Abstract
In insects that undergo complete metamorphosis, cell death is essential for reshaping or removing larval tissues and organs, thus contributing to formation of the adult's body structure. In the last few decades, the study of metamorphosis in Lepidoptera and Diptera has provided broad information on the tissue remodelling processes that occur during larva–pupa–adult transition and made it possible to unravel the underlying regulatory pathways. This review summarizes recent knowledge on cell death mechanisms in Lepidoptera and other holometabolous insects, highlighting similarities and differences with Drosophila melanogaster, and discusses the role of apoptosis and autophagy in this developmental setting.
This article is part of the theme issue ‘The evolution of complete metamorphosis'.
Keywords: apoptosis, autophagy, Lepidoptera, metamorphosis, programmed cell death
1. Introduction
Both in hemimetabolous and holometabolous insects, the body plan is modified during the transition from juvenile to adult stage, but in Holometabola these modifications are marked and a pupal stage is interposed between the last larval instar and the adult stage. Insect development and metamorphosis are controlled by 20-hydroxyecdysone (20E) and juvenile hormone (JH) [1,2]. The response triggered by these hormones in each tissue depends on receptors, activation and/or inhibition of precise pathways, and expression of specific factors that together drive the cell-specific response to the stimulus. As a consequence, according to their fate, cells and tissues during metamorphosis can: (i) degenerate if they belong to specific organs present only in immature stages (e.g. head glands); (ii) undergo remodelling without cells being completely replaced (e.g. fat body) or being replaced with those deriving from undifferentiated cells (e.g. midgut); or (iii) generate a new adult structure thanks to the proliferation and differentiation of undifferentiated cells present in the imaginal discs (e.g. wings and legs) [3]. Although we are far from having a complete picture, in the last few decades much effort has been devoted to elucidate the mechanisms involved in the reshaping of tissues during insect metamorphosis, and programmed cell death (PCD) has emerged as a key event that drives this process.
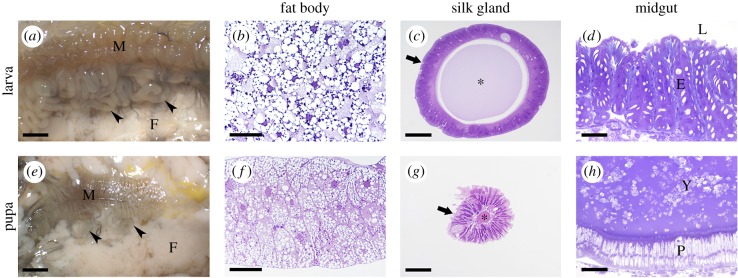
Among the insect organs that reshape more or less extensively, or even degenerate, during the larva–pupa transition, the midgut, the fat body, head glands and neurons are worthy of mention (figure 1). The larval midgut is composed of a monolayered epithelium in which columnar cells, involved in digestion and absorption of nutrients, are the most abundant cell type. When the larva enters metamorphosis, stem cells (small round cells located at the base of the monolayer) proliferate and differentiate, thus creating the midgut of the adult insect. Concomitantly, larval midgut cells are displaced by the newly forming epithelium and are pushed toward the lumen, forming a mass called the yellow body where they die [4]. The fat body is a multifunctional organ that plays a key role in nutrient storage and mobilization, but is also involved in hormonal regulation, immune response and detoxification. At metamorphosis, tissue remodelling involves partial dissociation of clusters of cells and differentiation of primordial cells to replace those cells undergoing PCD [5]. By contrast, head glands such as the silk glands (Bombyx mori), salivary glands (Drosophila melanogaster) and labial glands (Manduca sexta) completely (in the first two types of head glands) or almost completely (in the latter type) degenerate during metamorphosis and no remnants of these tissues are found in the adult insect [6,7]. Finally, some motoneurons, such as proleg motoneurons (the best-studied examples of dying neurons) undergo a segment-specific pattern of PCD during larval–pupal transition together with their target muscles [8]. It must be underlined that tissue remodelling and cell death are not limited to the early phase of metamorphosis, i.e. larva–pupa transition, but can also occur at later stages, during pupal–adult transition. For example, the intersegmental muscles of moths are giant syncytial fibres that begin to atrophy some days prior to adult eclosion; then, once they have played their part in the emergence of the adult from the pupa, they are no longer necessary and die within a few hours [9].
Figure 1.
Remodelling of silkworm organs during metamorphosis. (a,e) Stereomicroscopic images showing the internal anatomy of Bombyx mori; (b,f) cross-sections of the fat body; (c,g) cross-sections of the silk gland; (d,h) cross-sections of the midgut. E, larval midgut epithelium; F, fat body; Y, yellow body; L, midgut lumen; M, midgut; P, pupal midgut epithelium; arrowheads, silk glands; arrows, silk gland epithelium; asterisk, silk gland lumen. Bars: 25 mm (a,e); 50 µm (b,f); 200 µm (c); 100 µm (d,g,h). (Online version in colour.)
Although tissue remodelling involving cell death processes has been reported in many species that undergo complete metamorphosis, Lepidoptera and the dipteran D. melanogaster have provided the bulk of information on these processes and have made it possible to unravel the underlying regulatory mechanisms. Recent reviews describe in detail PCD during metamorphosis in Drosophila tissues and organs [7,10–12]. By contrast, a comprehensive description of the processes and regulatory mechanisms involved in tissue reshaping in Lepidoptera is lacking. For this reason, we report in this review the current knowledge on PCD during metamorphosis in this order, as well as in other holometabolous and hemimetabolous insects, highlighting similarities and differences with Drosophila.
2. Programmed cell death
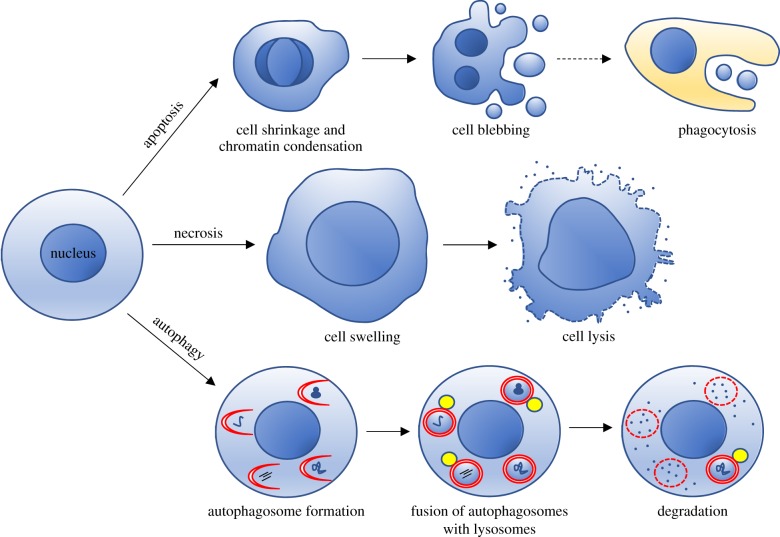
PCD plays a fundamental role in the normal development of metazoans as it is essential for removing damaged cells and for remodelling and sculpting tissues during morphogenesis. Many different forms of cell death, sometimes characterized by overlapping features, have been reported in the literature. Since the definition and the interpretation of the various cell death processes are complex and novel mechanisms that regulate cell death pathways are continuously being unveiled, the Nomenclature Committee on Cell Death has formulated detailed guidelines (see [13] for the last version). However, two cell death processes have been mainly described during metamorphosis, i.e. apoptosis and autophagic cell death. While the biochemical pathways that regulate apoptosis have been widely studied, less information about the mechanisms and genes regulating autophagic cell death is available [13,14].
Apoptosis is a caspase-dependent cell death process characterized by DNA fragmentation, cytoplasmic blebbing and engulfment and degradation of the apoptotic bodies by phagocytes (figure 2). Caspases are cysteine proteases that include two groups of enzymes: initiator caspases are triggered by the apoptotic stimulus and, in turn, activate effector caspases that cleave the majority of cell components and lead to the death of the cell [15]. The apoptotic pathway has been well characterized in Drosophila where it involves the intervention of seven caspases that show enzymatic function comparable to that characterized in Caenorhabditis elegans and mammals, although the regulation of apoptotic machinery is slightly different in this insect. Among the major differences, it is worth highlighting that in Drosophila cytochrome c is not required for the assembly of the apoptosome and caspases are mainly regulated by inhibitor of apoptosis proteins (IAPs) [16].
Figure 2.
Schematic of the apoptotic, necrotic and autophagic processes. (Online version in colour.)
On the other hand, cells that undergo autophagic cell death are capable of digesting their own contents without the aid of engulfing phagocytes. The morphological hallmark of autophagic cell death is the formation and the presence of autophagosomes in the dying cell. However, this does not necessarily mean that autophagy plays an active role in the death of the cell [14]. Autophagy is a catabolic process that leads to the degradation of proteins and organelles through their engulfment into the autophagosome, a double membrane structure which is formed from an isolation membrane whose origin is under debate. Once the autophagosome fuses with lysosomes, the inner content is degraded and the breakdown products are recycled back into the cytosol (figure 2). The autophagic pathway is highly conserved among eukaryotes and is mediated by more than 35 AuTophaGy-related (ATG) genes, originally identified from genetic screening for yeast mutants unable to survive under starvation. Autophagosome formation is triggered by activating the Atg1 kinase complex, which is obtained by relieving the inhibitory action of Target of rapamycin (Tor). The Atg6 complex then activates nucleation of the isolation membrane, while the elongation and expansion of the autophagosome membrane to form a closed autophagosome depends on two conserved ubiquitin-like conjugation systems, i.e. the Atg12-Atg5/Atg16 complex and Atg8, which mediate the lipidation and insertion of Atg8 in the growing autophagosome membrane [17].
In some developmental settings, a third type of cell death has been observed, i.e. necrosis, which is characterized by an increase in cell volume, swelling of cytoplasmic organelles, membrane rupture and release of cytoplasmic content in the extracellular environment (figure 2). Although necrosis is usually considered an uncontrolled form of cell death, its execution may be finely regulated at least in some contexts (e.g. necroptosis) [13].
It must be highlighted that the role of autophagy in tissue remodelling during insect metamorphosis is still widely debated, and, over the years, a copresence of autophagic and apoptotic features in insect organs during metamorphosis has been described (for an overview on the early age of autophagic and apoptotic studies in holometabolous insects, see [18–20]). Moreover, recent studies suggest an intimate relationship between the two processes and corroborate the hypothesis that they both have an active role in remodelling/removing larval tissues.
3. Programmed cell death in Lepidoptera
Lepidoptera, and in particular B. mori, Helicoverpa armigera and M. sexta, have been used in the last two decades to gain insight into the endocrinological and physiological aspects of cell death processes in insects. More recently, thanks to the development of genetic tools such as RNAi and CRISPR-Cas9, the first two species have also provided information on the regulatory mechanisms of cell death. Although knowledge on cell death in Drosophila might be exploited for studying the role of autophagy and apoptosis in normal and pathological conditions, it is worth underscoring the fact that some of the most important insect pest species belong to Lepidoptera and, therefore, an understanding of the processes that intervene during metamorphosis and identifying critical regulatory factors involved in this crucial process could be useful for application purposes.
(a). The apoptotic process in Lepidoptera
Caspase-1 represents the first caspase found in Lepidoptera. It was identified and characterized in Spodoptera frugiperda where it acts as the main effector caspase and is able to trigger apoptosis. The activation process requires cleavage of the p37 proenzyme in a two-step process to produce the p12 and p19 subunits [21]. Thereafter, homologues, characterized by conserved amino acid sequences and similar mechanisms of action, were found in Spodoptera littoralis (SlCaspase-1) [22], B. mori (BmCaspase-1) [23] and Galleria mellonella (GmCaspase-1) [24]. The complexity of the apoptotic pathway in Lepidoptera was brought to light thanks to a genome-wide analysis of apoptosis-related genes in B. mori that identified two initiator caspases, namely BmDronc and BmDreed, and three effector caspases, BmCaspase-N, BmCaspase-1 and BmICE [25]. Moreover, a number of homologues of both human and Drosophila apoptosis-related genes were reported and the presence of both the extrinsic and the intrinsic apoptotic pathway (i.e. initiated by extracellular and intracellular stimuli, respectively), described in other organisms, was also hypothesized for the silkworm [25]. Subsequently, an extensive survey of lepidopteran-derived expressed sequence tag datasets in which sequences from 27 different lepidopteran species were compared to Drosophila homologues, helped identify 63 putative caspase genes in Lepidoptera [26]. These caspases clustered into six distinct groups: caspase-1, -2 and -3 were considered homologues to Drosophila Dcp-1, Drice and Decay, respectively, and thus classified as effector caspases owing to their short prodomain; caspase-5 and -6 showed structural features typical of initiator caspases and shared similarities with Drosophila Dronc and Dreed, respectively; the function of caspase-4 as effector or initiator caspase remained uncertain.
20E represents one of the major regulators of apoptosis in Lepidoptera [27]. As in Drosophila, upon binding to its heterodimeric nuclear receptor complex, which consists of the ecdysone receptor (EcR) and ultraspiracle (USP), this hormone elicits the transcription of early and late genes and the activation of apoptosis. Accordingly, RNAi-mediated knockdown of USP decreases the expression of apoptosis-related genes and reduces apoptosis in B. mori larval fat body [27]. However, regulation of apoptosis by 20E in Lepidoptera may be more complex than expected. In fact, based on studies performed in the anterior silk gland of B. mori, it has been proposed that cell death is regulated by a double pulse of 20E, which peaks twice during larval–pupal transition [28]. In particular, the first ecdysone peak (named the commitment peak) drives a genomic response by upregulating the expression of apoptosis-related genes. This effect is mediated by the binding of 20E to the EcR/USP receptor complex. A second pulse of hormone (the so-called metamorphic peak) occurs later, at pupal stage, and is much stronger than the commitment peak. The effects of this second wave of ecdysone are instead mediated by a putative ecdysone membrane receptor, probably a G protein-coupled receptor (GPCR), which activates effector caspases and triggers apoptosis (non-genomic response) [28]. The involvement of GPCRs in mediating 20E signalling that leads to apoptosis has been reported in H. armigera as well [29]. Similar to the silk gland, a biphasic activation of the apoptotic process by 20E has also been observed in the silkworm midgut. In particular, Romanelli et al. [30] demonstrated that the first ecdysone peak promotes the transcriptional upregulation of apoptotic genes in the midgut, but a second 20E peak is necessary to obtain caspase cleavage and the subsequent activation of the apoptotic process. In this setting, IAPs, which act through binding to key apoptotic players and whose removal is necessary for caspase activation, could contribute to the regulation of the apoptotic process by 20E. In Lepidoptera, IAPs are highly expressed at the end of the last larval stage when the 20E commitment peak occurs [30–35] and the high levels of IAPs can likely block the activation of caspases. Conversely, after metamorphosis begins, and concomitantly with the occurrence of the 20E metamorphic peak, the expression of IAPs decreases, while the mRNA levels of death activator genes and caspase reach a maximum, a clear indication of the shift of the cells to the apoptotic condition. This evidence confirms the existence of a cross-link between IAPs and caspases in regulating the activation of apoptosis in Lepidoptera and suggests that these anti-apoptotic proteins may play a role in the two-step process needed to activate apoptosis following the 20E peaks. Recently, the Hippo signalling pathway, which controls the organ size by restricting cell proliferation and promoting apoptosis, was shown to be involved in regulating cell death through IAPs [36]. Hippo is highly expressed during metamorphosis by 20E induction to regulate the activity of the transcriptional coactivator Yorkie. In particular, following 20E stimulation, Yorkie is phosphorylated and retained in the cytoplasm as it interacts with the adaptor protein 14-3-3-ε. The cytoplasmic localization of Yorkie suppresses the transcriptional activation of IAP1 and releases its inhibitory effects on caspases, thus leading to apoptosis [36,37]. Interestingly, He et al. [38] showed that IAPs can intervene at later stages of metamorphosis as well. In fact, IAP survivin can preserve both the imaginal midgut that forms at late pupal stage and the imaginal fat body from cell death, thus allowing the midgut and fat body to develop in the adult moth. This evidence demonstrates that, similarly to Drosophila, IAPs are the main regulators of cell survival in Lepidoptera and prevent the inappropriate activation of caspases during development, and that various IAPs may play specific, distinct roles at different developmental stages.
To fully understand the regulatory process of apoptosis, JH must also be considered. In fact, studies based on the administration of methoprene, a JH analogue, indicate that JH plays an inhibitory role towards apoptosis in many tissues [33]. In particular, the anterior silk glands become competent to respond to 20E before the prepupal period and the commitment to die is preceded by a loss of sensitivity to JH [3]. Moreover, Wang et al. [39] demonstrated that the counteracting activity of 20E and JH in inducing cell death is mediated by different levels of calcium mobilization. In fact, upon binding to GPCRs, 20E induces high calcium levels in the cell activating caspases and inducing apoptosis, while lower calcium levels, induced by JH, have the opposite effect.
(b). The autophagic process in Lepidoptera
A genome-wide search of autophagy-related genes performed in 2009 paved the way for the molecular analysis of the autophagic process in Lepidoptera [40]. Homologues of several Atg proteins as well as factors involved in the PI3K signal transduction pathway were soon identified, thus demonstrating the existence of a well-organized autophagic pathway in these insects [19,30,40–44]. Zhou et al. [45] also identified two paralogous genes that code for Tor, a key regulatory factor of autophagy in eukaryotes. The upregulation of both BmTOR genes by starvation and 20E, two signals that promote autophagy in Drosophila by recruiting Tor, confirms that silkworm Tor is able to mediate the activation of the autophagic pathway triggered by nutrient conditions and hormones [45].
The autophagic pathway in the fat body is elicited by 20E through a twofold activation process. In fact, the 20E commitment peak during larval–pupal transition inhibits the PI3K/Torc1 (Target of rapamycin complex 1) pathway and activates the downstream Atg1/Atg13 complex, thus inducing autophagosome initiation. In parallel, 20E also contributes to autophagosome formation by upregulating the transcription of ATG genes: this effect is mediated by the action of Broad-Complex (Br-C) and downstream proteins, but also through direct interaction with the promoter of ATG genes, as demonstrated for BmATG1 [46]. Data on the midgut support the double regulatory effects of 20E on autophagy in lepidopteran organs. In fact, a single dose of ecdysone in this tissue can induce the transcription of ATG genes and, at the same time, activate the autophagic flux through Torc1 inhibition [30]. By using loss-of-function and gain-of-function approaches, it has been recently demonstrated that not only ecdysone biosynthesis but also 20E depletion is fundamental for regulating autophagy-mediated tissue degeneration in the silkworm. In fact, Li et al. [47] showed that the overexpression of ecdysone oxidase, a 20E inactivation enzyme, specifically inhibits autophagy and impairs the degeneration of the middle silk gland, confirming that 20E-triggered autophagy is required for tissue histolysis in the silkworm. In addition, the authors speculate that the tissue-specific differences in response to ecdysteroid, such as timing of autophagy and apoptosis activation, may be partially mediated by a differential expression of ecdysteroid inactivation enzymes.
Only limited information on the signalling pathway that links autophagy to JH is available for Lepidoptera. In this context, it has been shown that administration of JH to Mamestra brassicae during last larval instar inhibits autophagy in the fat body [48]. Moreover, RNAi of the putative JH receptor Met has a marked effect on the autophagic response during metamorphosis in B. mori [49]. This evidence suggests that autophagy is inhibited by JH, and it can be activated at metamorphosis by an increase in 20E concentration, when the JH titre is low.
(c). Apoptosis and autophagy in Lepidoptera: a matter of coexistence, cooperation or overlap?
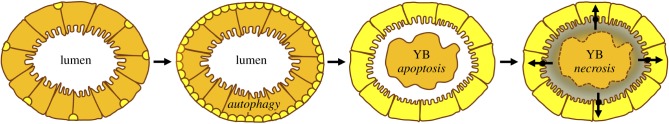
Coexistence of autophagic and apoptotic features within the same tissue or organ has been reported in both Lepidoptera [6,31,50,51] and Drosophila (for a review, see [7]). Research in these insects has been mainly devoted to unraveling the role of the two processes and to looking for potential factors that mediate the interaction between them. By using specific markers, it has been demonstrated that, in general, autophagy is activated in many lepidopteran organs at wandering stage and precedes apoptosis during metamorphosis [6,30,31,34]. As a double pulse of ecdysone is needed to activate apoptosis, as described above, the occurrence of apoptosis follows that of autophagy, but the precise timing of activation is organ-specific and is related to the role played by autophagy as follows. In the posterior silk gland of B. mori, apoptosis is activated 48–72 h after the autophagic process. In this tissue, glycogen stores need to be mobilized and the cytoplasmic components degraded by autophagy to produce the energy that the gland cells need to stay alive and complete the spinning of the cocoon. Conversely, apoptosis leads cells to death once the silk gland is no longer useful to the larva and apoptotic bodies can be removed by phagocytes in the larval haemocoel [6]. In the larval fat body, the temporal shift in the occurrence of autophagy and apoptosis is not as evident. However, the two processes seem to have different roles: autophagy is activated during metamorphosis to promote lipolysis and trophically support the insect [46], while apoptosis is necessary for remodelling this tissue [27,52]. Thus, the evidence collected seems to exclude an involvement of autophagy in the death of fat body and silk gland cells. The function of autophagy and apoptosis has been investigated in the silkworm midgut, too. In this setting, the administration of autophagy inhibitors to the larvae leads to an increased degeneration of the larval midgut compared to the control, thus suggesting a prosurvival role of autophagy in this tissue. Moreover, caspase inhibitors significantly delay larval midgut degeneration, thus demonstrating that apoptosis is sufficient to drive this process during metamorphosis [30]. The precise role of autophagy in this scenario can be appreciated by considering its intimate relationship with apoptosis and necrosis. In fact, autophagy is activated in larval midgut cells as soon as the larva stops feeding at the beginning of metamorphosis. This is initially necessary to cope with nutrient starvation, but the larval cells are then digested completely. Meanwhile, cells undergo apoptosis and, subsequently, secondary necrosis determines the release of the breakdown products, generated by the autophagic process, into the extracellular environment (i.e. the midgut lumen). These products can be directly absorbed by the surrounding cells that, in the meantime, are forming the new midgut epithelium of the adult (figure 3). Thus, this tissue can recycle products deriving from the degeneration of larval midgut cells that contribute in this manner to trophically sustain the silkmoth [4,53]. Therefore, as in fat body [46] and silk gland [6], autophagy has a prosurvival role in the larval midgut, while apoptosis is the major process responsible for the death of the larval cells, questioning the existence of autophagic cell death in Lepidoptera. This setting (in which autophagy guarantees cell survival, apoptosis plays a key function in driving the demise of larval tissues and cell death processes are caspase-dependent, is well supported by functional experiments in which the use of inhibitors [30] and gene silencing by RNAi [36,54,55] have been used to block the autophagic and the apoptotic pathway in the larval organs of B. mori and H. armigera. However, at least four different, alternative scenarios that confer further complexity to developmental cell death in Lepidoptera have been described in M. sexta and other insects belonging to this order. Importantly, though, some of them still require further investigation using functional or genetic tools. (1) In dying larval motoneurons, which exhibit an autophagic phenotype without features characteristic of apoptosis, the administration of caspase inhibitors impairs the cell death process [56], suggesting that caspase-dependent autophagic cell death occurs [8]. (2) Since the early studies of Lockshin [57–60], it has been evident that the death process causing intersegmental muscles to disappear soon after adult eclosion diplays typical autophagic features and does not show any apoptotic marker such as DNA condensation and fragmentation or caspase activation. According to this evidence, the term ‘PCD’ was introduced and this degenerative process classified as autophagic cell death [57,58]. The removal of these cells is indeed mediated by lysosomal enzymes as well as by proteasomal activity, as demonstrated by the upregulation of several genes associated with proteolysis, and increased protein catabolism through the ubiquitin-proteasome pathway [61–64]. This cell death scenario likely reflects the impossibility for these muscles to be phagocytosed owing to their large size. For this reason, they undergo autophagic cell death which not only removes unnecessary larval cells in the moth, but also provides amino acids that can serve as a nutrient source for the adult insect [9]. (3) Similarly to intersegmental muscles, the involution of labial glands involves autophagy-associated PCD without any sign of apoptotic features [65], although the apoptosis machinery is conserved and functional in this organ [66]. In this case, caspase activity is substituted by the proteolytic activity of lysosomal cathepsins [66]. (4) A recent view identified cathepsins as the cell death switch. Cathepsins are cysteine or aspartate proteases that, in Lepidoptera, play several roles in regulating metamorphic and cell death processes (for a review, see [67]). The expression of cathepsins B, D and L is induced by 20E and a correlation between their expression and degradation of larval midgut and fat body has been established [68–70]. A recent study clearly demonstrates that cathepsin L is able to cleave procaspase 1 into the mature peptide and activate apoptosis in H. armigera midgut [71]. In addition, cathepsin L knockdown in Antheraea pernyi decreases the expression of several apoptotic genes and impairs fat body dissociation, confirming that this cathepsin is located upstream of the apoptotic pathway [72]. Together, this evidence indicates that cathepsins play a major role in triggering apoptosis.
Figure 3.
Schematic of the remodelling process that occurs in silkworm midgut during metamorphosis. Larval epithelium is indicated in orange, pupal–adult epithelium in yellow.
In conclusion, the contrasting data obtained so far suggest that the involvement of caspases in PCD could be context-dependent in Lepidoptera. These results also leave the role of autophagy in the metamorphosis of these holometabolous insects still unresolved, and foreshadow a more complex picture of cell death processes than expected.
(d). Autophagy and autophagic cell death: learning from Drosophila
In contrast to Lepidoptera, strong evidence demonstrating an active role of autophagy in ecdysone-mediated PCD in specific tissues comes from Drosophila. Apoptosis and autophagy must act in parallel to remove salivary glands during metamorphosis. Following puparium formation, the high titre of 20E triggers the expression of genes that regulate both mechanisms. The impairment of one of these two processes results in partial salivary gland degradation, while only the combined inhibition of both processes can significantly delay their removal [73]. Salivary glands can be considered a unique organ in which both apoptosis and autophagy cooperate to trigger cell death, although the involvement of other cell death pathways cannot be excluded as the organ removal is not completely blocked when the two mechanisms are inhibited [74]. In the Drosophila larval midgut, although apoptosis is activated during PCD, autophagy is the process that causes cell demise. It must be highlighted that caspase activation occurs in the absence of the main initiator and effector caspases (i.e. Ark, Dronc, Drice, Dcp-1 and Strica) and the primary contributor of caspase activity in dying midgut is Decay [75], which is, however, not essential for PCD in this tissue [75]. Therefore, the canonical apoptotic pathway is not required for larval midgut removal. By contrast, inhibition of autophagy by using ATG loss-of-function mutants (including ATG1, ATG2 and ATG18) induces a severe delay in the tissue remodelling without a decrease in caspase activity [75], indicating a key role for autophagy in midgut cell death. More recently, different components of the autophagic machinery that are required during midgut removal have been identified and their role defined [76], but further studies are required to fully delineate the regulation and mechanisms that mediate autophagy-dependent cell death in the midgut. Interestingly, a comparison of the components of the autophagic pathway essential for midgut degradation and those involved in starvation-induced autophagy in the fat body reveals that only a subset of ATG genes involved in the latter are required for autophagy-dependent cell death in the midgut [10], indicating that the regulative pathways for the autophagic process may vary in the two settings. Drosophila fat body during metamorphosis is extensively remodelled by a balanced 20E-dependent activation of apoptosis and autophagy. The 20E primary response gene E93 plays a key role in the activation of both pathways [77], thanks to the inhibition of the PI3K–Torc1 pathway, but the precise role of apoptosis and autophagy in this organ is not completely understood.
Taken together, these data demonstrate that, during insect metamorphosis, apoptosis and autophagy play a key role in tissue remodelling, but their direct involvement in cell demise is highly tissue-, context- and species-specific; moreover, it is still unknown what determines whether autophagy has a prosurvival or prodeath function in this setting. Drosophila has proven to be a powerful model for studying regulative processes and understanding gene function in insects, but the knowledge derived from this insect cannot be extended to other species without the risk of missing important peculiarities. PCD in insect metamorphosis represents a perfect example of this concept. A comparative approach as well as functional studies and the use of large-scale screenings to evaluate the expression profile of autophagic and apoptotic genes in different insects and tissues will hopefully clarify the precise role of the pathways activated for reshaping or removing larval organs during metamorphosis and unravel why PCD is executed via different mechanisms in the same organ of insects belonging to diverse, or even to the same, orders. Moreover, great attention must be devoted to possible interactions among different PCD mechanisms. Some apparently contrasting results reported in the literature, which are still not completely understood (e.g. the different roles played by autophagy and apoptosis in tissue remodelling among different lepidopteran species, and between them and Drosophila), may derive from different experimental approaches, but may also reflect a real variability that depends on factors whose meaning is not completely clear or still need to be discovered. Indeed, we are still far from having exhaustive and complete knowledge of the complex pathways that regulate insect body remodelling and only after the genes and pathways activated in different tissues/organs/insects have been characterized in depth will we understand why different PCD mechanisms are set in motion in a context-specific manner during metamorphosis.
(e). Cross-talk between autophagy and apoptosis
In the search for potential cross-talk between autophagy and apoptosis in Lepidoptera, considerable effort has been devoted to identifying potential mediators between the two processes.
Different Atg proteins have been suggested as molecular links between autophagy and apoptosis in Lepidoptera. In B. mori cell lines, it was recently proposed that the induction of apoptosis, which follows an autophagic response triggered by 20E or starvation, could be mediated by BmAtg5 and BmAtg6 after their cleavage by calpains [78,79]. Although the association of cleaved BmAtg5 to anti-apoptotic Bcl-2 family proteins to trigger cytochrome c release from mitochondria and caspase activation (similarly to that observed in mammals) has not been investigated yet, this mechanism could explain why 20E and/or starvation cause cell death in the larval organs of Lepidoptera during metamorphosis, with autophagy preceding apoptosis [79]. Support for this hypothesis was obtained in H. armigera, where it was shown that the cleavage of Atg5 could promote the switch from autophagy to apoptosis in the larval midgut [54]. In particular, Atg5 mediates the activation of autophagy triggered by low levels of 20E, thus leading to the survival of the cell. High levels of ecdysone determine an increase in intracellular calcium levels, which results in the cleavage of Atg5, probably mediated by calpains, and finally to the activation of apoptosis, causing the cell to die. A subsequent study focused on the Atg12-Atg5 complex, which is required for Atg8 lipidation, demonstrated that a low concentration of 20E can promote the conjugation of Atg5–Atg12, thus activating autophagy. Conversely, higher levels of the hormone induce the cleavage of Atg5 and impair the Atg5–Atg12 conjugation process, thus switching to apoptosis [55].
A final consideration concerns the PI3K/Akt pathway, which plays an important role in regulating a number of cellular processes, such as cell proliferation, cell survival, cell death, signal transduction and protein synthesis, and which may also deserve attention as a potential point of interaction between autophagy and apoptosis. This signalling pathway in fact represents the mediator that leads to the activation of autophagy at metamorphosis via nutrient signals and hormones, at least in the larval fat body [5]. Furthermore, a recent study has reported that the expression of apoptosis-related genes, such as caspases, during the spinning period is regulated by PI3K/Akt, suggesting a possible role of this signalling pathway in activating apoptosis in the posterior silk gland of B. mori, too [80]. However, further investigation is needed to confirm this hypothesis for the PI3K/Akt pathway and, hopefully, to characterize the putative regulatory mechanism.
4. Cell death in other holometabolous insects
In addition to Lepidoptera and Drosophila, the involvement of cell death processes in tissue remodelling during metamorphosis has been reported in Coleoptera, Hymenoptera and other Diptera. Since in most cases only morphological studies were carried out and there is no general agreement on the features that are typical of the different cell death forms, it is difficult to get a clear picture even in species that belong to the same insect order. Moreover, only limited functional evidence has been provided to evaluate the role of cell death processes in this setting. In spite of the fact that information is fragmentary and that further considerations of the regulatory mechanisms are not possible, we included an overview of other Holometabola for the sake of completeness.
In the honeybee, Apis mellifera, the intervention of PCD has been described in many developmental processes [81], not strictly related to metamorphosis. During the larva–pupa transition, a concomitant activation of autophagy and apoptosis is necessary for the removal of the larval midgut [82], the hindgut [83], salivary glands [84,85] and Malpighian tubules [86]. In particular, in the salivary glands, autophagy and apoptosis act in a coordinated manner to reduce the thickness of the larval epithelium by releasing cytoplasmic protrusions from the apical side of the cells. This process leads to the progressive degeneration of the epithelium and the authors speculate that the remnants of the glands are recycled back into the haemolymph without the intervention of phagocytes [85]. Both autophagy and apoptosis are responsible for the removal of the larval midgut and fat body in another hymenopteran, the stingless bee Melipona quadrifasciata [87,88]. In the fat body of this insect, autophagy appears to be the predominant process, while only a few apoptotic cells can be observed; this pattern could be explained by considering that this larval tissue is not subjected to significant destruction during metamorphosis, but it is only remodelled to generate the adult fat body.
In haematophagous Diptera, the remodelling of the midgut during metamorphosis is fundamental to produce an organ that is able to cope with the change in dietary requirements between the larval and the adult stage. Malta et al. [89] demonstrated that metamorphic changes in the larval midgut epithelium of sand flies are associated with the upregulation of several ATG genes owing to the presence of ecdysone-responsive elements in their promoter, as occurs in the silkworm. This evidence confirms that the mechanisms controlling the onset of metamorphosis are conserved across multiple orders of holometabolous insects. In Aedes aegyptii, it has been demonstrated that the differential expression of EcR and USP isoforms, as well as transcription factors, allows 20E to finely regulate cell death in the midgut [90]. A precise regulation of the death processes by 20E has been confirmed in Rynchosciara americana [91]. In this case, the prepupal and pupal ecdysone peak seem to trigger salivary gland histolysis and fat body reorganization, respectively. Finally, Mittapalli & Shukle [92] showed that the anti-apoptotic factor DAD1 (defender against apoptotic cell death) is highly expressed up to pupation in order to inhibit unwanted cell death events prior to metamorphosis. This evidence confers further complexity to the mechanisms that inhibit apoptosis in insects, in addition to the involvement of IAPs, as discussed above for Lepidoptera.
Studies in Coleoptera are mainly limited to the mealworm, Tenebrio molitor. In this coleopteron, many ATG genes have been cloned and characterized and, despite some variations, they are expressed in various tissues during development [93–95]. In particular, ATG8 is highly expressed in the midgut at pupal stages, corroborating a role of autophagy in the remodelling process of this tissue. It is noteworthy that ATG5 silencing impairs Atg8 lipidation [94], thus demonstrating not only the presence of autophagy-related homologues in this insect, but also the high conservation of the whole autophagy machinery. Parthasarathy & Palli [96] demonstrated that cell death, essentially apoptosis, in the Tribolium castaneum midgut is tightly controlled by 20E, which triggers a cascade of early and late genes as in Drosophila, and that these effects are counteracted by JH. A peculiar structure that is remodelled during metamorphosis has been reported in horned beetles [97]. Many species present horns, a cuticular projection of the head and/or prothorax that are completely degraded once they have facilitated the shedding of the head capsule during the pupal stage, or are remodelled to generate interindividual diversity at the adult stage. This remodelling process of the horn ensues after a classical apoptotic pathway is activated, as demonstrated by a microarray screening. Thus, in this case, the metamorphic remodelling of a body structure is not associated with any physiological function, but is needed to generate morphological diversity within and between species.
5. Tissue remodelling in hemimetabolous insects and evolutionary perspectives
In hemimetabolous insects, the morphological changes occurring during the transition from juvenile (i.e. nymph) to adult stage are less extensive than in Holometabola. These apparently less dramatic changes probably explain why relatively little attention has been devoted to the mechanisms and pathways involved in the remodelling and removal of tissues and organs during this event of the life cycle of Hemimetabola compared to Holometabola. Nonetheless, considering the relevance of cell death in the metamorphosis in Holometabola, the characterization of the molecular pathways involved in tissue remodelling and removal during the transition from juvenile to adult stage in Hemimetabola can aid our understanding of how metamorphosis has evolved from less to more derived insect species, as pointed out by different authors in recent reviews [98–100].
Since the studies of Wigglesworth [101,102] on Rhodnius prolixus, it has been clear that the onset of metamorphosis is hormonally regulated. Later, it was suggested that hormone-dependent PCD is involved in restructuring the metathoracic pleuroaxillary muscles in Locusta migratoria immediately after the imaginal molt [103], but functional studies were not performed until more recently. By using RNAi, activators and inhibitor proteins that coordinate the degeneration of the prothoracic glands during the onset of the adult stage of Blattella germanica have been identified [104]. The inhibitor of apoptosis BgIAP1 is required to block premature cell death processes in this gland, suggesting that the mechanism involved in impairing inappropriate caspase activation during juvenile development is highly conserved in insects. The degeneration of prothoracic glands depends on the complex cascade triggered by 20E that converges on the activation of the nuclear receptor BgFtz-f1. BgE75 acts as a suppressor of apoptotic cell death by restricting the expression of BgFtz-f1 to the end of the last nymphal instar. Moreover, JH plays a key role in preventing prothoracic gland degeneration during nymphal development, probably exerting its effect directly on the ecdysteroid-dependent transcriptional cascade, because treating last instar nymphs with the JH analogue methoprene reduces the expression of FTZ-F1 and prevents degeneration of the gland. After this first functional study, efforts were devoted to identifying the early genes activated by JH and 20E during incomplete metamorphosis [105–107] and the event that triggers the decrease of JH at the beginning of the last nymphal instar [108]. By contrast, less attention has been dedicated to PCD in postembryonic development. The evidence obtained so far indicates that the pathways triggering metamorphosis are generally conserved, although some differences have been shown. Regarding the regulation of JH level in the haemolymph, which always remains low during the last nymphal instar, myoglianin plays a key role [109]. In Holometabola, myoglianin is not involved in regulating JH production, probably because this regulatory mechanism might not be appropriate to determine a rapid pattern of decrease–increase–decrease of JH production, typical of the prepupal stage in Holometabola. Another interesting peculiarity of Hemimetabola concerns Br-C [105]. BR-C is an early response gene in the 20E signalling pathway whose expression is also influenced by JH, but in a different manner in holometabolous and hemimetabolous insects. In the latter, JH stimulates BR-C transcription from the beginning to the end of the nymphal stages and Br-C seems to be involved in regulating wing primordia development during nymphal stages [109,110]. By contrast, JH inhibits BR-C expression in holometabolous insects during the juvenile stage and the transcript levels of this gene increase at the end of the last larval instar, in correspondence to the commitment peak, and remain high during the prepupal phase, triggering entrance to the pupal–adult stage [111,112]. Moreover, the evidence that the expression patterns triggering adult morphogenesis (i.e. decrease in KR-H1 transcripts and increase in E93 expression) occur at the beginning of the last instar nymph in Hemimetabola and in the prepupae of Holometabola suggests that the last nymphal instar of hemimetabolous insects is homologous to the holometabolous pupa [98,105]. An alternative theory, largely based on observations of developmental endocrinology, equates larva–pupa–adult of the holometabolous insects to pronymph–nymph–adult of Hemimetabola [100]. The hemimetabolous pronymph is a cryptic embryonic stage with unique endocrinology and behavioural modifications that evolved into the larva of Holometabola.
Some questions remain open. Data reported in the literature seem to indicate, at least in the tissues examined so far, that apoptosis is the mechanism involved in restructuring the body plan during metamorphosis in Hemimetabola. Autophagic cell death has never been described, but its involvement cannot be excluded a priori. Is autophagic cell death present only in insects undergoing complete metamorphosis? If so, how relevant is this evidence from an evolutionary point of view? The answers to these questions will shed light on the evolution of metamorphosis and processes that drive this event.
Acknowledgements
We thank Sherryl Sundell for editing the English.
Data accessibility
This article has no additional data.
Authors' contributions
G.T. and M.C. wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by FAR 2017-2018 (University of Insubria).
References
- 1.Jindra M, Palli SR, Riddiford LM. 2013. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 58, 181–204. ( 10.1146/annurev-ento-120811-153700) [DOI] [PubMed] [Google Scholar]
- 2.Yamanaka N, Rewitz KF, O'Connor MB. 2013. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu. Rev. Entomol. 58, 497–516. ( 10.1146/annurev-ento-120811-153608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kakei M, Iwami M, Sakurai S. 2005. Death commitment in the anterior silk gland of the silkworm, Bombyx mori. J. Insect Physiol. 51, 17–25. ( 10.1016/j.jinsphys.2004.10.012) [DOI] [PubMed] [Google Scholar]
- 4.Franzetti E, et al. 2015. The midgut of the silkmoth Bombyx mori is able to recycle molecules derived from degeneration of the larval midgut epithelium. Cell Tissue Res. 361, 509–528. ( 10.1007/s00441-014-2081-8) [DOI] [PubMed] [Google Scholar]
- 5.Li S, Yu X, Feng Q. 2019. Fat body biology in the last decade. Annu. Rev. Entomol. 64, 315–333. ( 10.1146/annurev-ento-011118-112007) [DOI] [PubMed] [Google Scholar]
- 6.Montali A, Romanelli D, Cappellozza S, Grimaldi A, de Eguileor M, Tettamanti G.. 2017. Timing of autophagy and apoptosis during posterior silk gland degeneration in Bombyx mori. Arthropod Struct. Dev. 46, 518–528. ( 10.1016/j.asd.2017.05.003) [DOI] [PubMed] [Google Scholar]
- 7.Nicolson S, Denton D, Kumar S. 2015. Ecdysone-mediated programmed cell death in Drosophila. Int. J. Dev. Biol. 59, 23–32. ( 10.1387/ijdb.150055sk) [DOI] [PubMed] [Google Scholar]
- 8.Kinch G, Hoffman KL, Rodrigues EM, Zee MC, Weeks JC. 2003. Steroid-triggered programmed cell death of a motoneuron is autophagic and involves structural changes in mitochondria. J. Comp. Neurol. 457, 384–403. ( 10.1002/Cne.10563) [DOI] [PubMed] [Google Scholar]
- 9.Schwartz LM. 2008. Atrophy and programmed cell death of skeletal muscle. Cell Death Differ. 15, 1163–1169. ( 10.1038/cdd.2008.68) [DOI] [PubMed] [Google Scholar]
- 10.Denton D, Kumar S. 2019. Autophagy-dependent cell death. Cell Death Differ. 26, 605–616. ( 10.1038/s41418-018-0252-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulakkal NC, Nagy P, Takats S, Tusco R, Juhasz G, Nezis IP. 2014. Autophagy in Drosophila: from historical studies to current knowledge. Biomed. Res. Int. 2014, 273473 ( 10.1155/2014/273473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yalonetskaya A, Mondragon AA, Elguero J, McCall K. 2018. I spy in the developing fly a multitude of ways to die. J. Dev. Biol. 6, 26 ( 10.3390/jdb6040026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galluzzi L, et al. 2018. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541. ( 10.1038/s41418-017-0012-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anding AL, Baehrecke EH. 2015. Autophagy in cell life and cell death. Curr. Top. Dev. Biol. 114, 67–91. ( 10.1016/bs.ctdb.2015.07.012) [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Lai Y, Hua ZC. 2019. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci. Rep. 39, pii. BSR20180992 ( 10.1042/BSR20180992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Accorsi A, Zibaee A, Malagoli D. 2015. The multifaceted activity of insect caspases. J. Insect Physiol. 76, 17–23. ( 10.1016/j.jinsphys.2015.03.007) [DOI] [PubMed] [Google Scholar]
- 17.Tettamanti G, Carata E, Montali A, Dini L, Fimia GM. 2019. Autophagy in development and regeneration: role in tissue remodelling and cell survival. Eur. Zool. J. 86, 113–131. ( 10.1080/24750263.2019.1601271) [DOI] [Google Scholar]
- 18.Tettamanti G, Cao Y, Feng Q, Grimaldi A, de Eguileor M.. 2011. Autophagy in Lepidoptera: more than old wine in new bottle. Inverteb. Surviv. J. 8, 5–14. [Google Scholar]
- 19.Romanelli D, Casati B, Franzetti E, Tettamanti G. 2014. A molecular view of autophagy in Lepidoptera. Biomed. Res. Int. 2014, 902315 ( 10.1155/2014/902315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockshin RA. 2016. Programmed cell death 50 (and beyond). Cell Death Differ. 23, 10–17. ( 10.1038/cdd.2015.126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Chejanovsky N. 2006. Activation pathways and signal-mediated upregulation of the insect Spodoptera frugiperda caspase-1. Apoptosis 11, 487–496. ( 10.1007/s10495-006-5059-5) [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Qi Y, Chejanovsky N. 2005. Spodoptera littoralis caspase-1, a Lepidopteran effector caspase inducible by apoptotic signaling. Apoptosis 10, 787–795. ( 10.1007/s10495-005-0365-x) [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Ju XL, Chen L, Chen KP. 2017. Caspase-1 from the silkworm, Bombyx mori, is involved in Bombyx mori nucleopolyhedrovirus infection. Z. Naturforsch. C. 72, 147–153. ( 10.1515/znc-2016-0133) [DOI] [PubMed] [Google Scholar]
- 24.Khoa DB, Trang LT, Takeda M. 2012. Expression analyses of caspase-1 and related activities in the midgut of Galleria mellonella during metamorphosis. Insect Mol. Biol. 21, 247–256. ( 10.1111/j.1365-2583.2011.01131.x) [DOI] [PubMed] [Google Scholar]
- 25.Zhang JY, Pan MH, Sun ZY, Huang SJ, Yu ZS, Liu D, Zhao DH, Lu C. 2010. The genomic underpinnings of apoptosis in the silkworm, Bombyx mori. BMC Genomics 11, 611 ( 10.1186/1471-2164-11-611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Courtiade J, Pauchet Y, Vogel H, Heckel DG. 2011. A comprehensive characterization of the caspase gene family in insects from the order Lepidoptera. BMC Genomics 12, 357 ( 10.1186/1471-2164-12-357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian L, Liu S, Liu H, Li S. 2012. 20-hydroxyecdysone upregulates apoptotic genes and induces apoptosis in the Bombyx fat body. Arch. Insect Biochem. 79, 207–219. ( 10.1002/arch.20457) [DOI] [PubMed] [Google Scholar]
- 28.Manaboon M, Iga M, Sakurai S. 2008. Nongenomic and genomic actions of an insect steroid coordinately regulate programmed cell death of anterior silk glands of Bombyx mori. Inverteb. Surviv. J. 5, 1–22. [Google Scholar]
- 29.Chen CH, Pan J, Di YQ, Liu W, Hou L, Wang JX, Zhao XF.. 2017. Protein kinase C delta phosphorylates ecdysone receptor B1 to promote gene expression and apoptosis under 20-hydroxyecdysone regulation. Proc. Natl Acad. Sci. USA 114, E7121–E7130. ( 10.1073/pnas.1704999114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romanelli D, Casartelli M, Cappellozza S, de Eguileor M, Tettamanti G.. 2016. Roles and regulation of autophagy and apoptosis in the remodelling of the lepidopteran midgut epithelium during metamorphosis. Sci. Rep. 6, 32939 ( 10.1038/srep32939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franzetti E, et al. 2012. Autophagy precedes apoptosis during the remodeling of silkworm larval midgut. Apoptosis 17, 305–324. ( 10.1007/s10495-011-0675-0) [DOI] [PubMed] [Google Scholar]
- 32.Khoa DB, Takeda M. 2012. Expression analysis of inhibitor of apoptosis and related caspases in the midgut and silk gland of the greater wax moth, Galleria mellonella, during metamorphosis and under starvation. Gene 510, 133–141. ( 10.1016/j.gene.2012.08.036) [DOI] [PubMed] [Google Scholar]
- 33.Parthasarathy R, Palli SR. 2007. Developmental and hormonal regulation of midgut remodeling in a lepidopteran insect, Heliothis virescens. Mech. Dev. 124, 23–34. ( 10.1016/j.mod.2006.09.002) [DOI] [PubMed] [Google Scholar]
- 34.Li QR, Deng XJ, Yang WY, Huang ZJ, Tettamanti G, Cao Y, Feng QL. 2010. Autophagy, apoptosis, and ecdysis-related gene expression in the silk gland of the silkworm (Bombyx mori) during metamorphosis. Can. J. Zool. 88, 1169–1178. ( 10.1139/Z10-083) [DOI] [Google Scholar]
- 35.Vilaplana L, Pascual N, Perera N, Belles X. 2007. Molecular characterization of an inhibitor of apoptosis in the Egyptian armyworm, Spodoptera littoralis, and midgut cell death during metamorphosis. Insect Biochem. Mol. Biol. 37, 1241–1248. ( 10.1016/J.Ibmb.2007.07.013) [DOI] [PubMed] [Google Scholar]
- 36.Dong DJ, Jing YP, Liu W, Wang JX, Zhao XF. 2015. The steroid hormone 20-hydroxyecdysone up-regulates Ste-20 family serine/threonine kinase Hippo to induce programmed cell death. J. Biol. Chem. 290, 24 738–24 746. ( 10.1074/jbc.M115.643783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang D, Li XR, Dong DJ, Huang H, Wang JX, Zhao XF. 2016. The steroid hormone 20-hydroxyecdysone promotes the cytoplasmic localization of Yorkie to suppress cell proliferation and induce apoptosis. J. Biol. Chem. 291, 21 761–21 770. ( 10.1074/jbc.M116.719856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He HJ, Hou L, Wang JX, Zhao XF. 2012. The apoptosis inhibitor survivin prevents insect midgut from cell death during postembryonic development. Mol. Biol. Rep. 39, 1691–1699. ( 10.1007/s11033-011-0909-9) [DOI] [PubMed] [Google Scholar]
- 39.Wang D, Pei XY, Zhao WL, Zhao XF. 2016. Steroid hormone 20-hydroxyecdysone promotes higher calcium mobilization to induce apoptosis. Cell Calcium 60, 1–12. ( 10.1016/j.ceca.2016.05.003) [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Hu ZY, Li WF, Li QR, Deng XJ, Yang WY, Cao Y, Zhou CZ. 2009. Systematic cloning and analysis of autophagy-related genes from the silkworm Bombyx mori. BMC Mol. Biol. 10, 50 ( 10.1186/1471-2199-10-50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khoa DB, Takeda M. 2012. Expression of autophagy 8 (Atg8) and its role in the midgut and other organs of the greater wax moth, Galleria mellonella, during metamorphic remodelling and under starvation. Insect Mol. Biol. 21, 473–487. ( 10.1111/j.1365-2583.2012.01152.x) [DOI] [PubMed] [Google Scholar]
- 42.Hu C, Zhang XA, Teng YB, Hu HX, Li WF. 2010. Structure of autophagy-related protein Atg8 from the silkworm Bombyx mori. Acta Crystallogr. F 66, 787–790. ( 10.1107/S1744309110018464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casati B, Terova G, Cattaneo AG, Rimoldi S, Franzetti E, de Eguileor M, Tettamanti G.. 2012. Molecular cloning, characterization and expression analysis of ATG1 in the silkworm, Bombyx mori. Gene 511, 326–337. ( 10.1016/j.gene.2012.09.086) [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Zheng S, Liu L, Tettamanti G, Cao Y, Feng Q. 2011. Expression of autophagy-related genes in the anterior silk gland of the silkworm (Bombyx mori) during metamorphosis. Can. J. Zool. 11, 1019–1026 ( 10.1139/Z11-075) [DOI] [Google Scholar]
- 45.Zhou S, Zhou Q, Liu Y, Wang S, Wen D, He Q, Wang W, Bendena WG, Li S. 2010. Two Tor genes in the silkworm Bombyx mori. Insect Mol. Biol. 19, 727–735. ( 10.1111/j.1365-2583.2010.01026.x) [DOI] [PubMed] [Google Scholar]
- 46.Tian L, Ma L, Guo E, Deng X, Ma S, Xia Q, Cao Y, Li S. 2013. 20-hydroxyecdysone upregulates Atg genes to induce autophagy in the Bombyx fat body. Autophagy 9, 1172–1187. ( 10.4161/auto.24731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, et al. 2015. Ectopic expression of ecdysone oxidase impairs tissue degeneration in Bombyx mori. Proc. R. Soc. B 282, 20150513 ( 10.1098/rspb.2015.0513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sass M, Kovacs J. 1975. Ecdysterone and an analogue of juvenile hormone on the autophagy in the cells of fat body of Mamestra brassicae. Acta Biol. Acad. Sci. Hung. 26, 189–196. [PubMed] [Google Scholar]
- 49.Guo E, et al. 2012. MET is required for the maximal action of 20-hydroxyecdysone during Bombyx metamorphosis. PLoS ONE 7, e53256 ( 10.1371/journal.pone.0053256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tettamanti G, et al. 2007. Programmed cell death and stem cell differentiation are responsible for midgut replacement in Heliothis virescens during prepupal instar. Cell Tissue Res. 330, 345–359. ( 10.1007/s00441-007-0449-8) [DOI] [PubMed] [Google Scholar]
- 51.Sumithra P, Britto CP, Krishnan M. 2010. Modes of cell death in the pupal perivisceral fat body tissue of the silkworm Bombyx mori L. Cell Tissue Res. 339, 349–358. ( 10.1007/S00441-009-0898-3) [DOI] [PubMed] [Google Scholar]
- 52.Kaneko Y, Yasanga T, Suzuki M, Sakurai S. 2011. Larval fat body cells die during the early pupal stage in the frame of metamorphosis remodelation in Bombyx mori. J. Insect Physiol. 57, 1715–1722. ( 10.1016/j.jinsphys.2011.09.013) [DOI] [PubMed] [Google Scholar]
- 53.Tettamanti G, Grimaldi A, Pennacchio F, de Eguileor M.. 2007. Lepidopteran larval midgut during prepupal instar: digestion or self-digestion? Autophagy 3, 630–631. ( 10.4161/auto.4908) [DOI] [PubMed] [Google Scholar]
- 54.Li YB, Li XR, Yang T, Wang JX, Zhao XF. 2016. The steroid hormone 20-hydroxyecdysone promotes switching from autophagy to apoptosis by increasing intracellular calcium levels. Insect Biochem. Mol. Biol. 79, 73–86. ( 10.1016/j.ibmb.2016.10.004) [DOI] [PubMed] [Google Scholar]
- 55.Li YB, Yang T, Wang JX, Zhao XF. 2018. The steroid hormone 20-hydroxyecdysone regulates the conjugation of autophagy-related proteins 12 and 5 in a concentration and time-dependent manner to promote insect midgut programmed cell death. Front. Endocrinol. 9, 28 ( 10.3389/fendo.2018.00028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffman KL, Weeks JC. 2001. Role of caspases and mitochondria in the steroid-induced programmed cell death of a motoneuron during metamorphosis. Dev. Biol. 229, 517–536. ( 10.1006/dbio.2000.9987) [DOI] [PubMed] [Google Scholar]
- 57.Lockshin RA, Williams CM. 1965. Programmed cell death—V. Cytolytic enzymes in relation to the breakdown of the intersegmental muscles of silkmoths. J. Insect Physiol. 11, 831–844. ( 10.1016/0022-1910(65)90186-1) [DOI] [PubMed] [Google Scholar]
- 58.Lockshin RA, Williams CM. 1965. Programmed cell death—I. Cytology of degeneration in the intersegmental muscles of the pernyi silkmoth. J. Insect. Physiol. 11, 123–133. ( 10.1016/0022-1910(65)90099-5) [DOI] [PubMed] [Google Scholar]
- 59.Beaulaton J, Lockshin R. 1977. Ultrastructural study of the normal degeneration of the intersegmental muscles of Anthereae polyphemus and Manduca sexta (Insecta, Lepidoptera) with particular reference of cellular autophagy. J. Morphol. 154, 39–58. ( 10.1002/jmor.1051540104) [DOI] [PubMed] [Google Scholar]
- 60.Lockshin R, Beaulaton J. 1979. Programmed cell death. Electrophysiological and ultrastructural correlations in metamorphosing muscles to lepidopteran insects. Tissue Cell 11, 803–819. ( 10.1016/0040-8166(79)90033-8) [DOI] [PubMed] [Google Scholar]
- 61.Schwartz LM, Myer A, Kosz L, Engelstein M, Maier C. 1990. Activation of polyubiquitin gene expression during developmentally programmed cell death. Neuron 5, 411–419. ( 10.1016/0896-6273(90)90080-Y) [DOI] [PubMed] [Google Scholar]
- 62.Haas AL, Baboshina O, Williams B, Schwartz LM. 1995. Coordinated induction of the ubiquitin conjugation pathway accompanies the developmentally programmed death of insect skeletal muscle. J. Biol. Chem. 270, 9407–9412. ( 10.1074/jbc.270.16.9407) [DOI] [PubMed] [Google Scholar]
- 63.Jones ME, Haire MF, Kloetzel PM, Mykles DL, Schwartz LM. 1995. Changes in the structure and function of the multicatalytic proteinase (proteasome) during programmed cell death in the intersegmental muscles of the hawkmoth, Manduca sexta. Dev. Biol. 169, 436–447. ( 10.1006/dbio.1995.1159) [DOI] [PubMed] [Google Scholar]
- 64.Fahrbach SE, Nambu JR, Schwartz LM. 2012. Programmed cell death in insects. In Insect molecular biology and biochemistry (ed. Gilbert LI.), pp. 419–449. London, UK: Academic Press. [Google Scholar]
- 65.Lockshin RA, Zakeri Z. 1994. Programmed cell death: early changes in metamorphosing cells. Biochem. Cell Biol. 72, 589–596. ( 10.1139/o94-078) [DOI] [PubMed] [Google Scholar]
- 66.Facey CO, Lockshin RA. 2010. The execution phase of autophagy associated PCD during insect metamorphosis. Apoptosis 15, 639–652. ( 10.1007/S10495-010-0499-3) [DOI] [PubMed] [Google Scholar]
- 67.Saikhedkar N, Summanwar A, Joshi R, Giri A. 2015. Cathepsins of lepidopteran insects: aspects and prospects. Insect Biochem. Mol. Biol. 64, 51–59. ( 10.1016/j.ibmb.2015.07.005) [DOI] [PubMed] [Google Scholar]
- 68.Gui ZZ, et al. 2006. Functional role of aspartic proteinase cathepsin D in insect metamorphosis. BMC Dev. Biol. 6, 49 ( 10.1186/1471-213X-6-49) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee KS, et al. 2009. Expression profile of cathepsin B in the fat body of Bombyx mori during metamorphosis. Comp. Biochem. Phys. B. Biochem. Mol. Biol. 154, 188–194. ( 10.1016/J.Cbpb.2009.06.002) [DOI] [PubMed] [Google Scholar]
- 70.Zhai X, Zhao XF. 2012. Participation of haemocytes in fat body degradation via cathepsin L expression. Insect Mol. Biol. 21, 521–534. ( 10.1111/j.1365-2583.2012.01157.x) [DOI] [PubMed] [Google Scholar]
- 71.Yang C, Lin XW, Xu WH. 2017. Cathepsin L participates in the remodeling of the midgut through dissociation of midgut cells and activation of apoptosis via caspase-1. Insect Biochem. Mol. Biol. 82, 21–30. ( 10.1016/j.ibmb.2017.01.010) [DOI] [PubMed] [Google Scholar]
- 72.Sun YX, Tang L, Wang P, Abbas MN, Tian JW, Zhu BJ, Liu CL. 2018. Cathepsin L-like protease can regulate the process of metamorphosis and fat body dissociation in Antheraea pernyi. Dev. Comp. Immunol. 78, 114–123. ( 10.1016/j.dci.2017.09.019) [DOI] [PubMed] [Google Scholar]
- 73.Berry DL, Baehrecke EH. 2007. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell 131, 1137–1148. ( 10.1016/j.cell.2007.10.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doherty J, Baehrecke EH. 2018. Life, death and autophagy. Nat. Cell Biol. 20, 1110–1117. ( 10.1038/s41556-018-0201-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Denton D, Shravage B, Simin R, Berry DL, Baehrecke EH, Kumar S. 2009. Autophagy, not apoptosis, is essential for midgut cell death in Drosophila. Curr. Biol. 19, 1741–1746. ( 10.1016/J.Cub.2009.08.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu T, Nicolson S, Denton D, Kumar S. 2015. Distinct requirements of autophagy-related genes in programmed cell death. Cell Death Differ. 22, 1792–1802. ( 10.1038/cdd.2015.28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu H, Wang J, Li S. 2014. E93 predominantly transduces 20-hydroxyecdysone signaling to induce autophagy and caspase activity in Drosophila fat body. Insect Biochem. Mol. Biol. 45, 30–39. ( 10.1016/j.ibmb.2013.11.005) [DOI] [PubMed] [Google Scholar]
- 78.Yi HY, Yang WY, Wu WM, Li XX, Deng XJ, Li QR, Cao Y, Zhong YJ, Huang YD. 2018. BmCalpains are involved in autophagy and apoptosis during metamorphosis and after starvation in Bombyx mori. Insect Sci. 25, 379–388. ( 10.1111/1744-7917.12417) [DOI] [PubMed] [Google Scholar]
- 79.Xie K, et al. 2016. BmATG5 and BmATG6 mediate apoptosis following autophagy induced by 20-hydroxyecdysone or starvation. Autophagy 12, 381–396. ( 10.1080/15548627.2015.1134079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu JH, Cheng XY, Li JX, Xue B, Tian JH, Hu JS, Li B. 2018. Apoptosis of posterior silk gland of Bombyx mori during spinning period and the role of PI3K/Akt pathway. Arch. Insect. Biochem. Physiol. 98, e21450 ( 10.1002/arch.21450) [DOI] [PubMed] [Google Scholar]
- 81.Malagoli D, et al. 2010. Autophagy and its physiological relevance in arthropods: current knowledge and perspectives. Autophagy 6, 575–588. ( 10.4161/auto.6.5.11962) [DOI] [PubMed] [Google Scholar]
- 82.Gregorc A, Bowen ID. 1997. Programmed cell death in the honey-bee (Apis mellifera L.) larvae midgut. Cell Biol. Int. 21, 151–158. ( 10.1006/cbir.1997.0127) [DOI] [PubMed] [Google Scholar]
- 83.Gonçalves WG, Fernandes KM, Santana WC, Martins GF, Zanuncio JC, Serrão JE. 2017. Post-embryonic changes in the hindgut of honeybee Apis mellifera workers: morphology, cuticle deposition, apoptosis, and cell proliferation. Dev. Biol. 431, 194–204. ( 10.1016/j.ydbio.2017.09.020) [DOI] [PubMed] [Google Scholar]
- 84.Silva-Zacarin EC, Tomaino GA, Brocheto-Braga MR, Taboga SR, De Moraes R.. 2007. Programmed cell death in the larval salivary glands of Apis mellifera (Hymenoptera, Apidae). J. Biosci. 32, 309–328. ( 10.1007/s12038-007-0031-2) [DOI] [PubMed] [Google Scholar]
- 85.Zacarin EC. 2007. Autophagy and apoptosis coordinate physiological cell death in larval salivary glands of Apis mellifera (Hymenoptera: Apidae). Autophagy 3, 516–518. ( 10.4161/auto.4735) [DOI] [PubMed] [Google Scholar]
- 86.Gonçalves WG, Fernandes KM, Santana WC, Martins GF, Zanuncio JC, Serrão JE. 2018. Post-embryonic development of the Malpighian tubules in Apis mellifera (Hymenoptera) workers: morphology, remodeling, apoptosis, and cell proliferation. Protoplasma 255, 585–599. ( 10.1007/s00709-017-1171-3) [DOI] [PubMed] [Google Scholar]
- 87.Cruz LC, Araujo VA, Fialho MCQ, Serrao JE, Neves CA. 2013. Proliferation and cell death in the midgut of the stingless bee Melipona quadrifasciata anthidioides (Apidae, Meliponini) during metamorphosis. Apidologie 44, 458–466. ( 10.1007/s13592-013-0196-7) [DOI] [Google Scholar]
- 88.Santos DE, Azevedo DO, Campos LA, Zanuncio JC, Serrão JE. 2015. Melipona quadrifasciata (Hymenoptera: Apidae) fat body persists through metamorphosis with a few apoptotic cells and an increased autophagy. Protoplasma 252, 619–627. ( 10.1007/s00709-014-0707-z) [DOI] [PubMed] [Google Scholar]
- 89.Malta J, Heerman M, Weng JL, Fernandes KM, Martins GF, Ramalho-Ortigão M. 2017. Midgut morphological changes and autophagy during metamorphosis in sand flies. Cell Tissue Res. 368, 513–529. ( 10.1007/s00441-017-2586-z) [DOI] [PubMed] [Google Scholar]
- 90.Parthasarathy R, Palli SR. 2007. Stage- and cell-specific expression of ecdysone receptors and ecdysone-induced transcription factors during midgut remodeling in the yellow fever mosquito, Aedes aegypti. J. Insect Physiol. 53, 216–229. ( 10.1016/J.Jinsphys.2006.09.009) [DOI] [PubMed] [Google Scholar]
- 91.Brandão AS, do Amaral JB, Rezende-Teixeira P, Hartfelder K, Siviero F, Machado-Santelli GM. 2014. Cell death and tissue reorganization in Rhynchosciara americana (Sciaridae: Diptera) metamorphosis and their relation to molting hormone titers. Arthropod Struct. Dev. 43, 511–522. ( 10.1016/j.asd.2014.05.001) [DOI] [PubMed] [Google Scholar]
- 92.Mittapalli O, Shukle RH. 2008. Molecular characterization and responsive expression of a defender against apoptotic cell death homologue from the Hessian fly, Mayetiola destructor. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 149, 517–523. ( 10.1016/j.cbpb.2007.12.001) [DOI] [PubMed] [Google Scholar]
- 93.Lee JH, Jo YH, Patnaik BB, Park KB, Tindwa H, Seo GW, Chandrasekar R, Lee YS, Han YS. 2015. Cloning, expression analysis, and RNA interference study of a HORMA domain containing autophagy-related gene 13 (ATG13) from the coleopteran beetle, Tenebrio molitor. Front. Physiol. 6, 180 ( 10.3389/fphys.2015.00180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tindwa H, Jo YH, Patnaik BB, Lee YS, Kang SS, Han YS. 2015. Molecular cloning and characterization of autophagy-related gene TmATG8 in Listeria-invaded hemocytes of Tenebrio molitor. Dev. Comp. Immunol. 51, 88–98. ( 10.1016/j.dci.2015.02.017) [DOI] [PubMed] [Google Scholar]
- 95.Tindwa H, et al. 2015. Depletion of autophagy-related genes ATG3 and ATG5 in Tenebrio molitor leads to decreased survivability against an intracellular pathogen, Listeria monocytogenes. Arch. Insect Biochem. Physiol. 88, 85–99. ( 10.1002/arch.21212) [DOI] [PubMed] [Google Scholar]
- 96.Parthasarathy R, Palli SR. 2008. Proliferation and differentiation of intestinal stem cells during metamorphosis of the red flour beetle, Tribolium castaneum. Dev. Dyn. 237, 893–908. ( 10.1002/dvdy.21475) [DOI] [PubMed] [Google Scholar]
- 97.Kijimoto T, Andrews J, Moczek AP. 2010. Programmed cell death shapes the expression of horns within and between species of horned beetles. Evol. Dev. 12, 449–458. ( 10.1111/j.1525-142X.2010.00431.x) [DOI] [PubMed] [Google Scholar]
- 98.Belles X. 2019. The innovation of the final moult and the origin of insect metamorphosis. Phil. Trans. R. Soc. B 374, 20180415 ( 10.1098/rstb.2018.0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jindra M. 2019. Where did the pupa come from? The timing of juvenile hormone signalling supports homology between stages of hemimetabolous and holometabolous insects. Phil. Trans. R. Soc. B 374, 20190064 ( 10.1098/rstb.2019.0064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Truman JW, Riddiford LM. 2019. The evolution of insect metamorphosis: a developmental and endocrine view. Phil. Trans. R. Soc. B 374, 20190070 ( 10.1098/rstb.2019.0070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wigglesworth VB. 1962. Hormones in relation to metamorphosis. Gen. Comp. Endocrinol. 1962(Suppl. 1), 316–321. ( 10.1016/0016-6480(62)90102-8) [DOI] [PubMed] [Google Scholar]
- 102.Wigglesworth VB. 1952. The thoracic gland in Rhodnius prolixus (Hemiptera) and its role in moulting. J. Exp. Biol. 29, 561–570. [Google Scholar]
- 103.Meuser S, Pflüger H-J. 1998. Programmed cell death specifically eliminates one part of a locust pleuroaxillary muscle after the imaginal moult. J. Exp. Biol. 201, 2367–2382. [DOI] [PubMed] [Google Scholar]
- 104.Mané-Padrós D, Cruz J, Vilaplana L, Nieva C, Ureña E, Bellés X, Martín D. 2010. The hormonal pathway controlling cell death during metamorphosis in a hemimetabolous insect. Dev. Biol. 346, 150–160. ( 10.1016/j.ydbio.2010.07.012) [DOI] [PubMed] [Google Scholar]
- 105.Belles X, Santos CG. 2014. The MEKRE93 (methoprene tolerant-Kruppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect Biochem. Mol. Biol. 52, 60–68. ( 10.1016/j.ibmb.2014.06.009) [DOI] [PubMed] [Google Scholar]
- 106.Santos CG, Fernandez-Nicolas A, Belles X. 2016. Smads and insect hemimetabolan metamorphosis. Dev. Biol. 417, 104–113. ( 10.1016/j.ydbio.2016.07.006) [DOI] [PubMed] [Google Scholar]
- 107.Ureña E, Manjón C, Franch-Marro X, Martín D. 2014. Transcription factor E93 specifies adult metamorphosis in hemimetabolous and holometabolous insects. Proc. Natl Acad. Sci. USA 111, 7024–7029. ( 10.1073/pnas.1401478111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kamsoi O, Belles X. 2019. Myoglianin triggers the pre-metamorphosis stage in hemimetabolan insects. FASEB J. 33, 3659–3669. ( 10.1096/fj.201801511R) [DOI] [PubMed] [Google Scholar]
- 109.Huang JH, Lozano J, Belles X. 2013. Broad-complex functions in postembryonic development of the cockroach Blattella germanica shed new light on the evolution of insect metamorphosis. Biochim. Biophys. Acta 1830, 2178–2187. ( 10.1016/j.bbagen.2012.09.025) [DOI] [PubMed] [Google Scholar]
- 110.Konopova B, Smykal V, Jindra M. 2011. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS ONE 6, e28728 ( 10.1371/journal.pone.0028728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Konopova B, Jindra M. 2008. Broad-Complex acts downstream of Met in juvenile hormone signaling to coordinate primitive holometabolan metamorphosis. Development 135, 559–568. ( 10.1242/dev.016097) [DOI] [PubMed] [Google Scholar]
- 112.Parthasarathy R, Tan A, Bai H, Palli SR. 2008. Transcription factor broad suppresses precocious development of adult structures during larval-pupal metamorphosis in the red flour beetle, Tribolium castaneum. Mech. Dev. 125, 299–313. ( 10.1016/j.mod.2007.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.