellular chromatin states using ultrafast trans-splicing inteins. Nat. Chem. 7, 394–402 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mootz HD Split inteins as versatile tools for protein semisynthesis. ChemBioChem 10, 2579–2589 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Shah NH & Muir TW Inteins: nature’s gift to protein chemists. Chem. Sci. 5, 446–461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens AJ et al. Design of a split intein with exceptional protein splicing activity. J. Am. Chem. Soc. 138, 2162–2165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorr BM, Ham HO, An CH, Chaikof EL & Liu DR Reprogramming the specificity of sortase enzymes. Proc. Natl. Acad. Sci. U. S. A. 111, 13343–13348 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens AJ et al. A promiscuous split intein with expanded protein engineering applications. Proc. Natl. Acad. Sci. U. S. A. 114, 8538–8543 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brocchieri L & Karlin S Protein length in eukaryotic and prokaryotic proteomes. Nucleic Acids Res. 33, 3390–3400 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harshman SW, Young NL, Parthun MR & Freitas MA H1 histones: current perspectives and challenges. Nucleic Acids Res. 41, 9593–9609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan Q & Danishefsky SJ Free-radical-based, specific desulfurization of cysteine: A powerful advance in the synthesis of polypeptides and glycopolypeptides. Angew. Chem.-Int. Edit. 46, 9248–9252 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Guy J, Cheval H, Selfridge, & J. Bird A The role of MeCP2 in the brain. Annu. Rev. Cell Dev. Biol. 27, 631–652 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Amir RE et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genet. 23, 185–188 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Bellini E et al. MeCP2 post-translational modifications: a mechanism to control its involvement in synaptic plasticity and homeostasis? Front. Cell. Neurosci. 8, 15 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen WG et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302, 885–889 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Pickart CM Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Liszczak GP et al. , Genomic targeting of epigenetic probes using a chemically tailored Cas9 system. Proc. Natl. Acad. Sci. U. S. A. 114, 681–686 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lockless SW & Muir TW Traceless protein splicing utilizing evolved split inteins. Proc. Natl. Acad. Sci. U. S. A. 106, 10999–11004 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carvajal-Vallejos P, Pallissé R, Mootz HD, Schmidt SR Unprecedented rates and efficiencies revealed for new natural split inteins from metagenomic sources. J. Biol. Chem. 287, 28686–28696 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen GK et al. Butelase 1: A Versatile Ligase for Peptide and Protein Macrocyclization. J. Am. Chem. Soc. 137, 15398–15401 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Nikghalb KD et al. Expanding the Scope of Sortase-Mediated Ligations by Using Sortase Homologues. ChemBioChem 19, 185–195 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Schmohl L, Bierlmeier J, Gerth F, Freund C & Schwarzer D Engineering sortase A by screening a second-generation library using phage display. J. Pept. Sci. 23, 631–635 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.