Abstract
Juvenile hormones and the genetic interaction between the transcription factors Krüppel homologue 1 (Kr-h1) and Broad (Br) regulate the transformation of insects from immature to adult forms in both types of metamorphosis (holometaboly with a pupal stage versus hemimetaboly with no pupal stage); however, knowledge about the exact instar in which this occurs is limited. Using the hemimetabolous cricket Gryllus bimaculatus (Gb), we demonstrate that a genetic interaction occurs among Gb′Kr-h1, Gb′Br and the adult-specifier transcription factor Gb′E93 from the sixth to final (eighth) nymphal instar. Gb′Kr-h1 and Gb′Br mRNAs were strongly expressed in the abdominal tissues of sixth instar nymphs, with precocious adult moults being induced by Gb′Kr-h1 or Gb′Br knockdown in the sixth instar. The depletion of Gb′Kr-h1 or Gb′Br upregulates Gb′E93 in the sixth instar. By contrast, Gb′E93 knockdown at the sixth instar prevents nymphs transitioning to adults, instead producing supernumerary nymphs. Gb′E93 also represses Gb′Kr-h1 and Gb′Br expression in the penultimate nymphal instar, demonstrating its important role in adult differentiation. Our results suggest that the regulatory mechanisms underlying the pupal transition in holometabolous insects are evolutionarily conserved in hemimetabolous G. bimaculatus, with the penultimate and final nymphal periods being equivalent to the pupal stage.
This article is part of the theme issue ‘The evolution of complete metamorphosis’.
Keywords: Gryllus bimaculatus, insect metamorphosis, Krüppel homologue 1, Broad complex, E93
1. Introduction
Holometabolous insects, such as butterflies, beetles and flies, undergo a complete morphological transformation from larva to adult via a pupal stage. The intermediate pupal stage is specific to holometabolous insects, and is needed to transform immature larvae to winged adults. The nymphs of hemimetabolous insects, like locusts, cockroaches and crickets, also undergo morphogenesis to form mature wings and external genitalia, as observed in the larva-to-pupa transition and pupa of holometabolous insects. However, the nymphs of hemimetabolous insects resemble miniature adult forms with wing pads, with the wings and genitalia outgrowths developing throughout the nymphal stages.
Despite these two types of metamorphosis being clearly distinct, both are regulated by two hormones, the steroid 20-hydroxyecdysone (20E) and the sesquiterpenoid juvenile hormones (JHs) [1–3]. 20E is the most active form of insect moulting hormone, ecdysone, and larval–larval moulting and larval–pupal–adult metamorphosis are provoked by pulses of 20E [4,5]. 20E binds to a heterodimer in the nuclear receptor complex, ecdysone receptor (EcR) and ultraspiracle protein (USP), and EcR-USP binds to 20E response elements (EcRE). 20E-EcR-USP triggers a transcriptional cascade, which includes transcription of the 20E primary response genes, such as Br-C, E74, E75 and E93, and subsequent events of 20E secondary response genes [4,5]. However, the type of moult is determined by JH levels. For instance, for larva-to-larva moults, high JH titres are needed. JH antagonizes 20E signalling to prevent precocious metamorphosis during the larval stages, and the metamorphic moult occurs when the JH titre drops during the final instar. JH acting through JH receptor Methoprene-tolerant (Met) [6–9], which is the bHLH-PAS protein family member, prevents adult differentiation during the pre-ultimate immature stages, by inducing the expression of the antimetamorphic transcription factor gene Krüppel homologue 1 (Kr-h1) [3,10–13]. JH-stimulated Kr-h1 expression prevents metamorphosis, whereas the noticeable decline in Kr-h1 expression, following a natural drop in the JH titre during the final juvenile stages, particularly last-instar nymphs in hemimetabolans and pupae in holometabolans, permits adult development [14–18].
A key regulatory gene in the metamorphosis of holometabolous insects is the pupal-specifier transcription factor Broad (Br), which specifies pupal development. The transient expression of Br is essential for the successful formation of pupae. The subsequent repression of Br during the pupal stage allows proper pupal–adult transition [19–22]. In Drosophila melanogaster (Dm), Dm’Br is predominantly expressed during the larval–pupal transition when 20E is high and JH is absent [23,24]. In comparison, the milkweed bug Oncopeltus fasciatus (Of) and cockroach Blattella germanica (Bg), which are hemimetabolous insects that lack pupal stages, Of’Br or Bg’Br are expressed during embryonic, pronymphal (first postembryonic form before hatching) and nymphal development, but then disappear at the moult to adult [25,26]. However, during the final nymphal instar of B. germanica nymphal instar, a small peak in Bg’Br expression was reported when ecdysone peaks. Thus, these compounds, at least, regulate gradual wing bud growth.
Recent studies have identified E93, which is a helix–turn–helix transcription factor containing a Pipsqueak (Psq) motif, as an important player downstream of Kr-h1 [27]. The depletion of E93 in final instar nymphs of B. germanica, as well as the pupae of Tribolium castaneum (T. castaneum) and D. melanogaster, prevents transition to the adult stage. Thus, in contrast with Kr-h1, it has been proposed that E93 is an adult specifier in both hemimetabolan and holometabolan species [28,29]. The effects of Kr-h1 and E93 are, to some extent, antagonistic during the prefinal nymphal instars of B. germanica, with Kr-h1 and E93 acting as mutual repressors [28].
Based on experimental data of B. germanica, T. castaneum and D. melanogaster, Bellés & Santos [30,31] proposed the MEKRE93 (Met-Kr-h1-E93) pathway to explain the regulation of insect metamorphosis. The MEKRE93 pathway appears to be central to the status quo action of JH, which switches adult morphogenesis off and on in a variety of insect species, ranging from cockroaches to flies. JH signals through Met to induce the expression of Kr-h1, which, in turn, blocks adult development, at least partly, by repressing the E93 gene.
The single short period of morphogenesis that arises in the larva-to-pupa transition of holometabolous insects evolved from progressive changes that occur during the nymphal series in basal insects. The hemimetabolous B. germanica and Hemiptera (true bugs) pass through six and five instar stages, respectively, before becoming adults. The levels of Kr-h1 mRNA in these insects are not detectable in the final nymphal stage, which allows adult development. In addition, Br levels are greatly reduced during the final instar.
To extend our further understanding of the conservation and diversification in the mechanisms of metamorphosis among hemimetabolous insects, this study focused on the two-spotted cricket, Gryllus bimaculatus, belonging to the order Orthoptera. In G. bimaculatus nymphs, the life stages following hatching progresses through eight instars, moulting into adults after the final (eighth) instar nymph [32,33]. Based on the MEKRE93 pathway, we propose a model to explain the evolution of pupal formation. In the hemimetabolous G. bimaculatus, RNA interference (RNAi)-mediated knockdown of Gb′Kr-h1 or Gb′Br during the nymphal stage causes the precocious upregulation of Gb′E93 and adult differentiation, bypassing the penultimate and the final nymphal instar stages. In addition to Gb′Kr-h1 and Gb′Br, we show that Gb′E93 is highly expressed in the penultimate nymphal instar, with RNAi knockdown of Gb′E93 preventing nymphal–adult transition, inducing an endless reiteration of nymphal development. Based on our findings, we propose a novel hypothesis for the evolutionary origin of the pupal homologous stage in the hemimetabolous insect, G. bimaculatus.
2. Material and methods
(a). Animals
All adults and nymphs of the two-spotted cricket, G. bimaculatus, were reared at 28°C and 70% under standard conditions, as described previously [34].
(b). Cloning
Gryllus cDNAs homologous to Kr-h1 (346 bp) and Br (978 bp) or E93 (1572 bp) were cloned by reverse transcription-polymerase chain reaction (RT-PCR) from abdomen cDNA samples of third or eighth instar nymphs, respectively, by using gene-specific primers listed in the electronic supplementary material, table S3. The design of all gene-specific primers was performed with the draft genomic sequences of G. bimaculatus (T. Mito et al. 2019, unpublished data). All PCR conditions were as follows: 98°C for 2.5 min, 40 cycles of 94°C for 30 s, 55°C for 30 , and 72°C for 1 min, followed by 72°C for 5 min. Following amplification, the PCR products were subcloned into a pGEM T-Easy vector (Promega, Madison, WI, USA) and were sequenced using an ABI-300 instrument (Applied Biosystems, Foster City, CA, USA). Recombinant vector containing the Gb′Kr-h1 cDNA fragment was used for double stranded RNA (dsRNA) synthesis.
(c). RNA interference
Template cDNA fragments for the synthesis of Gb′Br and Gb′E93 dsRNAs were prepared by RT-PCR by using gene-specific primers listed in the electronic supplementary material, table S4. The templates for Gb′Kr-h1 (346 bp), Gb′Br (448 bp) and Gb′E93 (492 bp) dsRNA synthesis were amplified with a T7 promoter sequence primer and a Sp6 promoter sequence primer with T7 on the 5′ end. dsRNAs were synthesized using the MEGAscript T7 Transcription Kit (Ambion, Austin, TX, USA). Within 24 h of ecdysis, nymphs were injected in the ventral abdomen with 20 µM dsRNA in a volume of 0.2–0.5 µl, as described previously [35]. In all RNAi experiments, DsRed2 dsRNA was injected as a negative control, as described previously [36]. The body length (cm) of RNAi-treated adults was measured from the anterior part of the head to the posterior of the anus, and weight (g) was measured in the whole body just after moulting to adult. The graphs of body size are created using the average values of measured body length and weight in each RNAi-treated adult. The obtained total numbers of survival are shown in the electronic supplementary material, tables S1 and S2.
(d). Quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted from the abdomen tissues, including peripheral tissues such as epidermis and fat body cells targeted by JH and 20E [12,24,37], using ISOGEN (Wako Pure Chemical Industries Ltd, Osaka, Japan). Total RNA was reverse transcribed to cDNA using the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA) with an oligo(dT)20 primer, according to the manufacturer's instructions. The quantitative PCR (qPCR) primers that were used are listed in the electronic supplementary material, table S5. qPCR was performed using the Power SYBR Green PCR Master Mix (Applied Biosystems) on an ABI 7900 Real-Time PCR System (Applied Biosystems). qPCR conditions were as follows: 95°C for 10 min and then 95°C for 15 s, 60°C for 30 s and 72°C for 30 s repeated 40 cycles with 0.4 µM concentration of each primer. The G. bimaculatus β-actin (Gb′β-actin) gene was detected as a reference gene. All qPCR reactions were performed in triplicate as technical replicates.
(e). Hormone treatment
20E (Sigma-Aldrich, Saint Louis, MO, USA) was dissolved in ethanol to a concentration of 1 µg µl−1, and then approximately 3 µl of this 20E solution was injected into the ventral abdomen of newly moulted fifth instar nymphs (approx. 3 µg of 20E per nymph). The same volume of ethanol was injected as a control.
3. Results
(a). Gb′Kr-h1 and Gb′Br prevent adult metamorphosis during late instar stages of the hemimetabolous Gryllus bimaculatus
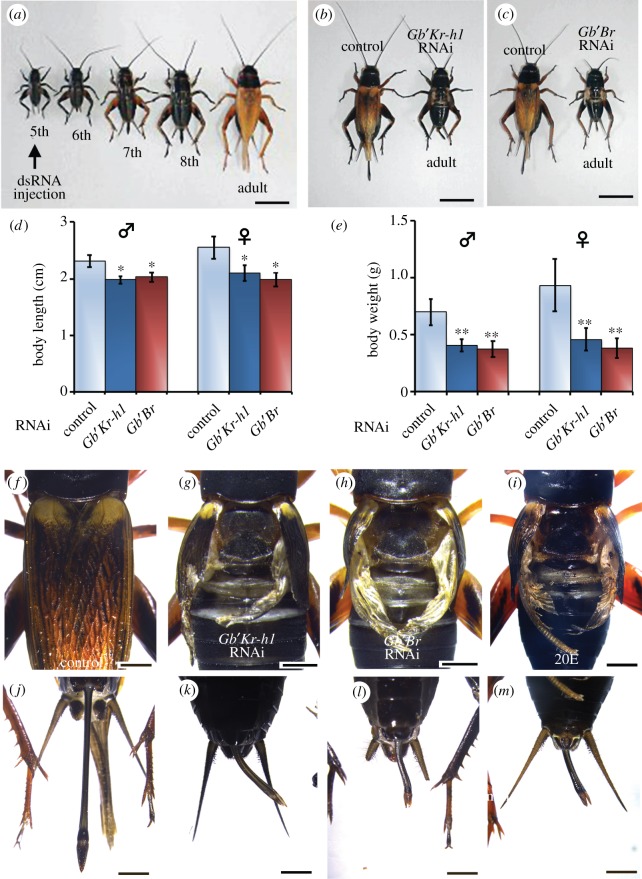
The cricket G. bimaculatus progresses through eight nymphal instars before adult differentiation, with each nymphal instar being distinguished by body size and shape (figure 1a). In particular, the morphological changes that occur between the sixth and the penultimate (seventh) instar are mainly characterized by the degree of development of the wing pads and ovipositor (electronic supplementary material, figure S1a–d). However, no significant changes were observed during the seventh and final (eighth) instar (electronic supplementary material, figure S1c–f). Following the injection of progressively younger fifth instar with dsRNA, we found that nymphs treated with RNAi against Gb′Kr-h1 moulted into normal sixth instar nymphs, showing precocious differentiation of adult features in the ensuing moult, instead of moulting into seventh instar nymphs, as observed for control nymphs in both sexes (figure 1b; electronic supplementary material, table S1). RNAi-mediated depletion of Gb′Br in fifth instar nymphs also caused precocious metamorphosis to adults, instead of normal moult to seventh instar (figure 1c; electronic supplementary material, table S1). In addition, the overall body size and weight of the treated precocious adults significantly declined (figure 1d,e). These animals were not able to moult again, and exhibited strikingly abnormal morphology of the wings (figure 1g,h; electronic supplementary material, figure S2) and ovipositor (figure 1k,l), when compared with the control (figure 1f,j). Interestingly, when fifth instar nymphs were treated with 20E, precocious metamorphosis was induced after the sixth instar, instead of normal moult to seventh instar. In addition, the abnormal development of the wing and ovipositor resembled that of animals subjected to Gb′Kr-h1 and Gb′Br RNAi (figure 1i,m). These results demonstrate that Gb′Kr-h1 and Gb′Br are required for the seventh instar moult, and that their functions during the sixth instar are essential to prevent the precocious differentiation of adult features.
Figure 1.
Phenotypes observed after RNAi-mediated depletion of Gb′Kr-h1 and Gb′Br in the nymph stage of G. bimaculatus. (a) dsRNA was injected into nymphs on day 1 of the fifth instar. After hatching, the life cycle of cricket nymphs progresses through eight instars, and the final instar nymph moults into an adult. (b,c) The effects of RNAi targeting DsRed2 (control), Gb′Kr-h1 or Gb′Br in nymphs on day 1 of the fifth instar. In each panel, the control adult is on the left side and the RNAi-treated on the right. RNAi-treated nymphs underwent precocious adult metamorphosis after the sixth instar. (d,e) Body length (d) and weight (e) of adults (male: ♂; and female: ♀) that developed following injections of dsRNA targeting DsRed2, Gb′Kr-h1 or Gb′Br. Data are presented as the mean ± s.d. *p < 0.05; **p < 0.005 according to Student's t-test. Wings of precocious adults produced following the injection of dsRNA were significantly smaller and were wrinkled (f–h). Following the injection of 20E at the fifth instar, the fifth instar nymphs underwent precocious adult metamorphosis after the sixth instar, and the wings of the 20E-treated adults were also significantly reduced and wrinkled (i). (j–m) Ovipositors of precocious adults produced following the injection of dsRNAs (j–l) or 20E-treated (m) were cleaved at the tip and they became abnormally short. Scale bars: 10 mm in (a–c); 2 mm in (f–m).
(b). Gb′E93 promotes adult differentiation in the last-instar stage
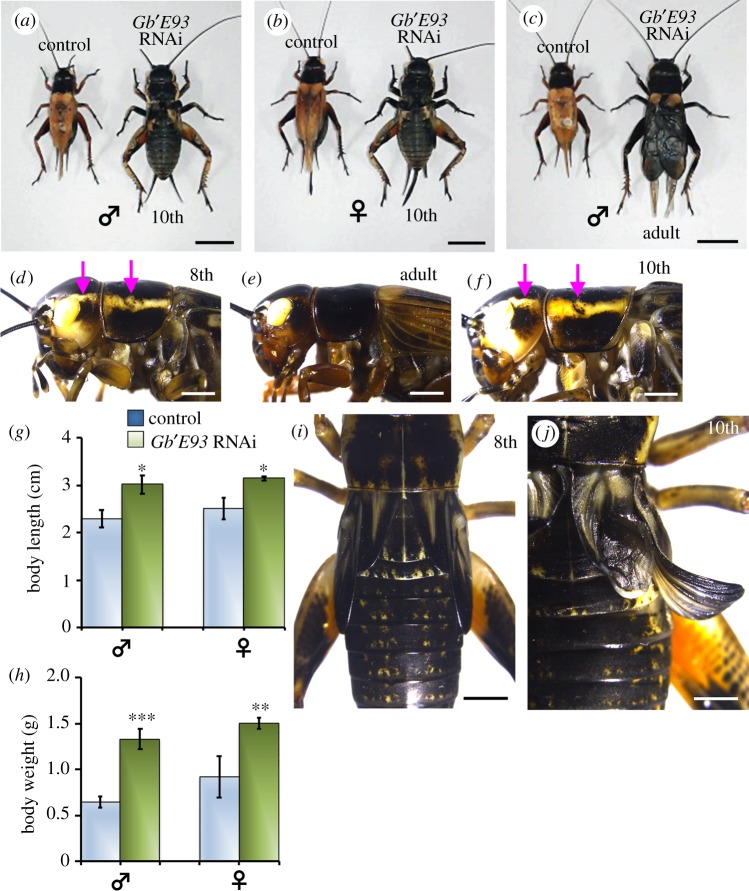
When Gb′E93 dsRNA was injected into fifth instar nymphs, all Gb′E93 RNAi nymphs successfully moulted to normal final instar nymphs, but subsequently failed to cause adult metamorphosis. Instead, the individuals of both sexes repeated the nymphal moult to the supernumerary instar. All of the supernumerary Gb′E93 RNAi nymphs continuously moulted until they became giant 10th instar nymphs (figure 2a,b). Although the development of many of these supernumerary nymphs was arrested without adult metamorphosis, several individuals subsequently moulted into adults (figure 2c; electronic supplementary material, table S2). However, instead of the normal adult pigmentation with a black cuticle, the supernumerary Gb′E93 RNAi 10th instar nymphs had the typical characteristics of control eighth instar nymphs. Specifically, they had thick lines of white melanin on the prothorax and head (figure 2d–f). Consistently, when nymphs moulted to the supernumerary 10th instar, their body size and weight were significantly larger than those of control adults (figure 2g,h). Furthermore, the wing pads of these 10th instar nymphs had similar proportions to those of eighth instar nymphs, and begin to bend to the outside (figure 2i,j). These Gb′E93 knockdown experiments indicate that Gb′E93 is required for the morphological transition from the eighth instar to adults. Thus, Gb′E93 is a critical factor that promotes adult metamorphosis in hemimetabolous G. bimaculatus.
Figure 2.
Phenotypes observed after Gb′E93 depletion using RNAi in G. bimaculatus. (a–c) dsRNA targeting DsRed2 (control) or Gb′E93 was injected into fifth instars on day 1. The fifth instar nymphs that received RNAi targeting Gb′E93 initiated supernumerary moults at the 8th–9th–10th instars (instead of eighth instar to adult), and subsequently developed into large-sized adults compared with the control adults. The nymphal instar and adult stage are indicated at the bottom of each panel (male: ♂; and female: ♀). (d–f) Lateral views of the eighth (d), adult (e) or 10th (f) instar nymphs that were injected with dsRNA targeting DsRed2 or Gb′E93 on day 1 of the fifth instar, respectively. Magenta arrows indicate the white pigmentation of the head and prothorax. (g,h) Body length (g) and weight (h) of adults that were treated with RNAi targeting DsRed2 or Gb′E93. Data are presented as the mean ± s.d. *p < 0.05; **p < 0.005; ***p < 0.001 according to Student's t-test. (i,j) Dorsal views of the eighth instar or a representative supernumerary 10th instar nymph that was injected with dsRNA targeting DsRed2 (i) or Gb′E93 (j) on day 1 of the fifth instar. Scale bars: 10 mm in (a–c); 2 mm in (d–f,i,j).
(c). Interplay between Gb′Kr-h1, Gb′Br and Gb′E93 is associated with sixth instar-to-adult transition of Gryllus bimaculatus
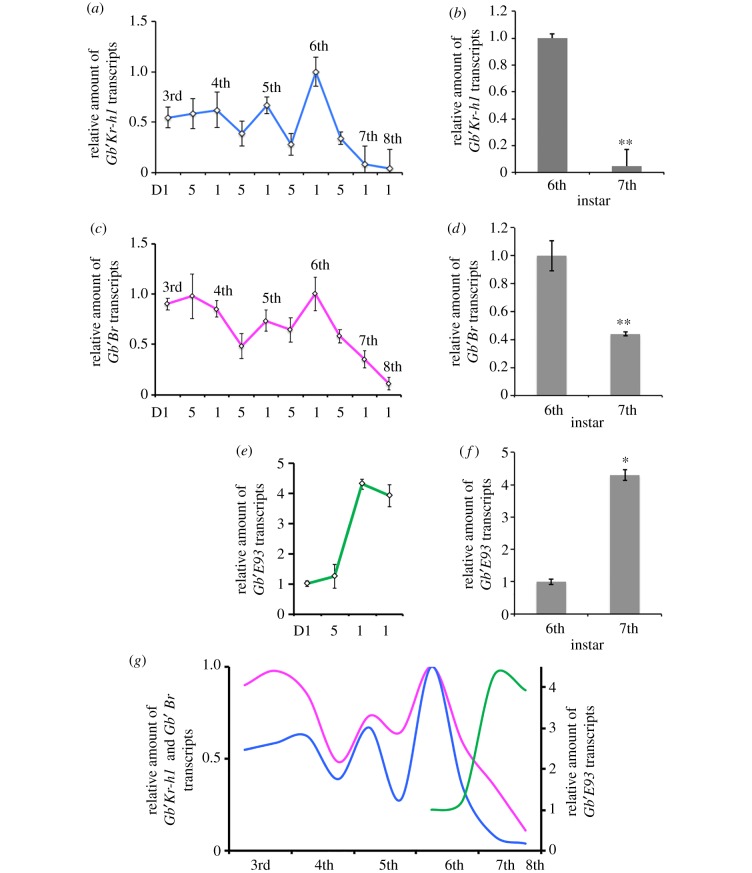
Gb′Kr-h1 and Gb′Br mRNAs were found to be constitutively expressed throughout the nymphal stages, while the levels of these mRNAs exhibited periodic changes in each of the instars (figure 3a,c). The peak in relative amount of Gb′Kr-h1 transcript was observed on day 1 of the sixth instar, but decreased until it could no longer be detected during the seventh instar (figure 3b). Similarly, higher Gb′Br mRNA level was detected in the sixth instar, and then mRNA level decreased during the seventh instar (figure 3d). Conversely, the expression of Gb′E93 transcript significantly increased after moulting to the seventh instar, and the high transcript level was also detected in the eighth instar (figure 3e,f). Declines in Gb′Kr-h1 and Gb′Br expression, and an increased expression of Gb′E93, suggest that the cross-talk between these genes contributes towards regulating metamorphosis in G. bimaculatus (figure 3g).
Figure 3.
Expression profiles of Gb′Kr-h1 Gb′Br and Gb′E93 transcripts in G. bimaculatus during development. (a,c,e) Temporal expression of Gb′Kr-h1 (a), Gb′Br (c), and Gb′E93 (e) in the abdomen tissues of nymphs based on qPCR analyses. Relative fold changes in mRNA levels were plotted, and the average expression levels on day 1 of the sixth instar were set at 1. mRNA levels were normalized to Gb′β-actin mRNA levels. Developmental stages are defined as days (D) after moulting. Data are presented as the mean ± s.d. (b,d,f) Transcript levels of Gb′Kr-h1 (b), Gb′Br (d) and Gb′E93 (f) were determined on day 1 of the sixth and seventh instars. The transcript levels determined on day 1 of the sixth instar control nymphs were set to 1. The data presented are the mean ± s.d. *p < 0.05; **p < 0.005 according to Student's t-test. (g) Comparison between expression levels of Gb′Kr-h1, Gb′Br and Gb′E93 during the development of nymphs. Units in the ordinates reflect the relative mRNA levels at each moment. Each value measured in individuals on day 1 of the sixth instar were set to 1. The blue, magenta and green curved lines represent the Gb′Kr-h1, Gb′Br and Gb′E93 mRNA levels, respectively.
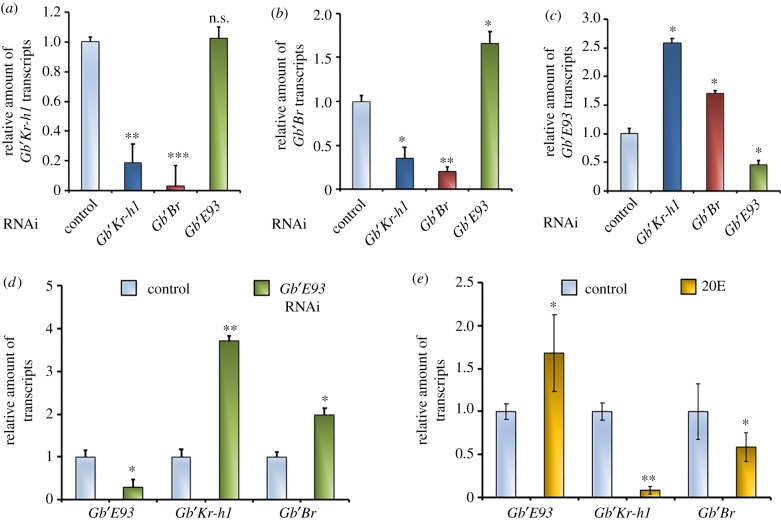
At the sixth instar, the level of Gb′Kr-h1 mRNA in Gb′Kr-h1 RNAi nymphs was significantly lower than that in the control (figure 4a). The knockdown of Gb′Kr-h1 caused the mRNA level of Gb′Br to decline in comparison to the control (figure 4b). Thus, the expression of Gb′Br during the sixth instar might be controlled by Gb′Kr-h1. However, Gb′E93 expression during the sixth instar was upregulated by Gb′Kr-h1 RNAi knockdown (figure 4c). Similarly, the RNAi-mediated depletion of Gb′Br (figure 4b) also caused the expression of Gb′E93 to increase during the sixth instar when compared with its expression in the control (figure 4c). Thus, the Gb′E93 transcript during the sixth instar had already been repressed by Gb′Kr-h1 and Gb′Br.
Figure 4.
Effect of RNAi-mediated knockdown of Gb′Kr-h1, Gb′Br or Gb′E93 and 20E treatment on expression patterns. (a–c) dsRNA targeting DsRed2 (control), Gb′Kr-h1, Gb′Br or Gb′E93 was injected on day 1 of the fifth instar. Transcript levels of Gb′Kr-h1 (a), Gb′Br (b), and Gb′E93 (c) were subsequently determined in the abdomens of the sixth instar. The transcript levels determined at the sixth instar of each control nymph for (a–c) were set to 1. Data are presented as the mean ± s.d. (d) Transcript levels of Gb′Kr-h1, Gb′Br and Gb′E93 were also determined for the seventh instar, following the injection of dsRNA targeting Gb′E93. The transcript levels of these genes in control nymphs on day 1 of the seventh instar were set to 1. Data are presented as the mean ± s.d. (e) 20E was injected into the fifth instar nymphs. Transcript levels of Gb′Kr-h1, Gb′Br and Gb′E93 were subsequently determined on day 1 in the sixth instars. The transcript levels of these genes on day 1 of the control sixth instar nymphs were set to 1. Data presented are the mean ± s.d. n.s., not significant; *p < 0.05; **p < 0.005; ***p < 0.001 according to Student's t-test.
Of note, the expression levels of Gb′Kr-h1 and Gb′Br progressively declined during the seventh instar, just after Gb′E93 upregulation. When sixth instar nymphs received Gb′E93 RNAi, Gb′Br mRNA levels in Gb′E93 RNAi nymphs were significantly higher than those of control nymphs (figure 4b), despite Gb′Kr-h1 not being upregulated in these sixth instar nymphs (figure 4a). These results suggest that Gb′Br expression might be negatively affected by Gb′E93 in the sixth instar nymphs. Accordingly, the precocious increase in Gb′E93 expression caused by the RNAi-mediated Gb′Kr-h1 knockdown might lead to reduced expression of Gb′Br during the sixth instar. Interestingly, under the Gb′Br RNAi treatment, Gb′Kr-h1 expression during the sixth instar was downregulated in these nymphs (figure 4a). Consequently, the observed decrease in Gb′Kr-h1 might be owing to increased Gb′E93 expression, which results in Gb′Br knockdown. Thereafter, Gb′E93 depletion during the seventh instar prevented the downregulation of Gb′Kr-h1 and Gb′Br (figure 4d). Thus, the Gb′Kr-h1 and Gb′Br transcripts during the seventh instar can be tightly repressed by Gb′E93.
In previous studies in D. melanogaster and Bombyx mori [27,38,39], E93 has been found to be a primary response gene that is positively regulated by 20E. In the present study, we showed that the injection of 20E into the fifth instar nymphs causes precocious metamorphosis to adults, instead of moulting into seventh instar nymphs. Consistent with the precocious adult metamorphosis that had received 20E treatment, Gb′E93 mRNA levels were upregulated at the sixth instar nymphs, while Gb′Kr-h1 and Gb′Br were downregulated (figure 4e). Thus, the expression of Gb′E93 might be positively affected by an excess of 20E during the sixth instar.
Overall, our results indicate that the precocious adult moult in Gb′Kr-h1 and Gb′Br RNAi nymphs depends on the precocious upregulation of Gb′E93 expression during the sixth instar. Furthermore, Gb′E93 causes morphological changes to form adults through the repression of Gb′Kr-h1 and Gb′Br during the penultimate and final nymphal instars, as adult metamorphosis is prevented by RNAi depletion of Gb′E93.
(d). Gb′Kr-h1, Gb’′Br and Gb′E93 expression are influenced by juvenile hormone biosynthesis signalling
Based on our recent study, Gb′Myoglianin (Gb′Myo) and Gb′Decapentaplegic (Gb′Dpp)/Gb′Glass bottom boat (Gb′Gbb) signalling are involved in JH synthesis by mediating the transcriptional regulation of Gb′jhamt [33]. Gb′jhamt is a key enzyme for JH biosynthesis in the corpora allata (CA) of G. bimaculatus. Because the RNAi-mediated depletion of Gb′Myo and Gb′Dpp/Gb′Gbb signalling molecules altered the JH titre to increase and decrease, respectively, we examined how the expression of Gb′Kr-h1, Gb′Br and Gb′E93 is altered by Gb′myo or Gb′mothers against dpp (Gb′mad) knockdown.
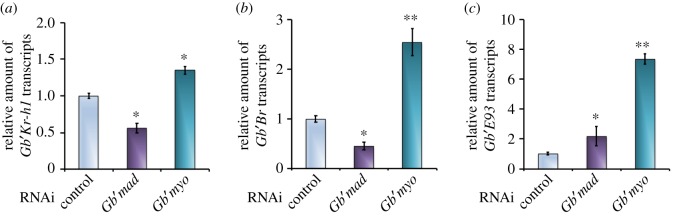
We have previously shown that RNAi targeting Gb′mad results in significant reductions in Gb′jhamt transcript and JH titre in the sixth instar nymphs and consequently causes precocious adult metamorphosis [33]. Here, we found that the levels of Gb′Kr-h1 and Gb′Br mRNA in Gb′mad RNAi-treated nymphs were lower than those in the controls during the sixth instar (figure 5a,b). Thus, the decline of Gb′Kr-h1 and Gb′Br mRNA levels might be largely attributed to the absence of JH by the depletion of Gb′mad. Furthermore, the knockdown of Gb′mad caused the expression of Gb′E93 to increase during the sixth instar (figure 5c). By contrast, Gb′Kr-h1 and Gb′Br mRNA levels were upregulated in Gb′myo RNAi nymphs during the sixth instar (figure 5a,b). Furthermore, we also found that Gb′myo RNAi allows the strong upregulation of Gb′E93 (figure 5c). Thus, Gb′Kr-h1, Gb′Br-C and Gb′E93 are probably regulated by JH in hemimetabolous G. bimaculatus.
Figure 5.
Effects of RNAi-mediated depletion of Gb′myo and Gb′mad on the expression of Gb′Kr-h1, Gb′Br and Gb′E93. (a–c) Gb′Kr-h1, Gb′Br and Gb′E93 mRNA levels in the abdomen of sixth instar nymphs after injecting dsRNA targeting Gb′myo or Gb′mad into fifth instars. The transcript levels of the control sixth instar nymphs were set to 1. Data are presented as the mean ± s.d. *p < 0.05; **p < 0.005 according to Student's t-test.
4. Discussion
Previous studies showed that the downregulation of Kr-h1 and the upregulation of E93 in the final nymph stages of hemimetabolous insects and in the pupae of holometabolous insects are crucial for adult development in both types of metamorphosis [14,29–31,40]. The present study showed that RNAi-mediated depletion of Gb′Kr-h1 during the sixth nymphal instar of G. bimaculatus induces Gb′E93 and suppresses Gb′Br expression. Consequently, Gb′Kr-h1 RNAi animals showed the precocious differentiation of adult features. We also showed that RNAi knockdown of Gb′E93 during the penultimate (seventh) nymphal instar prevents adult metamorphosis and promotes supernumerary nymphal moults. In addition, Gb′E93 is required to prevent the expression of Gb′Kr-h1 and Gb′Br during the penultimate nymphal instar. Overall, the mechanism of the functional interactions between Kr-h1 and E93 for metamorphosis is conserved in G. bimaculatus. Consequently, the regulation of the MEKRE93 pathway is common throughout hemimetabolous and holometabolous insects. However, based on data reported from other hemimetabolous insects (including Pyrrhocoris apterus, Rhodnius prolixus, Cimex lectularius and B. germanica), changes to the timing of expression and regulation of cross-talk between Kr-h1, Br and E93 usually occur during the penultimate and final nymphal period [14,17,29,41] as they do during the final larval and pupal stage in holometabolous insects. In comparison, the functional relationship between Gb′Kr-h1, Gb′Br and Gb′E93 is already present in the antepenultimate (sixth) instar nymphs of G. bimaculatus. Consequently, Gb′Kr-h1 RNAi-dependent induction of Gb′E93 expression during the sixth instar initiates the precocious development of adult structures. Previous reports showed that high JH levels prevent the incomplete metamorphosis, by inducing the expression of Kr-h1, in early instars, whereas its subsequent disappearance allows metamorphosis to occur [2,3,5,13,42,43]. This is because the elevated expression level of Gb′myo is essential for the arrest of JH biosynthesis in G. bimaculatus [33]. Thus, the shift in the timing of regulation of cross-talk between Gb′Kr-h1, Gb′Br and Gb′E93 might be attributed to the mechanism for regulating stepwise expressions of Gb′myo.
Gb′Myo and Gb′Dpp/Gb′Gbb signalling might be associated with the expression of Gb′Kr-h1, Gb′Br-C and Gb′E93 through regulating JH biosynthesis. Interestingly, it has previously been demonstrated that myo is also expressed in the prothoracic glands (PG) in G. bimaculatus [33] and B. germanica [44]. Thus, Myo might be independently associated with both JH and ecdysone biosynthesis. Consequently, increased Gb′E93 expression caused by Gb′myo RNAi might be related to changes in 20E levels. Thus, Gb′Kr-h1, Gb′Br and Gb′E93 are probably regulated by JH and 20E in hemimetabolous G. bimaculatus, assuming that Gb′Myo and Gb′Dpp/Gb′Gbb signalling in the CA or Gb′Myo signalling in the PG are responsible for regulating JH or ecdysone biosynthesis, respectively.
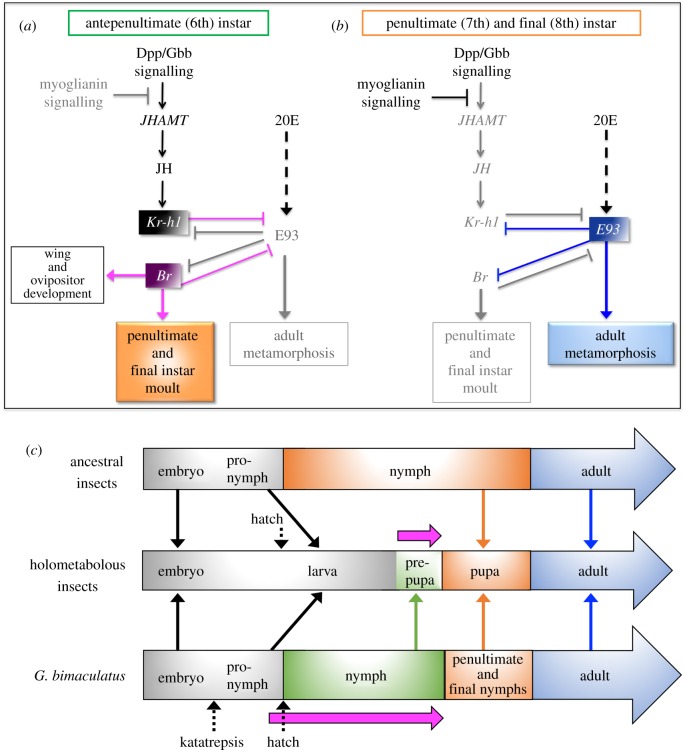
In this study, we propose a model showing the interactions of Gb′Kr-h1, Gb′Br and Gb′E93 during the antepenultimate (sixth) instar (figure 6a) with the penultimate (seventh) and final (eighth) nymphal instars (figure 6b). First, Gb′Kr-h1-dependent repression of Gb′E93 might be essential for the proper moulting of the penultimate and final nymphal instars, but prevents adult differentiation (figure 6a). Subsequently, after moulting into the penultimate nymphal instar, Gb′E93 represses the expression of Gb′Kr-h1 and Gb′Br; thus, ensuring metamorphosis into adults (figure 6b). Therefore, our present results suggest that the expression profiles and the functions of Gb′Kr-h1, Gb′Br and Gb′E93 during the sixth-to-penultimate instars of G. bimaculatus closely resemble those of the final larval-to-pupal period in holometabolous insects [14,29,40].
Figure 6.
Schematics of the mechanisms regulating adult characteristics in the hemimetabolous insect G. bimaculatus. (a,b) Schematic diagrams of the regulatory mechanisms in controlling adult differentiation [29] based on the results obtained from experiments targeting Gb′Kr-h1, Gb′Br and Gb′E93 genes using RNAi. The proposed models depict the regulatory interaction between these metamorphic genes in the antepenultimate (sixth), penultimate (seventh) and final (eighth) nymphal instars of G. bimaculatus. Grey colours denote gene depletion and transcriptional regulatory effects that are absent during each phase. Magenta and blue lines indicate the findings of the present study. The characters illustrating the functions of TGF-β signalling in controlling JH biosynthesis are from G. bimaculatus [33]. The role of 20E still remains unclear (dotted lines). (c) Schematic of the hypothesis for the stages of ancestral insects, holometabolous insects and hemimetabolous (G. bimaculatus) counterparts [45,46]. Magenta arrows indicate the periods of Br expression in holometabolous insects and G. bimaculatus.
Several theories have been proposed to explain the pupal formation and the evolutionary transition from hemimetabolous to holometabolous insects [45,47]. Truman & Riddiford [46,48] proposed a hypothesis that the endocrinology of the larvae of holometabolous insects corresponds to the final hemimetabolous embryonic stage, which the authors termed pronymphs (the pronymph hypothesis). The three stages (pronymph, nymph and adult) of ancestral insect species are equivalent to the larva, pupa and adult stages of insects that exhibit complete metamorphosis. The authors speculated that the progressive changes which occur in a number of nymphal series in basal insects are compressed to a single short period of morphogenesis that is seen in the larva-to-pupa transition of holometabolous insects. This interpretation might support the pronymph hypothesis [25].
However, this hypothesis is subject to controversy. Huang et al. [26] proposed a hypothesis on the evolution from hemimetaboly to holometaboly regarding Br (the wing-to-pupa hypothesis). In this hypothesis, JH action on Br expression shifts from being stimulatory (hemimetaboly) to inhibitory (holometaboly) during the young larval stages. Thus, Br expression is inhibited in the young larvae of holometabolous ancestors, suppressing Br-dependent wing development and patterning. Accordingly, the roles of Br culminated in the morphogenesis of pupae in extant holometabolous species.
In both hypotheses, because Br expression is regulated by JH, the evolution of metamorphosis might be attributed to heterochronic change in the timing of JH activation and/or suppression or changes in the target organs of JH action. In addition, Br functions might have radically diverged and changed from the progressive development of hemimetabolous nymphs to specifying holometabolous pupa [14,19–22,24–26].
Indeed, we also found that Gb′Kr-h1 mRNA levels were significantly reduced in Gb′Br RNAi-treated sixth instar nymphs. The RNAi knockdown of Gb′Br also induced precocious Gb′E93 expression and promoted precocious adult development in our study. Thus, the expression of Gb′E93 might be negatively affected by Gb′Br in the sixth instar nymphs of G. bimaculatus. Therefore, Gb′Kr-h1 and Gb′Br might have to interact for nymphs to transition to the penultimate instar. However, the phenotypes of Gb′Br RNAi knockdown exhibited irregular morphology, especially in the wing pads and ovipositor (electronic supplementary material, figure S2). Nymphs which were given Gb′Br RNAi in the third instar showed abnormal development of Br-dependent tissues, such as the wing pads in the sixth instar nymphs and the wings and ovipositor in the precocious adults (electronic supplementary material, figure S3). Therefore, the function of Gb′Br related to regulating wing size and form is conserved from the progressively nymphal stages in hemimetabolous insects (like P. apterus [14], O. fasciatus [25] and B. germanica [26]) to the final larval stage in holometabolous insects (like T. castaneum [19,21], lacewing Chrysopa perla [22] and D. melanogaster [24]). Interestingly, the role of Gb′Br in the wing and ovipositor transition that occurs at the penultimate instar seems similar to those of Br in the imaginal leg and eye primordia in the final instar just prior to the onset of their morphogenic growth for metamorphosis in Manduca sexta [49]. Thus, Br specializes in wing development, and retains the pupal specifying function in these periods. Ultimately, Gb′Br is required to prevent adult metamorphosis and to allow the anisometric growth of developing wings and ovipositors in G. bimaculatus.
5. Conclusion
We demonstrated that the parallel timing of the critical interaction between Kr-h1 and Br is conserved in hemimetabolous and holometabolous insects. This interaction underlies the transition to the penultimate instar nymph in G. bimaculatus and the formation of pupae in holometabolous insects. Thus, these periods might represent evolutionarily homologous units. Gb′Br was shown to regulate progressive wing development during nymphal stages. In addition, the interaction between Gb′Kr-h1 and Gb′Br is related to the transition that occurs during later (antepenultimate-to-penultimate) instars, preventing metamorphosis to adults in G. bimaculatus (figure 6a). In holometabolous insects, Br might fulfil both function of regulating wing development and preventing adult metamorphosis in the single short period of pupal transition.
We hypothesize that three stages, pronymph (first postembryonic stage), nymphs and penultimate nymph, of the hemimetabolous insect G. bimaculatus are equivalent to the larva, final larva and pupa stages of insects with complete metamorphosis (figure 6c). Notably, the prepupa (late phase of the final larva)-to-pupa transitional stages of holometabolous insects might be evolutionarily homologous to the antepenultimate-to-penultimate nymph transitional stages of G. bimaculatus, supported by the conservation of the mechanisms underlying insect metamorphosis. In addition, wing formation and development from the larva to the pupa might originate from the periods of pronymph-to-penultimate nymph in hemimetabolous insects.
Supplementary Material
Acknowledgements
We thank Kayoko Tada for providing technical assistance.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material. Gb′Kr-h1 (accession number LC476894), Gb′Br (accession number LC476892) and Gb′E93 (accession number LC476893) cDNA sequences have been deposited in DNA Data Bank of Japan.
Authors' contributions
Y.I., S.N. and T.M. designed this study. Y.I. performed all of the experiments. Y.I., S.T., T.W., S.N. and T.M. analysed the data. Y.I., S.N. and T.M. prepared all of the figures and wrote the main text of the manuscript. All of the authors critically assessed the manuscript.
Competing interests
We have no competing interests.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI grant nos JP16K1506800 to Y.I., JP26292176 to T.M. and JP17H03945 to T.M. and Y.I.
References
- 1.Dubrovsky EB. 2005. Hormonal cross talk in insect development. Trends Endocrinol. Metab. 16, 6–11. ( 10.1016/j.tem.2004.11.003) [DOI] [PubMed] [Google Scholar]
- 2.Truman JW, Riddiford LM. 2002. Endocrine insights into the evolution of metamorphosis in insects. Annu. Rev. Entomol. 47, 467–500. ( 10.1146/annurev.ento.47.091201.145230) [DOI] [PubMed] [Google Scholar]
- 3.Jindra M, Palli SR, Riddiford LM. 2013. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 58, 181–204. ( 10.1146/annurev-ento-120811-153700) [DOI] [PubMed] [Google Scholar]
- 4.Riddiford LM, Cherbas P, Truman JW. 2000. Ecdysone receptors and their biological actions. Vitam. Horm. 60, 1–73. ( 10.1016/S0083-6729(00)60016-X) [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka N, Rewitz KF, O'Connor MB. 2013. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu. Rev. Entomol. 58, 497–516. ( 10.1146/annurev-ento-120811-153608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y, Fang F, Chu Y, Jones D, Jones G. 2002. Activation of transcription through the ligand-binding pocket of the orphan nuclear receptor ultraspiracle. Eur. J. Biochem. 269, 6026–6036. ( 10.1046/j.1432-1033.2002.03293.x) [DOI] [PubMed] [Google Scholar]
- 7.Ashok M, Turner C, Wilson TG. 1998. Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc. Natl Acad. Sci. USA. 95, 2761–2766. ( 10.1073/pnas.95.6.2761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charles JP, et al. 2011. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl Acad. Sci. USA. 108, 21 128–21 133. ( 10.1073/pnas.1116123109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jindra M, Uhlirova M, Charles JP, Smykal V, Hill RJ. 2015. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet. 11, e1005394 ( 10.1371/journal.pgen.1005394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riddiford LM, Truman JW, Mirth CK, Shen YC. 2010. A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development 137, 1117–1126. ( 10.1242/dev.037218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kayukawa T, et al. 2012. Transcriptional regulation of juvenile hormone-mediated induction of Kruppel homolog 1, a repressor of insect metamorphosis. Proc. Natl Acad. Sci. USA. 109, 11 729–11 734. ( 10.1073/pnas.1204951109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smykal V, et al. 2014. Importance of juvenile hormone signaling arises with competence of insect larvae to metamorphose. Dev. Biol. 390, 221–230. ( 10.1016/j.ydbio.2014.03.006) [DOI] [PubMed] [Google Scholar]
- 13.Jindra M, Bellés X, Shinoda T. 2015. Molecular basis of juvenile hormone signaling. Curr. Opin. Insect. Sci. 11, 39–46. ( 10.1016/j.cois.2015.08.004) [DOI] [PubMed] [Google Scholar]
- 14.Konopova B, Smykal V, Jindra M. 2011. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS ONE 6, e28728 ( 10.1371/journal.pone.0028728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minakuchi C, Namiki T, Shinoda T. 2009. Kruppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev. Biol. 325, 341–350. ( 10.1016/j.ydbio.2008.10.016) [DOI] [PubMed] [Google Scholar]
- 16.Minakuchi C, Zhou X, Riddiford LM. 2008. Kruppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech. Dev. 125, 91–105. ( 10.1016/j.mod.2007.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lozano J, Bellés X. 2011. Conserved repressive function of Kruppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci. Rep. 1, 163 ( 10.1038/srep00163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minakuchi C, Tanaka M, Miura K, Tanaka T. 2011. Developmental profile and hormonal regulation of the transcription factors broad and Kruppel homolog 1 in Hemimetabolous thrips. Insect. Biochem. Mol. Biol. 41, 125–134. ( 10.1016/j.ibmb.2010.11.004) [DOI] [PubMed] [Google Scholar]
- 19.Parthasarathy R, Tan A, Bai H, Palli SR. 2008. Transcription factor broad suppresses precocious development of adult structures during larval-pupal metamorphosis in the red flour beetle, Tribolium castaneum. Mech. Dev. 125, 299–313. ( 10.1016/j.mod.2007.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uhlirova M, et al. 2003. Use of Sindbis virus-mediated RNA interference to demonstrate a conserved role of broad-complex in insect metamorphosis. Proc. Natl Acad. Sci. USA. 100, 15 607–15 612. ( 10.1073/pnas.2136837100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki Y, Truman JW, Riddiford LM. 2008. The role of broad in the development of Tribolium castaneum: implications for the evolution of the holometabolous insect pupa. Development 135, 569–577. ( 10.1242/dev.015263) [DOI] [PubMed] [Google Scholar]
- 22.Konopova B, Jindra M. 2008. Broad-complex acts downstream of Met in juvenile hormone signaling to coordinate primitive holometabolan metamorphosis. Development 135, 559–568. ( 10.1242/dev.016097) [DOI] [PubMed] [Google Scholar]
- 23.DiBello PR, Withers DA, Bayer CA, Fristrom JW, Guild GM. 1991. The Drosophila broad-complex encodes a family of related proteins containing zinc fingers. Genetics 129, 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X, Riddiford LM. 2002. Broad specifies pupal development and mediates the ‘status quo’ action of juvenile hormone on the pupal-adult transformation in Drosophila and Manduca. Development 129, 2259–2269. [DOI] [PubMed] [Google Scholar]
- 25.Erezyilmaz DF, Riddiford LM, Truman JW. 2006. The pupal specifier broad directs progressive morphogenesis in a direct-developing insect. Proc. Natl Acad. Sci. USA. 103, 6925–6930. ( 10.1073/pnas.0509983103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang JH, Lozano J, Bellés X. 2013. Broad-complex functions in postembryonic development of the cockroach Blattella germanica shed new light on the evolution of insect metamorphosis. Biochim. Biophys. Acta. 1830, 2178–2187. ( 10.1016/j.bbagen.2012.09.025) [DOI] [PubMed] [Google Scholar]
- 27.Baehrecke EH, Thummel CS. 1995. The Drosophila E93 gene from the 93F early puff displays stage- and tissue-specific regulation by 20-hydroxyecdysone. Dev. Biol. 171, 85–97. ( 10.1006/dbio.1995.1262) [DOI] [PubMed] [Google Scholar]
- 28.Urena E, Manjon C, Franch-Marro X, Martin D. 2014. Transcription factor E93 specifies adult metamorphosis in hemimetabolous and holometabolous insects. Proc. Natl Acad. Sci. USA 111, 7024–7029. ( 10.1073/pnas.1401478111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urena E, Chafino S, Manjon C, Franch-Marro X, Martin D. 2016. The occurrence of the holometabolous pupal stage requires the interaction between E93, Kruppel-homolog 1 and broad-complex. PLoS Genet. 12, e1006020 ( 10.1371/journal.pgen.1006020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellés X, Santos CG. 2014. The MEKRE93 (Methoprene tolerant-Kruppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect. Biochem. Mol. Biol. 52, 60–68. ( 10.1016/j.ibmb.2014.06.009) [DOI] [PubMed] [Google Scholar]
- 31.Belles X. 2019. The innovation of the final moult and the origin of insect metamorphosis. Phil. Trans. R. Soc. B 374, 20180415 ( 10.1098/rstb.2018.0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mito T, Noji S. 2008. The two-spotted cricket Gryllus bimaculatus: an emerging model for developmental and regeneration studies. CSH Prot. 110, pdb.emo110 ( 10.1101/pdb.emo110) [DOI] [PubMed] [Google Scholar]
- 33.Ishimaru Y, et al. 2016. TGF-beta signaling in insects regulates metamorphosis via juvenile hormone biosynthesis. Proc. Natl Acad. Sci. USA 113, 5634–5639. ( 10.1073/pnas.1600612113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niwa N, Saitoh M, Ohuchi H, Yoshioka H, Noji S. 1997. Correlation between distal-less expression patterns and structures of appendages in development of the two-spotted cricket, Gryllus bimaculatus. Zool. Sci. 14, 115–125. ( 10.2108/Zsj.14.115) [DOI] [Google Scholar]
- 35.Ishimaru Y, et al. 2015. Involvement of dachshund and distal-less in distal pattern formation of the cricket leg during regeneration. Sci. Rep. 5, 8387 ( 10.1038/srep08387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyawaki K, et al. 2004. Involvement of Wingless/Armadillo signaling in the posterior sequential segmentation in the cricket, Gryllus bimaculatus (Orthoptera), as revealed by RNAi analysis. Mech. Dev. 121, 119–130. ( 10.1016/j.mod.2004.01.002) [DOI] [PubMed] [Google Scholar]
- 37.Cao JQ, et al. 2017. The role of MicroRNAs in Drosophila regulation of insulin-like peptides and ecdysteroid signalling: where are we now? Adv. Insect. Physiol. 53, 55–85. ( 10.1016/bs.aiip.2017.02.002) [DOI] [Google Scholar]
- 38.Mou X, Duncan DM, Baehrecke EH, Duncan I. 2012. Control of target gene specificity during metamorphosis by the steroid response gene E93. Proc. Natl Acad. Sci. USA 109, 2949–2954. ( 10.1073/pnas.1117559109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, et al. 2015. 20-Hydroxyecdysone (20E) primary response gene E93 modulates 20E signaling to promote Bombyx larval–pupal metamorphosis. J. Biol. Chem. 290, 27 370–27 383. ( 10.1074/jbc.M115.687293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jindra M. 2019. Where did the pupa come from? The timing of juvenile hormone signalling supports homology between stages of hemimetabolous and holometabolous insects. Phil. Trans. R. Soc. B 374, 20190064 ( 10.1098/rstb.2019.0064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gujar H, Palli SR. 2016. Kruppel homolog 1 and E93 mediate Juvenile hormone regulation of metamorphosis in the common bed bug, Cimex lectularius. Sci. Rep. 6, 26092 ( 10.1038/srep26092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riddiford LM. 1994. Cellular and molecular actions of juvenile hormone. I. General considerations and premetamorphic actions. Adv. Insect. Physiol. 24, 213–274. ( 10.1016/S0065-2806(08)60084-3) [DOI] [Google Scholar]
- 43.Hiruma K, Kaneko Y. 2013. Hormonal regulation of insect metamorphosis with special reference to juvenile hormone biosynthesis. Curr. Top. Dev. Biol. 103, 73–100. ( 10.1016/B978-0-12-385979-2.00003-4) [DOI] [PubMed] [Google Scholar]
- 44.Kamsoi O, Bellés X. 2019. Myoglianin triggers the premetamorphosis stage in hemimetabolan insects. FASEB J. 33, 3659–3669. ( 10.1096/fj.201801511R) [DOI] [PubMed] [Google Scholar]
- 45.Cheong SP, Huang J, Bendena WG, Tobe SS, Hui JH. 2015. Evolution of ecdysis and metamorphosis in arthropods: the rise of regulation of juvenile hormone. Integr. Comp. Biol. 55, 878–890. ( 10.1093/icb/icv066) [DOI] [PubMed] [Google Scholar]
- 46.Truman JW, Riddiford LM. 2019. The evolution of insect metamorphosis: a developmental and endocrine view. Phil. Trans. R. Soc. B 374, 20190070 ( 10.1098/rstb.2019.0070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellés X. 2011. Origin and evolution of insect metamorphosis. In Encyclopedia of life sciences (ELS). Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- 48.Truman JW, Riddiford LM. 1999. The origins of insect metamorphosis. Nature 401, 447–452. ( 10.1038/46737) [DOI] [PubMed] [Google Scholar]
- 49.Truman JW, et al. 2006. Juvenile hormone is required to couple imaginal disc formation with nutrition in insects. Science 312, 1385–1388. ( 10.1126/science.1123652) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material. Gb′Kr-h1 (accession number LC476894), Gb′Br (accession number LC476892) and Gb′E93 (accession number LC476893) cDNA sequences have been deposited in DNA Data Bank of Japan.