Abstract
Metamorphosis and, in particular, holometaboly, the development of organisms through a series of discrete stages (egg, larva, pupa, adult) that hardly resemble one another but are finely adapted to specific roles in the life cycle of the organism, has fascinated and mystified humans throughout history. However, it can be difficult to visualize the dramatic changes that occur during holometaboly without destructive sampling, traditionally through histology. However, advances in imaging technologies developed mainly for medical sciences have been applied to studies of insect metamorphosis over the past couple of decades. These include micro-computed tomography, magnetic resonance imaging and optical coherence tomography. A major advantage of these techniques is that they are rapid and non-destructive, enabling virtual dissection of an organism in any plane by anyone who has access to the image files and the necessary software. They can also be applied in some cases to visualize metamorphosis in vivo, including the periods of most rapid and dramatic morphological change. This review focusses on visualizing the intra-puparial holometabolous metamorphosis of cyclorraphous flies (Diptera), including the primary model organism for all genetic investigations, Drosophila melanogaster, and the blow flies of medical, veterinary and forensic importance, but also discusses similar studies on other insect orders.
This article is part of the theme issue ‘The evolution of complete metamorphosis’.
Keywords: metamorphosis, holometaboly, intra-puparial, micro-computed tomography, magnetic resonance imaging, blow fly
1. History
The two major forms of insect metamorphosis are hemimetaboly (incomplete or partial metamorphosis) and holometaboly (complete metamorphosis) [1]. In hemimetabolous metamorphosis, the insect transforms to an adult by passing through a number of nymphal stages, shedding the exoskeleton during a moult between each stage. At each stage, the nymph increasingly resembles the final adult form, but even the least developed nymphs, which hatch from the eggs, are of the general adult form. In holometabolous metamorphosis, the changes from one stage to another are more dramatic—egg to larva to pupa to adult—and the larval stages have no physical resemblance to the adult stages, although there are changes akin to incomplete metamorphosis when one larval instar moults to the next. Therefore, holometabolous metamorphosis has long been a subject that has captured the imagination of both scientific and public realms, from the works of naturalist and artist Maria Sibylla Merian [2], including Metamorphosis Insectorum Surinamensium published in 1705 [3] (figure 1), to the present day. The mystery of metamorphosis remains a powerful stimulus in education—encouraging students to learn about scientific observation and techniques [4]—also in art, with an art/science video of blow fly metamorphosis winning the 2018 Waterhouse Natural Science Art Prize of the South Australian Museum [5]. How does an organism bound to the earth or aquatic environments, frequently tube-shaped and without obvious sensory and locomotory structures, transform into a graceful adult that can take to the air on wings? Early visualizations of metamorphosis illustrated just the different stages of development (e.g. figure 1). The highly acclaimed children's book, The Very Hungry Caterpillar [6], also focussed on the main developmental stages. While it vividly illustrates the transformation of a rather fat and ugly caterpillar into a beautiful butterfly through the pupal stage, what takes place within the pupal cocoon remains a mystery.
Figure 1.
Plate 19 from Metamorphosis Insectorium Surinamensium [3] showing a branch of guava, Psidium guineense, with complete and dissected fruit, featuring the larva, empty pupal case with cocoon and adult of the moth, Megalopyge lanata (Lepidoptera: Megalopygidae). The butterfly in flight is Thyridia psidii (Lepidoptera: Nymphalidae). The blue coloured adult fly (coloured green in the original painting) at the top of the picture is unidentified, but the larval and puparial stages of metamorphosis are depicted on the branches below it.
Visualizing hemimetabolous metamorphosis is relatively straightforward—you can witness it by eye under a standard binocular microscope. In the same way, the changes between larval instars in holometabolous metamorphosis can be visualized. Often the changes are slight: externally an increase in size and physical changes to the respiratory spiracles while, internally, there are changes to the cephalopharyngeal skeleton associated with enlarged mouthparts. These changes have been visualized in many ways, from standard line drawings, to histology slides, to highly focussed photographs composed from a stack of images using a microscope-mounted camera and suitable automontage software, to scanning electron microscopy and confocal laser microscopy (e.g. [7–9]). Where pigmented insect cuticle prevents the use of light microscopy techniques such as confocal laser microscopy, initial clearing of the cuticle to render it effectively transparent can facilitate three dimensional imaging of underlying organs [10].
By contrast, visualizing holometabolous metamorphosis from larva to adult is something that has long been a significant challenge, simply because the cuticle of the pupal stages is often opaque and one cannot see the events that occur within without destructive opening of the outer layers. Indeed Ammonite Films uploaded a fine video of blow fly metamorphosis to the Web, but the details of what happens inside the puparium could not be imaged [11]. However, even by the late 1800s microscopy and histology techniques had advanced sufficiently to produce detailed illustrations of the changes that were taking place within the puparium, but only after removing the outer cuticle (figure 2a [12]).
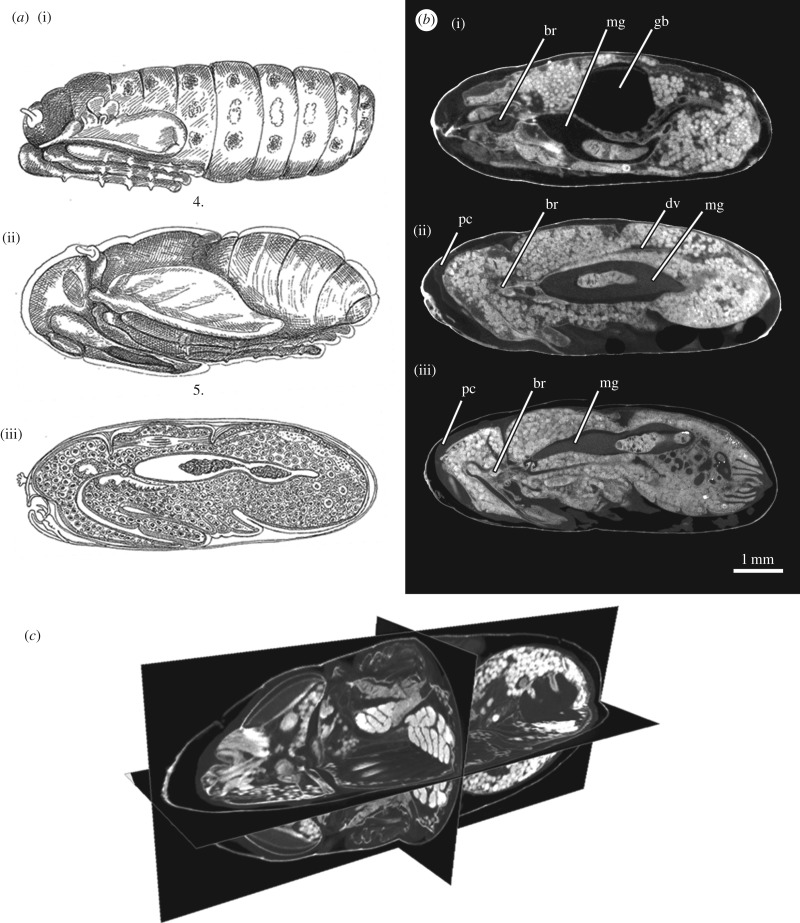
Figure 2.
(a) Figures taken from Lowne [12] showing a crypocephalic pupa (i) and phanerocephalic pupa (ii and iii). The original captions are as follows: (i) the pronymph at the end of the 3rd day, showing the position of the abdominal imaginal discs (from Plate XX, Fig. 4); (ii) the nymph on the 6th day of the pupa stage (from Plate XX, Fig. 5); (iii) a median section of the pupa on the 5th day, showing the proboscis, neuroblast, dorsal muscles, archenteron and dorsal vessel of the young nymph (from Fig. 45, p. 321). (b) Calliphora vicina (Diptera: Calliphoridae): micro-computed tomography (μCT)-based virtual medial-sagittal sections of a cryptocephalic pupa (i, equivalent to the ‘pronymph’ illustrated by Lowne [12]), a phanerocephalic pupa (ii) and a female pharate adult at 50% of the total intra-puparial development (iii, equivalent to the ‘nymph on the 5th/6th day’ illustrated by Lowne [12]). The specimens were stained in 0.5 M iodine in aqueous solution for two weeks and scanned in a Nikon Metrology HMX ST 225 system. Detailed descriptions and additional information from this dataset can be found in Martín-Vega et al. [20]. (c) μCT-based virtual sections of a pharate adult of Calliphora vicina at the end of the intra-puparial period (i.e. near emergence from the puparium), showing a cross, horizontal and sagittal section of the same specimen. The specimens were stained in 0.5 M iodine in aqueous solution for two weeks and scanned in a Nikon Metrology HMX ST 225 system. Detailed descriptions and additional information from this dataset can be found in Martín-Vega et al. [20]. br, brain; dv, dorsal vessel; gb, gas bubble; mg, adult midgut or archenteron (containing the yellow body); pc, pupal cuticle.
While studies to visualize metamorphosis have included the insect orders Lepidoptera [13], Mecoptera [14], Coleoptera [15] and Hymenoptera [16], the focus of our review is on the so-called higher Diptera [17], the monophyletic Cyclorrhapha. In these flies, metamorphosis from legless larvae to legged and winged adults takes place within the puparium, a dark case formed from the cuticle of the final larval instar [18,19]. Metamorphosis within a protective puparium also occurs in a few other insects (e.g. Strepsiptera or the non-cyclorrhaphous Diptera family Stratiomyidae) and it enables an extensive and complete histolysis of most larval tissues [20]. Cyclorrhaphous Diptera include the fruit fly, Drosophila melanogaster (Diptera: Drosophilidae), which has been studied intensely as a model for genetic systems [21] and the blow flies (Diptera: Calliphoridae), which have been studied in order to be able to age them as a tool for estimating minimum post-mortem interval in forensic investigations of death [22]. While not normally considered a part of metamorphosis, even after emergence of the adult fly from its puparium changes occur in the morphology associated with assuming the full adult coloration, cuticle hardness and configuration, e.g. inflation of the wings which are folded within the puparium—these are easily visualized by light microscopy (e.g. [23]).
2. Techniques
(a). Light microscopy
Visualization of metamorphosis within the puparium of cyclorraphous flies under a standard binocular microscope is possible for some species, most significantly Drosophila, which has a semi-transparent cuticle, the transparency of which is enhanced by wetting [24]. Indeed, Bainbridge and Bownes [24] recorded the most detailed staging of metamorphic events of any insect in their study of intra-puparial metamorphosis of Drosophila melanogaster, describing 24 convenient stages for use in experimental analysis of metamorphosis among a sequence of 51 visible changes. The changes could be observed through the cuticle, but the cuticle was removed for photography. This technique of viewing intra-puparial changes through the cuticle has been used to image and reveal much information on the hormonal and genetic control of metamorphosis and impact of mutations on Drosophila metamorphosis (e.g. [25–31]). Some features of intra-puparial metamorphosis can also be similarly visualized through the cuticle of blow flies by applying mineral oil or microscopy immersion oil to the surface of the puparium (e.g. [32,33]).
The non-destructive techniques mentioned above enable visualization of metamorphosis in real time, using living specimens. However, where this is not possible, then snap-shots of metamorphosis are possible at prescribed time intervals by opening the puparium to reveal the developing pupa or pharate adult within (e.g. [32,34]). During the 1960s–1970s, the flesh fly Sarcophaga bullata was used for a wide range of developmental studies; to aid in such studies Sivasubramanian & Biagi [35] provided a standardized description of the pupal developmental stages based on a constant temperature. Although those authors had no forensic objective in their study, similar destructive methods of visualizing metamorphic events have been used increasingly in the last decade to present data useful for ageing of blow fly puparia in forensic entomology investigations, because the intra-puparial period was hitherto the most neglected in a forensic context. These studies frequently resulted in a series of images of the intra-puparial specimens, imaged from one or more orientation (most often dorsally), to show the morphological and colour changes that accompany metamorphosis. Examples of these, with varying degrees of detail and time resolution but all focussing entirely on external morphology of the intra-puparial specimens, have provided data for: Calliphora vicina [36], Lucilia sericata [37,38], Lucilia cuprina [39], Lucilia illustris [40], Chrysomya albiceps [41,42], Chrysomya megacephala [43], Chrysomya putoria [44] and Cochliomyia macellaria [39]. Similar studies have been undertaken on the forensically relevant scuttle flies, Megaselia spiracularis and Megaselia scalaris (Diptera: Phoridae) [45,46], and on the soldier fly, Hermetia illuscens (Diptera: Stratiomyidae) [47,48]. In their study of the intra-puparial development of Calliphora vicina, Zajac & Amendt [49] included information from traditional histology techniques to supplement that from external morphology. Information from internal changes revealed by histology or non-destructive imaging techniques (see below) can complement information from external changes, because there are more changes to observe and they can occur more rapidly, enabling a finer temporal resolution.
The complex terminology of the intra-puparial period, often used incorrectly, has been recently reviewed [50]. One of the most frequent sources of confusion in the use of concepts and terminology in intra-puparial development studies is the determination of the completion of the larval-pupal apolysis (the separation of the epidermal cells of the pupa from the larval cuticle or puparium) and the pupal-adult apolysis (i.e. the separation of the adult epidermal cells from the pupal cuticle). Determining the completion of those two developmental events is essential to correctly identify the developmental stage (prepupa, pupa or pharate adult) of an individual. However, determining the completion of an apolytic event requires either traditional or virtual (see below) histological sections (figure 2) [50,51]. Traditional histology is a highly informative technique but it is also time-consuming and particularly challenging in the case of cyclorrhaphous fly puparia owing to the profusion of fat bodies and fatty droplets, often resulting in fragmented sections and loss of information [52]. This may explain the limited use of traditional histology in intra-puparial development studies in the recent years.
Sometimes rather than the entire organism, research concentrates on changes during metamorphosis of a single organ system. An example of this is a study of development of the compound eyes of the blow fly Calliphora vicina, using light microscopy and scanning electron microscopy to study surface changes and traditional histology to examine contemporaneous internal changes [53]. Other studies have examined the development of the tracheal system and how it adapts to ensure adequate air supply to the rapidly developing flight muscles [54], one of the organ systems that shows greatest increase in volume during the intra-puparial period [20,55].
(b). X-ray
Perhaps the earliest published account of the visualization of holometabolous metamorphosis within the puparium of a blow fly is that of Thévenard [56] who, decades before anyone else, used the process of kineradiography (cineradiography), recording X-ray radiographs at up to 32 frames per second so that they could be used in a movie. His paper publishes single radiographs in sequence to show some of the events within the puparium of Calliphora vicina, without a timescale but with resolution equivalent to that of the X-ray study of Hall et al. [57]. Thévenard [56] demonstrated movement of an intra-puparial gas bubble which is vital to metamorphosis during the change from the cryptocephalic (hidden head) to the phanerocephalic (visible head) pupa. In their report of the staging of metamorphosis of Drosophila, Bainbridge & Bownes [24] first described the role of this bubble in metamorphosis, but its appearance at the posterior end of the puparium as it exits the pupal abdomen was first noted by Tate [33], who described it as a first sign of the subsequent withdrawal of the dorsal tracheal trunks from the body of the pupa. The gas bubble's critical role in pupation was described and visualized in line drawings by Chadfield & Sparrow [58].
Langley & Ely [59] used single X-ray images to visualize formation within the tsetse fly, Glossina morsitans (Diptera: Glossinidae), of the gas bubble that Thévenard [56] and Tate [33] had observed in the blow fly and Drosophila, respectively. From dissections of puparia they judged that the gas bubble lay free in the haemocoel of the pre-pupa and, owing to its position close to one of the longitudinal tracheal trunks, they believed that it entered and exited the abdomen along these trachea. When the gas exited the pupa they suggested that it played an important role in separating the pupal cuticle from the third instar larval cuticle (i.e. the cuticle of the puparium).
It was not until over 60 years after Thévenard's study that essentially the same technique was used to publish videos of metamorphosis of the same blow fly species [57]. Two-dimensional time-lapse videos of the eversion of the head, during transition from the cryptocephalic to the phanerocephalic pupal stages, were made by taking radiographs at intervals of 1 min and greater using the X-ray of a micro-computed tomography (μCT) system. These videos are freely available to view in the online publication [57]. Because the specimens were living they could not be stained and therefore the contrast between tissue types was poor and the resolution low. Nevertheless, the videos revealed the major obvious changes in physical structure that took place within an interval equivalent to just 0.5% of the entire intra-puparial period (ca 10 days at 24°C) and the role of movement of the aforementioned intra-abdominal gas bubble during this rapid period of change, in agreement with the suggestions of Langley & Ely [59]. Of course, significant changes occur throughout the other 99.5% of that period, but they are less visually dramatic.
(c). Micro-computed tomography
Computed tomography (CT) is the application of X-ray imaging to produce three-dimensional images that reveal both the external and internal characters of the specimens being scanned. In essence, a series of X-rays is taken from different angles in a complete circle around one plane of the specimen, either by rotating the X-ray source, as in a standard hospital CT suite, or by rotating the specimen in front of a static X-ray source, as for most μCT scientific applications. μCT has enormous potential for studies of insect science, addressing a range of questions from taxonomy [60,61], to anatomy [62], to in vivo muscle function [63], to exploring the host-parasite interface [64]. Two clear advantages of μCT over traditional histological techniques are that μCT is less time-consuming and, also, less invasive, allowing the virtual dissection of a sample in any plane (figure 2b,c).
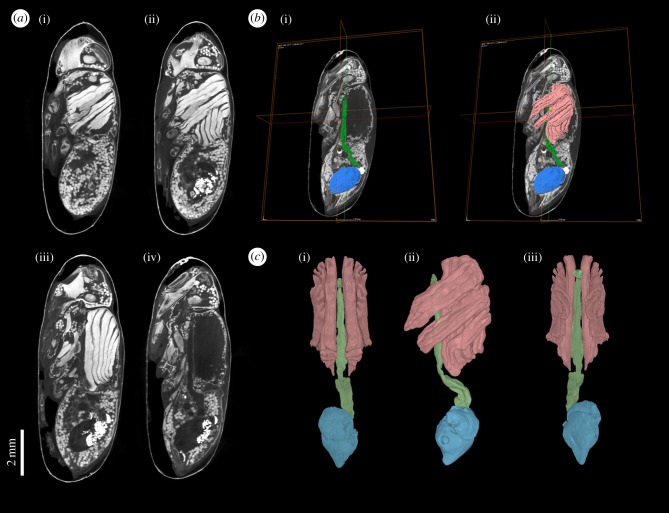
Among the first advocates of the benefits of μCT for the study of insect morphology, comparing it to a renaissance, were Friedrich and Beutel in 2008 [65]. To our knowledge, the first published µCT image of a blow fly pharate adult within the puparium was that of Metscher [66], whose study highlighted the possibility of using µCT to study metamorphosis. Soon afterwards, Richards et al. [67] published a study of the potential forensic application of this work. In the same way that virtopsy (virtual autopsy) is performed on the bodies of crime victims by CT scanning in forensic pathology studies [68,69], so virtopsy of the puparia of blow flies developing on decomposing tissues was undertaken, to establish their developmental stage for ageing, thereby providing a minimum post-mortem interval in forensic investigations [22]. These initial studies compared development at intervals equivalent to approximately 25% of the intra-puparial development interval [67]. More recent studies have refined this to just 10% intervals, equivalent to 1 day of development of Calliphora vicina at 24°C [20,55]. These studies not only tracked development qualitatively but the use of μCT enabled volumetric measurement of organ systems so that quantitative data could also be provided. Tracking the changes in volume of selected organs during metamorphosis has been applied to a range of holometabolous insect orders [13,15,16,70] and it has provided interesting insights into developmental allometries [20]. By selecting and defining a particular region of tomographic data through the process of segmentation, a label is assigned to the voxels (i.e. the smallest volume elements) included within that region (figure 3). Thus the number of voxels can be counted and, because the voxel size is set prior to scan, the volume of the segmented region can be calculated. Just like with pixels (smallest picture elements within an image), the smaller the voxel size the greater the spatial resolution of the μCT images. There is a variety of segmentation tools, from manual to automatic, and their availability changes between different visualization and analysis software packages. Published workflows (e.g. [71–73]) can help the users to choose the most appropriate software and segmentation tool depending on the data analysed.
Figure 3.
Calliphora vicina (Diptera: Calliphoridae) female pharate adult at the end of the intra-puparial period (i.e. near emergence from the puparium), stained in 0.5 M iodine in aqueous solution for two weeks and scanned in a Nikon Metrology HMX ST 225 system. Detailed descriptions and additional information from this dataset can be found in Martín-Vega et al. [20]. (a) μCT-based virtual sagittal sections from more lateral (i) to medial (iv, in which the section is between the muscle blocks, hence the apparent absence of tissue). (b) Segmentation of selected internal structures (green, pre-helicoidal portion of the adult midgut; blue, rectal pouch; pink, indirect flight muscles). (c) 3D volume renderings of the segmented structures in dorsal (i), lateral (ii) and ventral (iii) views.
All of the above μCT studies of blow flies used stained specimens to increase the contrast between different tissues. However, prior to the latest studies of blow flies, Lowe et al. [13] published a study of the metamorphic changes of living specimens of the painted lady, Vanessa cardui (Lepidoptera: Nymphalidae). These specimens were not stained, but the development of the midgut and of, in particular, the tracheal system could be followed in detail because the air inside the tracheal trunks contrasted strongly with the pupal tissues. This can hardly be achieved with other organ systems with low inherent contrast, for example the muscles and central nervous system were not resolved in the painted lady study, so the use of staining solutions is generally required. The most widely used stains are iodine and phosphotungstic acid (PTA) solutions, as both provide excellent contrast between soft tissues [66,74]. While PTA provides a sharper discrimination between adjacent tissues, iodine is much more rapid at penetrating the tissues [66]. Nonetheless, in the case of hard-cuticled insects, as happens with the puparium of cyclorrhaphous flies, piercing the cuticle to enhance the penetration of the solution may be required [20,55]. On the other hand, iodine may also overstain some mineralized tissues [66]. Hence, just like with the selection of the analysis software and tools, it is the researcher who ultimately should choose the most appropriate staining solution depending on the nature of the study material and the organ(s) or structure(s) of interest. There is certainly a strong need for further studies to develop optimized staining protocols for different types of insect samples.
(d). Other imaging methods: magnetic resonance imaging, optical coherence tomography and hyperspectral imaging
Most of the studies to visualize insect metamorphosis have used techniques described above. However, other methods are being explored that have significant future potential. These include three dimensional magnetic resonance imaging (MRI) techniques, as used to study the post-embryonic development of the flesh fly, Sarcophaga peregrina [75]. Specimens were imaged while alive without staining: the resolution was better than single X-ray images but not as good as μCT; nevertheless, good visualization of the dynamic changes that occur during gas bubble loss and head eversion in the intra-puparial development was possible. MRI has also been used to investigate the development of the alimentary tract of a Lepidopteran, Manduca sexta [76]. The success of this technique for larvae was enhanced by feeding these stages on diets containing a contrast agent (CA, gadodiomide) before imaging. If sufficiently high levels were used a significant amount of CA remained in the alimentary tract of post-feeding stages even after gut purging. Interestingly, both the silk and mandibular glands were strongly visualized without CA, suggesting that they contain high levels of a paramagnetic material. Other organ systems could be visualized in future by using tissue-specific or non-specific CA in the haemocoel. This study described the dynamic natures of changes in the morphology of the alimentary tract during transition from the fifth instar larva through to the adult for the first time, relating them to endocrinological events. Clearly, MRI is a powerful, non-invasive tool for revealing insect anatomy during development, complementing μCT.
One technique showing initial promise for imaging development in living specimens is optical coherence tomography (OCT), a method developed more recently than MRI and μCT. While many morphological changes in blow fly intra-puparial development (e.g. mouthparts, brain, bristles) were visible, unfortunately, the imaging lacked detail owing to the significant absorption of light by the tissues, especially by the puparial cuticle [77]. Penetration of lasers was limited to 1–2 mm and resolution was lost. The technique was used with more success to reveal the daily development of lepidopteran wings which lie just beneath the surface of the pupal cuticle [78], but the authors did highlight the limitations owing to scattering and absorption of light. Nevertheless, the advantages of being able to work with live specimens is clear and further developments are possible, with a bidirectional imaging modality with a single spectrometer almost doubling the penetration depths achieved with standard OCT [79]. Another optical system that suffers from lack of penetration is digital micromirror device-based LED-illumination structured illumination microscopy (LED-SIM), however, it is relatively inexpensive compared to μCT and has potential for imaging of small insects (see [80] for a detailed comparison of LED-SIM with other techniques).
A method that does not actually visualize metamorphosis yet reflects temporal developmental changes in a meaningful way is hyperspectral imaging (HIS). HIS has been applied non-destructively to blow fly puparia and a direct puparial age relationship was shown between external puparial reflectance and internal morphological development [81]. This could be further developed as a proxy for staging metamorphic events.
3. Future research applications of micro-computed tomography in studies of metamorphosis
While adults of Calliphoridae feed, indeed females of most blow flies need a protein intake in order to develop their eggs, the adults of flies in the family Oestridae do not feed [82]. They have vestigial mouthparts and so are physically unable to intake food. Therefore, they emerge from the puparia with a ‘single tank of fuel’ which is filled entirely during the larval stages. Evolutionary adaptations to this strategy include the possession of a set of fully developed eggs in females at adult emergence, so that only mating and consequent fertilization is required before they can begin to oviposit or larviposit [83]. This life strategy has some advantages over the calliphorid blow fly strategy in that egg-laying can begin sooner after emergence. The downside of a strict limit to their longevity is probably not as serious as one might think, because most calliphorids probably live for not much longer than oestrids owing to the hazards of life for a fly such as predation [84]. However, we hypothesize that for the oestrid strategy of intra-puparial reproductive development, it is more likely that the larvae need to develop to a certain critical size before they can pupariate than for calliphorids. The environment occupied by oestrids within a mammalian host, with optimal food source and relatively limited competition, would facilitate development to a critical size; certainly more so than the intensely competitive environment on carrion that calliphorids exploit. This hypothesis needs to be tested, but it is supported by the observation that if oestrid larvae are removed from the host before they ‘mature’, they often fail to pupariate [83,85]. On the other hand, calliphorids appear to be very developmentally flexible as long as they reach the third instar; small larvae produce small flies which develop full-sized viable eggs, just fewer in number than in larger flies [86,87].
If adult oestrids do not feed then it follows that they have no need for a full digestive tract, apart from the rectal pouch which is a store for metabolic waste from pupariation, from which the meconium is excreted soon after adult emergence [88]. Despite seeming to be obvious, this has not been explored in detail until recently when μCT-scanning was used to visualize the digestive tract of two species of adult oestrid, which were then compared to that of adult Calliphora vicina [89]. This study showed that adults of both Oestrus ovis (Diptera: Oestridae) and Hypoderma lineatum (Diptera: Oestridae) showed significant reduction in their digestive tracts compared to Calliphora vicina. Hence there was neither salivary gland, nor crop for food storage, the cardia and diameter of the digestive tract were significantly reduced and there was an absence of the helicoidal portion of the midgut.
The reduced state of development of the digestive tract of adult oestrid flies in comparison to that of blow flies, discussed above [89], must be owing to changes effected during intra-puparial metamorphosis, but at what stage? This is clearly an area for future study, comparable to studies of wing reduction in females of bagworm moths [90]. All of the components of the adult alimentary tract of blow flies are fully developed by the end of the intra-puparial period, before adult emergence from the puparium [20]. However, during a morphological and histological study of the intra-puparial development of Oestrus ovis it was recorded that the adult mouthparts ‘appeared transitorily, but never reached a developed appearance and then regressed during the early pharate adulthood’ [91]. The mouthparts appearance and regression followed head eversion, but no observations of changes in the gut were made. It will be fascinating to use non-invasive μCT to determine if organs of the digestive tract start to develop and then regress (as the external mouthparts appear to do), or start to develop and then stop or just never develop. Future research will track the development of the alimentary tract within the puparium of various species of Oestridae, ideally combining morphological studies with gene expression analysis. At least two studies of blow flies have tracked changes in the profiles of expressed gene during the intra-puparial period [40,92]. The digestive tract of Drosophila melanogaster represents a model system [93,94] and the genetic control of its intestinal stem cell specification and development is increasingly well understood [95] and the gene activity can be visualized [96]. This should provide a basis for what genes to look for in the development of the gut of Oestrus ovis. Another example of the benefits of combining changes in external morphology and in internal tissues with studies of gene expression in metamorphosis is provided by studies of the embryology of blow flies [97–99].
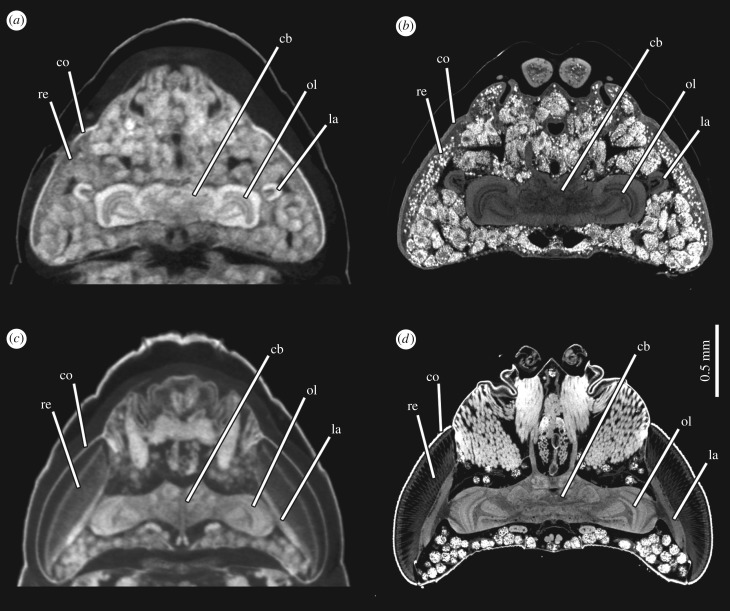
One organ system that does not undergo histolysis during intra-puparial development is the brain, although there is considerable remodelling [100]. This remodelling was imaged for the sphinx moth, Manduca sexta using confocal laser scanning microscopy [70]. However, it was not possible to image the brains in situ; it was necessary to dissect them out and, for larger brains, to image them from more than one orientation to overcome the limited working distance of the objectives. The non-destructive imaging afforded by μCT would be an advantage. Indeed, µCT has been used to image the brain of adult bumblebees, Bombus terrestris, enabling volumetric measurements of independently segmented structures [72]. Nevertheless, the advancement of resolution of μCT imaging systems continues apace and the difference in quality of the images between earlier machines and those with more recently developed modes of magnification is striking, as exemplified by images of the brain of a pharate adult of Calliphora vicina still inside its puparium taken with a Nikon Metrology HMX ST 225 system (used in [72]) and a Zeiss Versa 520 (figure 4). The higher resolution of the neuropils with the Zeiss system is achieved mainly with secondary optical magnification between the scintillator and photodiode detector (a charge coupled device (CCD) camera). Improved contrast and more defined material boundaries are also achieved by using a longer exposure time, providing a higher signal to noise ratio. This has enabled an ongoing study of intra-puparial brain development, showing changes from larva to adult. For example, the lamina, one of the neuropils within the optic lobe, is folded during the early phases of metamorphosis (resembling a horseshoe in horizontal section) but then unfolds and extends parallel to the layer of cells of the retina (figure 4). The use of high-resolution μCT scans has enabled the visualization of this and other cases of large-scale tissue remodelling in the optic lobes during metamorphosis, including the construction of three dimensional models and quantitative volumetric analyses. However, even that study will not be able to achieve the resolution of the adult brain available for Drosophila melanogaster through the online ‘virtual fly brain’, a compilation of imaging data relating to neural anatomy [101], let alone the astonishing synaptic resolution of the entire 100 000 neurons in the brain of Drosophila melanogaster revealed by volume electron microscopy [102].
Figure 4.
Remodelling of the brain and eye development during metamorphosis in Calliphora vicina (Diptera: Calliphoridae) illustrated with μCT-based virtual horizontal sections of the head. (a) Female pharate adult at 50% of the total intra-puparial period, stained in 0.5 M iodine in aqueous solution for two weeks without removing the puparium and scanned in a Nikon Metrology HMX ST 225 system. (b) Female pharate adult of the same developmental stage, stained in 1% PTA solution in 70% ethanol for 7 days after removing the puparium and scanned in a Zeiss Versa 520 system. (c) Female pharate adult at the end of the intra-puparial period (i.e. near emergence from the puparium), stained in 0.5 M iodine in aqueous solution for two weeks without removing the puparium and scanned in a Nikon Metrology HMX ST 225 system. (d) Female pharate adult of the same developmental stage, stained in 1% PTA solution in 70% ethanol for 7 days after removing the puparium and scanned in a Zeiss Versa 520 system. cb, central brain; co, cornea; la, lamina; ol, optic lobe; re, retina.
Perhaps the most exciting future studies that visualize metamorphosis will be those undertaken in vivo. The metamorphosis of Drosophila, in particular, is a process that is amenable to in vivo imaging, combined with cell biology and genetics for studies of, for example, muscle remodelling [103,104], morphological changes of the nervous system [105] and imaginal disc development [106,107].
This review is certainly not exhaustive, but it has highlighted some of the developments in visualization of insect metamorphosis through history, focussing on intra-puparial development of cyclorrhaphous Diptera. The advantages and disadvantages of various techniques are described, but a fuller treatment of these including a table of comparative resolutions is provided by Wipfler et al. [108]. The phrase ‘a picture is worth a thousand words' is overused and not always appropriate, because images and text are different types of data, not necessarily interchangeable. Nevertheless, in the case of studies of metamorphosis, pictures certainly complement, enhance and extend traditional descriptions made using the written word, especially those pictures that are generated by µCT applications. For example, our use of µCT imaging enabled clear visualization of the larval-pupal and pupal-adult apolysis in the blow fly intra-puparial period, helping to resolve confusion in the complex terminology of developmental events in metamorphosis [50]. The use of three dimensional rendering of dynamic changes enables a more complete understanding of developmental events than is possible by any other imaging technique, as they can be viewed at any angle in three dimensions and even, by moving backwards and forwards through time, in four dimensions (e.g. [5]), a technique that in vivo studies have the potential to excel at. Without question, visualization of metamorphosis will continue to play an increasingly important role in the understanding of this critical process in the evolution of insects [109].
Acknowledgements
We are grateful to Paul Cooper, John Rose and Grace Touzel of the Natural History Museum Library for their help and advice with some of the older references and to Brett Clark of the Natural History Museum Core Laboratories for advice on µCT. The identifications of insects in figure 1 follow those provided by Julie Harvey [110].
Data accessibility
This article has no additional data.
Authors' contributions
Both authors contributed equally to this review.
Competing interests
We have no competing interests.
Funding
Some of the research that stimulated this review was funded by an EC Marie Curie Intra-European fellowship award to D.M.-V. (FP7-PEOPLE-2013-IEF no: 624575) and through an award from the Mactaggart Third Fund.
References
- 1.Truman JW, Riddiford LM. 2019. The evolution of insect metamorphosis: a developmental and endocrine view. Phil. Trans. R. Soc. B 374, 20190070 ( 10.1098/rstb.2019.0070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todd K. 2007. Chrysalis: Maria Sibylla Merian and the secrets of metamorphosis, 330 pp New York, NY: Harvest, Harcourt Inc. [Google Scholar]
- 3.Merian MS. 1705. Metamorphosis insectorum Surinamensium, 60 pp. Amsterdam, The Netherlands: Gerard Valk. [Google Scholar]
- 4.Bowen A, Bell RL. 2004. Winging it – using digital imaging to investigate butterfly metamorphosis. Learn. Leading Technol. 31, 24–27. [Google Scholar]
- 5.Seccombe E. 2018. Metamorphosis. See https://www.ericaseccombe.co,/metamorphosis-1/ (accessed 29 January 2019).
- 6.Carle E. 1969. The very hungry caterpillar, 22 pp New York, NY: World Publishing Company. [Google Scholar]
- 7.Wipfler B, Schneeberg K, Löffler A, Hünefeld F, Meier R, Beutel RG. 2013. The skeletomuscular system of the larva of Drosophila melanogaster (Drosophilidae, Diptera) - a contribution to the morphology of a model organism. Arthrop. Struct. Dev. 42, 47–68. ( 10.1016/j.asd.2012.09.005) [DOI] [PubMed] [Google Scholar]
- 8.Grzywacz A, Góral T, Szpila K, Hall MJR. 2014. Confocal laser scanning microscopy as a valuable tool in Diptera larval morphology studies. Parasitol. Res. 113, 4297–4302. ( 10.1007/s00436-014-4125-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grzywacz A, Hall MJR, Pape T, Szpila K. 2017. Muscidae (Diptera) of forensic importance - an identification key to third instar larvae of the western Palaearctic region and a catalogue of the muscid carrion community. Int. J. Legal Med. 131, 855–866. ( 10.1007/s00414-016-1495-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smolla M, Ruchty M, Nagel M, Kleineidam CJ. 2014. Clearing pigmented insect cuticle to investigate small insects' organs in situ using confocal laser-scanning microscopy (CLSM). Arthrop. Struct. Dev. 43, 175–181. ( 10.1016/j.asd.2013.12.006) [DOI] [PubMed] [Google Scholar]
- 11.Ammonite Films. 2010. Maggot to fly transformation. See www.ammonite.co.uk; https://vimeo.com/42759483 (accessed 23 January 2019).
- 12.Lowne BT. 1890-92 The anatomy, physiology, morphology, and development of the blow-fly. (Calliphora erythrocephala), volume 1, ix + 350 pp London, UK: RH Porter. [Google Scholar]
- 13.Lowe T, Garwood RJ, Simonsen TJ, Bradley RS, Withers PJ. 2013. Metamorphosis revealed: time-lapse three-dimensional imaging inside a living chrysalis. J. R. Soc. Interface 10, 20130304 ( 10.1098/rsif.2013.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saltin BD, Haug C, Haug JT. 2016. How metamorphic is holometabolous development? Using microscopical methods to look inside the scorpionfly (Panorpa) pupa. Spixiana 39, 105–118. [Google Scholar]
- 15.Ras M, Iwan D, Kamiński MJ. 2018. The tracheal system in post-embryonic development of holometabolous insects: a case study using the mealworm beetle. J. Anat. 232, 997–1015. ( 10.1111/joa.12808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helm BR, Payne S, Rinehart JP, Yocum GD, Bowsher JH, Greenlee KJ. 2018. Micro-computed tomography of pupal metamorphosis in the solitary bee Megachile rotundata. Arthrop. Struct. Dev. 47, 521–528. ( 10.1016/j.asd.2018.05.001) [DOI] [PubMed] [Google Scholar]
- 17.Wiegmann BM, et al. 2011. Episodic radiations in the fly tree of life. Proc. Natl Acad. Sci. USA 108, 5690–5695. ( 10.1073/pnas.1012675108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Žďárek J, Fraenkel G. 1972. The mechanism of puparium formation in flies. J. Exp. Zool. 179, 315–324. ( 10.1002/jez.1401790304) [DOI] [Google Scholar]
- 19.Denlinger DL, Žďárek J. 1994. Metamorphosis behaviour of flies. Annu. Rev. Entomol. 39, 243–266. ( 10.1146/annurev/en.39.010194.001331) [DOI] [PubMed] [Google Scholar]
- 20.Martín-Vega D, Simonsen T, Hall MJR. 2017. Looking into the puparium: micro-CT visualization of the internal morphological changes during metamorphosis of the blow fly, Calliphora vicina, with the first quantitative analysis of organ development in cyclorraphous dipterans. J. Morphol. 278, 629–651. ( 10.1002/jmor.20660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohr SE. 2018. First in fly: Drosophila research and biological discovery, 272 pp Cambridge, MA: Harvard University Press. [Google Scholar]
- 22.Hall M, Whitaker A, Richards C. 2012. Forensic entomology, Chapter 8. In Forensic ecology handbook: from crime scene to court (eds Márquez-Grant N, Roberts J), pp. 111–140. Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 23.Cottrell CB. 1962. General observations on the imaginal ecdysis of blowflies. Trans. R. Ent. Soc. Lond. 114, 317–333. ( 10.1111/j.1365-2311.1962.tb01069.x) [DOI] [Google Scholar]
- 24.Bainbridge SP, Bownes M. 1981. Staging the metamorphosis of Drosophila melanogaster. J. Embryol. Exp. Morph. 66, 57–80. [PubMed] [Google Scholar]
- 25.Lam G, Hall BK, Bender M, Thummel CS. 1999. DHR3 is required for the prepupal-pupal transition and differentiation of adult structures during Drosophila metamorphosis. Dev. Biol. 212, 204–216. ( 10.1006/dbio.1999.9343) [DOI] [PubMed] [Google Scholar]
- 26.Lam G, Thummel CS. 2000. Inducible expression of double-stranded RNA directs specific genetic interference in Drosophila. Curr. Biol. 10, 957–963. ( 10.1016/S0960-9822(00)00631-X) [DOI] [PubMed] [Google Scholar]
- 27.Ji Y, Clark DV. 2006. The purine synthesis gene Prat2 is required for Drosophila metamorphosis as revealed by inverted-repeat-mediated RNA interference. Genetics 172, 1621–1631. ( 10.1534/genetics.105.045641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bashirullah A, Lam G, Yin VP, Thummel CS. 2007. dTrf2 is required for transcriptional and developmental responses to ecdysone during Drosophila metamorphosis. Dev. Dyn. 236, 3173–3179. ( 10.1002/dvdy.21350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rayburn LYM, Rhea J, Jocoy SR, Bender M. 2009. The proprotein convertase amontillado (amon) is required during Drosophila pupal development. Dev. Biol. 333, 48–56. ( 10.1016/j.ydbio.2009.06.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rewitz KF, Yamanaka N, O'Connor MB. 2010. Steroid hormone inactivation is required during the juvenile-adult transition in Drosophila. Dev. Cell 19, 895–902. ( 10.1016/j.devcel.2010.10.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipinszki Z, Pál M, Nagy O, Deák P, Hunyadi-Gulyas E, Udvardy A. 2011. Overexpression of Dsk2/dUbqln results in severe developmental defects and lethality in Drosophila melanogaster that can be rescued by overexpression of the p54/Rpn10/S5a proteasomal subunit. FEBS J. 278, 4833–4844. ( 10.1111/j.1742-4658.2011.08383.x) [DOI] [PubMed] [Google Scholar]
- 32.Žďárek J, Friedman S. 1986. Pupal ecdysis in flies: mechanisms of evagination of the head and expansion of the thoracic appendages. J. Insect Physiol. 32, 917–923. ( 10.1016/0022-1910(86)90139-3) [DOI] [Google Scholar]
- 33.Tate P. 1953. Prepupal moult in the blowfly (Calliphora erythrocephala). Nature 171, 341–342. ( 10.1038/171341a0) [DOI] [PubMed] [Google Scholar]
- 34.Whitten JM. 1957. The supposed pre-pupa in Cyclorrhaphous Diptera. Q. J. Micros. Sci. 98, 241–250. [Google Scholar]
- 35.Sivasubramanian P, Biagi M. 1983. Morphology of the pupal stages of the fleshfly, Sarcophaga bullata (Parker) (Diptera: Sarcophagidae). Int. J. Insect Morphol. 12, 355–359. ( 10.1016/0020-7322(83)90029-6) [DOI] [Google Scholar]
- 36.Brown K, Thorne A, Harvey M. 2015. Calliphora vicina (Diptera: Calliphoridae) pupae: a timeline of external morphological development and a new age and PMI estimation tool. Int. J. Legal Med. 129, 835–850. ( 10.1007/s00414-014-1068z) [DOI] [PubMed] [Google Scholar]
- 37.Karabey T, Sert O. 2014. The analysis of pupal development period in Lucilia sericata (Diptera: Calliphoridae) forensically important insect. Int. J. Legal Med. 132, 1185–1196. ( 10.1007/s00414-014-0968-2) [DOI] [PubMed] [Google Scholar]
- 38.Grisendi A., Defilippo F, Gatti F, Dottori M, Bonilauri P. 2015. Estimation of accumulated degree day value of six landmarks within the pupal stage of Lucilia sericata. J. Life Sci. 9, 311–317. ( 10.17265/1934-7391/2015.07.003) [DOI] [Google Scholar]
- 39.Barros-Cordeiro KB, Pujol-Luz JR, Name KPO, Báo SN. 2016. Intra-puparial development of the Cochliomyia macellaria and Lucilia cuprina (Diptera: Calliphoridae). Rev. Bras. Entomol. 60, 334–340. ( 10.1016/j.rbe.2016.06.009) [DOI] [Google Scholar]
- 40.Wang Y, Gu Z, Xia S, Wang J, Zhang Y, Tao L. 2018. Estimating the age of Lucilia illustris during the intrapuparial period using two approaches: morphological changes and differential gene expression. Forensic Sci. Int. 287, 1–11. ( 10.1016/j.forsciint.2018.02.025) [DOI] [PubMed] [Google Scholar]
- 41.Pujol-Luz JR, Barros-Cordeiro KB. 2012. Intra-puparial development of the females of Chrysomya albiceps (Wiedemann) (Diptera, Calliphoridae). Rev. Bras. Entomol. 56, 269–272. ( 10.1590/S0085-56262012005000038) [DOI] [Google Scholar]
- 42.Salazar-Souza M, Couri MS, Aguiar VM. 2018. Chronology of the intrapuparial development of the blowfly Chrysomya albiceps (Diptera: Calliphoridae): application in forensic entomology. J. Med. Entomol. 55, 825–832. ( 10.1093/jme/tjy054) [DOI] [PubMed] [Google Scholar]
- 43.Sinha SK, Mahato S.. 2018. Study on intra-puparial development of latrine fly Chrysomya megacephala (Fabricius) (Diptera, Calliphoridae). Int. J. Creative Res. Thoughts 6, IJCRT1812804. [Google Scholar]
- 44.Proença B, Ribeiro AC, Luz RT, Aguiar VM, Maia VC, Couri MS. 2014. Intrapuparial development of Chrysomya putoria (Diptera: Calliphoridae). J. Med. Entomol. 51, 908–914. ( 10.1603/MEI13205) [DOI] [PubMed] [Google Scholar]
- 45.Feng D, Liu G. 2013. Pupal age estimation of forensically important Megaselia spiracularis Schmitz (Diptera: Phoridae). Forensic Sci. Int. 231, 199–203. ( 10.1016/j.forsciint.2013.05.008) [DOI] [PubMed] [Google Scholar]
- 46.Feng D, Liu G. 2014. Pupal age estimation of forensically important Megaselia scalaris (Loew) (Diptera: Phoridae). Forensic Sci. Int. 236, 133–137. ( 10.1016/j.forsciint.2014.01.002) [DOI] [PubMed] [Google Scholar]
- 47.Barros-Cordeiro KB, Báo SN, Pujol-Luz JR. 2014. Intra-puparial development of the black soldier-fly, Hermetia illucens. J. Insect Sci. 14, 83 ( 10.1673/031.014.83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Wang Y, Wang J. 2016. Intra-puparial development and age estimation of forensically important Hermetia illucens (L.). J. Asia-Pac. Entomol. 19, 233–237. ( 10.1016/j.aspen.2016.01.006) [DOI] [Google Scholar]
- 49.Zajac BK, Amendt J. 2012. Bestimmung des Alters forensisch relevanter Fliegenpuppen – Morphologische und histologische Methoden. Rechtsmedizin 22, 456–465. ( 10.1007/s00194-012-0854-5) [DOI] [Google Scholar]
- 50.Martín-Vega D, Hall MJR, Simonsen T. 2016. Resolving confusion in the use of concepts and terminology in intra-puparial development studies of cyclorrhaphous Diptera. J. Med. Entomol. 53, 1249–1251 ( 10.1093/jme/tjw081) [DOI] [PubMed] [Google Scholar]
- 51.Fraenkel G, Bhaskaran G. 1973. Pupariation and pupation in cyclorrhaphous flies (Diptera): terminology and interpretation. Ann. Entomol. Soc. Am. 66, 418–422. ( 10.1093/aesa/66.2.418) [DOI] [Google Scholar]
- 52.Davies K, Harvey M. 2013. Internal morphological analysis for age estimation of blow fly pupae (Diptera: Calliphoridae) in postmortem interval estimation. J. Forensic Sci. 58, 79–84. ( 10.1111/j.1556-4029.2012.02196.x) [DOI] [PubMed] [Google Scholar]
- 53.Finell N, Järvilehto M. 1983. Development of the compound eyes of the blowfly Calliphora erythrocephala: changes in morphology and function during metamorphosis. Ann. Zool. Fennici 20, 223–234. [Google Scholar]
- 54.Houlihan DF, Newton JRL. 1979. The tracheal supply and muscle metabolism during muscle growth in the puparium of Calliphora vomitoria. J. Ins. Physiol. 25, 33–44. ( 10.1016/0022-1910(79)90034-9) [DOI] [Google Scholar]
- 55.Martín-Vega D, Simonsen TJ, Wickelin M, Hall MJR. 2017. Age estimation during the blow fly intra-puparial period: a qualitative and quantitative approach using micro-computed tomography. Int. J. Legal Med. 131, 1429–1448. ( 10.1007/s00414-017-1598-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thevenard P. 1955. Direct kineradiography on 35 mm film and application in biological research. Med. Biol. Eng. 5, 66–70. [PubMed] [Google Scholar]
- 57.Hall MJR, Simonsen TJ, Martín-Vega D. 2017. The ‘dance’ of life: visualizing metamorphosis during pupation in the blow fly Calliphora vicina by X-ray video imaging and micro-computed tomography. R. Soc. open sci. 4, 160699 ( 10.1098/rsos.160699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chadfield CG, Sparrow JC. 1985. Pupation in Drosophila melanogaster and the effect of the Lethalcryptocephal mutation. Dev. Genet. 5, 103–114. ( 10.1002/dvg.1020050206) [DOI] [Google Scholar]
- 59.Langley PA, Ely R. 1978. X-ray investigation of gas bubble formation and water loss in tsetse fly pupae. Physiol. Entomol. 3, 303–307. ( 10.1111/j.1365-3032.1978.tb00163.x) [DOI] [Google Scholar]
- 60.Garcia FH, et al. 2017. X-ray microtomography for ant taxonomy: an exploration and case study with two new Terataner (Hymenoptera, Formicidae, Myrmicinae) species from Madagascar. PLoS ONE 12, e0172641 ( 10.1371/journal.pone.0172641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia FH, Fischer G, Liu C, Audisio TL, Economo EP. 2017. Next-generation morphological character discovery and evaluation: an X-ray micro-CT enhanced revision of the ant genus Zasphinctus Wheeler (Hymenoptera, Formicidae, Dorylinae) in the Afrotropics. ZooKeys 693, 33–93. (doi:103897/zookeys.693.13012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galinskaya TV, Gafurova (Gilyazetdinova) D, Ovtshinnikova OG. 2018. X-ray microtomography (microCT) of male genitalia of Nothybus kuznetsovorum (Nothybidae) and Cothornbata sp. (Micropezidae). Zookeys 744, 139–147. ( 10.3897/zookeys.744.22347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker SM, et al. 2014. In vivo time-resolved microtomography reveals the mechanics of the blowfly flight motor. PLoS ONE 12, e1001823 ( 10.1371/journal.pbio.1001823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martín-Vega D, et al. 2018. 3D virtual histology at the host/parasite interface: visualisation of the master manipulator, Dicrocoelium dendriticum, in the brain of its ant host. Sci. Rep. –UK 8, 8587 ( 10.1038/s41598-018-26977-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedrich F, Beutel RG. 2008. Micro-computer tomography and a renaissance of insect morphology. In Developments in X-ray tomography VI (ed. Stock SR.). Proc. SPIE 7078: 70781U-1–6 ( 10.1117/12.794057) [DOI] [Google Scholar]
- 66.Metscher BD. 2009. MicroCT for developmental biology: a versatile tool for high-contrast 3D imaging at histological resolutions. Dev. Dyn. 238, 632–640. ( 10.1002/dvdy.21857) [DOI] [PubMed] [Google Scholar]
- 67.Richards CS, Simonsen TJ, Abel RL, Hall MJR, Schywn DA, Wicklein M. 2012. Virtual forensic entomology: improving estimates of minimum post-mortem interval with 3D micro-computed tomography. Forensic Sci. Int. 220, 251–264. ( 10.1016/j.forsciint.2012.03.012) [DOI] [PubMed] [Google Scholar]
- 68.Bolliger SA, Thali MJ. 2015. Imaging and virtual autopsy: looking back and forward. Phil. Trans. R. Soc. B 370, 20140253 ( 10.1098/rstb.2014.0253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krentz BV, Alamo L, Grimm J, Dédouit F, Bruguier C, Chevallier C, Egger C, Da Silva LFF, Grabherr S.. 2016. Performance of postmortem CT compared to autopsy in children. Int. J. Legal Med. 130, 1089–1099. ( 10.1007/s00414-016-1370-z) [DOI] [PubMed] [Google Scholar]
- 70.Huetteroth W, el Jundi B, el Jundi S, Schachtner J. 2010. 3D reconstructions and virtual 4D-visualisation to study metamorphic brain development in the sphinx moth Manduca sexta. Front. Syst. Neurosci. 4, 7 ( 10.3389/fnsys.2010.00007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abel RL, Laurini CR, Richter M. 2012. A palaeobiologist's guide to ‘‘virtual’’ micro-CT preparation. Palaeontol . Electron. 15, 1–17. ( 10.26879/284) [DOI] [Google Scholar]
- 72.Smith DB, et al. 2016. Exploring miniature insect brains using micro-CT scanning techniques. Sci. Rep. 6, 21768 ( 10.1038/srep21768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Semple TL, Peakall R, Tatarnic NJ. 2019. A comprehensive and user-friendly framework for 3D-data visualisation in invertebrates and other organisms. J. Morphol. 280, 223–231. ( 10.1002/jmor.20938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swart P, Wicklein M, Sykes D, Ahmed F, Krapp HG. 2016. A quantitative comparison of micro-CT preparations in dipteran flies. Sci. Rep. UK 6, 39380 ( 10.1038/srep39380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Price WS, Kobayashi A, Ide H, Natori S, Arata Y. 1999. Visualizing the postembryonic development of Sarcophaga peregrina (flesh fly) by NMR microscopy. Physiol. Entomol. 24, 386–390. ( 10.1046/j.1365-3032.1999.00156.x) [DOI] [Google Scholar]
- 76.Rowland IJ, Goodman WG. 2016. Magnetic resonance imaging of alimentary tract development in Manduca sexta. PLoS ONE 11, e0157124 (doi:10/1371/journal/pone.0157124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brown K, Harvey M. 2014. Optical coherence tomography: age estimation of Calliphora vicina pupae in vivo? Forensic Sci. Int. 242, 157–161. ( 10.1016/j.forsciint.2014.07.001) [DOI] [PubMed] [Google Scholar]
- 78.Kambe M, Kinoshita S, Ohmi M, Haruna M. 2008. In-vivo imaging of developing wings in butterfly pupa by using optical coherence tomography. J. Korean Phys. Soc. 53, 1290–1294. ( 10.3938/jkps.53.1290) [DOI] [Google Scholar]
- 79.Ravichandran NK, et al. 2016. Depth enhancement in spectral domain optical coherence tomography using bidirectional imaging modality with a single spectrometer. J. Biomed. Opt. 21, 076005 ( 10.1117/1.JBO.21.7.076005) [DOI] [PubMed] [Google Scholar]
- 80.Ruan Y, et al. 2016. Visualization of the 3D structures of small organisms via LED-SIM. Front. Zool. 13, 26 ( 10.1186/s12983-016-0158-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Voss SC, Magni P, Dadour I, Nansen C. 2017. Reflectance-based determination of age and species of blowfly puparia. Int. J. Legal Med. 131, 263–274. ( 10.1007/s00414-016-1458-5) [DOI] [PubMed] [Google Scholar]
- 82.Anderson JR. 2006. Adult biology. Chapter 10. In The oestrid flies. Biology, host–parasite relationships, impact and management (eds Colwell DD, Hall MJR, Scholl PJ), pp. 140–166. Wallingford, UK: CABI Publishing. [Google Scholar]
- 83.Cepeda-Palacios R, Angulo Valadez CE, Scholl PJ, Ramírez-Orduña R, Jacquiet P, Dorchies P. 2011. Ecobiology of the sheep nose bot fly (Oestrus ovis L.): a review. Revue Méd. Vét. 162, 503–507. [Google Scholar]
- 84.Pitts KM, Wall R. 2004. Adult mortality and oviposition rates in field and captive populations of the blowfly Lucilia sericata. Ecol. Entomol. 29, 727–734. ( 10.1111/j.0307-6946.2004.00653.x) [DOI] [Google Scholar]
- 85.Nilssen AC. 2006. Pupal biology and metamorphosis behaviour. Chapter 9. In The oestrid flies. Biology, host–parasite relationships, impact and management (eds Colwell DD, Hall MJR, Scholl PJ), pp. 124–139. Wallingford, UK: CABI Publishing. [Google Scholar]
- 86.Saunders DS, Bee A. 1995. Effects of larval crowding on size and fecundity of the blow fly, Calliphora vicina (Diptera: Calliphoridae). Eur. J. Entomol. 92, 615–622. [Google Scholar]
- 87.Saunders DS, Wheeler I, Kerr A. 1999. Survival and reproduction of small blow flies (Calliphora vicina; Diptera: Calliphoridae) produced in severely overcrowded short-day larval cultures. Eur. J. Entomol. 96, 19–22. [Google Scholar]
- 88.Rivers D, Geiman T. 2017. Insect artefacts are more than just altered bloodstains. Insects 8, 37 ( 10.3390/insects8020037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martín-Vega D, et al. 2018. Micro-computed tomography visualization of the vestigial alimentary canal in adult oestrid flies. Med. Vet. Entomol. 32, 378–382. ( 10.1111/mve.12301) [DOI] [PubMed] [Google Scholar]
- 90.Niitsu S, Sims I, Ishizaki T. 2011. Morphology and ontogeny of wing bud development during metamorphosis in females of the wingless bagworm moth Epichnopterix plumella (Denis & Schiffermüller, 1775) (Psychidae). Nota Lepid. 34, 103–110. [Google Scholar]
- 91.Cepeda-Palacios R, Scholl PJ. 2000. Intra-puparial development in Oestrus ovis (Diptera: Oestridae). J. Med. Entomol. 37, 239–245. ( 10.1603/0022-2585-37.2.239) [DOI] [PubMed] [Google Scholar]
- 92.Zajac BK, Amendt J, Verhoff MA, Zehner R. 2018. Dating pupae of the blow fly Calliphora vicina Robineau-Desvoidy 1830 (Diptera: Calliphoridae) for post mortem interval-estimation: validation of molecular markers. Genes-Basel 9, 153 ( 10.3390/genes9030153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lengyel JA, Iwaki DD. 2002. It takes guts: the Drosophila hindgut as a model for organogenesis. Dev. Biol. 243, 1–19. ( 10.1006/dbio.2002.0577) [DOI] [PubMed] [Google Scholar]
- 94.Lemaitre B, Miguel-Aliaga I. 2013. The digestive tract of Drosophila melanogaster. Annu. Rev. Genet. 47, 377–404. ( 10.1146/annurev-genet-111212-133343) [DOI] [PubMed] [Google Scholar]
- 95.Takashima S, Hartenstein V. 2012. Genetic control of intestinal stem cell specification and development: a comparative view. Stem Cell Rev. 8, 597–608. ( 10.1007/s12015-012-9351-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takashima S, Aghajanian P, Younossi-Hartenstein A, Hartenstein V. 2016. Origin and dynamic lineage characteristics of the developing Drosophila midgut stem cells. Dev. Biol. 416, 347–360. ( 10.1016/j.ydbio.2016.06.018) [DOI] [PubMed] [Google Scholar]
- 97.Tarone AM, Jennings KC, Foran DR. 2007. Aging blow fly eggs using gene expression: a feasibility study. J. Forensic Sci. 52, 1350–1354. ( 10.1111/j.1556-4029.2007.00587.x) [DOI] [PubMed] [Google Scholar]
- 98.Martín-Vega D, Hall MJR. 2016. Estimating the age of Calliphora vicina eggs (Diptera: Calliphoridae): determination of embryonic morphological landmarks and preservation of egg samples. Int. J. Legal Med. 130, 845–854. ( 10.1007/s00414-015-1308-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pais M, Archer MS. 2018. Histological age estimation of the eggs of Calliphora vicina Robineau Desvoidy (Diptera: Calliphoridae). Forensic Sci. Res. 3, 40–51. ( 10.1080/20961790.2017.1404707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Langen M, Agi E, Altschuler DJ, Wu LF, Altschuler SJ, Hiesinger PR. 2015. The developmental rules of neural superposition in Drosophila. Cell 162, 120–133. ( 10.1016/j.cell.2015.05.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Milyaev N, Osumi-Sutherland D, Reeve S, Burton N, Baldock RA, Armstrong JD. 2012. The virtual fly brain browser and query interface. Bioinformatics 28, 411–415. ( 10.1093/bioinformatics/btr677) [DOI] [PubMed] [Google Scholar]
- 102.Zheng Z, et al. 2018. A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell 174, 730–743. ( 10.1016/j.cell.2018.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chinta R, Tan JH, Wasser M. 2012. The study of muscle remodelling in Drosophila metamorphosis using in vivo microscopy and bioimage informatics. BMC Bioinf. 13, S14 ( 10.1186/1471-2105-13-S17-S14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuleesha Y, Puah WC, Wasser M. 2016. Live imaging of muscle histolysis in Drosophila metamorphosis. BMC Dev. Biol. 16, 12 ( 10.1186/s12861-016-0113-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun B, Xu P, Salvaterra PM. 1999. Dynamic visualisation of nervous system in live Drosophila. Proc. Natl Acad. Sci. USA 96, 10 438–10 443. ( 10.1073/pnas.96.18.10438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aldaz S, Escudero LM, Freeman M. 2010. Live imaging of Drosophila imaginal disc development. Proc. Natl Acad. Sci. USA 107, 14 217–14 222. ( 10.1073/pnas.1008623107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aldaz S, Escudero LM, Freeman M. 2013. Dual role of myosin II during Drosophila imaginal disc metamorphosis. Nat. Commun. 4, 1761 ( 10.1038/ncomms2763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wipfler B, Pohl H, Yavorskaya MI, Beutel RG. 2016. A review of methods for analysing insect structures – the role of morphology in the age of phylogenomics. Curr. Opin. Insect Sci. 18, 60–68. ( 10.1016/j.cois.2016.09.004) [DOI] [PubMed] [Google Scholar]
- 109.Cheong SPS, Huang J, Bendena WG, Tobe SS, Hui JHL. 2015. Evolution of ecdysis and metamorphosis in arthropods: the rise and regulation of juvenile hormone. Integr. Comp. Biol. 55, 878–890. ( 10.1093/icb/icv066) [DOI] [PubMed] [Google Scholar]
- 110.Harvey J. 2006. Maria Sibylla Merian, the Surinam album, commentary, 87 pp London, UK: The Folio Society. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.