Abstract
The three modes of insect postembryonic development are ametaboly, hemimetaboly and holometaboly, the latter being considered the only significant metamorphosis mode. However, the emergence of hemimetaboly, with the genuine innovation of the final moult, represents the origin of insect metamorphosis and a necessary step in the evolution of holometaboly. Hemimetaboly derives from ametaboly and might have appeared as a consequence of wing emergence in Pterygota, in the early Devonian. In extant insects, the final moult is mainly achieved through the degeneration of the prothoracic gland (PG), after the formation of the winged and reproductively competent adult stage. Metamorphosis, including the formation of the mature wings and the degeneration of the PG, is regulated by the MEKRE93 pathway, through which juvenile hormone precludes the adult morphogenesis by repressing the expression of transcription factor E93, which triggers this change. The MEKRE93 pathway appears conserved in extant metamorphosing insects, which suggest that this pathway was operative in the Pterygota last common ancestor. We propose that the final moult, and the consequent hemimetabolan metamorphosis, is a monophyletic innovation and that the role of E93 as a promoter of wing formation and the degeneration of the PG was mechanistically crucial for their emergence.
This article is part of the theme issue ‘The evolution of complete metamorphosis’.
Keywords: evolution of insect metamorphosis, juvenile hormone, E93, prothoracic gland degeneration, mekre93 pathway, origin of insect final moult
1. Introduction
The main modes of postembryonic development in insects are ametaboly, hemimetaboly and holometaboly. Ametabolan insects do not undergo significant morphological transformations during their life cycle, and they continue to moult after becoming reproductively competent. This is seen in the Archaeognatha and Zygentoma. Hemimetabolan species hatch as nymphs that are morphologically similar to the adults and grow progressively until the last nymphal instar, which moults into the adult stage. The adults differ from the nymphs mainly by having wings and genitalia, and because they no longer moult. Hemimetaboly is characteristic of the Palaeoptera, Polyneoptera and Paraneoptera. Holometabolan insects hatch as larvae that are morphologically very divergent from the adults; they progressively grow through successive moults until the last larval instar and the pupa, often quiescent and similar to the adult, and then to the adult stage, with flying wings and functional genitalia, which no longer moults. All the Endopterygota are holometabolous [1–5].
Evolutionarily, ametaboly is the most ‘ancestral’ mode of postembryonic development. Ametaboly gave rise to hemimetaboly, which in turn gave rise to holometaboly [3–5]. Often, holometaboly is considered the only significant type of metamorphosis [6–8], and some authors even consider metamorphosis and holometaboly to be synonymous [9]. For this reason, studies have focused predominantly on the evolution of holometaboly [1,2]. However, the emergence of hemimetaboly, consubstantial with the innovation of the final moult, represents the origin of insect metamorphosis and a necessary step in the evolution of holometaboly, the process through which more than 80% of extant insects metamorphose [10], even if this was not the case during the Palaeozoic. In this context, the present essay contends that the emergence of hemimetaboly should be considered one of the great innovations in insect evolution.
Hemimetaboly emerged with the clade Pterygota (viz. presence of wings), as we have no fossil record of any apterous hemimetabolan insects in the Devonian [11,12]. The innovation of hemimetabolan metamorphosis after the emergence of wings [13–15] is probably not coincidental, but rather hemimetaboly could have come about as a consequence of wing acquisition. Ametabolan species, like the firebrat Thermobia domestica (Zygentoma), undergo morphological modifications throughout their life cycle, including the apparition of scales between the third and the fourth nymphal instars [16]. Therefore, the most genuine innovation after the emergence of wings is the final moult, mainly achieved through the degeneration of the prothoracic gland (PG), which produces the moulting hormone, and perhaps also through the adult commitment of epidermal cells, which become incapable of producing a new cuticle. This would happen after the full formation of the flying and reproductively competent adult stage. This work aims to explore the circumstances that surrounded and facilitated the transition from ametaboly to hemimetaboly, and the presumptive mechanisms that made this transition possible.
2. The phylogenetic and Palaeontological framework
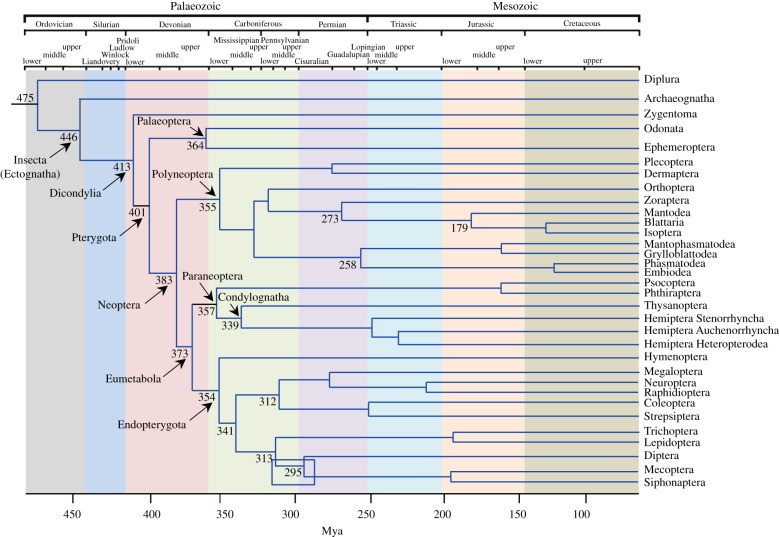
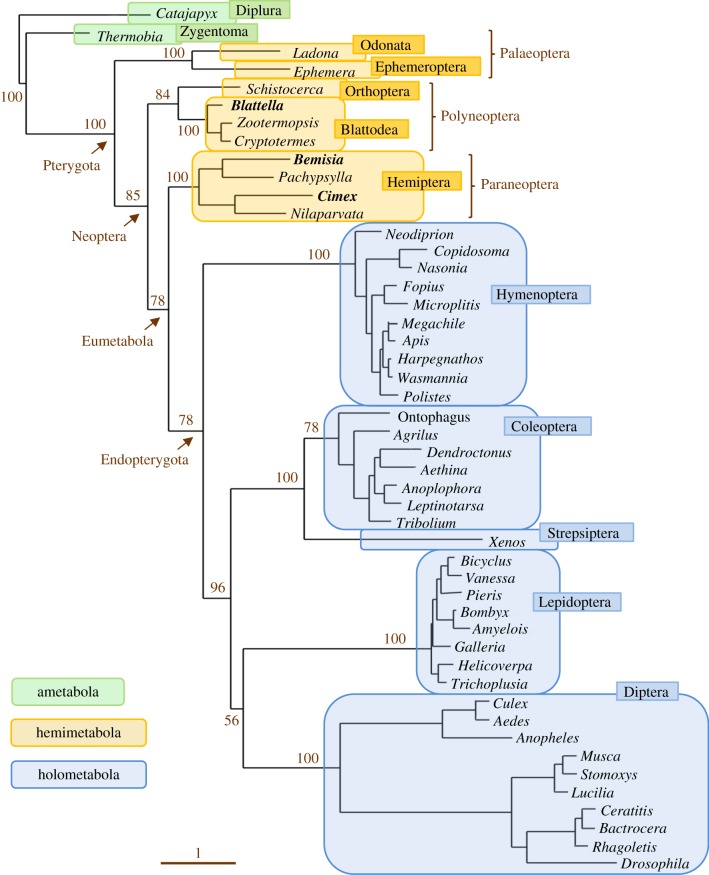
Phylogenetic and palaeontological data indicate that wings and hemimetaboly originated during the early–middle Devonian, some 400 Ma [11,12] (figure 1). The fact that extant pterygotes are monophyletic [11,12], and that the wing types of all insect orders are constructed in the same way, both in extant and fossil species [17], strongly suggest that wings evolved only once. The monophyly of Pterygota, in turn, suggests that there was a single origin of hemimetaboly and that this could be linked to the emergence of wings.
Figure 1.
Cladogenesis of the main insect groups in a chronological context. The phylogenetic reconstruction is based on Misof et al. [11] and Wang et al. [12]. The main discrepancy between these two proposals is the situation of Paraneoptera, which is monophyletic for Wang et al. and polyphyletic for Misof et al. Divergence times are generally similar in both proposals. Those indicated here are based on the average values reported by Wang et al. [12]. Modified from [12]. (Online version in colour.)
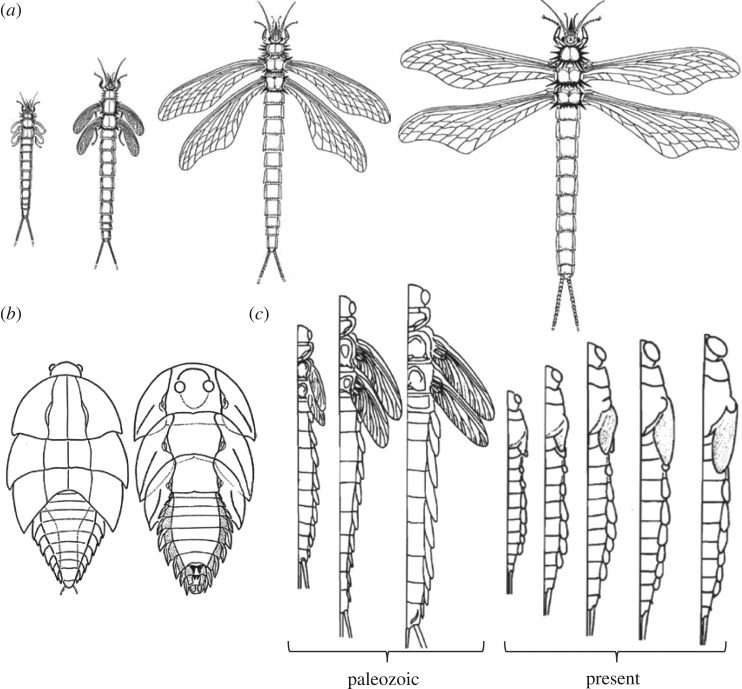
Fossils can provide direct evidence of the origin and morphological evolution of metamorphosis. Nevertheless, studies documenting the fossils of successive stages of the same species are scarce [18]. The most complete series of Palaeozoic fossils of successive nymphal phases are of Megasecoptera and Ephemeroptera, as reported, for example, by Kukalová-Peck [13–15]. These series show how the wings of the early nymphs were small and curved backwards. With successive moults, the wings increased in length, became less curved and tended to adopt a position perpendicular to the body. In the adult, the wings were slender, with the basal region narrower than the distal, and practically perpendicular to the body axis [13–15,18] (figure 2a). Another type of wing development is exemplified by Palaeozoic roachoid nymphs, early representatives of the Blattodea lineage, which broadly resemble extant cockroach nymphs (figure 2b). In extant cockroaches, the wing pads develop posteriorly and the attachment is latero-posterior, whereas in Carboniferous forms, the wing pads protruded to the sides and the attachment area was restricted to the lateral tergum [18].
Figure 2.
Palaeozoic and extant insects. (a) Selected developmental stages of Palaeozoic Mischoptera (Megasecoptera) species, from left to right, young nymph with articulated wings arched backwards (wing venation is still absent), older nymph with larger wings already showing wing venation, mature nymph or subadult with straighter wings and adult, with the wings practically perpendicular to the body axis. (b) Two Carboniferous roachoid nymphs, one in dorsal (left) and the other in ventral position, showing long wing pads. (c) Wing development in Palaeozoic and extant Ephemeroptera. Note the articulated and detached winglets in Palaeozoic mayflies and the winglets attached to the body in extant species. From [14].
In those fossil groups with external nymphal winglets, such as the Ephemeroptera and Megasecoptera, selective pressures might have acted to facilitate the concealment of these in juveniles (figure 2c) [13]. It is possible that, among other advantages, concealed winglets facilitated moulting by reducing mechanical problems during ecdysis and the shedding off the exuvia. The evolution of Palaeozoic roachoids could have followed the same tendency, with the winglets concealed by being retracted backwards and attached to the body. Although it is difficult to discern in fossil samples, the winglet morphology of Palaeozoic roaches suggests they were not membranous. They are more reminiscent of the pterotheca or cuticular pocket that envelops the wing primordia of extant cockroaches [19].
3. The final moult as a crucial innovation in the origin of the Pterygota
Fossils of Palaeozoic nymphs suggest that ‘ancestral’ Pterygota had a remarkable diversity of morphologies and life cycles. This led Kukalová-Peck [13,14] to argue that, in the Carboniferous, the insect life cycle comprised a high number of moults, and that older nymphs had articulated, functional wings. Kukalová-Peck [14] suggested that a number of late nymphal stages could have become ‘condensed’ into the adult stage, and that also younger nymphs would have undergone various degrees of ‘condensation’. The general tendency would have been to reduce the number of moults, and differences in the juvenile/imaginal specialization in the various stages would have produced the life cycle diversity observed in the fossils.
The sometimes disparate nymphal morphologies (as between mayflies and roachoids, for example), and the different life cycles revealed by the Palaeozoic fossils suggested to Kukalová-Peck [13,14] that metamorphosis evolved independently and at distinct rates in the diverging hemimetabolan lineages. However, although hemimetabolan life cycles can be very different in terms of number of moults and nymphal morphologies, in all of them the development ends with a final moult. It is proposed here that this final moult was a crucial innovation in the evolution of insects, consubstantial with the origin of hemimetabolan metamorphosis. We also contend that there was a single origin of the final moult, in the origin of the monophyletic Pterygota. This hypothesis is supported by data on the mechanisms determining the degeneration of the PG and the final moult, as explained below.
The hypothesis of the monophyletic origin of the final moult is compatible with the diverse life cycles observed in the different hemimetabolan groups, which would have evolved independently in each lineage. It is also compatible with scenarios of life cycle evolution through the ‘condensation’ of late nymphal stages with articulated wings, as proposed by Kukalová-Peck [14]. The final moult could have been fixed immediately after the formation of the mature wings, membranous, without active epidermal cells and fully efficient for flying, although there may have been intermediate subimaginal stages with (precariously) flying wings, like in extant Ephemeroptera.
4. The case of Ephemeroptera
The Ephemeroptera are unique among extant insects in that they moult once more after forming flying wings. Thus, the last moult of the nymphal period does not achieve the definitive adult form, but rather a winged stage, known as a subimago, which morphologically resembles the adult. The outer and hind edges of wings in the subimago present a row of cilia, and their surface is covered with microtrichia, whereas the adult wings of most species lack these hairy structures [20]. The regulation of the subimago to imago moult is intriguing, because in some cases, the subimago stage lasts for minutes or a few hours before the ecdysis to the imago. Ecdysone measurements might help to explain how two so closely following moults can be regulated.
The evolutionary and functional sense of the subimago has been widely discussed. It has been proposed that the hydrofuge properties of the hairy surface of the subimago body and wings have a particular function, as these could help to overcome the hazards of transitioning from an aquatic nymph to a winged aerial form at the water–air interface [21]. Maiorana [22] conjectured that the subimaginal stage would allow the necessary growth to form a full-sized and reproductively competent adult. The palaeontological data (see above) suggest that the subimago is a ‘primitive’ feature, conserved in extant species as a kind of relic of preadult winged instars of ‘ancestral’ mayflies. Thus, according to the currently accepted phylogenies (figure 1), the subimago appears to be a plesiomorphy conserved in extant mayflies. Then, the subimago (or the imago) would have been convergently lost in other extant Pterygota.
A few examples where the moult from subimago to adult is incomplete or absent are worth mentioning. In females of at least two species of Leptophlebiidae, the apolysis of the subimago to the adult occurs but the ecdysis does not. Oligoneuriinae mayflies are also peculiar because when moulting to the adult stage they shed off the exuvia from the body but retain the subimaginal cuticle on the wings. Finally, females of a few specialized species of Palingeniinae seem to have lost the adult stage, and the subimago is the last one [20]. Significantly, this feature was first reported by Swammerdam in 1675, when he discovered that adult males of Palingenia longicauda moult twice but females do so only once [23]. These examples suggest that evolution may favour the merging of winged stages in the life cycle of mayflies, as Kukalová-Peck [14] proposed, and as must have happened in other insect lineages where there is a single winged stage.
5. E93 and the MEKRE93 pathway
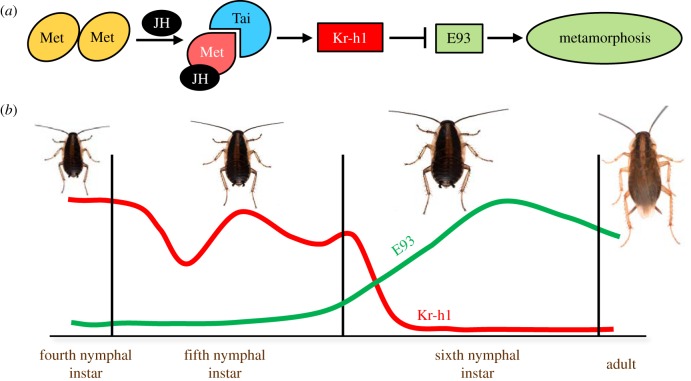
In extant hemimetabolan insects, the PG degenerates with the imaginal moult (see, for example [24,25]). The cell death process is associated with the hormonal and molecular mechanisms that regulate the metamorphosis, in general, which are condensed in the MEKRE93 pathway [26]. The metamorphosis-triggering action of E93 occurs in both hemimetabolan and holometabolan species [27], but in hemimetabolan metamorphosis, the MEKRE93 pathway is simpler: juvenile hormone (JH) bound to its receptor Methoprene tolerant (Met) [28] induces the expression of Krüppel homolog 1 (Kr-h1), and Kr-h1 represses the expression of E93, thus precluding metamorphosis [26,29] (figure 3a). As exemplified in the German cockroach, Blattella germanica, JH production ceases early in the last nymphal stage, the expression of Kr-h1 decreases and that of E93 increases, bringing about the metamorphic moult (figure 3b). The study of mature wing formation in Drosophila melanogaster shows that E93 acts as a chromatin modifier, enabling or preventing expression in certain genetic regions [30]. This helps to explain the pleiotropic action of E93, which simultaneously triggers the development of the different tissues and organs that configure the adult morphology.
Figure 3.
The MEKRE93 pathway exemplified by the cockroach Blattella germanica. (a) Juvenile hormone (JH) disrupts the homodimer Methoprene tolerant (Met)-Met, binds to Met, recruits the co-receptor Taiman (Tai), and the complex JH-Met + Tai induces the expression of Krüppel homolog 1 (Kr-h1), and Kr-h1 represses the expression of E93, which codes for the metamorphosis-triggering factor. (b) Early in the last (sixth) nymphal instar, the production of JH ceases, the expression of Kr-h1 decreases and that of E93 increases, thus bringing about the metamorphic moult.
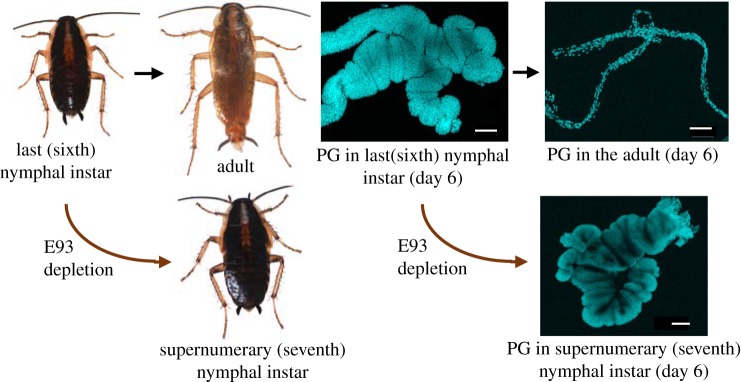
Additionally, E93 is a key factor regulating cell death processes, such as midgut and fat body remodelling, and salivary gland degeneration at the onset of metamorphosis in D. melanogaster [31–34]. These antecedents suggest the hypothesis that E93 might also be involved in the degeneration of the PG in holometabolan and hemimetabolan species. In the hemimetabolan B. germanica, RNAi depletion of E93 in the last nymphal instar precludes metamorphosis, and the nymphs moult to successive supernumerary nymphs [26,27]. As expected, the PG does not degenerate in E93-depleted supernumerary nymphs (figure 4).
Figure 4.
Depletion of E93 in the last nymphal instar of the cockroach Blattella germanica inhibits metamorphosis. The last nymphal instar (sixth) moults into a supernumerary (seventh) nymphal instar, with full nymphal features, including the absence of wings, instead moulting to a winged adult (left). After the imaginal moult, from the sixth nymphal instar, the prothoracic gland (PG) degenerates within the first days of adult life, but the E93-depleted, supernumerary (seventh) nymphal instar keeps an active PG and can moult again (right). Photos from Carolina G. Santos (left) and Orathai Kamsoi (right).
Among the hemimetabolan insects, the MEKRE93 pathway operates in Polyneoptera [26,27,35] and Paraneoptera [36,37]. In Palaeoptera, a transcriptomic study conducted on the Ephemeroptera Cloeon viridulum revealed that the expression of Kr-h1 decreases progressively from young to mature nymphs, and to the subimago [38], suggesting that Kr-h1 represses metamorphosis. Unfortunately, expression of E93 was not reported. In the neotenic Hemiptera Coccomorpha (Paraneoptera), male postembryonic development includes the quiescent stages of ‘prepupa’ and ‘pupa’ that precede the adult. Interestingly, the expression of Kr-h1 decreases in the ‘prepupa’ phase, while the expression of E93 increases [39,40]. This suggests that in these hemimetabolan species with complex life cycles, the MEKRE93 pathway may operate similarly to the way it does in other hemimetabolan species.
6. The origin of metamorphosis from a mechanistic perspective
Possibly, the ancestral role of the JH in insects was related to reproductive functions, a role that is still played in Zygentoma [41,42] (see below) and in most metamorphosing insects [43], and even in crustaceans [44], the sister group of the hexapods [45]. Subsequently, JH would have been further co-opted to perform functions related to development, in particular, to prevent metamorphosis. In this context, data reported above suggest that the MEKRE93 pathway regulates metamorphosis in the extant hemimetabolan clades Palaeoptera, Polyneoptera and Paraneoptera and that it would have operated in the last common ancestor of the hemimetabolan groups. If so, then a single mechanism, the MEKRE93 pathway and E93 in particular, might have simultaneously regulated wing formation and PG degeneration, and hence the final moult (i.e. hemimetabolan metamorphosis), at the origin of the Pterygota.
Much progress has been made in explaining the evolutionary origin of insect wings. The theory that currently enjoys the greatest consensus postulates a dual origin, tergal and pleural, of the wings (see [46] for a review). However, there are no theories as to which factors and mechanisms might have triggered and regulated the formation and maturation of the ‘ancestral’ wing. What we currently know about E93 makes it a prime candidate to be one of the key factors. From a mechanistic point of view, one of the first innovations in the ‘ancestor’ of the Pterygota could have been the upregulation of E93 expression in mature nymphs, mediated by the ecdysone signalling. An increase in the expression of E93 could have contributed to the maturation of the wings, and, in turn, to the degeneration of the PG. E93 could also contribute to the adult commitment of epidermal cells, thus making them incompetent to produce a new cuticle, even in the presence of the circulating ecdysteroids produced by the adult ovary (reviewed in [47]). In extant insects, the pleiotropic action of E93 appears to be facilitated by its properties as a chromatin modifier, enabling or preventing expression in certain genomic regions [30]. If these properties are ‘ancestral’, then they could have been a versatile way to turn the appropriate genes on (or off) to regulate new processes, including wing maturation and PG degeneration. Another necessary requirement would be for JH to play an inhibitory role in metamorphosis, possibly through Kr-h1 repressing E93, as in extant insects. This would have allowed proper growth of the nymph. Refinements of this incipient MEKRE93 pathway, driven by natural selection, would have adjusted the regulation of the genes involved, mainly Kr-h1 and E93, in different tissues and at the appropriate times, producing in a more precise system of regulation. Differential solutions for ‘condensing’ juvenile and/or subadult stages in different lineages [14] would have generated the life cycle diversity that we observe today among the hemimetabolan insects. The same Kr-h1–E93 axis could have regulated the tendency to attenuate the development of the wing pads in the juvenile phases [13] (figure 5).
Figure 5.
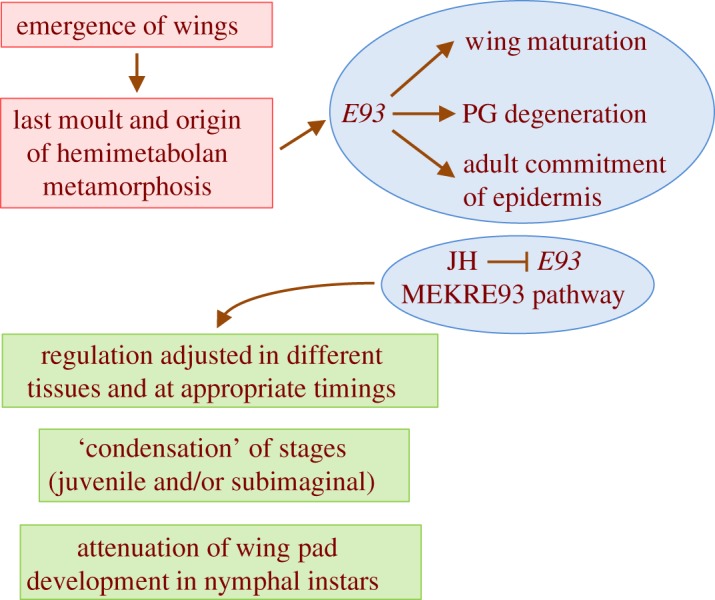
The emergence of wings may have triggered the innovation of the last moult and the origin of (hemimetabolan) metamorphosis. One of the first innovations could have been the upregulation of E93 expression in mature nymphs. This could have contributed to wing maturation, prothoracic gland degeneration and adult commitment of epidermis. Another necessary requirement would be for JH to play an inhibitory role in metamorphosis, repressing E93, possibly through Kr-h1, as in extant insects. Refinements of this incipient MEKRE93 pathway would have adjusted the regulation of the genes involved, mainly Kr-h1 and E93, in different tissues and at the appropriate times. Differential solutions for ‘condensing’ juvenile and/or subadult stages in different lineages would have produced the life cycle diversity that we observe today in hemimetabolan species. The same Kr-h1–E93 axis could have regulated the tendency to attenuate the development of the wing pads in nymphal instars. (Online version in colour.)
7. The components of the MEKRE93 pathway predate the origin of Pterygota
The Met and Kr-h1 genes are present in T. domestica [48], a representative of the Zygentoma, the sister group of pterygote insects and which has ametabolan development. JH is involved in vitellogenesis in T. domestica [41,42], therefore, Met, and perhaps Kr-h1, might operate in this reproductive function. Indeed, there is functional evidence that T. domestica Met actually binds JH III [28]. Moreover, the degree of amino acid sequence conservation of the two corresponding proteins, Met and Kr-h1, in T. domestica, as compared with the corresponding orthologues in pterygote insects, is very high [48], suggesting conservation of general functions. Intriguingly, we have found that T. domestica also has the E93 gene (electronic supplementary material, figure S1), the last component of the MEKRE93 pathway. Thermobia domestica E93 contains the typical two helix-turn-helix DNA binding motifs of the pipsqueak family (RHF-1 and RHF-2), and, in general, the sequence is highly conserved with respect to other pterygote orthologues. Indeed, the phylogenetic analysis of representative insect E93 orthologues, including T. domestica, as well as the Diplura Catajapyx aquilonaris as an hexapodan external group, reveals a topology which perfectly matches that recovered in the most recent and robust phylogenetic studies in insects [11,12] (figure 6). The conservation of T. domestica E93 is greatest in the RHF-1 and RHF-2 motifs, which are, respectively, 97% and 93% identical to those of B. germanica E93, and 89% and 98% to those of Nilaparvata lugens (electronic supplementary material, figure S2). In the hemimetabolan species B. germanica and N. lugens, it has been experimentally demonstrated that E93 acts as a metamorphosis trigger [26,27,37].
Figure 6.
Phylogenetic analysis of E93 orthologues of Palaeoptera, Polyneoptera, Paraneoptera and Endopterygota species, as well as Thermobia domestica, as a representative of the ametabolan Zygentoma, and Catajapyx aquilonaris (Hexapoda, Diplura) as an hexapodan external group. Alignments were carried out with ClustalX [49] and phylogenetic reconstruction with RAxML [50], based on the maximum-likelihood principle, a JTT matrix, a gamma model of heterogeneity rate, and using empirical base frequencies and estimating proportions. The data were bootstrapped for 100 replicates. Bootstrap values are indicated in the subclass, superorder and order nodes. Scale bar indicates the number of substitutions per site. Complete species names and accession data of the respective sequences are detailed in the electronic supplementary material, table S1.
The Zygentoma do not metamorphose, but they do undergo changes throughout their life cycle, the most apparent of which is the formation of scales in early instars. In T. domestica, scales appear towards the middle of the third nymphal instar [16], and integument transplantation experiments, as well as corpora allata (CA; the JH producing glands) volume and activity measurements [51], suggest that these scales appear shortly after a transient decrease in JH. According to Watson [51], when a fragment of integument from a first nymphal instar of T. domestica was implanted in an adult that was going to moult, scales formed in the implanted fragment, suggesting that scale formation is determined by a humoral factor. CA measurements showed that the minimal gland volume is found in the early–mid third nymphal instar, in other words prior to scale formation. It is worth noting that CA volume correlates approximately with CA activity, as demonstrated by techniques for measuring JH production [52]. Finally, the JH activity of the CA, when tested using the Gilbert and Schneiderman bioassay [53], was shown to be minimal in the third stage [51]. The data as a whole suggest that the formation of scales in early nymphs of T. domestica is repressed by JH.
8. Testable hypotheses
A point to be tested is whether the MEKRE93 pathway operates in Palaeoptera, to confirm that it is conserved in the three extant insect superorders. Ephemeroptera may be the choice case study, as it allows us to look at not only the regulation of metamorphosis in general but also that of the exceptional subimago. Expression patterns and functional genomics experiments would show whether Kr-h1 transduces the antimetamorphic signal of JH, and whether E93 promotes metamorphosis and is repressed by Kr-h1. It should also provide an insight into how PG degeneration is prevented in the subimago.
The life cycle of Thysanoptera and Hemiptera Coccomorpha, which are paraneopteran and hemimetabolan groups, includes one or more preadult, pupa-like quiescent stages. Understanding how these stages are determined could reveal whether there are similar mechanisms that regulate the holometabolan pupa. If so, this would suggest that the quiescent states of these Paraneopera represent an exaptation in the evolution of holometabolan metamorphosis and the pupal stage. In Thysanoptera and male Coccomorpha, the expression of Kr-h1 decreases progressively from nymphs to the preadult stages and ceases in the adult [39,54]. This suggests that Kr-h1 represses metamorphosis in these insects. In male Coccomorpha, moreover, E93 expression increases in the preadult stages, at the same time that of Kr-h1 decreases [40]; this suggests that Kr-h1 represses E93, whereas E93 triggers metamorphosis [26]. Although there are clear technical difficulties owing to the minute size of these insects, it would be interesting to undertake functional studies where Kr-h1 and E93 were depleted and suppressed, in order to confirm that the MEKRE93 pathway operates in these groups. Functional studies would also shed light on how Kr-h1 and E93, and perhaps Broad complex, a factor that determines the pupal stage in holometabolan species [55], interact with each other to determine these pupa-like stages.
The main hypotheses of this work are that the final moult, and the consequent hemimetabolan metamorphosis, is a monophyletic innovation and that the role of E93 as a promoter of wing formation and the degeneration of the PG was mechanistically crucial for this innovation. Demonstrating that E93 is fundamental in wing formation and PG degeneration is therefore essential. The role of E93 in the formation of mature wings is part of the morphogenetic action of this factor in metamorphosis [26,27], and the role of E93 on wing formation has been specifically described in D. melanogaster [30]. Data available on PG degeneration in cockroaches show that supernumerary nymphs obtained in E93-depleted insects have active PG (figure 4), and moult again. The survival of PG in these nymphs must be considered in the context of metamorphosis inhibition, in general. It would be interesting to uncouple the effect of E93 on adult morphogenesis from its possible direct role in PG degeneration. This could be undertaken in D. melanogaster by suppressing the expression of E93 specifically in the PG cells. In hemimetabolan species, the possible specific role of E93 in PG degeneration could be studied using systemic RNAi, but depleting the expression of E93 towards the end of the last nymphal stage, when its morphogenetic effects have already been implemented. The same approach could be followed to study whether E93 is involved in the adult commitment of the epidermal cells.
The study of the role of Kr-h1 and E93 in Zygentoma has an obvious evolutionary interest. The formation of scales in young nymphs of T. domestica might be determined by a transient decrease of JH [51]. Treatment of pre-scaled juveniles with JH would allow us to assess whether the formation of scales is prevented by this hormone. Moreover, expression studies could suggest whether Kr-h1 is involved in the transduction of the JH signal, and whether E93 is involved in scale formation. The depletion or suppression of Kr-h1 and E93 expression could show whether these factors play any role in regulating scale formation, or any other morphological transformation, such as the formation of functional genitalia. Heritable targeted mutagenesis based on CRISPR/Cas9 approaches has been recently developed in T. domestica [56], which makes possible the above functional studies. If morphogenetic functions of E93 are demonstrated in the Zygentoma, this would represent an exaptation for the roles of E93 in the formation of the adult in hemimetabolan metamorphosis. The degeneration of the PG (thus determining the last moult), and the possible role of E93 in this process, would therefore be the most genuine innovation in the transition from ametaboly to hemimetaboly.
Supplementary Material
Acknowledgements
Thanks are due to Jarmila Kukalová-Peck for her hospitality during the week I spent in Ottawa and for showing me her insect fossils collection. Thanks are also due to André Nel, from the Muséum national d'Histoire naturelle (Paris), and Takahiro Ohde, from Kyoto University, for valuable suggestions after critically reading the first version of the manuscript.
Data accessibility
The data supporting this review are included in the original articles listed as references. The sequences used to study the phylogeny of E93 are indicated in the electronic supplementary material, table S1.
Competing interests
I declare I have no competing interests.
Funding
This work was supported by the Spanish Ministry of Economy and Competitiveness (grant nos. CGL2012–36251 and CGL2015–64727-P) and the Catalan Government (grant 2017 SGR 1030). It also received financial assistance from the European Fund for Economic and Regional Development (FEDER funds).
References
- 1.Sehnal F, Svacha P, Zrzavy J. 1996. Evolution of insect metamorphosis. In Metamorphosis. Postembryonic reprogramming of gene expression in amphibian and insect cells (eds Gilbert LI, Tata JR, Atkinson BG), pp. 3–58. San Diego, CA: Academic Press. [Google Scholar]
- 2.Truman JW, Riddiford LM. 1999. The origins of insect metamorphosis. Nature 401, 447–452. ( 10.1038/46737) [DOI] [PubMed] [Google Scholar]
- 3.Belles X. 2011. Origin and evolution of insect metamorphosis. In Encyclopedia of life sciences (ELS), pp. 1–11. Chichester, UK: John Wiley & Sons, Ltd; ( 10.1002/9780470015902.a0022854) [DOI] [Google Scholar]
- 4.Truman JW, Riddiford LM. 2019. The evolution of insect metamorphosis: a developmental and endocrine view. Phil. Trans. R. Soc. B 374, 20190070 ( 10.1098/rstb.2019.0070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jindra M. 2019. Where did the pupa come from? The timing of juvenile hormone signalling supports homology between stages of hemimetabolous and holometabolous insects. Phil. Trans. R. Soc. B 374, 20190064 ( 10.1098/rstb.2019.0064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rainford JL, Hofreiter M, Nicholson DB, Mayhew PJ. 2014. Phylogenetic distribution of extant richness suggests metamorphosis is a key innovation driving diversification in insects. PLoS ONE 9, e109085 ( 10.1371/journal.pone.0109085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson DB, Ross AJ, Mayhew PJ. 2014. Fossil evidence for key innovations in the evolution of insect diversity. Proc. R. Soc. B 281, 20141823 ( 10.1098/rspb.2014.1823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clapham ME, Karr JA, Nicholson DB, Ross AJ, Mayhew PJ. 2016. Ancient origin of high taxonomic richness among insects. Proc. R. Soc. B 283, 20152476 ( 10.1098/rspb.2015.2476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danley PD, Mullen SP, Liu F, Nene V, Quackenbush J, Shaw KL. 2007. A cricket Gene Index: a genomic resource for studying neurobiology, speciation, and molecular evolution. BMC Genomics 8, 109 ( 10.1186/1471-2164-8-109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristensen NP. 1999. Phylogeny of endopterygote insects, the most successful lineage of living organisms. Eur. J. Entomol. 96, 237–253. [Google Scholar]
- 11.Misof B, et al. 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767. ( 10.1126/science.1257570) [DOI] [PubMed] [Google Scholar]
- 12.Wang Y-H, Engel MS, Rafael JA, Wu H-Y, Rédei D, Xie Q, Wang G, Liu X-G, Bu W-J. 2016. Fossil record of stem groups employed in evaluating the chronogram of insects (Arthropoda: Hexapoda). Sci. Rep. 6, 38939 ( 10.1038/srep38939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kukalová-Peck J. 1978. Origin and evolution of insect wings and their relation to metamorphosis, as documented by the fossil record. J. Morphol. 156, 53–125. ( 10.1002/jmor.1051560104) [DOI] [PubMed] [Google Scholar]
- 14.Kukalová-Peck J. 1991. Fossil history and the evolution of hexapod structures. In The insects of Australia, pp. 141–179. Carlton, Australia: Melbourne University Press. [Google Scholar]
- 15.Kukalová-Peck J. 1997. Mazon Creek insect fossils. The origin of insect wings and clues about the origin of insect metamorphosis. In Richardson's guide to the fossil fauna of Mazon creek (eds Shabica CW, Hay AA), pp. 194–207. Chicago, IL: Northeastern Illinois University. [Google Scholar]
- 16.Delany MJ. 1957. Life histories in the Thysanura. Acta Zool. Cracoviensia 2, 61–90. [Google Scholar]
- 17.Prokop J, Pecharová M, Nel A, Hörnschemeyer T, Krzemińska E, Krzemiński W, Engel MS. 2017. Paleozoic nymphal wing pads support dual model of insect wing origins. Curr. Biol. 27, 263–269. ( 10.1016/j.cub.2016.11.021) [DOI] [PubMed] [Google Scholar]
- 18.Haug JT, Haug C, Garwood RJ. 2016. Evolution of insect wings and development – new details from Palaeozoic nymphs. Biol. Rev. 91, 53–69. ( 10.1111/brv.12159) [DOI] [PubMed] [Google Scholar]
- 19.Huang J-H, Lozano J, Belles X. 2013. Broad-complex functions in postembryonic development of the cockroach Blattella germanica shed new light on the evolution of insect metamorphosis. Biochim. Biophys. Acta Gen. Subj. 1830, 2178–2187. ( 10.1016/j.bbagen.2012.09.025) [DOI] [PubMed] [Google Scholar]
- 20.Edmunds GF, McCafferty WP. 1988. The mayfly subimago. Annu. Rev. Entomol. 33, 509–527. ( 10.1146/annurev.en.33.010188.002453) [DOI] [Google Scholar]
- 21.Ide FP. 1937. The subimago of Ephoron leukon Will., and a discussion of the imago instar (Ephem.). Can. Entomol. 69, 25–29. ( 10.4039/Ent6925-2) [DOI] [Google Scholar]
- 22.Maiorana VC. 1979. Why do adult insects not moult? Biol. J. Linn. Soc. 11, 253–258. ( 10.1111/j.1095-8312.1979.tb00037.x) [DOI] [Google Scholar]
- 23.Swammerdam J. 1675. Ephemeri vita of afbeeldingh van ‘s menschen leven, vertoont in de wonderbaarelijcke en nooyt gehoorde historie van het vliegent ende een-daghlevent haft of oever-aas. Amsterdam, The Netherlands: Abraham Wolfgang. [Google Scholar]
- 24.Romañá I, Pascual N, Belles X. 1995. The ovary is a source of circulating ecdysteroids in Blattella germanica (Dictyoptera: Blattellidae). Eur. J. Entomol. 93, 93–103. [Google Scholar]
- 25.Mané-Padrós D, Cruz J, Vilaplana L, Nieva C, Ureña E, Belles X, Martín D. 2010. The hormonal pathway controlling cell death during metamorphosis in a hemimetabolous insect. Dev. Biol. 346, 150–160. ( 10.1016/j.ydbio.2010.07.012) [DOI] [PubMed] [Google Scholar]
- 26.Belles X, Santos CG. 2014. The MEKRE93 (Methoprene tolerant-Krüppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect Biochem. Mol. Biol. 52, 60–68. ( 10.1016/j.ibmb.2014.06.009) [DOI] [PubMed] [Google Scholar]
- 27.Ureña E, Manjón C, Franch-Marro X, Martín D. 2014. Transcription factor E93 specifies adult metamorphosis in hemimetabolous and holometabolous insects. Proc. Natl Acad. Sci. USA 111, 7024–7029. ( 10.1073/pnas.1401478111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charles J-P, Iwema T, Epa VC, Takaki K, Rynes J, Jindra M. 2011. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl Acad. Sci. USA 108, 21 128–21 133. ( 10.1073/pnas.1116123109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jindra M, Belles X, Shinoda T. 2015. Molecular basis of juvenile hormone signaling. Curr. Opin. Insect Sci. 11, 39–46. ( 10.1016/j.cois.2015.08.004) [DOI] [PubMed] [Google Scholar]
- 30.Uyehara CM, Nystrom SL, Niederhuber MJ, Leatham-Jensen M, Ma Y, Buttitta LA, McKay DJ. 2017. Hormone-dependent control of developmental timing through regulation of chromatin accessibility. Genes Dev. 31, 862–875. ( 10.1101/gad.298182.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C-Y, Cooksey BAK, Baehrecke EH. 2002. Steroid regulation of midgut cell death during Drosophila development. Dev. Biol. 250, 101–111. ( 10.1006/dbio.2002.0784) [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Wang J, Li S. 2014. E93 predominantly transduces 20-hydroxyecdysone signaling to induce autophagy and caspase activity in Drosophila fat body. Insect Biochem. Mol. Biol. 45, 30–39. ( 10.1016/j.ibmb.2013.11.005) [DOI] [PubMed] [Google Scholar]
- 33.Lee CY, Wendel DP, Reid P, Lam G, Thummel CS, Baehrecke EH. 2000. E93 directs steroid-triggered programmed cell death in Drosophila. Mol. Cell 6, 433–443. ( 10.1016/S1097-2765(00)00042-3) [DOI] [PubMed] [Google Scholar]
- 34.Tettamanti G, Casartelli M.. 2019. Cell death during complete metamorphosis. Phil. Trans. R. Soc. B 374, 20190065 ( 10.109/rstb.2019.0065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishimaru Y, Tomonari S, Watanabe T, Noji S, Mito T.. 2019. Regulatory mechanisms underlying the specification of the pupal-homologous stage in a hemimetabolous insect. Phil. Trans. R. Soc. B 374, 20190225 ( 10.109/rstb.2019.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gujar H, Palli SR. 2016. Krüppel homolog 1 and E93 mediate juvenile hormone regulation of metamorphosis in the common bed bug, Cimex lectularius. Sci. Rep. 6, 26092 ( 10.1038/srep26092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li KL, Yuan SY, Nanda S, Wang WX, Lai FX, Fu Q, Wan PJ. 2018. The roles of E93 and Kr-h1 in metamorphosis of Nilaparvata lugens. Front. Physiol. 9, 1677 ( 10.3389/fphys.2018.01677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Si Q, Luo J-Y, Hu Z, Zhang W, Zhou C-F. 2017. De novo transcriptome of the mayfly Cloeon viridulum and transcriptional signatures of Prometabola. PLoS One 12, e0179083 ( 10.1371/journal.pone.0179083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vea IM, Tanaka S, Shiotsuki T, Jouraku A, Tanaka T, Minakuchi C. 2016. Differential juvenile hormone variations in scale insect extreme sexual dimorphism. PLoS ONE 11, e0149459 ( 10.1371/journal.pone.0149459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vea IM, Tanaka S, Tsuji T, Shiotsuki T, Jouraku A, Minakuchi C. 2019. E93 expression and links to the juvenile hormone in hemipteran mealybugs with insights on female neoteny. Insect Biochem. Mol. Biol. 104, 65–72. ( 10.1016/j.ibmb.2018.11.008) [DOI] [PubMed] [Google Scholar]
- 41.Bitsch C, Baehr JC, Bitsch J. 1985. Juvenile hormones in Thermobia domestica females: identification and quantification during biological cycles and after precocene application. Experientia 41, 409–410. ( 10.1007/BF02004532) [DOI] [Google Scholar]
- 42.Bitsch C, Bitsch J. 1988. 20-Hydroxyecdysone and ovarian maturation in the firebrat Thermobia domestica (Thysanura: Lepismatidae). Arch. Insect Biochem. Physiol. 7, 281–293. ( 10.1002/arch.940070406) [DOI] [Google Scholar]
- 43.Raikhel AS, Brown MR, Belles X. 2005. Hormonal control of reproductive processes. In Comprehensive molecular insect science (eds Gilbert LI, Iatrou K, Gill SS), pp. 432–491. San Diego, CA: Elsevier. [Google Scholar]
- 44.Laufer H, Biggers WJ. 2001. Unifying concepts learned from methyl farnesoate for invertebrate reproduction and post-embryonic development. Am. Zool. 41, 442–457. ( 10.1093/icb/41.3.442) [DOI] [Google Scholar]
- 45.Schwentner M, Combosch DJ, Pakes Nelson J, Giribet G. 2017. A phylogenomic solution to the origin of insects by resolving crustacean-hexapod relationships. Curr. Biol. 27, 1818–1824.e5. ( 10.1016/j.cub.2017.05.040) [DOI] [PubMed] [Google Scholar]
- 46.Clark-Hachtel CM, Tomoyasu Y. 2016. Exploring the origin of insect wings from an evo-devo perspective. Curr. Opin. Insect Sci. 13, 77–85. ( 10.1016/j.cois.2015.12.005) [DOI] [PubMed] [Google Scholar]
- 47.Nijhout HF. 1994. Insect hormones. Princeton, NJ: Princeton University Press. [Google Scholar]
- 48.Konopova B, Smykal V, Jindra M. 2011. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS ONE 6, e28728 ( 10.1371/journal.pone.0028728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larkin MA, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948. ( 10.1093/bioinformatics/btm404) [DOI] [PubMed] [Google Scholar]
- 50.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. ( 10.1093/bioinformatics/btu033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watson JAL. 1967. The growth and activity of the corpora allata in the larval firebrat, Thermobia domestica (Packard) (Thysanura, Lepismatidae). Biol. Bull. 132, 277–291. ( 10.2307/1539895) [DOI] [PubMed] [Google Scholar]
- 52.Belles X, Casas J, Messeguer A, Piulachs MD. 1987. In vitro biosynthesis of JH III by the corpora allata of adult females of Blattella germanica (L). Insect Biochem. 17, 1007–1010. ( 10.1016/0020-1790(87)90111-9) [DOI] [Google Scholar]
- 53.Gilbert LI, Schneiderman HA. 1960. The development of a bioassay for the juvenile hormone of insects. Trans. Amer. Micr. Soc. 79, 38–67. ( 10.2307/3223973) [DOI] [Google Scholar]
- 54.Minakuchi C, Tanaka M, Miura K, Tanaka T. 2011. Developmental profile and hormonal regulation of the transcription factors broad and Krüppel homolog 1 in hemimetabolous thrips. Insect Biochem. Mol. Biol. 41, 125–134. ( 10.1016/j.ibmb.2010.11.004) [DOI] [PubMed] [Google Scholar]
- 55.Zhou X, Riddiford LM. 2002. Broad specifies pupal development and mediates the ‘status quo’ action of juvenile hormone on the pupal-adult transformation in Drosophila and Manduca. Development 129, 2259–2269. [DOI] [PubMed] [Google Scholar]
- 56.Ohde T, Takehana Y, Shiotsuki T, Niimi T. 2018. CRISPR/Cas9-based heritable targeted mutagenesis in Thermobia domestica: a genetic tool in an apterygote development model of wing evolution. Arthropod. Struct. Dev. 47, 362–369. ( 10.1016/j.asd.2018.06.003) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this review are included in the original articles listed as references. The sequences used to study the phylogeny of E93 are indicated in the electronic supplementary material, table S1.