Abstract
Many animals depend on microbial symbionts to provide nutrition, defence or other services. Holometabolous insects, as well as other animals that undergo metamorphosis, face unique constraints on symbiont maintenance. Microbes present in larvae encounter a radical transformation of their habitat and may also need to withstand chemical and immunological challenges. Metamorphosis also provides an opportunity, in that symbiotic associations can be decoupled over development. For example, some holometabolous insects maintain the same symbiont as larvae and adults, but house it in different tissues; in other species, larvae and adults may harbour entirely different types or numbers of microbes, in accordance with shifts in host diet or habitat. Such flexibility may provide an advantage over hemimetabolous insects, in which selection on adult-stage microbial associations may be constrained by its negative effects on immature stages, and vice versa. Additionally, metamorphosis itself can be directly influenced by symbionts. Across disparate insect taxa, microbes protect hosts from pathogen infection, supply nutrients essential for rebuilding the adult body and provide cues regulating pupation. However, microbial associations remain completely unstudied for many families and even orders of Holometabola, and future research will undoubtedly reveal more links between metamorphosis and microbiota, two widespread features of animal life.
This article is part of the theme issue ‘The evolution of complete metamorphosis’.
Keywords: development, microbiota, transmission, endosymbiont, gut microbiome, bacteria
1. Introduction
A large part of terrestrial biodiversity and biomass is made up of beetles, flies, wasps, ants, bees, butterflies, moths and other insects that undergo complete metamorphosis [1,2]. The success of holometabolous insects is thought to be driven largely by the ‘reset button’ effect of metamorphosis, which enables their larval and adult life stages to differ radically in form and function [2,3]. This process, known as adaptive decoupling, allows larvae and adults to specialize more independently for distinct tasks, such as growth versus reproduction, achieving greater efficiency at each [4]. Another prominent feature of many holometabolous insects is an association with microbial symbionts, or microbiota, which can influence numerous aspects of insect development, physiology and ecology [5–7]. How does the holometabolous lifestyle shape microbial symbioses, and vice versa?
First, metamorphosis and the accompanying divergence in physiology, morphology and ecology between larvae and adults constrain the evolution of symbionts vertically transmitted within the insect body. We hypothesize that hemimetabolous insects, whose body plan is more stable over development, may be more likely to acquire such symbionts, and less likely to use alternate transmission strategies that bypass metamorphosis. Second, holometaboly enables temporal flexibility in the structure, abundance and activity of microbiota across metamorphosis. These dynamics have a number of implications, from whether microbially mediated larval phenotypes carry over into the adult stage, to the genome evolution of symbionts passing through bottlenecks at metamorphosis. We speculate that such flexibility is a special case of adaptive decoupling and could play a role in the success of Holometabola. Third, the process of metamorphosis entails challenges which microbial symbionts could help insects to meet. In this section, we review systems in which microbes protect hosts from pathogens as they transition from larva to adult, provide nutrients needed to rebuild the adult body or serve as cues triggering the onset of metamorphosis.
As may be expected given their approximately 350 My age [8] and extreme diversity, holometabolous insects are highly variable in the nature of their microbial associations and in whether and how such associations change across development. No rules appear to hold across Holometabola, much less within individual orders; even closely related insect species can behave quite differently with respect to symbioses and their dynamics during metamorphosis [9]. Although we focus on insects, many of the principles we discuss could apply to other animals that undergo metamorphosis or organisms with complex life cycles in general. We also focus on microbes that are beneficial in some way to their insect hosts, noting that effects of metamorphosis on parasites and pathogens are important components of host immunity and may conflict with the need to transmit mutualists across life stages [10–12]. Finally, we note that the fate of human pathogens, plant pathogens or spoilage microorganisms during metamorphosis of their insect vectors is also of interest (e.g. [13–15]) but is not specifically covered here.
2. Complete metamorphosis constraining the acquisition of vertically transmitted symbionts
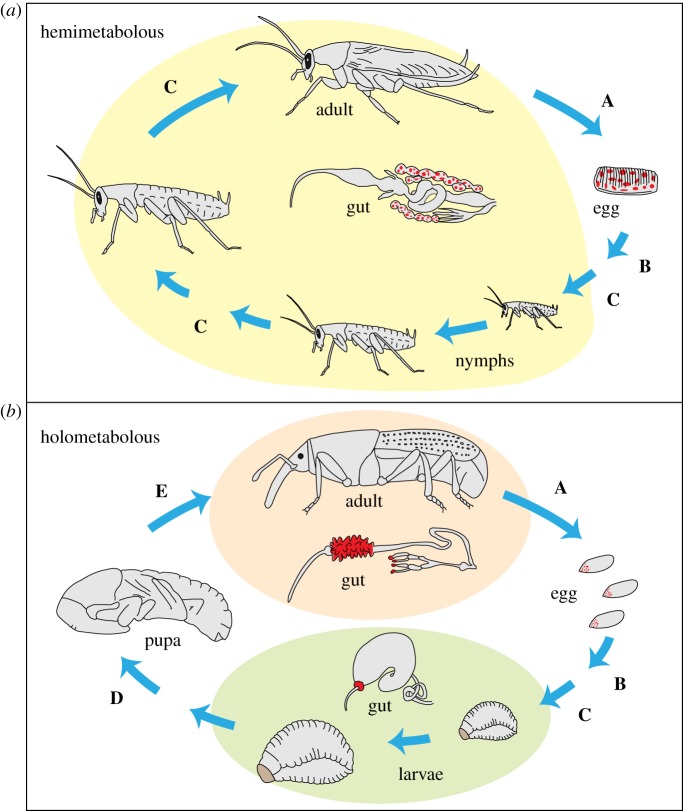
Transmitting symbionts vertically has certain advantages over acquiring them from the environment: it more closely aligns symbiont fitness interests with those of the host, guarantees an inoculum for offspring, and ultimately provides an opportunity for host–symbiont coevolution [16–18]. But as not all microbiota are vertically transmitted, it is worth considering features of host biology that may prevent this type of symbiosis from forming. Some barriers are common among the life cycles of both holo- and hemimetabolous insects (figure 1), as well as other animals. For example, moulting of immature insects can be one such barrier, as insects typically shed the lining of the foregut and hindgut and eliminate most or all gut contents with each moult. This disturbance may cause gut-associated symbionts to relocate within the host, to be suppressed, or to be lost entirely [22–24]. The sometimes-elaborate means by which parent insects inoculate offspring (e.g. [18,25,26]) highlight the gap between generations as another important barrier. In holometabolous insects, however, complete metamorphosis and the often extreme divergence of life stages present yet another hurdle to vertical transmission (figure 1). We suggest that holometabolous insects may thus be less likely to evolve strictly vertically transmitted symbioses than other insects, and perhaps more likely to use alternate strategies for inter-generational transmission (discussed below) or to acquire symbionts from the environment.
Figure 1.
Barriers to persistence of microbial symbionts across hemimetabolous and holometabolous insect life cycles. (a) Cockroaches possess obligate endosymbionts within fat body cells that are transmitted through eggs and which contribute to host nutrition [19] and also hindgut microbiota that are acquired through a faecal–oral route [20]. The gut structure and bacteriomes remain largely unchanged during development, enabling consistent habitat for both symbiont types. (b) In Sitophilus grain weevils, amino acid-provisioning intracellular symbionts migrate during metamorphosis, from bacteriocytes at the foregut/midgut junction in larvae to cells located on midgut caecae in adults, and then to ovarioles where they are transmitted to eggs [21]. Both bacteriomes and gut structure undergo restructuring, requiring symbionts to shift locations and adjust to novel habitats. Letters indicate barriers to symbiont transmission: (A) transmission from female to egg at oviposition, followed by maintenance of symbionts on or in the egg; (B) incorporation into the embryo during development or acquisition at hatching by ingestion; (C) transmission across larval moults including shedding of cuticle and lining of foregut and hindgut; (D,E) migration to new locations during organ remodelling during metamorphosis. Gut morphologies corresponding to different stages are shown in the centre. Red = location of intracellular, vertically transmitted endosymbionts. (Online version in colour.)
(a). Challenges posed by holometaboly
For a microbial symbiont of holometabolous insects, a primary, but not exclusive challenge of metamorphosis is the dissolution and restructuring of its habitat [7,9,12]. During complete metamorphosis, larval organs—including the gut and often bacteriomes, sites that can house vertically transmitted symbionts—break down and may not be reformed in the adult insect [9]. To persist into the adult stage and subsequent host generation, symbionts in either the gut or other sites may need to transition between intra- and extracellular phases and/or migrate to and colonize new structures. Adult versions of these structures may be less conducive to microbial growth than those in larvae; in dung beetles, for example, an enlarged pouch in the larval hindgut—which likely houses digestive symbionts—disappears during metamorphosis and is replaced with a simple tube in the adult stage [27]. These disturbances are much more pronounced in holometabolous insects as compared with hemimetabolous insects (figure 1). As noted by Buchner, the maintenance of symbionts even in unsheltered sites such as the gut lumen ‘offers no difficulties of any kind in the hemimetabolic insects. In fact, [symbiont-housing structures in nymphs] are simply taken unchanged into the imaginal organization’ ([9], p. 681).
In addition to disruption of their habitat, gut-associated symbionts may need to withstand harsh conditions during metamorphosis. In many insects, the larval gut (including microbes in the gut lumen) is purged prior to pupation [28–31]. Remaining microbes may be exposed to lysozyme and antimicrobial peptides secreted into the pupal gut [32–34] and fluctuations in pH [35]. Newly emerging adults will then discharge the remnants of the larval gut as meconium [36]. These factors, which likely help prevent infection by harmful microbes during metamorphosis, could also reduce or eliminate beneficial symbionts. Thus, holometabolous insects may face a tradeoff between creating a friendlier environment for symbiont persistence and limiting the spread of parasites and pathogens [12].
Microbes surviving metamorphosis may also find themselves in a very different biotic and abiotic environment from that of the larva, facing new stressors, competitors or nutrient deficiencies. As insects transition from larval to adult stages, new niches for microbes will be opened and others closed as the host adopts a new diet or ceases feeding altogether, alters its internal chemical conditions or adjusts physiological processes. For example, adult insects may ingest secondary metabolites with antimicrobial properties [37,38], upregulate immune activity [39,40], alter gut pH [41] and present differing levels of circulating lipids or other nutrients [42] that could limit the growth of microbes dependent on them (e.g. [43]). Reorganization of the microbiota, and the arrival of new microbes ingested by the adult, could also create new competitive or antagonistic interactions for symbionts persisting into the adult stage.
(b). Transmission strategies that bypass metamorphosis
The challenge of maintaining symbionts into the adult stage, while also maintaining an internal environment hostile to pathogens, is likely one reason for the prevalence of alternate transmission strategies that circumvent metamorphosis. For eusocial insects, in which generations overlap within a shared nest, host-specific and coevolved symbionts can be transferred among adult nest-mates [44]. With this transmission mode, symbionts do not need to be able to colonize the larval stage, nor persist through metamorphosis. Such is the case for gut bacteria in turtle ants, honeybees and bumblebees, and for cuticular bacteria of Acryomyrmex leafcutter ants [45–49]. Solitary insects may also use external routes: in beewolves (Sphecidae, Philanthus), larvae incorporate symbionts from the walls of their brood cell into the cocoon; adults emerge sterile and take up symbionts from the cocoon material into reservoirs in the antennae [50]. Likewise, larval gut microbes in the burying beetle Nicrophorus vespilloides disappear upon metamorphosis and apparently recolonize the adult from residual populations on the shed larval cuticle or the wall of the pupal chamber [51].
An environment shared between life stages creates another opportunity for indirect transmission outside the host. This factor is an important component of microbial symbiosis in Drosophila species, many of which both feed on, and breed in, decomposing plant material [52]. For example, in a population of D. melanogaster collected from figs, adults are readily colonized by a mutualistic gut bacterium (Acetobacter thailandicus); adult flies also disperse the symbiont to new figs, where they feed and oviposit [53]. While internal persistence through the pupal stage is also a possibility [31], A. thailandicus transmission to newly emerging adults likely occurs through prior inoculation of the substrate [53]. Foodborne transmission may also explain the presence of characteristic gut bacteria in wild Drosophila adults that consume decaying mushroom or cactus tissue [54,55]. By regurgitating, defaecating and ovipositing into a resource consumed by both larvae and adults, Drosophila and other insects with similar habits may be provided with a reliable means of symbiont transmission across life stages.
Social or external transmission modes are not exclusive to holometabolous insects; for example, they have also evolved in gregarious or subsocial cockroaches and their eusocial termite relatives [56]. However, these routes may be particularly important when metamorphosis makes transmission through the pupal stage less likely to evolve.
(c). Examples of symbiont transmission through metamorphosis
Despite the challenges posed by metamorphosis and life stage divergence, many holometabolous insects do possess strictly vertically transmitted symbionts (i.e. transmitted through the pupa as well as other life stages). Bacteria that infect germline tissues intracellularly and/or occur in haemolymph, such as Wolbachia [57] and Spiroplasma [58], are particularly common among holometabolous insects. For example, in broad surveys of Lepidoptera and Drosophila species, Wolbachia and Spiroplasma were the only heritable symbionts detected [59,60]. The within-host localization of these taxa is likely a major factor enabling their persistence through the life cycle of diverse holometabolous insect lineages: intracellular symbionts are more sheltered from harsh conditions than extracellular symbionts. Also, symbionts in the germline do not need to relocate for transmission to offspring. Similarly, symbionts circulating freely through the body cavity in haemolymph are less affected by the disappearance of specific organs during metamorphosis and can avoid the often volatile conditions occurring within the gut.
On the other hand, some symbionts do persist despite colonizing comparatively less stable or sheltered microhabitats. In the larvae of reed beetles (Chrysomelidae, Donaciinae) for example, bacterial symbionts colonize the lumen and cells of midgut-associated sacs; perhaps because these sacs (and the symbionts remaining within) disintegrate during metamorphosis, prior to pupation, some of the symbionts migrate posteriorly to colonize hypertrophied sections of two specialized Malpighian tubules [61,62]. In the adult females, symbionts are then expelled into the hindgut and later deposited on eggs. And in certain louse flies (Hippoboscidae), symbionts must also escape from degenerating larval tissues and reinfect the appropriate location in the adult for subsequent persistence. In this case, intracellular gut symbionts are discharged into the lumen as the larval gut degrades during metamorphosis and then reinfect the adult gut epithelium [9].
(d). Host/symbiont adaptations and the value of persistence
These latter examples do not discount the importance of complete metamorphosis as a barrier to the vertical transmission of symbionts. Rather, they likely reflect adaptations on the part of hosts and/or microbes to favour maintenance of the symbiosis between life stages. When symbionts are particularly important to host fitness, hosts may evolve to modify immune responses or the biochemical environment, guide symbiont migration to new sites (e.g. [63]) or create special structures to house and protect them. On the part of the microbe, persistence within the host may also be beneficial and one class of adaptations could involve resistance to host-derived antimicrobial peptides or physico-chemical changes. In particular, an intact cell envelope may be an important trait for survival during host metamorphosis. This possibility is suggested by Wigglesworthia and Blochmannia, endosymbionts of tsetse flies and carpenter ants (respectively), which—despite having highly reduced genomes—retain genes necessary for normal outer membrane and cell wall synthesis [64,65]. By contrast, endosymbionts of multiple hemimetabolous insect lineages have lost many of these genes [66].
Other microbial adaptations to host metamorphosis could take the form of chemosensory and motility capabilities for migration to new sites [67] and molecular tools to colonize newly formed host tissues. As an example of the latter, obligate intracellular symbionts of Sitophilus grain weevils are expelled as the larval bacteriome, adjacent to the foregut, disappears during metamorphosis; symbionts must then invade new bacteriocytes associated with midgut caecae of adults (figure 1). This migration and reentry into host cells appear to depend on a bacterial type III secretion system [68].
Is there anything special about the holometabolous insect species whose symbionts are faithfully maintained through metamorphosis? Solitary species without parental care or shared feeding sites may simply have less ready access to alternate transmission routes. And in hosts obligately dependent on particular symbionts for survival and reproduction, selection may favour direct transmission as a more reliable strategy for ensuring that the association is preserved in subsequent life stages. Additionally, in contrast to the aforementioned cases of ants and bees, where host-restricted symbionts are only present in adults, services provided by the symbionts may be required in larvae as well. For example, the tortoise beetle Cassida rubiginosa feeds on leaves as both larvae and adults, and its symbiont—upon which it relies to metabolize the pectin present in plant cell walls—is present throughout the life cycle [69]. Symbiont maintenance through metamorphosis may be particularly common in holometabolous insects in which larvae and adults overlap in diet or other ecological traits.
3. Holometaboly enabling flexibility in microbial associations between life stages
Microbiota can change dramatically, and in multiple ways, between larvae and adults of holometabolous insects. Although below we describe each separately, multiple types of change can occur within the same host. For example, in the beetle Lagria villosa, bacterial symbionts simultaneously vary in abundance, function and within-host localization across the host life cycle [70]. While such changes do not always occur, holometaboly may facilitate their evolution when favoured by natural selection. Microbiota may thus generally be more dynamic across host development for holometabolous insects as compared with hemimetabolous insects. In firebugs and cockroaches, for example, even complex gut microbiota are highly similar between nymphs and adults [71,72]. As we argue further below, the dynamism afforded by complete metamorphosis could be adaptive, in allowing hosts to flexibly respond to stage-specific selection pressures by regulating the colonization and activity of particular symbionts.
(a). Changes in microbiota composition
Analogously to the host's own morphology, microbiota composition often restructures during metamorphosis. While microbial diversity per se may or may not differ [27,73,74], larvae and adults of a variety of holometabolous insects host distinct microbial taxa and/or different relative abundances of shared taxa [27,73–79]. Similar microbiota restructuring also occurs in other animals that undergo metamorphosis, such as lampreys [80], frogs [81] and sponges [82]. These dynamics likely reflect concomitant changes in absolute abundance, metabolic activity and functions within hosts (discussed below), though these latter metrics are less commonly reported.
We do not yet know why holometabolous insect species vary in the stability of their microbiota across development [74,83]. One factor could be the extent to which feeding ecology differs between larvae and adults [73]. Another might be the opportunity for transmission from larval to adult stages. For example, feeding in groups on shared resources may enable newly emerged adults to reacquire symbionts from neighbours even when they have eliminated their own during metamorphosis, as proposed for Drosophila [53,54].
Moreover, within a given microbiota, microbial taxa can vary widely in their persistence and dynamics across host metamorphosis. For example, in the butterfly Heliconius erato, bacteria in the genera Enterococcus and Enterobacter are present in larvae, pupae and newly emerged adults, indicating that they either survive metamorphosis or are reacquired rapidly from the environment throughout the life cycle [73]. By contrast, Acinetobacter is present in larvae—possibly ingested with leaves—yet virtually disappears after metamorphosis. Orbus shows a third pattern: it is very rare in larvae and undetectable in pupae, yet becomes abundant in newly emerged adults prior to feeding [73]. Most likely, similar combinations of comings and goings are the norm for holometabolous insects with multi-species microbiota. But as these communities are often a mixture of beneficial symbionts, commensals, transients, parasites and pathogens, with intergradations and context-dependent shifts among these categories, the outcome of these dynamics will depend strongly on the microbe in question.
Whether, and if so how, microbiota or specific subsets thereof change between larvae and adults is also relevant to the mechanisms of ‘carryover’ or ‘latent’ effects—when phenotypic variation occurring in one life stage is retained through transitions to a subsequent life stage [84,85]. Such effects are of major interest to life history theory and to the evolution of complex life cycles, as they bear on the degree to which discrete life stages, such as larvae and adults, are decoupled [85–88]. In insects whose microbiota restructures or is replaced during metamorphosis, interindividual variation in larval microbiota may be erased, whereas insects without such restructuring are more likely to retain interindividual variation into the adult stage. These differences in microbial dynamics across metamorphosis may then partly explain the degree to which carryover effects occur, at least for host phenotypes that are specifically affected by the presence and composition of microbiota (e.g. [89]).
(b). Changes in abundance
Symbionts persisting from larvae to adults can undergo dramatic fluctuations in their absolute abundance (titre), with potential consequences for their population structure and evolution. Such fluctuations are also characteristic of other transitions in insect life cycles (e.g. [23,90]), but those occurring across complete metamorphosis constitute an additional, and potentially severe, bottleneck. For example, in mosquitoes, the burying beetle Nicrophorus vespilloides and Drosophila melanogaster, gut bacterial titres in larvae decrease strongly upon pupation [30,31,51]. Major bottlenecks can also occur for symbionts transmitted externally to the adult stage. In beewolves, from a population of approximately 100 000 symbiont cells in the cocoon, only approximately 100–1000 colonize the newly emerged adult females [50]. The aforementioned tortoise beetle Cassida rubiginosa illustrates how metamorphosis-associated bottlenecks may favour genome degradation and loss of non-essential functions in vertically transmitted symbionts. Here, numbers of the gut symbiont Stammera drop approximately four orders of magnitude from mature larvae to pupae—an even larger decrease than what occurs from adult females to eggs [69]. The change in symbiont population structure caused by this bottleneck [91] has likely contributed to the highly reduced genome and metabolic repertoire observed in Stammera [69].
While bottlenecks may ultimately affect symbiont evolution and function, even huge reductions in microbial abundance may not immediately affect the maintenance of the symbiosis, as a tiny inoculum is often sufficient to colonize the next generation (e.g. [92]). Complete loss associated with metamorphosis can occur when the symbionts are no longer needed, and selection on hosts to retain them has been relaxed. For example, if symbionts have no role in the adult stage other than subsequent transmission to offspring, they may be lost after metamorphosis in males, which are usually dead ends [9]. Elimination and recycling of symbionts after their main purpose has been served can also allow hosts to acquire nutrients present in microbial biomass [21]. There are, however, holometabolous insects in which symbiont numbers do not decrease but rather increase during metamorphosis, such as Camponotus and Cardiocondyla ants [93,94]. Here, symbiont-produced metabolites are thought to contribute to pupal-stage nutrition and synthesis of the adult cuticle, as discussed further below. In other cases where metamorphosis itself relies on symbiont activity, hosts may encourage symbionts to grow or protect them from degradation.
(c). Changes in within-host localization
In a number of insects, symbionts migrate during metamorphosis to inhabit a different host tissue in the adult than in the larva. This migration occurs in the reed beetles described earlier, whose symbionts migrate from larval midgut sacs into adult Malpighian tubules, and in Sitophilus grain weevils, whose symbionts relocate from bacteriomes near the foregut periphery to temporarily occupy bacteriocytes attached to caecae in the midgut and then to ovariole-associated bacteriocytes that provide inocula to eggs (figure 1, [95]). Symbionts sometimes transition between stable intra- and extracellular modes across metamorphosis, as occurs in olive flies [96]; these modes have implications for their specificity and genome evolution [26]. In lagriine beetles, symbionts pass the larval stage within curious ‘dorsal compartments’—invaginations of the cuticle that close during embryonic development [70,97]. During metamorphosis, the symbionts are released from the compartments and colonize reproductive glands of the adult females, where they produce metabolites that protect the eggs from fungal attack [70,97]. This kind of arrangement, in which symbionts are cordoned off during stages when they are not needed, could serve to control symbiont proliferation and/or protect them from degradation during metamorphosis.
(d). The potentially adaptive value of flexible symbioses in Holometabola
Changes in microbiota structure, abundance or within-host localization typically reflect parallel changes in the host insect's need for microbial services. In some insects, symbionts are only, or mainly, required for a particular phase of the insect life cycle, such as protecting eggs [70] or overwintering larvae [98], digestion of a larva- or adult-specific diet (e.g. [99,100]) or provisioning nutrients in demand during a specific developmental window [21,101]. Even when present throughout the life cycle, symbiont presence in some life stages may only serve for transmission to subsequent stages. Their proliferation, if not needed and costly, may be suppressed or contained. And for many Lepidoptera and some bees and ants, specific symbionts are largely absent from larvae [47,49,102–104], underscoring that microbial roles in insect biology can be highly life-stage-specific. Analogous gains or losses of symbionts during metamorphosis occur in other animals as well. For example, a hydrothermal vent snail undergoes a form of metamorphosis in which it transitions from grazing on free-living microbes to a total dependency on internal chemosynthetic symbionts, for which it grows a specialized bacteria-housing organ in the process [105].
Thus, in many (but not all) holometabolous insects and other metamorphosing species, there is a decoupling not just in morphology, ecology and other traits, but also in the nature of their interactions with microbial symbionts. As with these other aspects of organismal biology [4], metamorphosis could be adaptive in allowing each life stage to more independently respond to selection on phenotypes related to microbial associations. Given that hosting symbionts incurs costs (e.g. [10,106–108]), it may be advantageous to support symbionts as larvae but not as adults, or vice versa. For example, in the coral Orbicella faveolata, photosynthetic endosymbionts are beneficial when acquired by the sessile adults, but neutral or harmful when acquired as planktonic larvae [109]. Alternatively, an insect may require different symbionts as larvae and adults specialized for life stage-specific diet, physiology and ecology. Many animals have mechanisms for precisely regulating the composition or growth of their symbionts [110–113], and complete metamorphosis facilitates the decoupling of these mechanisms to regulate symbionts differently between life stages. By enabling hosts to discard, restructure or obtain new microbiota, it may generally allow a finer degree of control over engagement or disengagement with symbionts over the course of the life cycle. If such modular evolvability [114] increases population growth and lineage diversification, it could be a contributing factor to the extraordinary success of holometabolous insects.
4. Microbial mediation of metamorphosis
Metamorphosis clearly can affect symbionts, but can symbionts affect metamorphosis? As described in more detail below, metamorphosis entails immunological and nutritional challenges that are—in some insects—met with the aid of microbes. Although less well-studied, microbes might also provide defense from abiotic stressors during the transition from larva to adult. For example, symbiont-derived secretions are used by aquatic reed beetles, which pass the pupal stage buried in sediment for several months, to construct a rigid cocoon that allows gas exchange and serves as physical protection [61].
(a). Disease risk
Pupae may be especially vulnerable to disease as physical barriers to pathogen spread are broken down and as behavioural defenses (e.g. [115]) are unavailable. Honeybees, and perhaps some other insects, also lack the ability to upregulate immune responses as pupae [116]. Hence, many insects have evolved sterilizing mechanisms prior to pupation, as discussed earlier. However, the strength of this cleansing process may be constrained for those particular hosts that need to transmit symbionts internally from larvae to adults. In addition to external transmission mechanisms, a solution may be to rely on the symbionts themselves for pathogen suppression. For example, in beewolves, whose larvae overwinter and pupate in the soil, antibiotic-producing Streptomyces symbionts incorporated into the cocoon provide protection from opportunistic fungal pathogens [98,117]. In a laboratory population of the wax moth Galleria mellonella, the presence of antimicrobial peptide-producing Enterococcus during metamorphosis suppresses infection and host mortality from co-occurring bacterial pathogens [12,118]. Pathogen protection might be a common benefit of keeping symbionts through metamorphosis.
(b). Nutritional demands
Holometabolous insects must largely rebuild an adult body using only nutrients and energy accumulated during the larval stage, a process which could benefit from microbes. One such role has been clearly demonstrated in weevils, which grow a particularly thick exoskeleton as adults. Many weevils, including Sitophilus grain weevils, harbour specialized intracellular endosymbionts that proliferate in newly emerged adults and produce tyrosine needed for cuticle biosynthesis; symbiont-free weevils develop thin, light-coloured cuticles [21,119]. A role for symbiont-produced tyrosine in adult cuticle biosynthesis has also been suggested for Cardiocondyla ants [94]. Another potential example is the carpenter ant Camponotus floridanus: the midgut epithelium greatly expands and fills with intracellular bacterial symbionts during metamorphosis, after which symbiont titres decrease in older adults [120]. This symbiont proliferation in pupae is thought to provide amino acids (including tyrosine) and to recycle nitrogenous waste, thus helping to meet nutritional demands [120,121]. Likewise, high expression of symbiont genes for thiamine and haem biosynthesis in the pupal stage of tsetse flies suggests a role in nutrient provisioning during host metamorphosis [122].
(c). Microbes as metamorphosis-inducing stimuli
In insects that consistently interact with microbes, metamorphosis and other developmental programmes may evolve to rely on microbial cues or activity for normal function (e.g. [123,124]). In a number of marine invertebrates, including corals, tubeworms, polychaetes and many other groups, microbes are well known to serve as stimuli for settling and metamorphosis [125,126], through sometimes intricate mechanisms. For example, unique bacterial molecules trigger tubeworm metamorphosis by upregulating expression of tissue-remodelling enzymes and by activating key developmental signalling pathways [127,128]. Similar phenomena also occur in some insects. For example, in the stingless bee Scaptotrigona depilis, larvae need to consume an ergosterol-producing fungal symbiont to pupate. Fungus-derived ergosterol appears to be used by the bee larva as a precursor for the biosynthesis of hormones known to regulate insect moulting and metamorphosis [101]. Mosquitoes present an analogous example: here, larval moulting is triggered by oxygen depletion resulting from respiration by gut microbes [129,130]. In these latter cases, it is unclear whether microbes actually provide novel benefits, as moulting and metamorphosis in general can function without microbes (as exemplified by successful axenic rearing of some insects). These may instead represent evolutionary addiction [131,132]: animals for whom particular microbes are a constant feature of their external or internal environment may evolve dependence and thus lose the ability to carry out normal developmental processes in their absence.
5. Literature on insect microbiota variation across metamorphosis
In electronic supplementary material, table S1, we provide references for studies that compare microbiota in holometabolous insect larvae and adults or assess symbiont transmission or dynamics across metamorphosis. These are intended to highlight a selection of relevant literature and are not exhaustive. Buchner [9] and references therein are still a highly valuable source of information for the localization and transmission of endosymbionts across insect life stages. We do not include Raphidioptera, Strepsiptera, Megaloptera, Trichoptera and Mecoptera, as, to our knowledge, no pertinent studies on these orders exist. Furthermore, within each order listed in the table, microbial associations for many families are entirely unknown. Wider exploration of microbiota among Holometabola (including paedomorphic species [133]), as well as quantitative comparisons of their transmission routes and developmental dynamics with hemimetabolous insects, would help us better understand the evolution of metamorphosis-microbiota feedbacks.
6. Conclusion and outlook
As our summary illustrates, the microbial symbionts of holometabolous insects show a wide range of responses to metamorphosis. Some insects maintain a single, highly specialized symbiont through metamorphosis, though its services may be limited to a single life stage. Others have more diffuse interactions with microbes and may have completely different kinds of symbiotic associations as larvae and adults. Microbes themselves may directly influence metamorphosis, either by providing nutritional or defensive services or by serving as cues upon which host developmental programmes evolve dependence.
However, our understanding of these relationships is still in an early larval stage. The exact nature of biochemical and immunological changes occurring during metamorphosis is well known for only a handful of species, predominantly those with less specialized symbioses. Studies of symbiont gene expression across holometabolous insect development [122,134] are relatively rare. An especially critical area is how plasticity in immune function across development affects symbioses, and how larval exposure to symbionts or pathogens affects microbial associations in subsequent life stages [89,135–137]. Our understanding is also limited in its phylogenetic breadth, as microbial symbioses remain completely unstudied for many holometabolous groups. As we begin to explore these groups and strengthen connections between studies of insects with those of other hosts with complex life cycles, we will gain further insights into the links between metamorphosis and microbiota.
Supplementary Material
Acknowledgements
We thank the reviewers for comments that improved the manuscript and Kim Hammond for preparing the figure. The authors were supported by NIH award R01GM108477 to N.A.M.
Data accessibility
This article has no additional data.
Authors' contributions
Both authors contributed ideas and wrote the manuscript.
Competing interests
We declare we have no competing interests.
References
- 1.Kristensen NP. 1999. Phylogeny of endopterygote insects, the most successful lineage of living organisms. Eur. J. Entomol. 96, 237–253. [Google Scholar]
- 2.Whiting MF. 2003. Phylogeny of the holometabolous insects: the most successful group of terrestrial organisms. In Assembling the tree of life (eds Cracraft J, Donoghue MJ), pp. 345–361. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Grimaldi D, Engel MS. 2005. Evolution of the insects. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.Moran NA. 1994. Adaptation and constraint in the complex life cycles of animals. Annu. Rev. Ecol. Syst. 25, 573–600. ( 10.1146/annurev.es.25.110194.003041) [DOI] [Google Scholar]
- 5.Feldhaar H. 2011. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 36, 533–543. ( 10.1111/j.1365-2311.2011.01318.x) [DOI] [Google Scholar]
- 6.Douglas AE. 2015. Multiorganismal insects: diversity and function of resident microorganisms. Annu. Rev. Entomol. 60, 17–34. ( 10.1146/annurev-ento-010814-020822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engel P, Moran NA. 2013. The gut microbiota of insects—diversity in structure and function. FEMS Microbiol. Rev. 37, 699–735. ( 10.1111/1574-6976.12025) [DOI] [PubMed] [Google Scholar]
- 8.Misof B, et al. 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767. ( 10.1126/science.1257570) [DOI] [PubMed] [Google Scholar]
- 9.Buchner P. 1965. Endosymbiosis of animals with plant microorganisms. New York, NY: John Wiley & Sons. [Google Scholar]
- 10.Salem H, Onchuru TO, Bauer E, Kaltenpoth M. 2015. Symbiont transmission entails the risk of parasite infection. Biol. Lett. 11, 20150840 ( 10.1098/rsbl.2015.0840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinotte V, Freedman S, Ugelvig L, Seid M. 2018. Camponotus floridanus ants incur a trade-off between phenotypic development and pathogen susceptibility from their mutualistic endosymbiont Blochmannia. Insects 9, 58 ( 10.3390/insects9020058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston PR, Rolff J. 2015. Host and symbiont jointly control gut microbiota during complete metamorphosis. PLoS Pathog. 11, e1005246 ( 10.1371/journal.ppat.1005246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tebbutt H. 1912. On the influence of the metamorphosis of Musca domestica upon bacteria administered in the larval stage. Epidemiol. Infect. 12, 516–526. ( 10.1017/S0022172400005179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flury P, Vesga P, Dominguez-Ferreras A, Tinguely C, Ullrich CI, Kleespies RG, Keel C, Maurhofer M. 2019. Persistence of root-colonizing Pseudomonas protegens in herbivorous insects throughout different developmental stages and dispersal to new host plants. ISME J. 13, 860–872. ( 10.1038/s41396-018-0317-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crotti E, et al. 2010. Acetic acid bacteria, newly emerging symbionts of insects. Appl. Environ. Microbiol. 76, 6963–6970. ( 10.1128/AEM.01336-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewald PW. 1987. Transmission modes and evolution of the parasitism-mutualism continuum. Ann. N. Y. Acad. Sci. 503, 295–306. ( 10.1111/j.1749-6632.1987.tb40616.x) [DOI] [PubMed] [Google Scholar]
- 17.Herre E, Knowlton N, Mueller U, Rehner S. 1999. The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol. Evol. 14, 49–53. ( 10.1016/S0169-5347(98)01529-8) [DOI] [PubMed] [Google Scholar]
- 18.Bright M, Bulgheresi S. 2010. A complex journey: transmission of microbial symbionts. Nat. Rev. Microbiol. 8, 218–230. ( 10.1038/nrmicro2262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabree ZL, Kambhampati S, Moran NA. 2009. Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proc. Natl Acad. Sci. USA 106, 19 521–19 526. ( 10.1073/pnas.0907504106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosas T, García-Ferris C, Domínguez-Santos R, Llop P, Latorre A, Moya A. 2018. Rifampicin treatment of Blattella germanica evidences a fecal transmission route of their gut microbiota. FEMS Microbiol. Ecol. 94, 1–12. ( 10.1093/femsec/fiy002) [DOI] [PubMed] [Google Scholar]
- 21.Vigneron A, et al. 2014. Insects recycle endosymbionts when the benefit is over. Curr. Biol. 24, 2267–2273. ( 10.1016/j.cub.2014.07.065) [DOI] [PubMed] [Google Scholar]
- 22.Nalepa C. 2017. What kills the hindgut flagellates of lower termites during the host molting cycle? Microorganisms 5, 82 ( 10.3390/microorganisms5040082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JK, Heum S, Kim C, Hun Y, Futahashi R, Kikuchi Y, Fukatsu T, Luel B. 2014. Molting-associated suppression of symbiont population and up-regulation of antimicrobial activity in the midgut symbiotic organ of the Riptortus–Burkholderia symbiosis. Dev. Comp. Immunol. 43, 10–14. ( 10.1016/j.dci.2013.10.010) [DOI] [PubMed] [Google Scholar]
- 24.Prado SS, Rubinoff D, Almeida RPP. 2006. Vertical transmission of a pentatomid caeca-associated symbiont. Ann. Entomol. Soc. Am. 99, 577–585. ( 10.1603/0013-8746(2006)99[577:VTOAPC]2.0.CO;2) [DOI] [Google Scholar]
- 25.Hosokawa T, Hironaka M, Inadomi K, Mukai H, Nikoh N, Fukatsu T. 2013. Diverse strategies for vertical symbiont transmission among subsocial stinkbugs. PLoS ONE 8, e65081 ( 10.1371/journal.pone.0065081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salem H, Florez L, Gerardo N, Kaltenpoth M. 2015. An out-of-body experience: the extracellular dimension for the transmission of mutualistic bacteria in insects. Proc. R. Soc. B 282, 20142957 ( 10.1098/rspb.2014.2957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shukla SP, Sanders JG, Byrne MJ, Pierce NE. 2016. Gut microbiota of dung beetles correspond to dietary specializations of adults and larvae. Mol. Ecol. 25, 6092–6106. ( 10.1111/mec.13901) [DOI] [PubMed] [Google Scholar]
- 28.Nijhout HF, Williams CM. 1974. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): growth of the last-instar larva and the decision to pupate. J. Exp. Biol. 61, 481–491. [DOI] [PubMed] [Google Scholar]
- 29.Michener CD. 2007. Bees of the world, 2nd edn Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 30.Moll RM, Romoser WS, Modrakowski MC, Moncayo AC, Lerdthusnee K. 2001. Meconial peritrophic membranes and the fate of midgut bacteria during mosquito (Diptera: Culicidae) metamorphosis. J. Med. Entomol. 38, 29–32. ( 10.1603/0022-2585-38.1.29) [DOI] [PubMed] [Google Scholar]
- 31.Bakula M. 1969. The persistence of a microbial flora during postembryogenesis of Drosophila melanogaster. J. Invertebr. Pathol. 14, 365–374. ( 10.1016/0022-2011(69)90163-3) [DOI] [PubMed] [Google Scholar]
- 32.Russell V, Dunn PE. 1996. Antibacterial proteins in the midgut of Manduca sexta during metamorphosis. J. Insect Physiol. 42, 65–71. ( 10.1016/0022-1910(95)00083-6) [DOI] [PubMed] [Google Scholar]
- 33.Tryselius Y, Samakovlis C, Kimbrell DA, Hultmark D, Hind I. 1992. CecC, a cecropin gene expressed during metamorphosis in Drosophila pupae. Eur. J. Biochem. 399, 395–399. ( 10.1111/j.1432-1033.1992.tb16648.x) [DOI] [PubMed] [Google Scholar]
- 34.Wu S, Zhang X, He Y, Shuai J, Chen X, Ling E. 2010. Expression of antimicrobial peptide genes in Bombyx mori gut modulated by oral bacterial infection and development. Dev. Comp. Immunol. 34, 1191–1198. ( 10.1016/j.dci.2010.06.013) [DOI] [PubMed] [Google Scholar]
- 35.Agrell I. 1948. The fluctuation of pH, buffer capacity, and pH-dependance of hydrogen activating enzyme systems during insect metamorphosis. Acta Physiol. Scand. 16, 9–19. ( 10.1111/j.1748-1716.1948.tb00521.x) [DOI] [Google Scholar]
- 36.Hakim RS, Baldwin K, Smagghe G. 2010. Regulation of midgut growth, development, and metamorphosis. Annu. Rev. Entomol. 55, 593–608. ( 10.1146/annurev-ento-112408-085450) [DOI] [PubMed] [Google Scholar]
- 37.Hammer TJ, Bowers MD. 2015. Gut microbes may facilitate insect herbivory of chemically defended plants. Oecologia 179, 1–14. ( 10.1007/s00442-015-3327-1) [DOI] [PubMed] [Google Scholar]
- 38.Nishida R. 2002. Sequestration of defensive substances by Lepidoptera. Annu. Rev. Entomol. 47, 57–92. ( 10.1146/annurev.ento.47.091201.145121) [DOI] [PubMed] [Google Scholar]
- 39.Laughton AM, Boots M, Siva-Jothy MT. 2011. The ontogeny of immunity in the honey bee, Apis mellifera L. following an immune challenge. J. Insect Physiol. 57, 1023–1032. ( 10.1016/j.jinsphys.2011.04.020) [DOI] [PubMed] [Google Scholar]
- 40.Zerofsky M, Harel E, Silverman N, Tatar M. 2005. Aging of the innate immune response in Drosophila melanogaster. Aging Cell 4, 103–108. ( 10.1111/j.1474-9728.2005.00147.x) [DOI] [PubMed] [Google Scholar]
- 41.Waterhouse DF. 1949. The hydrogen ion concentration in the alimentary canal of larval and adult Lepidoptera. Aust. J. Biol. Sci. 2, 428–437. ( 10.1071/BI9490428) [DOI] [Google Scholar]
- 42.Beenakkers AMT, Van der Horst DJ, Van Marrewijk WJA. 1985. Insect lipids and lipoproteins, and their role in physiological processes. Prog. Lipid Res. 24, 19–67. ( 10.1016/0163-7827(85)90007-4) [DOI] [PubMed] [Google Scholar]
- 43.Herren JK, Paredes JC, Schüpfer F, Arafah K, Bulet P, Lemaitre B. 2014. Insect endosymbiont proliferation is limited by lipid availability. Elife 3, e02964 ( 10.7554/eLife.02964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onchuru TO, Martinez AJ, Ingham CS, Kaltenpoth M. 2018. Transmission of mutualistic bacteria in social and gregarious insects. Curr. Opin. Insect Sci. 28, 50–58. ( 10.1016/j.cois.2018.05.002) [DOI] [PubMed] [Google Scholar]
- 45.Powell JE, Martinson VG, Urban-Mead K, Moran NA. 2014. Routes of acquisition of the gut microbiota of the honey bee Apis mellifera. Appl. Environ. Microbiol. 80, 7378–7387. ( 10.1128/AEM.01861-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koch H, Abrol DP, Li J, Schmid-Hempel P. 2013. Diversity and evolutionary patterns of bacterial gut associates of corbiculate bees. Mol. Ecol. 22, 2028–2044. ( 10.1111/mec.12209) [DOI] [PubMed] [Google Scholar]
- 47.Martinson VG, Moy J, Moran NA. 2012. Establishment of characteristic gut bacteria during development of the honeybee worker. Appl. Environ. Microbiol. 78, 2830–2840. ( 10.1128/AEM.07810-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poulsen M, Bot ANM, Currie CR, Nielsen MG, Boomsma JJ. 2003. Within-colony transmission and the cost of a mutualistic bacterium in the leaf-cutting ant Acromyrmex octospinosus. Funct. Ecol. 17, 260–269. ( 10.1046/j.1365-2435.2003.00726.x) [DOI] [Google Scholar]
- 49.Lanan MC, Rodrigues PAP, Agellon A, Jansma P, Wheeler DE. 2016. A bacterial filter protects and structures the gut microbiome of an insect. ISME J. 10, 1866–1876. ( 10.1038/ismej.2015.264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaltenpoth M, Goettler W, Koehler S, Strohm E. 2010. Life cycle and population dynamics of a protective insect symbiont reveal severe bottlenecks during vertical transmission. Evol. Ecol. 24, 463–477. ( 10.1007/s10682-009-9319-z) [DOI] [Google Scholar]
- 51.Wang Y, Rozen DE. 2017. Gut microbiota colonization and transmission in the burying beetle Nicrophorus vespilloides throughout development. Appl. Environ. Microbiol. 83, e03250-16 ( 10.1128/aem.03250-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markow TA, O'Grady P. 2008. Reproductive ecology of Drosophila. Funct. Ecol. 22, 747–759. ( 10.1111/j.1365-2435.2008.01457.x) [DOI] [Google Scholar]
- 53.Pais IS, Valente RS, Sporniak M, Teixeira L. 2018. Drosophila melanogaster establishes a species-specific mutualistic interaction with stable gut-colonizing bacteria. PLoS Biol. 16, e2005710 ( 10.1371/journal.pbio.2005710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinson VG, Douglas AE, Jaenike J. 2017. Community structure of the gut microbiota in sympatric species of wild Drosophila. Ecol. Lett. 20, 629–639. ( 10.1111/ele.12761) [DOI] [PubMed] [Google Scholar]
- 55.Martinson VG, Carpinteyro-Ponce J, Moran NA, Markow TA. 2017. A distinctive and host-restricted gut microbiota in populations of a cactophilic Drosophila species. Appl. Environ. Microbiol. 83, e01551-17 ( 10.1128/AEM.01551-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brune A, Dietrich C. 2015. The gut microbiota of termites: digesting the diversity in the light of ecology and evolution. Annu. Rev. Microbiol. 69, 145–166. ( 10.1146/annurev-micro-092412-155715) [DOI] [PubMed] [Google Scholar]
- 57.Dobson SL, Bourtzis K, Braig HR, Jones BF. 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. 29, 153–160. [DOI] [PubMed] [Google Scholar]
- 58.Anbutsu H, Fukatsu T. 2011. Spiroplasma as a model insect endosymbiont. Environ. Microbiol. Rep. 3, 144–153. ( 10.1111/j.1758-2229.2010.00240.x) [DOI] [PubMed] [Google Scholar]
- 59.Mateos M, Castrezana SJ, Nankivell BJ, Estes AM, Markow TA, Moran NA. 2006. Heritable endosymbionts of Drosophila. Genetics 174, 363–376. ( 10.1534/genetics.106.058818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russell JA, Funaro CF, Giraldo YM, Goldman-Huertas B, Suh D, Kronauer DJC, Moreau CS, Pierce NE. 2012. A veritable menagerie of heritable bacteria from ants, butterflies, and beyond: broad molecular surveys and a systematic review. PLoS ONE 7, e51027 ( 10.1371/journal.pone.0051027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kölsch G, Matz-Grund C, Pedersen BV. 2009. Ultrastructural and molecular characterization of endosymbionts of the reed beetle genus Macroplea (Chrysomelidae, Donaciinae), and proposal of ‘Candidatus Macropleicola appendiculatae’ and ‘Candidatus Macropleicola muticae’. Can. J. Microbiol. 55, 1250–1260. ( 10.1139/W09-085) [DOI] [PubMed] [Google Scholar]
- 62.Stammer HJ. 1935. Studien an Symbiosen zwischen Kafern und Mikroorganismen. I. Die Symbiose der Donaciinen (Coleopt. Chrysomel.). Z. Morphol. Okol. Tiere 29, 585–608. ( 10.1007/BF00407434) [DOI] [Google Scholar]
- 63.Nawroth JC, Guo H, Koch E, Heath-Heckman EAC, Hermanson JC, Ruby EG, Dabiri JO, Kanso E, McFall-Ngai M. 2017. Motile cilia create fluid-mechanical microhabitats for the active recruitment of the host microbiome. Proc. Natl Acad. Sci. USA 114, 9510–9516. ( 10.1073/pnas.1706926114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. 2002. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat. Genet. 32, 402–407. ( 10.1038/ng986) [DOI] [PubMed] [Google Scholar]
- 65.Gil R, et al. 2003. The genome sequence of Blochmannia floridanus: comparative analysis of reduced genomes. Proc. Natl Acad. Sci. USA 100, 9388–9393. ( 10.1073/pnas.1533499100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCutcheon JP, Moran NA. 2012. Extreme genome reduction in symbiotic bacteria. Nat. Rev. Microbiol. 10, 13–26. ( 10.1038/nrmicro2670) [DOI] [PubMed] [Google Scholar]
- 67.Raina J-B, Fernandez V, Lambert B, Stocker R, Seymour JR. 2019. The role of microbial motility and chemotaxis in symbiosis. Nat. Rev. Microbiol. 17, 284–294. ( 10.1038/s41579-019-0182-9) [DOI] [PubMed] [Google Scholar]
- 68.Dale C, Plague GR, Wang B, Ochman H, Moran NA. 2002. Type III secretion systems and the evolution of mutualistic endosymbiosis. Proc. Natl Acad. Sci. USA 99, 12 397–12 402. ( 10.1073/pnas.182213299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salem H, et al. 2017. Drastic genome reduction in an herbivore's pectinolytic symbiont. Cell 171, 1520–1531. ( 10.1016/j.cell.2017.10.029) [DOI] [PubMed] [Google Scholar]
- 70.Flórez LV, Scherlach K, Gaube P, Ross C, Sitte E, Hermes C, Rodrigues A, Hertweck C, Kaltenpoth M. 2017. Antibiotic-producing symbionts dynamically transition between plant pathogenicity and insect-defensive mutualism. Nat. Commun. 8, 15172 ( 10.1038/ncomms15172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carrasco P, Pérez-Cobas AE, van de Pol C, Baixeras J, Moya A, Latorre A. 2014. Succession of the gut microbiota in the cockroach Blattella germanica. Int. Microbiol. 17, 99–109. ( 10.1093/femsec/fiv022) [DOI] [PubMed] [Google Scholar]
- 72.Sudakaran S, Salem H, Kost C, Kaltenpoth M. 2012. Geographical and ecological stability of the symbiotic mid-gut microbiota in European firebugs, Pyrrhocoris apterus (Hemiptera. Pyrrhocoridae). Mol. Ecol. 21, 6134–6151. ( 10.1111/mec.12027) [DOI] [PubMed] [Google Scholar]
- 73.Hammer TJ, McMillan WO, Fierer N. 2014. Metamorphosis of a butterfly-associated bacterial community. PLoS ONE 9, e86995 ( 10.1371/journal.pone.0086995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yun J-H, et al. 2014. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl. Environ. Microbiol. 80, 5254–5264. ( 10.1128/AEM.01226-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vasanthakumar A, Delalibera I, Handelsman J, Klepzig KD, Schloss PD, Raffa KF. 2009. Characterization of gut-associated bacteria in larvae and adults of the southern pine beetle, Dendroctonus frontalis Zimmermann. Environ. Entomol. 35, 1710–1717. ( 10.1603/0046-225X(2006)35[1710:COGBIL]2.0.CO;2) [DOI] [Google Scholar]
- 76.Mason CJ, Campbell A, Scully ED, Hoover K. 2018. Bacterial and fungal midgut community dynamics and transfer between mother and brood in the Asian longhorned beetle (Anoplophora glabripennis), an invasive xylophage. Microb. Ecol. 77, 230–242. ( 10.1007/s00248-018-1205-1) [DOI] [PubMed] [Google Scholar]
- 77.Wong CNA, Ng P, Douglas AE. 2011. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ. Microbiol. 13, 1889–1900. ( 10.1111/j.1462-2920.2011.02511.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang X, Gao Q, Wang W, Wang X, Lei C, Zhu F. 2018. The gut bacteria across life stages in the synanthropic fly Chrysomya megacephala. BMC Microbiol. 18, 131 ( 10.1186/s12866-018-1272-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Gilbreath TM, Kukutla P, Yan G, Xu J. 2011. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS ONE 6, e24767 ( 10.1371/journal.pone.0024767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tetlock A, Yost CK, Stavrinides J, Manzon RG. 2012. Changes in the gut microbiome of the sea lamprey during metamorphosis. Appl. Environ. Microbiol. 78, 7638–7644. ( 10.1128/AEM.01640-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kohl KD, Cary TL, Karasov WH, Dearing MD. 2013. Restructuring of the amphibian gut microbiota through metamorphosis. Environ. Microbiol. Rep. 5, 899–903. ( 10.1111/1758-2229.12092) [DOI] [PubMed] [Google Scholar]
- 82.Fieth RA, Gauthier MA, Bayes J, Green KM, Degnan SM. 2016. Ontogenetic changes in the bacterial symbiont community of the tropical demosponge Amphimedon queenslandica: metamorphosis is a new beginning. Front. Mar. Sci. 3, 1–20. ( 10.3389/fmars.2016.00228) [DOI] [Google Scholar]
- 83.Colman DR, Toolson EC, Takacs-Vesbach CD. 2012. Do diet and taxonomy influence insect gut bacterial communities? Mol. Ecol. 21, 5124–5137. ( 10.1111/j.1365-294X.2012.05752.x) [DOI] [PubMed] [Google Scholar]
- 84.Pechenik JA. 2006. Larval experience and latent effects—metamorphosis is not a new beginning. Integr. Comp. Biol. 46, 323–333. ( 10.1093/icb/icj028) [DOI] [PubMed] [Google Scholar]
- 85.Podolsky RD, Moran AL. 2006. Integrating function across marine life cycles. Integr. Comp. Biol. 46, 577–586. ( 10.1093/icb/icl026) [DOI] [PubMed] [Google Scholar]
- 86.De Block M, Stoks R. 2005. Fitness effects from egg to reproduction: bridging the life history transition. Ecology 86, 185–197. ( 10.1890/04-0116) [DOI] [Google Scholar]
- 87.Crean AJ, Monro K, Marshall DJ. 2011. Fitness consequences of larval traits persist across the metamorphic boundary. Evolution 65, 3079–3089. ( 10.1111/j.1558-5646.2011.01372.x) [DOI] [PubMed] [Google Scholar]
- 88.Fellous S, Lazzaro BP. 2011. Potential for evolutionary coupling and decoupling of larval and adult immune gene expression. Mol. Ecol. 20, 1558–1567. ( 10.1111/j.1365-294X.2011.05006.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dickson LB, et al. 2017. Carryover effects of larval exposure to different environmental bacteria drive adult trait variation in a mosquito vector. Sci. Adv. 3, e1700585 ( 10.1126/sciadv.1700585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mira A, Moran NA. 2002. Estimating population size and transmission bottlenecks in maternally transmitted endosymbiotic bacteria. Microb. Ecol. 44, 137–143. ( 10.1007/s00248-002-0012-9) [DOI] [PubMed] [Google Scholar]
- 91.Moran NA. 1996. Accelerated evolution and Muller's rachet in endosymbiotic bacteria. Proc. Natl Acad. Sci. USA 93, 2873–2878. ( 10.1073/pnas.93.7.2873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kikuchi Y, Yumoto I. 2013. Efficient colonization of the bean bug Riptortus pedestris by an environmentally transmitted Burkholderia symbiont. Appl. Environ. Microbiol. 79, 2088–2091. ( 10.1128/AEM.03299-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolschin F, Hölldobler B, Gross R, Zientz E. 2004. Replication of the endosymbiotic bacterium Blochmannia floridanus is correlated with the developmental and reproductive stages of its ant host. Appl. Environ. Microbiol. 70, 4096–4102. ( 10.1128/AEM.70.7.4096-4102.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klein A, et al. 2016. A novel intracellular mutualistic bacterium in the invasive ant Cardiocondyla obscurior. ISME J. 10, 376–388. ( 10.1038/ismej.2015.119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nardon P, Grenier AM. 1988. Genetical and biochemical interactions between the host and its endocytobiotes in the weevils Sitophilus (Coleoptere, Curculionidae) and other related species. In Cell to cell signals in plant, animal and microbial symbiosis (eds Scannerini S, Smith D, Bonfante-Fasolo P, Gianinazzi-Pearson V), pp. 255–270. Berlin, Germany: Springer Verlag. [Google Scholar]
- 96.Estes AM, Hearn DJ, Bronstein JL, Pierson EA. 2009. The olive fly endosymbiont, ‘Candidatus Erwinia dacicola,’ switches from an intracellular existence to an extracellular existence during host insect development. Appl. Environ. Microbiol. 75, 7097–7106. ( 10.1128/AEM.00778-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stammer HJ. 1929. Die Symbiose der Lagriiden (Coleoptera). Zoomorphology 15, 1–34. [Google Scholar]
- 98.Kaltenpoth M, Göttler W, Herzner G, Strohm E. 2005. Symbiotic bacteria protect wasp larvae from fungal infestation. Curr. Biol. 15, 475–479. ( 10.1016/j.cub.2004.12.084) [DOI] [PubMed] [Google Scholar]
- 99.Weiss B, Kaltenpoth M. 2016. Bacteriome-localized intracellular symbionts in pollen-feeding beetles of the genus Dasytes (Coleoptera. Dasytidae). Front. Microbiol. 7, 1486 ( 10.3389/fmicb.2016.01486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gibson CM, Hunter MS. 2005. Reconsideration of the role of yeasts associated with Chrysoperla green lacewings. Biol. Control 32, 57–64. ( 10.1016/j.biocontrol.2004.06.006) [DOI] [Google Scholar]
- 101.Paludo CR, et al. 2018. Stingless bee larvae require fungal steroid to pupate. Sci. Rep. 8, 122 ( 10.1038/s41598-018-19583-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hammer TJ, Janzen DH, Hallwachs W, Jaffe SL, Fierer N. 2017. Caterpillars lack a resident gut microbiome. Proc. Natl Acad. Sci. USA 114, 9641–9646. ( 10.1073/pnas.1707186114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dobson HEM, Peng YS. 1997. Digestion of pollen components by larvae of the flower-specialist bee Chelostoma florisomne (Hymenoptera: Megachilidae). J. Insect Physiol. 43, 89–100. ( 10.1016/S0022-1910(96)00024-8) [DOI] [PubMed] [Google Scholar]
- 104.Hu Y, et al. 2017. By their own devices: invasive Argentine ants have shifted diet without clear aid from symbiotic microbes. Mol. Ecol. 26, 1608–1630. ( 10.1111/mec.13991) [DOI] [PubMed] [Google Scholar]
- 105.Chen C, Linse K, Uematsu K, Sigwart JD. 2018. Cryptic niche switching in a chemosymbiotic gastropod. Proc. R. Soc. B 285, 1–6. ( 10.1098/rspb.2018.1099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Russell JA, Moran NA. 2006. Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc. R. Soc. B 273, 603–610. ( 10.1098/rspb.2005.3348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kohl KD, Brun A, Bordenstein SR, Caviedes-Vidal E, Karasov WH. 2018. Gut microbes limit growth in house sparrow nestlings (Passer domesticus) but not through limitations in digestive capacity. Integr. Zool. 13, 139–151. ( 10.1111/1749-4877.12289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mason KL, Stepien TA, Blum JE, Holt JF, Labbe NH, Rush JS, Raffa KF, Handelsman J. 2011. From commensal to pathogen: translocation of Enterococcus faecalis from the midgut to the hemocoel of Manduca sexta. MBio 2, e00065-11 ( 10.1128/mBio.00065-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hartmann AC, et al. 2019. Acquisition of obligate mutualist symbionts during the larval stage is not beneficial for a coral host. Mol. Ecol. 28, 141–155. ( 10.1111/mec.14967) [DOI] [PubMed] [Google Scholar]
- 110.Price DRG, Feng H, Baker JD, Bavan S, Luetje CW, Wilson ACC. 2014. Aphid amino acid transporter regulates glutamine supply to intracellular bacterial symbionts. Proc. Natl Acad. Sci. USA 111, 320–325. ( 10.1073/pnas.1306068111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Login FH, Balmand S, Vallier A, Vincent-Monégat C, Vigneron A, Weiss-Gayet M, Rochat D, Heddi A. 2011. Antimicrobial peptides keep insect endosymbionts under control. Science 334, 362–365. ( 10.1126/science.1209728) [DOI] [PubMed] [Google Scholar]
- 112.Nyholm SV, McFall-Ngai M. 2004. The winnowing: establishing the squid–Vibrio symbiosis. Nat. Rev. Microbiol. 2, 632–642. ( 10.1038/nrmicro957) [DOI] [PubMed] [Google Scholar]
- 113.Ohbayashi T, et al. 2015. Insect's intestinal organ for symbiont sorting. Proc. Natl Acad. Sci. USA 112, E5179–E5188. ( 10.1073/pnas.1511454112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang AS. 2001. Modularity, evolvability, and adaptive radiations: a comparison of the hemi- and holometabolous insects. Evol. Dev. 72, 59–72. ( 10.1046/j.1525-142x.2001.003002059.x) [DOI] [PubMed] [Google Scholar]
- 115.de Roode JC, Lefèvre T. 2012. Behavioral immunity in insects. Insects 3, 789–820. ( 10.3390/insects3030789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gätschenberger H, Azzami K, Tautz J, Beier H. 2013. Antibacterial immune competence of honey bees (Apis mellifera) is adapted to different life stages and environmental risks. PLoS ONE 8, e66415 ( 10.1371/journal.pone.0066415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Engl T, Kroiss J, Kai M, Nechitaylo TY, Svatoš A, Kaltenpoth M. 2018. Evolutionary stability of antibiotic protection in a defensive symbiosis. Proc. Natl Acad. Sci. USA 115, E2020–E2029. ( 10.1073/pnas.1719797115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jarosz J. 1979. Gut flora of Galleria mellonella suppressing ingested bacteria. J. Invertebr. Pathol. 198, 192–198. ( 10.1016/0022-2011(79)90101-0) [DOI] [PubMed] [Google Scholar]
- 119.Anbutsu H, et al. 2017. Small genome symbiont underlies cuticle hardness in beetles. Proc. Natl Acad. Sci. USA 114, E8382–E8391. ( 10.1073/pnas.1712857114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stoll S, Feldhaar H, Fraunholz MJ, Gross R. 2010. Bacteriocyte dynamics during development of a holometabolous insect, the carpenter ant Camponotus floridanus. BMC Microbiol. 10, 308 ( 10.1186/1471-2180-10-308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zientz E, Beyaert I, Gross R, Feldhaar H. 2006. Relevance of the endosymbiosis of Blochmannia floridanus and carpenter ants at different stages of the life cycle of the host. Appl. Environ. Microbiol. 72, 6027–6033. ( 10.1128/AEM.00933-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rio RVM, et al. 2012. Insight into the transmission biology and species-specific functional capabilities of tsetse (Diptera: Glossinidae) obligate symbiont Wigglesworthia. MBio 3, e00240-11 ( 10.1128/mbio.00240-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dedeine F, Vavre F, Fleury F, Loppin B, Hochberg ME, Bouletreau M. 2001. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl Acad. Sci. USA 98, 6247–6252. ( 10.1073/pnas.101304298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sullivan W. 2017. Wolbachia, bottled water, and the dark side of symbiosis. Mol. Biol. Cell 28, 2343–2346. ( 10.1091/mbc.e17-02-0132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hadfield MG. 2011. Biofilms and marine invertebrate larvae: what bacteria produce that larvae use to choose settlement sites. Ann. Rev. Mar. Sci. 3, 453–470. ( 10.1146/annurev-marine-120709-142753) [DOI] [PubMed] [Google Scholar]
- 126.Webster NS, Smith LD, Heyward AJ, Watts JEM, Webb RI, Blackall LL, Negri AP. 2004. Metamorphosis of a scleractinian coral in response to microbial biofilms. Appl. Environ. Microbiol. 70, 1213–1221. ( 10.1128/AEM.70.2.1213-1221.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shikuma NJ, Pilhofer M, Weiss GL, Hadfield MG, Jensen GJ, Newman DK. 2014. Marine tubeworm metamorphosis induced by arrays of bacterial phage tail-like structures. Science 343, 529–533. ( 10.1126/science.1246794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shikuma NJ, Antoshechkin I, Medeiros JM, Pilhofer M, Newman DK. 2016. Stepwise metamorphosis of the tubeworm Hydroides elegans is mediated by a bacterial inducer and MAPK signaling. Proc. Natl Acad. Sci. USA 113, 10 097–10 102. ( 10.1073/pnas.1603142113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Coon KL, Valzania L, McKinney DA, Vogel KJ, Brown MR, Strand MR. 2017. Bacteria-mediated hypoxia functions as a signal for mosquito development. Proc. Natl Acad. Sci. USA 114, E5362–E5369. ( 10.1073/pnas.1702983114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Valzania L, Martinson VG, Harrison RE, Boyd BM, Coon KL, Brown MR, Strand MR. 2018. Both living bacteria and eukaryotes in the mosquito gut promote growth of larvae. PLoS Negl. Trop. Dis. 12, e0006638 ( 10.1371/journal.pntd.0006638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moran NA. 2002. The ubiquitous and varied role of infection in the lives of animals and plants. Am. Nat. 160, S1–S8. ( 10.1086/342113) [DOI] [PubMed] [Google Scholar]
- 132.Douglas AE. 2010. The symbiotic habit. Princeton, NJ: Princeton University Press. [Google Scholar]
- 133.McMahon DP, Hayward A. 2016. Why grow up? A perspective on insect strategies to avoid metamorphosis. Ecol. Entomol. 41, 505–515. ( 10.1111/een.12313) [DOI] [Google Scholar]
- 134.Stoll S, Feldhaar H, Gross R. 2009. Transcriptional profiling of the endosymbiont Blochmannia floridanus during different developmental stages of its holometabolous ant host. Environ. Microbiol. 11, 877–888. ( 10.1111/j.1462-2920.2008.01808.x) [DOI] [PubMed] [Google Scholar]
- 135.Mondotte JA, Gausson V, Frangeul L, Blanc H, Lambrechts L, Saleh M-C. 2018. Immune priming and clearance of orally acquired RNA viruses in Drosophila. Nat. Microbiol. 3, 1394–1403. ( 10.1038/s41564-018-0265-9) [DOI] [PubMed] [Google Scholar]
- 136.Critchlow JT, Norris A, Tate AT. 2019. The legacy of larval infection on immunological dynamics over metamorphosis. Phil. Trans. R. Soc. B 374, 20190066 ( 10.1098/rstb.2019.0066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Johnston PR, Paris V, Rolff J. 2019. Immune gene regulation in the gut during metamorphosis in a holo- versus a hemimetabolous insect. Phil. Trans. R. Soc. B 374, 20190073 ( 10.1098/rstb.2019.0073) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.