Abstract
Patient: Female, 53
Final Diagnosis: Muscular mucormycosis
Symptoms: Arm pain • leg pain • swelling
Medication: —
Clinical Procedure: —
Specialty: Infectious Diseases
Objective:
Unusual clinical course
Background:
Mucormycosis is a serious, potentially fatal fungal infection caused by species in the Mucorales order. Together with candidiasis and aspergillosis, it is one of the most significant fungal infection that carries a high rate of mortality. Early detection and initiation of antifungal therapy with adequate surgical debridement improves the clinical outcome.
Case Report:
We describe a case of mucormycosis in a patient with acute myeloid leukemia who developed disseminated lung disease with muscular involvement without any cutaneous manifestation. Successful treatment was achieved with surgical debridement, amphotericin B lipid-complex and posaconazole step-down therapy.
Conclusions:
Mucormycosis can present in various clinical scenarios. Key to diagnosis depends on tissues diagnosis from the affected system, as was done with lung and muscle biopsy in our patient. Clinicians should maintain high suspicion for early diagnosis and prompt treatment.
MeSH Keywords: Antifungal Agents; Cunninghamella; Lung Diseases, Fungal; Mucormycosis; Myositis; Rhizomucor
Background
Mucormycosis is a potentially fatal fungal infection occurring in patients with well-established predisposing factors, but is often misdiagnosed. We report a case of disseminated mucormycosis in the lungs with muscle involvement without any cutaneous findings. Isolated muscle involvement is a rare form of the disease. Biopsy is the preferred method of diagnosis but may not be an option in the early course of the disease, resulting in a delay in the diagnosis and increased mortality [1]. Awareness of this rare entity leads to early diagnosis and better outcomes
Case Report
A 53-year-old female with no significant past medical history presented with shortness of breath and lightheadedness and was found to have severe pancytopenia on her initial workup. She subsequently underwent a bone marrow biopsy leading to the diagnosis of acute myeloid leukemia (AML). A Hickman catheter was placed, and the patient was started on 7 days of induction chemotherapy with cytarabine and idarubicin.
On day 10 of admission, the patient developed a neutropenic fever. Due to penicillin allergy, she was started on vancomycin and aztreonam. Neutropenic fever workup was unrevealing, including blood cultures, beta-D-glucan and Aspergillus galactomannan antigen levels. Patient continued to spike high-grade temperatures despite broad spectrum antibiotics. Voriconazole was added and aztreonam was changed to imipenem. On day 13, the patient started to complain of pain in the right lower extremity with calf tenderness. A venous doppler was done and was negative for deep vein thrombosis. The patient also had computed tomography (CT) scan of the chest which, showed 2.1×2.5 cm spiculated mass in left posterior lung lobe and 2.2×1.4 cm mass in right upper lobe (Figure 1). QuantiFERON was ordered and was negative. On day 15, she underwent lung biopsy of the left lung mass. The pathology report was consistent with angio-invasive aspergillosis by morphology (Figure 2). Due to initial presumed diagnosis of aspergillosis, the patient was continued on voriconazole at 200 mg every 12 hours. The patient continued to spike fevers and started to complain of pain and swelling in the right upper and lower extremities. A venous Doppler revealed partial thrombosis of right internal jugular vein. The patient was placed on enoxaparin; however, this was discontinued due to thrombocytopenia. The patient also underwent ultrasound for her other extremities that showed edema without any evidence of fluid collections.
Figure 1.
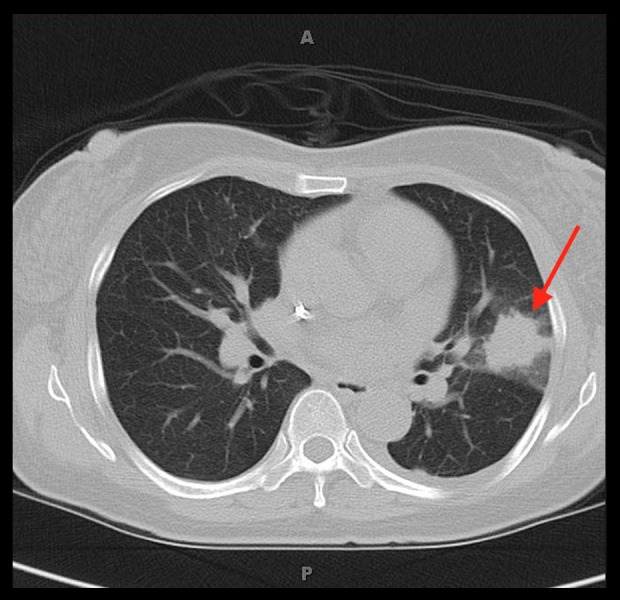
Computed tomography scan of the chest showed 2.1×2.5 cm spiculated mass in left posterior lung lobe and 2.2×1.4 cm mass in right upper lobe.
Figure 2.
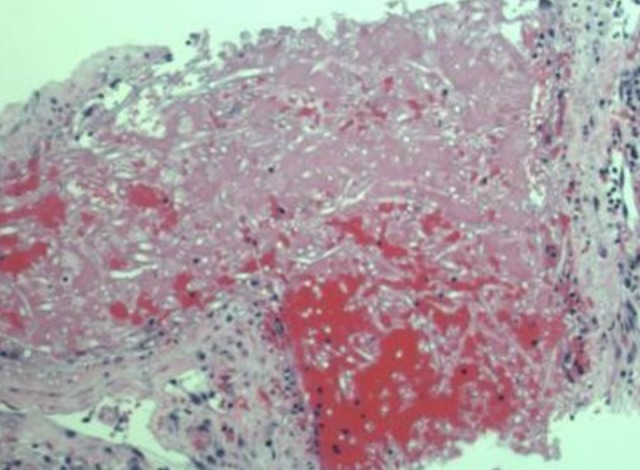
Lung biopsy of spiculated mass showing angio-invasive species.
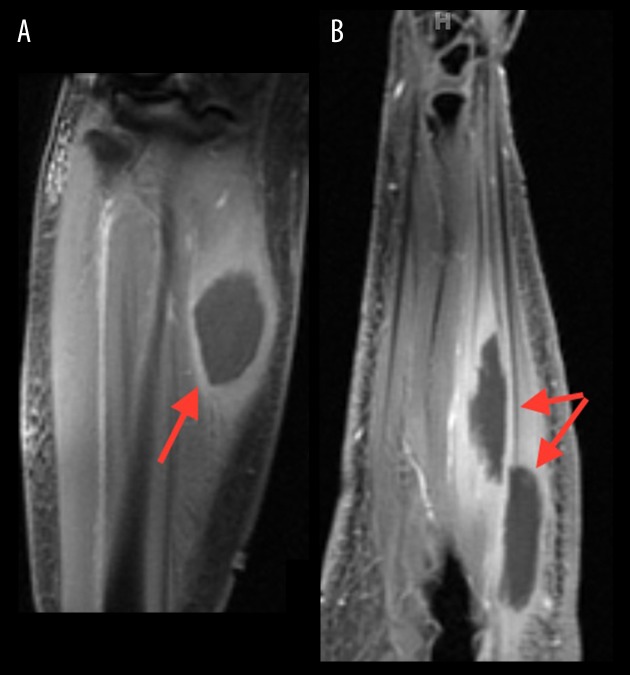
On Day 20 of admission, the patient continued to have increased right forearm swelling and pain; magnetic resonance imaging (MRI) was done which showed a 2.5×1.4×5.7 cm mass within the flexor carpi ulnaris and a 2.7×1.6×5.8 cm mass within the flexor digitorum profundus, with surrounding edema (Figure 3). On Day 24, MRI was also done for the right lower extremity, which showed 2.4×3.2×5.4 cm mass in the anteromedial aspect of the medial gastrocnemius muscle belly at the level of the proximal shaft of the tibia with surrounding edema (Figure 3).
Figure 3.
(A) 2.4×3.2×5.4 cm mass in anteromedial aspect of the medial gastrocnemius muscle belly at the level of the proximal shaft of the tibia with surrounding edema. (B) 2.5×1.4×5.7 cm mass within the flexor carpi ulnaris and 2.7×1.6×5.8 cm mass within the flexor digitorum profundus, with surrounding edema (right).
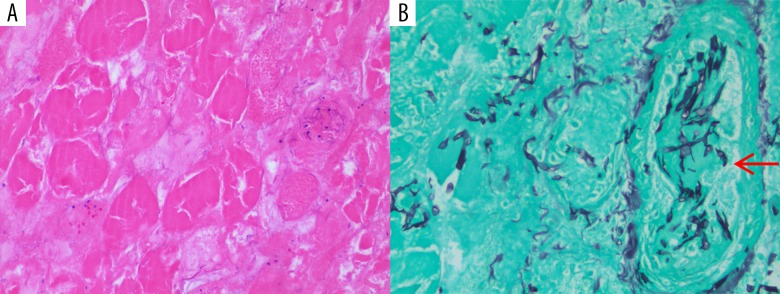
On Day 29, the patient developed worsening of edema of the right upper extremity with paresthesia. A surgical consult was placed emergently for a concern for compartment syndrome. Surgical debridement of the forearm showed myonecrosis, and clindamycin was added to the treatment regimen. Muscle biopsies were consistent with muscle necrosis. Routine cultures were negative. At the request of the infectious disease consultant, the pathology of muscle biopsy was re-reviewed and special stains were performed. Gomori methenamine-silver nitrate (GMS) stain showed broad wide-angle branching hyphae, morphologically consistent with mucormycosis (Figure 4). Voriconazole was changed to amphotericin B lipid complex. Cunninghamella bertholletiae, a Mucorales species, was later identified via 16S sequencing. The initial lung biopsy was re-examined and confirmed the diagnosis of mucormycosis. The patient’s persistent fevers resolved within 24 hours after starting amphotericin B lipid complex.
Figure 4.
(A) Skeletal muscle tissue debridement (hematoxylin and eosin stain, 200×) showing skeletal muscle necrosis with loss of nuclei and striation. (B) Angio-invasive (arrow pointing to a necrotic vessel) fungal organisms consistent with mucormycosis (Gomori methenamine-silver nitrate stain, 400×) showing broad width and wide angle branching fungal hyphae.
The rest of the hospital course was significant for a second surgical debridement with wound vacuum-assisted closure of the right upper and lower extremities. Repeat bone marrow biopsy showed acellular, chemo-ablated marrow. This was followed by filgrastim injections leading to the resolution of the neutropenia. The patient developed a left sided pleural effusion that was consistent with an exudative effusion per Light’s criteria; however, on further analysis, cytology and cultures were unremarkable. The patient was eventually switched to oral posaconazole and discharged to rehabilitation facility.
The patient’s AML remained in remission following discharge. Serial CT scans of the chest showed complete resolution of the lung masses approximately 3 years following the initial biopsy. The patient was maintained on posaconazole for 3 years until complete resolution was seen on the latest CT scan.
Discussion
Mucormycosis is a serious, potentially fatal fungal infection caused by species in the Mucorales order [1,2]. It is one of the most important causes of invasive fungal infections alongside candidiasis, aspergillosis, and cryptococcosis [2,3]. The genera Rhizopus, Rhizomucor, LichtheimiaI, and Mucor are responsible for the majority of the cases of invasive mucormycosis while other genera including Cunninghamella, Apophysomyces, Saksenaea, Cokeromyces, Actinomucor, and Syncephalastrum are encountered in less than 5% of cases [2,4]. Mucorales organisms are ubiquitous in nature, rapidly growing, and usually identified by wide non-septate hyphae branching at nearly 90° angles [1,4]. They are notorious vasotropic and angio-invasive organisms causing extensive tissue infarction and local invasion and destruction of multiple anatomical structures [5,6].
Mucormycosis is rare, with an incidence of 1.7 per million per year in the United States with higher predilection for males [7,8]. It is 42-fold less frequent than invasive candidiasis and 7.2-fold less frequent than aspergillosis [7]. The prevalence of the disease appears to differ between developed and developing countries [5]. Those with mucormycosis in developed countries tend be diabetic and have hematological malignancy, while those in developing countries have diabetes or trauma [1,2,5,8]. Other factors include solid tumors, solid organ transplant, iron overload, deferoxamine chelation therapy, trauma, burns, or iatrogenic inoculations [1,4,7,8]. These ubiquitous fungi are found in the soil and decaying organic matter and have an optimal growth temperature of 28°C to 30°C, which might explain the seasonal variation and the higher incidence in hematology patients between the months of August and November [9–11]. Given the widespread usage of voriconazole, aspergillosis infections in oncology patients has diminished. However, Mucorales is usually resistant to voriconazole, which might explain why mucormycosis in hematology units in increasing [12,13].
The organism is acquired through inhalation of spores, ingestion, or direct percutaneous inoculation [2]. The host’s mononuclear and polymorphonuclear phagocytes eliminate Mucorales via the generation of oxidative metabolite and cationic peptides defensins [9]. Hyperglycemia and ketoacidosis impair the function of phagocytes, resulting in an invasive fungal infection [12]. In patients with AML, neutrophil dysfunction leads to lack of production of proper immune mediators that play a vital role in fighting mucormycosis [14]. Treatment of AML with myeloablative chemotherapy and corticosteroid therapy causes profound and long-lasting neutropenia, also leaving the body susceptible to fungemia [14]. The incidence of mucormycosis in patients with AML is approximately 2%, with pulmonary involvement being the most common presentation [11,15].
Mucormycosis has a wide range of clinical presentations depending on the involved anatomical location typically classified into rhino-cerebral, pulmonary, cutaneous, gastrointestinal, disseminated, and other forms of mucormycosis [4]. Retrospective analysis of 929 cases of mucormycosis by Roden et al. showed sinus disease in 39% of cases, pulmonary disease in 24%, of cases, cutaneous disease in 19% of cases, and disseminated mucormycosis in 23% of cases [8]. Rhino-cerebral disease was found to be the most common disease and was encountered more in diabetic patients [9]. Sinus involvement typically presents with symptoms of sinusitis or periorbital cellulitis [9]. Progression can lead to the involvement of deeper structures such as the cavernous sinus and internal carotid artery, causing cavernous sinus thrombosis and central nervous system infections [12,16]. Vision loss, ophthalmoplegia, and cranial nerve involvement carry grave prognosis [12]. Pulmonary infection occurs after inhalation of spores or secondary to hematogenous or lymphatic spread [5,8,12]. Symptoms include cough, fevers, dyspnea, chest pain, and hemoptysis when there is vascular involvement [5,9].
Patient with cutaneous mucormycosis commonly have no underlying conditions, but rather suffered trauma, burns, or falls [8]. Cutaneous mucormycosis at insulin injection sites in diabetic patients and around central venous catheters in immunocompromised patients have also been reported [17,18]. A typical, primary cutaneous infection presents with erythematous to purple-colored papules with induration that become necrotic and forms into eschars that can ultimately extend into deeper structures to involve muscles, tendons, and bone [8,18–20]. In patients with cutaneous involvement, 24% of cases reported deep extensive into bone, tendon, or muscle [8].
Gastrointestinal mucormycosis is a rare form of the disease and mainly occurs in extremely malnourished infants and children and presents usually with abdominal pain, distension, nausea, and vomiting from fungal enterocolitis [5,12]. This can lead to bowel necrosis and ultimately perforation [21]. The disseminated form of the disease can originate from any site of infection, but pulmonary mucormycosis in neutropenic patients has the highest incidence of dissemination [8]. Metastatic lesions can involve any organ, but cerebral metastatic lesions are mostly commonly encountered [5,12]. Cases of endocarditis and pyelonephritis have also been described [22–24].
Our case represents a dissemination of pulmonary mucormycosis that was initially misdiagnosed on the initial biopsy as aspergillus. The antifungal chosen was voriconazole that has excellent aspergillus activity but does not treat mucormycosis. The patient did not have any significant cutaneous findings, other than edema and mild erythema, however, this was likely due to underlying myositis. Muscle involvement is usually due to local invasion of cutaneous infection, however, in our case it was likely hematogenously spread. [12]. Another case report described similar findings involving a single isolated muscle in a 9-year-old child with acute leukemia without any evidence of trauma or local inoculation [24]. A case reported by Patel et al. described an immunocompromised patient being treated for acute blast phase of chronic myelogenous leukemia. The patient developed ecchymotic papules with distinct violaceous borders and progressed to cellulitis and myositis [20]. This patient underwent debridement, however, extensive involvement of forearm muscles lead to an above elbow amputation [20]. In contrast, a case reported by Desai et al. described an immunocompetent patient with an initial thigh bacterial infection, who was readmitted for Mucorales myositis infection 2 weeks after incision and drainage [25]. Presumed pathophysiology in this case was due surgical inoculation [25].
Given the fact invasive aspergillosis is more common, clinicians rarely suspect mucormycosis and presume the diagnosis of aspergillosis, especially in the absence of typical disease presentation such as rhino-cerebral or cutaneous involvement [1,2]. Distinguishing mucormycosis from aspergillosis early in the course of the disease is of great importance and allows the delivery of the appropriate antifungal agent. and reduces mortality [4,26]. This is because most of the antimicrobial agents used to treat invasive aspergillosis have no activity against mucormycosis [26].
The preferred method of diagnosis is biopsy, but this is usually limited in most of the cases especially during the early stages of the disease [1]. Imaging with CT scans and MRI can help identifying infected foci and guide biopsy [1,4,9]. In other diagnostic options, such as blood cultures, sputum cultures, and bronchoalveolar lavage without a transbronchial biopsy, the circulating antigens like galactomannan are low yield in most cases [1,2,27]. Quantitative polymerase chain reaction (qPCR) assay is gaining more interest for its use in the early detection of mucormycosis [4,28,29]. A retrospective study by Millon et al. showed at least 81% sensitivity of detecting Mucorales DNA with qPCR [4]. Aggressive diagnostic strategy using CT scan, biopsy with the utilization of Calcofluor-white staining with surrogate markers like PCR and galactomannan enzyme immunoassay (EIA) might result in higher rate of early detection [30].
The mainstay of therapy includes the correction of the predisposing factors, surgical debridement, and the use of appropriate antifungal agents [2]. Combined surgical intervention and antifungal medical therapy is superior to medical therapy alone and is associated with lower mortality rates [9,27]. This is possibly due to the fact that Mucorales are extremely angioinvasive, causing thrombosis and infarction of target tissue, impairing the delivery of the antifungal agent even when the disease-causing strain is susceptible [12]. The choices for systemic antifungal agents are limited and most of the treatment recommendations are derived from retrospective studies and studies on murine models [12,31,32]. These data suggest superiority of amphotericin B when compared to other antifungal agents [12,31]. Further analysis showed higher rates of survival when using the liposomal form [12,31,32]. Another study showed that central nervous system (CNS) penetration was highest in the liposomal form and lowest in the lipid-complex form [31].
Posaconazole is a promising alternative and currently used as a salvage therapy for refractory mucormycosis or as step-down therapy [12,33]. Multiple retrospective studies evaluated the utilization of posaconazole as salvage therapy in patients with refractory mucormycosis or intolerance to amphotericin B and showed a favorable response in around 60% of patients [34–36]. A new azole, isavuconazole, is the only approved oral antifungal for the treatment of mucormycosis [33,37]. Two case reports describe refractory mucormycosis to traditional salvage therapy with posaconazole that had a good response to isavuconazole [37–39]. In our case, we describe a good response to the amphotericin B lipid complex as well as to posaconazole as a step-down therapy. Based on the available data so far, the use of the liposomal form of amphotericin B empirically and the utilization of posaconazole or isavuconazole as salvage therapy or step-down therapy is appropriate.
The overall survival rate is variable due the fact that different forms of the disease carry different prognosis, but it appears to be at least 50% [12]. Survival rates are higher in patients with rhino-cerebral mucormycosis compared to pulmonary and disseminated mucormycosis. This is likely due to the fact that these are easier to recognize and are associated with earlier onset of antifungal therapy and surgical intervention [12].
Conclusions
Mucormycosis is a rare but often fatal fungal infection affecting patient with well-established predisposing factors. The presentation can be nonspecific. Often, the diagnosis of mucormycosis is delayed as other more common invasive fungal infections such as aspergillosis are assumed. Correct diagnosis depends on expert pathologic review of tissue biopsies in order to differentiate mucormycosis from other invasive molds. Maintaining high index of suspicion, early detection, delivery of antifungal therapy and surgical debridement are associated with overall better outcomes with this disease.
Footnotes
Conflict of interest
None.
References:
- 1.Prabhu M, Patel R. Mucormycosis and entomophthoramycosis: A review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect. 2004;10(Suppl. 1):31–47. doi: 10.1111/j.1470-9465.2004.00843.x. [DOI] [PubMed] [Google Scholar]
- 2.Bouza E, Munoz P, Guinea J. Mucormycosis: An emerging disease? Clin Microbiol Infect. 2006;12:7–23. [Google Scholar]
- 3.Pfaller MA, Pappas P, Wingard JR. Invasive fungal pathogens: Current epidemiological trends. Clin Infect Dis. 2006;43:S3–14. [Google Scholar]
- 4.Millon L, Herbecht R, Grenouillet F, et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: Retrospective analysis of 44 cases collected through the French surveillance network of invasive fungal infections. Clin Microbiol Infect. 2016;22(9):810.e1–e8. doi: 10.1016/j.cmi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(Suppl. 1):S23–34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 6.Kauffman CA. Fungal infections. Proc Am Thorac Soc. 2006;3(1):35–40. doi: 10.1513/pats.200510-110JH. [DOI] [PubMed] [Google Scholar]
- 7.Rees JR, Pinner RW, Hajjeh RA, et al. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992–1993: Results of population-based laboratory active surveillance. Clin Infect Dis. 1998;27:1138–47. [PubMed] [Google Scholar]
- 8.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 9.Wang X-M, Guo L-C, Xue S-L, et al. Pulmonary mucormycosis: A case report and review of the literature. Oncol Lett. 2016;11(5):3049–53. doi: 10.3892/ol.2016.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talmi YP, Goldschmied-Reouven A, Bakon M, et al. Rhino-orbital and rhino-orbito-cerebral mucormycosis. Otolaryngol Head Neck Surg. 2002;127(1):22–31. doi: 10.1067/mhn.2002.126587. [DOI] [PubMed] [Google Scholar]
- 11.Funada H, Matsuda T. Pulmonary mucormycosis in a hematology ward. Intern Med. 1996;35(7):540–44. doi: 10.2169/internalmedicine.35.540. [DOI] [PubMed] [Google Scholar]
- 12.Spellberg B, Edward J, Jr, Ibrahim A. Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556–69. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kontoyiannis DP, Lionakis MS, Lewis RS, et al. Zygomycosis in a tertiary-care cancer center in the era of aspergillus-active antifungal therapy: A case-control observational study of 27 recent cases. J Infect Dis. 2005;191(8):1350–60. doi: 10.1086/428780. [DOI] [PubMed] [Google Scholar]
- 14.Sarvestani AS, Phisdad G, Bolandparvaz S. Epidemiology and clinical characteristics of mucormycosis in patients with leukemia; A 21-year experience from southern Iran. Bull Emerg Trauma. 2014;2(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- 15.Pak J, Tucci V, Vincent A, et al. Mucormycosis in immune-challenged patients. J Emerg Trauma Shock. 2008;1(2):106–13. doi: 10.4103/0974-2700.42203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fogarty C, Regennitter FF, Viozzi CF. Invasive fungal infection of the Maxilla following dental extractions in a patient with chronic obstructive pulmonary disease. J Can Dent Assoc. 2006;72(2):149–52. [PubMed] [Google Scholar]
- 17.Kerr OA, Bong C, Wallis C, Tidman MJ. Primary cutaneous mucormycosis masquerading as pyoderma gangrenosum. Br J Dermatol. 2004;50(6):1212–13. doi: 10.1111/j.1365-2133.2004.05826.x. [DOI] [PubMed] [Google Scholar]
- 18.Castrejón-Pérez AD, Welsh E, Miranda I, et al. Cutaneous mucormycosis. An Bras Dermatol. 2017;92(3):304–11. doi: 10.1590/abd1806-4841.20176614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonifaz A, Vázquez-González D, Tirado-Sánchez A, Ponce-Olivera RM. Cutaneous zygomycosis. Clin Dermatol. 2012;30:413–19. doi: 10.1016/j.clindermatol.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Patel V, Squires S, Shaath T, et al. Explosive cutaneous mucormycosis requiring limb amputation: Case report and literature review. J Dermatol Res Ther. 2015;1(1):005. [Google Scholar]
- 21.Termos S, Othman F, Alali M, et al. Total gastric necrosis due to mucormycosis: A rare case of gastric perforation. Am J Case Rep. 2018;19:527–33. doi: 10.12659/AJCR.908952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vesa J, Bielsa O, Arango O, et al. Massive renal infarction due to mucormycosis in an AIDS patient. Infection. 1992;20(4):234–36. doi: 10.1007/BF02033068. [DOI] [PubMed] [Google Scholar]
- 23.Virmani R, Connor DH, Mcallister HA. Cardiac mucormycosis: A report of five patients and review of 14 previously reported cases. Am J Clin Pathol. 1982;78(1):42–47. doi: 10.1093/ajcp/78.1.42. [DOI] [PubMed] [Google Scholar]
- 24.Kang CM, Kim HS, Song KS, et al. Localized muscular mucormycosis in a child with acute leukemia. Jpn J Clin Oncol. 1997;27(5):357–60. doi: 10.1093/jjco/27.5.357. [DOI] [PubMed] [Google Scholar]
- 25.Desai RP, Joseph NM, Ananthakrishnan N, Ambujam S. Subcutaneous zygomycosis caused by Mucor hiemalis in an immunocompetent patient. Australas Med J. 2013;6(7):374–77. doi: 10.4066/AMJ.2013.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamilos G, Lewis RE, Kontoyiannis D. Delaying amphotericin b-based front-line therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503–9. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 27.Tedder M, Spratt JA, Anstadt MP, et al. Pulmonary mucormycosis: Results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044–50. doi: 10.1016/0003-4975(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 28.Einsele H, Hebart H, Roller G, et al. Detection and identification of fungal pathogens in blood by using molecular probes. J Clin Microbiol. 1997;35(6):1353–60. doi: 10.1128/jcm.35.6.1353-1360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rickerts V, Loeffler J, Bohme A, et al. Diagnosis of disseminated zygomycosis using a polymerase chain reaction assay. Eur J Clin Microbiol Infect Dis. 2001;20(10):744–45. doi: 10.1007/s100960100599. [DOI] [PubMed] [Google Scholar]
- 30.Lass-Flörl C, Resch G, Nachbaur D, et al. The value of computed tomography – guided percutaneous lung biopsy for diagnosis of invasive fungal infection in immunocompromised patients. Clin Infect Dis. 2007;45(7):e101–4. doi: 10.1086/521245. [DOI] [PubMed] [Google Scholar]
- 31.Gleissner B, Schilling A, Anagnostopolous I, et al. Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma. 2004;45(7):1351–60. doi: 10.1080/10428190310001653691. [DOI] [PubMed] [Google Scholar]
- 32.Groll AH, Giri N, Petraitis V, et al. Comparative efficacy and distribution of lipid formulations of amphotericin b in experimental Candida albicans infection of the central nervous system. J Infect Dis. 2000;182(1):274–82. doi: 10.1086/315643. [DOI] [PubMed] [Google Scholar]
- 33.Riley TT, Muzny CA, Swiatlo E, Legendre DP. Breaking the mold: A review of mucormycosis and current pharmacological treatment options. Ann Pharmacother. 2016;50(9):747–57. doi: 10.1177/1060028016655425. [DOI] [PubMed] [Google Scholar]
- 34.Van Burik JA, Hare RS, Solomon HF, et al. Posaconazole is effective as salvage therapy in zygomycosis: A retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61–65. doi: 10.1086/500212. [DOI] [PubMed] [Google Scholar]
- 35.Vehreschild JJ, Birtel A, Vehreschild MJ, et al. Mucormycosis treated with posaconazole: review of 96 case reports. Crit Rev Microbiol. 2013;39(3):310–24. doi: 10.3109/1040841X.2012.711741. [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Kali Williams K. Posaconazole salvage treatment for invasive fungal infection. Mycoses. 2016;59(11):726–33. doi: 10.1111/myc.12524. [DOI] [PubMed] [Google Scholar]
- 37.Marty F, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infec Dis. 2016;16(7):828–37. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- 38.Peixoto D, Gagne LS, Hammond SP, et al. Isavuconazole treatment of a patient with disseminated mucormycosis. J Clin Microbiol. 2014;52(3):1016–19. doi: 10.1128/JCM.03176-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ervens J, Ghannoum M, Graf B, Schwartz S. Successful isavuconazole salvage therapy in a patient with invasive mucormycosis. Infection. 2014;42(2):429–32. doi: 10.1007/s15010-013-0552-6. [DOI] [PubMed] [Google Scholar]