Abstract
Insect metamorphosis boasts spectacular cases of postembryonic development when juveniles undergo massive morphogenesis before attaining the adult form and function; in moths or flies the larvae do not even remotely resemble their adult parents. A selective advantage of complete metamorphosis (holometaboly) is that within one species the two forms with different lifestyles can exploit diverse habitats. It was the environmental adaptation and specialization of larvae, primarily the delay and internalization of wing development, that eventually required an intermediate stage that we call a pupa. It is a long-held and parsimonious hypothesis that the holometabolous pupa evolved through modification of a final juvenile stage of an ancestor developing through incomplete metamorphosis (hemimetaboly). Alternative hypotheses see the pupa as an equivalent of all hemimetabolous moulting cycles (instars) collapsed into one, and consider any preceding holometabolous larval instars free-living embryos stalled in development. Discoveries on juvenile hormone signalling that controls metamorphosis grant new support to the former hypothesis deriving the pupa from a final pre-adult stage. The timing of expression of genes that repress and promote adult development downstream of hormonal signals supports homology between postembryonic stages of hemimetabolous and holometabolous insects.
This article is part of the theme issue ‘The evolution of complete metamorphosis’.
Keywords: metamorphosis, evolution, juvenile hormone, signal transduction, hormone receptor, transcription factor
The primitive holometabolous development appears much less different from the hemimetaboly than is usually assumed from the highly derived holometabolan models. This means that some developmental traits, e.g., endocrinological, of the Holometabola may be rather primitive, and we may safely compare developmental regulation of holometabolous and hemimetabolous insects.
Sehnal et al. [1]
1. What is metamorphosis?
When asked this question at a symposium held on the subject, 14 representatives of relevant areas of biology each gave a definition considering developmental transitions observed in multicellular forms across the kingdoms [2]. Their answers varied vastly, indicating that metamorphosis is an elastic term, adaptable to various life-history scenarios as long as these involve a ‘marked’ change in morphology and/or lifestyle. There seemed to be a consensus that a metamorphosis should be a postembryonic event and that it typically entails physiological adaptation, such as to a new habitat. How marked a change needs to be in order to qualify seems to depend on a subjective view. This also applies to insects, where we distinguish between a polyphyletic conglomerate of ‘incompletely’ metamorphosing (hemimetabolous) orders and the monophyletic Holometabola with a ‘complete’ metamorphosis, marked by the presence of a non-feeding pupal stage [3,4]. While the participants could not agree whether or not hemimetabolous insects metamorphose, some explicitly highlighted mayflies and dragonflies with their aquatic larvae as an example of metamorphosis. In this review all insects except the primarily wingless Archaeognatha (bristletails) and Zygentoma (silverfish) will be regarded as metamorphosing.
2. The historical position of the pupa
The origin of the pupa and its homology to any stage(s) in hemimetabolans has long been debated (see [1,5–7] for reviews). The issue may be largely artificial and partly caused by the natural human inclination to study the most dramatic, rather than basal but less conspicuous examples. Our most important model insect, Drosophila melanogaster, is an example in which the mode of metamorphosis is highly derived.
Considering relatively minor differences among larvae, pupae and adults in at least some basal holometabolans such as the snakeflies (Raphidioptera) (figure 1) makes it easier to perceive holometaboly as a continuous process, rather than an abrupt transformation. The snakefly larvae do not differ considerably from typical hemimetabolous larvae except that, being endopterygotes, they do not bear external wing pads [5]. Their wings develop concealed under the cuticle of the final-instar larva [1,8]. A single moult converts a snakefly larva to a relatively motile pupa with external wings and free mandibles and legs (figure 1), in appearance similar to a late hemimetabolous larva; the next moult liberates the already walking pharate adult from the pupal cuticle.
Figure 1.
Metamorphosis in a snakefly (Raphidioptera), a basal holometabolan. (a) A late (possibly final) larval instar. (b) A mid-stage male pupa from lateral (left) and ventro-lateral (right) views. (c) Adult male. Scale bars, 5 mm.
Holometaboly, characterized by postponed wing development and its internalization, has undergone a marked evolution towards specialized larvae that became increasingly different from the adults [1,5]. In Drosophila and other cyclorrhaphous flies, wings and most other parts of the future adult fly develop from invaginated primordia called imaginal discs that form as early as during embryogenesis [9]. This innovation appears to have greatly accelerated development as the wing, leg, eye-antennal, and other internalized precursors of the adult structures can proliferate already during the larval stage [1,5]. Meanwhile, Drosophila larval tissues grow via increasing the cell ploidy and volume, and most will degenerate while in the pupa. Whether basal or derived, holometabolans share a common feature: as observed by Hinton [10, p. 83], ‘two moults—no more and no less—are required for the metamorphosis of a larva with internal wings to an adult with functional wings regardless of the degree of difference between the two stages’.
While metamorphosis can be extremely complex, the origin of the holometabolous pupa can be found in the gradual evolution of an ancestral, late-stage hemimetabolous larva into a non-feeding, progressively less-mobile form. Similar evolution has evidently occurred outside the Holometabola, as exemplified by the non-feeding ‘propupae’ and ‘pupae’ of thrips (Thysanoptera), or the immobile ‘pupae’ in which adult whiteflies or males of scale insects (both within the Hemiptera) acquire their wings [5]. The latter examples represent adaptation of the juveniles to sessile feeding. Such metamorphoses, encountered in the paraneopteran orders, have collectively been termed neometaboly (see [1] for a review). While these neometabolous ‘pupae’ evolved independently of the true pupae of endopterygotes, it is noteworthy that the body plan rebuilding that takes place during neometaboly can be far more extensive than metamorphosis in a typical basal holometabolan. Furthermore, holometaboly and the pupal stage can be secondarily lost, again through environmental adaptation. This is the case in some net-winged beetles (Lycidae) whose neotenic wingless females become reproductive as permanent ‘larvae’ without undergoing a metamorphic moult [11].
Interestingly, the endopterygote pupa is not always the site of the most dramatic morphological change. In numerous species of several holometabolan orders, larvae have adapted to various phases of endoparasitic lifestyle, leading to successive larval instars that bear little resemblance to each other, a condition dubbed hypermetamorphosis [1,5]. An example of the beetle Rhipidius quadriceps [12] shows that, while inside the host, the parasitic larva can even lose and then regain functional thoracic legs in four successive moults. This remarkable sequential polymorphism combines in the same species with sexual dimorphism as the females develop wingless.
The few examples above show that within the great diversity of insect life histories, resting stages (pupae) are just some of many adaptations. Given the morphological plasticity of the endopterygote larva, holometaboly is better characterized by differences between adults and larvae than by developmental stagnation during the larval stage [1]. The developmental potential in endopterygote larvae, including their ability to sexually mature into neotenic females, does not harmonize with theories that view these larvae as ‘de-embryonised’, developmentally arrested forms [13–15].
3. Alternative hypotheses for the origin of the holometabolous pupa
The fundamental concepts proposed during the twentieth century by Berlese, Heslop-Harrison, Poyarkoff, and Hinton to explain the relationships between hemimetaboly and holometaboly and the origin of the pupa have been repeatedly summarized [1,5–7]. There were several amendments that applied additional aspects including ecological, palaeontological, phylogenetic or endocrine (for details see [5]), which all in effect sided either with the original view of Berlese or with that of Hinton. The several theories can thus be aggregated into two, mutually exclusive main hypotheses (figure 2).
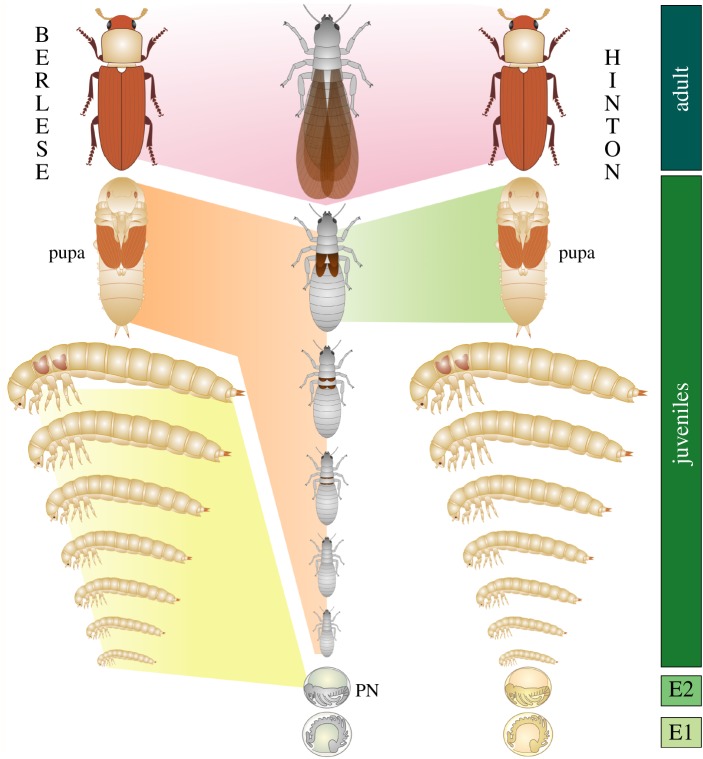
Figure 2.
Alternative hypotheses for evolutionary relationships between holometabolous stages and those of a hypothetical hemimetabolan ancestor. The green boxes on the right mark the successive embryonic stages (E1, E2) enclosed in the first and second embryonic cuticles, respectively. Left: the ‘de-embryonisation’ concept of Berlese, updated with the pronymph hypothesis [13]. The holometabolous larva hatches at a stage corresponding to E2, which becomes the hemimetabolous pronymph (PN). The pronymph expands into all holometabolous larval instars (yellow) whereas all hemimetabolous juvenile instars are compressed to the pupa (orange). Right: the Hinton concept of homology between the stages. Hemi- and holometabolous larvae hatch at an equal stage (in the third embryonic cuticle). The pupa is a modified late-stage hemimetabolous larva (although not necessarily precisely equal to the single, final instar). Note that the individual instars cannot be homologized.
One (‘Hinton’) maintains homology between hemimetabolous and holometabolous (endopterygote) larvae. Both represent equal ontogenetic stages except the latter have postponed and internalized their wing development. The holometabolous pupa arose via modification of a final pre-adult stage (late larva) of a hemimetabolous ancestor (figure 2).
The other (‘Berlese’) hypothesis derives holometabolous larvae by ‘de-embryonisation’, assuming that these larvae hatch at a stage of embryogenesis that is earlier relative to the stage at which hemimetabolans leave their eggs (figure 2). The holometabolous larvae then grow as free-living embryos while their development is limited until it resumes at metamorphosis, during the pupal stage. The consequence of ‘de-embryonisation’ is non-homology in the ‘Berlese’ view between the holometabolous and hemimetabolous larvae. The latter are called nymphs and undergo successive moults. In the Holometabola, the formerly nymphal instars are reduced to a single stage that does not moult and is called the pupa (figure 2).
The ‘Hinton’ hypothesis [10] was endorsed by many, even if with minor modifications, and received support based on palaeontology, eco-physiological simulation modelling, and mainly on phylogeny and endocrinology (reviewed in [1,5,16]). Early studies of Wigglesworth [17] had uncovered a blood borne ‘inhibitory hormone’, later identified as the sesquiterpenoid juvenile hormone (JH), which determines the juvenile character of insect moults. While a ‘moulting hormone’ (ecdysone) induces a moult, the presence of JH ensures that a larva is succeeded by another larval instar. Only when larvae attain adequate size, does JH subside to permit a metamorphic moult. As recognized by Wigglesworth, JH prevents precocious metamorphosis equally in hemimetabolans and holometabolans [18], suggesting that their juvenile stages are likewise equivalent. As discussed below, this notion and the ‘Hinton’ concept gained molecular, functional support once JH signalling genes and their roles in controlling metamorphosis became known [19,20] (figure 3) and were compared between holometabolous and hemimetabolous species [21–25].
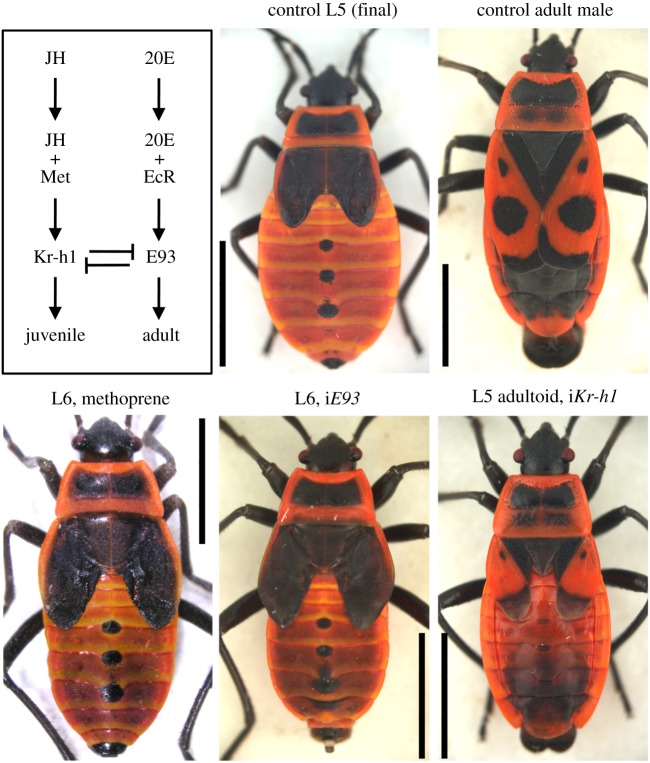
Figure 3.
Hormones effect juvenile and adult developmental programmes through mutual interactions of transcription factors. Bottom: phenotypes in Pyrrhocoris supporting the pathway scheme. An extranumerary larval instar (L6) can be induced either with the JH mimic methoprene or with E93 RNAi (iE93) early (on the first day) during the final larval instar (L5). E93 knockdown was achieved by injecting 3 µg per animal of double-stranded RNA encompassing the coding region of E93. Note that the wing pads of the aberrant L6 animals are larger relative to control L5 but similarly dark and unarticulated, external genitals undeveloped, and the notum retains the larval pattern. Conversely, precocious development of adult characters at the fifth instar was induced by knocking down Kr-h1 (iKr-h1) early during the L4 instar [21]. For details of RNAi treatments see Smykal et al. [22]. E93 and Kr-h1 are mutual repressors [23–25].
An endocrine aspect has also been the source of revival for the ‘Berlese’ concept. In 1999, Truman & Riddiford [13] proposed that after laying down the second embryonic cuticle (EC2), hemimetabolous insects (such as grasshoppers) enter a fleeting stage of a late embryo to hatchling, termed by them the ‘pronymph’, from which the animal moults/hatches into the first-instar nymph (figure 2). The authors have suggested that a shift in JH synthesis to an earlier phase of embryogenesis led to an early formation of structures needed for life outside the egg, and to hatching at the pronymph stage. The pronymph then presumably gave rise to the holometabolous larva which could specialize on growth while its development would be stalled until metamorphosis [13–15,26]. For instance, neuroblasts in the central nervous system of a moth embryo do not produce all of their progeny as neuroblasts in grasshopper embryos do, but resume proliferation while in larvae to generate adult neurons (reviewed in [15], this issue). A role for JH was supported by application of JH mimics to insect embryos, which caused severe growth arrest combined with premature tanning of mandibles and formation of the nymphal cuticle in hemimetabolans such as locusts [13,27] or crickets [28] but not in embryos of moths [14]. However, it is unclear how JH might block embryonic growth and accelerate nymphal maturation while its role after hatching is precisely the opposite—to promote growth and prevent morphogenesis regardless of the type of metamorphosis. Moreover, evidence of an early onset of JH synthesis in holometabolous versus hemimetabolous embryos remains limited [29], and some studies place the JH peak at a later phase of embryogenesis [30].
As has been acknowledged in a current update of the pronymph hypothesis [15] (this issue), the original concept collided with the finding that, except for cyclorrhaphous flies, both hemimetabolans and holometabolans sequentially deposit three embryonic cuticles and therefore hatch at an equivalent stage [31,32]. Embryonic cuticles EC1 and EC2 are shed within the embryo; EC3 becomes the cuticle of the first-instar larva (figure 2). As EC2 is strongly reduced in some holometabolous species [32], Truman and Riddiford speculate that EC2 is in the process of evolutionary loss [15].
4. Does juvenile hormone play a role in embryogenesis?
The pronymph theory presumes a critical role for JH in embryogenesis, wherein an early onset of JH synthesis forms the holometabolous larva [13,14]. Indeed, both holometabolans [29] and hemimetabolans [33] show a JH peak by mid- to late embryogenesis, respectively, resulting from JH synthesis by the newly formed corpora allata (CA) glands. This is consistent with zygotic expression of JH acid methyltransferase (JHAMT) [34,35], which is essential for JH synthesis. The JH peak is tightly mirrored in both types of insects by expression of the immediate JH-response gene Krüppel-homolog 1 (Kr-h1) [21,34–39]. JH induces transcription of Kr-h1 through the JH receptor Methoprene-tolerant (Met) [21,38,40–42]. However, what JH actually does in the embryos remains unclear. As discussed below, current evidence does not reveal a vital requirement for JH between oviposition and hatching.
Functional genetic data come from the silkworm, Bombyx mori. Through genome editing, Daimon et al. [34] generated null mutants for JHAMT and for both of the Bombyx JH receptors, Met1 and Met2. While JHAMT−/− embryos lost Kr-h1 expression, they still formed normal larvae. The only anomaly resulting from JH depletion was difficulty to hatch, which could be mitigated either by JH application or simply by breaking the eggshells. Embryos lacking both Met1 and Met2 also formed normal larvae [34]. Similarly, a complete zygotic loss of the JH receptor Met and its paralogue Gce in double-null mutants of Drosophila kills the animals no earlier than at the outset of metamorphosis [43,44]. Although Drosophila embryos homozygous for some alleles of Kr-h1 have difficulty hatching, the larvae form normally and even null Kr-h1 mutants predominantly die as prepupae [36]. The apparent lack of an essential role for JH in holometabolous embryos is disturbing given that, according to the pronymph hypothesis, embryonic JH should instate the larval programme [13–15].
Significance of JH for embryogenesis was also studied in hemimetabolans. In embryos of the migratory locust, where JH was reliably depleted using chemical destruction of the CA with a compound precocene, development was unaffected until after the second postembryonic moult when larvae arrested with hallmarks of precocious metamorphosis [45]. When applied to embryos of a cockroach (Nauphoeta cinerea), precocene reduced the endogenous JH titre, thus affecting gut morphogenesis, fat body, and formation of the first-instar larval cuticle [46,47].
A genetic approach was recently taken to address the issue using maternal RNAi-mediated knockdown of JHAMT, Met, and Kr-h1 in another cockroach, Blattella germanica [35]. The study yielded a collection of mostly low-penetrance phenotypes occurring at various phases of embryogenesis. Some anomalies, such as defective germ band formation, by far preceded the mid-embryonic increase in JH titre [35]. There was a reduced hatching rate, reminiscent of the Bombyx JHAMT−/− embryos [34]. Perhaps the most compelling defect, observed for all three tested genes, was premature tanning of the cuticle, which correlated with early upregulation of the expression of the laccase 2 gene [35]. Overall, the observed phenotypes were not reconciled with the results of precocene application in Nauphoeta and did not point to a specific, vital developmental function of JH signalling in the hemimetabolous cockroach.
5. Postembryonic hormone signalling supports stage homology
(a). Kr-h1, the guardian of the juvenile status
The idea that the parallel timing of action of the anti-metamorphic gene Kr-h1 in last-instar hemimetabolous larvae and holometabolous pupae argues for homology between these final pre-adult stages was first articulated in 2011 by Konopová et al. [21]. They noticed a striking similarity between the final-instar larva of a typical hemimetabolan, the linden bug Pyrrhocoris apterus (Heteroptera), and the holometabolous pupa of the beetle Tribolium castaneum [38] in that the Kr-h1 gene was completely suppressed for most of the instar duration (figure 4). The same observations were made independently in other hemimetabolans. In the cockroach B. germanica [39], the downregulation of Kr-h1 expression during the last larval instar coincided with the lowest titre of JH [48] (figure 4); in the bed bug Cimex lecticularius it correlated with minimal JHAMT expression [51]. A current study shows that in the cricket, Gryllus bimaculatus, Kr-h1 mRNA declines already by the penultimate larval instar, leading the authors to propose that not one but two hemimetabolous final juvenile instars are homologous to the pupa [52] (this issue).
Figure 4.
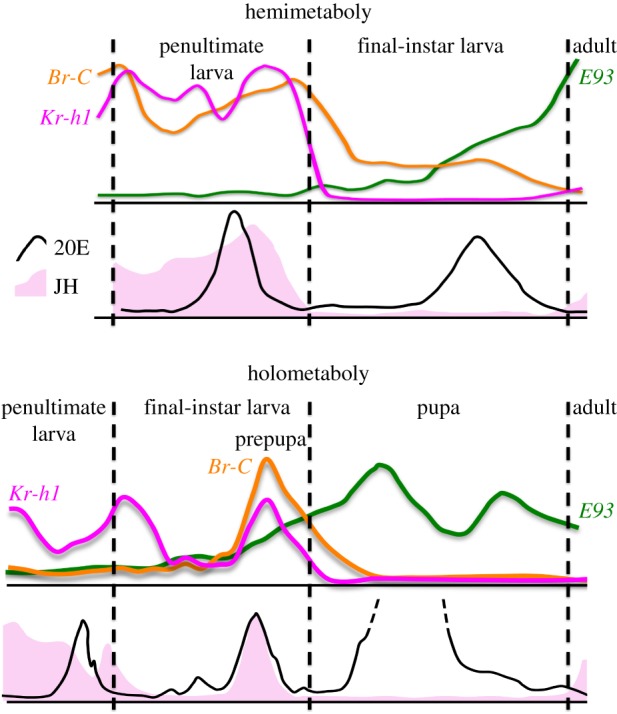
Temporal profiles of the JH titre and expression of the larval determinant Kr-h1 and of the adult determinant E93 support homology of final pre-adult instars, i.e. the pupa (bottom) and the last-instar hemimetabolous larva (top). The recruitment of Br-C to effect the pupal development is a novelty of the Holometabola. Compiled based on published data: representative hormone titres are from [24,48] for hemimetaboly (Blattella germanica) and from [49] for holometaboly (Manduca sexta); approximate mRNA levels are based on [21–23,39,50] for hemimetaboly (Blattella germanica, Pyrrhocoris apterus) and from [23,38] for holometaboly (Tribolium castaneum).
Two types of experiments were done repeatedly to demonstrate that the suppression of Kr-h1 was required and sufficient for metamorphosis to occur. First, reducing Kr-h1 level prematurely through RNAi at penultimate or earlier larval instars triggered precocious metamorphosis, i.e. development of adult characters in the following larval instars [21,22,39,51,52] (figure 3). Second, treatment with JH or its mimic methoprene early during the final instar induced ectopic Kr-h1 expression, causing the animals to moult into a supernumerary larval instar rather than to adults [21,39] (figure 3).
Importantly, knockdown of either Kr-h1 or the JH receptor Met prior to methoprene application averted the ectopic Kr-h1 induction and restored the normal moult to an adult [21], thus providing the causal evidence that Kr-h1 represses metamorphosis by acting downstream of Met. Similar experiments had been done with the holometabolous Tribolium pupae: when given native JH or methoprene, the beetle pupae moulted to a second pupal instar, but prior removal of Met or Kr-h1 prevented this status quo phenotype and rescued adult development [38,53]. Presently missing evidence that applied JH mimics act upon a defined pathway in embryos would be much needed to make any conclusions on JH effects during embryogenesis.
Like the last-instar hemimetabolous larva, the holometabolous pupa is also a JH-free stage [49,54] (figure 4). Accordingly, Kr-h1 mRNA levels plummet in prepupae and reach their lowest levels in pupae not only of Tribolium [25,38] (figure 4) but of highly derived holometabolans such as Bombyx [34,55] and Drosophila [37,56] as well. Even in the notoriously ‘JH-insensitive’ fly, exposure of prepupae or early pupae to ectopic JH prevents adult development, although the effect is mostly limited to blocking differentiation of the abdominal epidermis [37,57,58] which, unlike the adult head and thorax, derives from histoblasts rather than from imaginal discs. Our recent data show that JH exerts this effect on Drosophila metamorphosis in a manner highly dependent on the specific binding and activation of the JH receptor Gce [59].
Homology between postembryonic hemi- and holometabolous stages may not be suggested only by the timing of JH (and Kr-h1) disappearance at the final pre-adult instars, but additionally by the time when JH signalling first becomes critical to prevent precocious metamorphosis. In both Pyrrhocoris and Bombyx, each having five larval instars, the earliest signs of precocious metamorphosis could be provoked by blocking the JH signal at the third larval instar [22,34]. The two youngest instars in both species appear to lack competence to metamorphose and hence not to rely on JH to safeguard their larval status. Using epidermal implantation assays in Bombyx, Inui & Daimon [60] have recently demonstrated that the absence of JH or its receptor Met1 is in fact required, but not sufficient, to trigger premature development already in the first-instar larva. This early JH role in Bombyx corresponds to the effect achieved by exposing the epidermis of first-instar hemimetabolous larvae to the JH-free environment of last-instar hosts [17,18].
(b). Br-C, the maker of the pupa
The situation is only slightly complicated in holometaboly where two moults, the first to a pupa, are needed to convert a larva to an adult. Midway through the final instar, Tribolium larvae see a temporary and incomplete downregulation of Kr-h1 [25,38], clearly a signal for metamorphosis to commence (be it a hemimetabolan, Kr-h1 activity would not come back and the adult programme would ensue). However, as Tribolium enters the prepupal phase, Kr-h1 becomes re-expressed together with another gene, the Broad-Complex (Br-C) [25,38,61–63] (figure 4). A similar reappearance of Kr-h1 and a debut of Br-C expression occur in Bombyx prepupae, being induced by concomitant increase in JH and ecdysteroid titres, respectively [22,34,55].
Br-C was originally described in Drosophila as an ecdysteroid-response gene required for metamorphosis [64]; its co-regulation by 20-hydroxyecdysone (20E) and JH was later unveiled in the moth Manduca sexta [58,65]. Employing Drosophila genetics, Zhou and Riddiford have demonstrated that Br-C, whose repression by JH is temporarily lifted at the end of the last larval instar, is both required and sufficient to activate the pupal programme [58]. They dubbed Br-C a ‘pupal specifier’. Indeed, the indispensable role of Br-C in the formation of the pupa has been confirmed in Bombyx [34,66] and in basal holometabolans including the neuropteran lacewing [61] and the beetle Tribolium [25,61–63]. Intriguingly, once pupation is complete, the repressive effect of JH on Br-C can turn into activation if a JH agonist is given to pupae [58,61]. While this normally does not happen because pupae are devoid of their own JH, these experiments have demonstrated that the ectopic re-induction of Br-C leads to reiteration of the pupal programme. In other words, Br-C not only instructs pupal development but also prevents further metamorphosis to the adult.
It is important to note that one function of the episode of Kr-h1 and Br-C expression in holometabolous prepupae (figure 4) is to block precocious adult development. Upon Kr-h1 or Br-C knockdown in Tribolium larvae, mosaic intermediates with characters such as adult-like wings, compound eyes, legs, antennae, mouthparts and the cuticle were described [38,61,63] before the issue was systematically addressed by Ureña et al. [25]. The fact that knocking down the larval determinant Kr-h1 or the pupal determinant Br-C alone caused Tribolium to skip the pupal stage, albeit partially, seems incompatible with the notion that the pupa should comprise all juvenile instars of a hemimetabolous ancestor as proposed in the pronymph hypothesis [13–15] (figure 2). If the pupa indeed represented all hemimetabolous larval instars, the Kr-h1 or Br-C loss-of-function phenotypes would imply that the entire hemimetabolous larval stage could be omitted, at least experimentally.
While well conserved across insects, Br-C obviously plays other roles than making a pupa in hemimetabolans. In the true bugs (Heteroptera) Oncopeltus fasciatus or P. apterus, and in the cockroach B. germanica, Br-C expression continues from embryos to larvae, then it declines early during the last larval instar [21,50,67] (figure 4). Rather than being linked with metamorphosis, in these species Br-C is required during embryogenesis [68,69] and for growth and development of the larval wing pads [21,50,67]. Interestingly, similar to Kr-h1 RNAi, knockdown of Br-C has been shown to elicit precocious development of adult features (the wings and ovipositor) when deployed in juvenile females of the cricket G. bimaculatus [52].
Regardless of variations in Br-C functions among diverse hemimetabolous insects, it seems clear that during the evolution from hemimetaboly to holometaboly, Br-C has been recruited for the specific task of pupal morphogenesis [50]. In contrast to hemimetabolans where the action of Br-C in morphogenetic growth is spread over multiple larval instars, in the Holometabola it is concentrated around the prepupal phase of the final larva. This temporal compression of Br-C activity has been interpreted in favour of the pronymph hypothesis [15,67,68]. However, Huang et al. [50] have presented an alternative view according to which the regulation of Br-C by JH has turned from activation in hemimetabolous to repression in holometabolous larvae up until the prepupal phase when Br-C assumes its complex function in pupal development.
(c). E93, the gateway to adulthood
Having established that activities of the larval determinant Kr-h1 and of the pupal determinant Br-C must cease to permit adult development, the stage was open for a new player to determine the adult fate. Who this player was became evident through work in the Martín laboratory [23] combining Blattella, Tribolium and Drosophila. In all three models, the respective orthologues of the ecdysone-induced protein 93F (E93) [70] are expressed at low levels up until the latter half of the final larval instar (Blattella) or until the prepupal phase in the two holometabolans, after which the E93 mRNA rises for the adult development (figure 4). In the cricket G. bimaculatus, E93 expression increases in the penultimate rather than last larval instar [52]. Nonetheless, in all cases the timing of both E93 and Kr-h1 expression strongly suggests homology between the final juvenile stages in both types of metamorphosis.
Removal of E93 prevents adult development. In the cockroach, E93 RNAi delivered to the penultimate larval instar caused perpetuation of the larval status, which was retained for several additional moults beyond the normal number [23]; a similar phenotype was observed in G. bimaculatus [52] or C. lecticularius [51]. Using Pyrrhocoris, we produced an extranumerary larval instar, reminiscent of JH mimic application, by inducing E93 RNAi in early, final-instar larvae (V. Smýkal & M. Jindra 2015, unpublished) (figure 3). When Tribolium larvae received E93 RNAi during their final instar, the phenotype was manifested as reiteration of the pupal stage [23], again similar to the effect of ectopic JH application [38,53,61]. Finally, transgenic E93 RNAi driven in Drosophila ubiquitously throughout development resulted in a pupal arrest with the adult development blocked and the pupal status reinforced as Br-C expression failed to decline [23].
These data establish E93 as an adult determinant with a function opposing that of Kr-h1 but likewise common to hemi- and holometabolans. Being conserved across the different modes of insect metamorphosis, the regulatory axis comprising the key components (Met, Kr-h1 and E93) has been dubbed the MEKRE93 pathway [24]. More recently an acronym MGN (for metamorphic gene network) has been proposed [25,71] that reflects the nonlinear relationships among these players. A current review by Bellés [72] (this issue) explains how the pathway might have led to the origin of metamorphosis, the hemimetaboly.
(d). The conserved metamorphic gene network
Remarkably, the hormone signalling genes (Met, Kr-h1, Br-C, and E93) discussed so far encode transcription factors, each belonging to a different family. They all can engage in multiple protein interactions and bind DNA in order to activate or repress specific target genes. The JH receptor Met is additionally activated by hormonal ligands [59], and Kr-h1 is its best-characterized direct target gene. Besides being regulated by JH, Kr-h1, Br-C and E93 also respond to the steroid (20E) signalling downstream of the ecdysone receptor EcR [55,56,65,70]. Both of the major insect hormonal signalling systems thus converge and interact at this regulatory network.
There are intricate relationships among the hormone signalling proteins. At least part of the effect of Kr-h1 on preventing metamorphosis involves transcriptional repression of E93 [23–25,52]. Conversely, E93 effects the adult programme by repressing Kr-h1 and additionally Br-C in the case of holometabolans [23–25] and the hemimetabolous G. bimaculatus [52]. Therefore, Kr-h1 and E93 are mutual antagonists (figure 3).
The above RNAi studies in the diverse insects have laid down the basic genetic epistasis among the hormone signalling components. Concurrent genetic [34] and molecular studies undertaken by the Shinoda group using their silkworm model have confirmed the key roles of these proteins in metamorphosis and established their mutual interactions. They have demonstrated direct transcriptional repression of Br-C [73] and E93 [74] by the Kr-h1 protein binding to the upstream regulatory regions of both genes. These studies have also explained how the JH-dependent regulation of Br-C and E93 can be modulated by 20E through adjacent DNA elements for binding of EcR.
6. Conclusion
While the pronymph hypothesis is ingenious and attractive for its elegant explanations, particularly of the discontinuity in the development of the holometabolous nervous system [13–15], it builds on certain assumptions. One is the stage of embryogenesis at which hemimetabolans and holometabolans hatch. The comparative study by Konopová & Zrzavý [32] has shown that this stage is the same in both, thus refuting the ‘de-embryonisation’ concept. The pronymph hypothesis appears to fit derived holometabolans with secondarily simplified larvae but less so basal holometabolans whose larvae resemble adults in appearance and lifestyle [1]. Further, the abilities of holometabolous larvae in certain species to either become reproductive without metamorphosing (neoteny) or undergo major morphological transitions (hypermetamorphosis), contradict their embryonic character. Conversely, pupa-like resting stages are not unique to the Holometabola as they have independently evolved in some hemimetabolans. Another key assumption concerns the postulated role of an advanced JH production in ‘de-embryonisation’ [13,14]. Evidence for an early onset of JH appearance in holometabolous embryos is isolated [29], while a role for JH, particularly in holometabolous embryogenesis, remains elusive [34].
In contrast, the well-established function of JH signalling during postembryonic development grants strong genetic support to the alternative hypothesis for stage homology [10] (figure 2). Defining the holometabolous pupa as all hemimetabolous larval instars compressed to one contradicts the expression profiles, common to both types of metamorphosis, of genes determining the juvenile (Kr-h1) and the adult (E93) states [21–25,38,52] (figure 4). The fact that the pupal stage can be omitted when Kr-h1 is experimentally removed is difficult to reconcile considering the pupa as being equivalent to all rather than just one or two juvenile instars.
Together, the findings from the complementary insect models characterize the pupa as a late juvenile stage most similar to the last-instar hemimetabolous larva in a sense proposed by Hinton [1,10] (figure 2). Recent work covered in this issue [52] suggests that the pupa may be equivalent to the last two pre-adult instars in crickets, illustrating that a simple one-to-one homology cannot be applied to hemi- and holometabolous juvenile instars. In either case the final juvenile stage must experience a JH-free period during which the absence of Kr-h1 is requisite for adult development, executed by E93 (figure 4). These roles of Kr-h1 and E93 are universal to hemi- and holometabolans. Based primarily on the nature of JH signalling that precedes metamorphosis, it would follow that the pupal stage has evolved as an adaptation to the increasing diversification between the larval and adult forms, and that it represents a modified equivalent of a late developmental phase of an ancestral hemimetabolous juvenile.
7. What next
The convergence of developmental, endocrine, and mainly of functional genetic evidence on hormonal regulation of metamorphosis presented in this review strongly argues for homology between the final pre-adult stages in hemimetaboly and holometaboly. The regulatory circuitry of transcription factors downstream of JH and 20E has been outlined in detail allowing us to address the remaining issues.
One question is how do the systemic hormonal signals instruct individual tissues and cells to undergo the timely orchestrated programmes of morphogenesis, cell death and remodelling during highly evolved metamorphosis? What are the genes downstream of the JH and 20E receptors that effect such diverse tissue-specific responses? And how is heterochrony achieved, namely in the wing and other imaginal discs of Drosophila that are allowed to proliferate but not to commence developing adult structures during larval growth?
A great gap waits to be filled at the other end of development – embryogenesis. Understanding the role of hormones, particularly of JH and its response genes, in early development from maternal deposition to hatching remains largely obscure. Current genetic tools should afford us the insight. These techniques are now available even for the most primitive, ametabolous insects [75], enabling us to send a deep probe into the evolution of insect metamorphosis.
Acknowledgement
I thank my former students, Barbora Konopová for her inspiring thoughts on the evolution of insect metamorphosis, and Vlastimil Smýkal for unpublished work on Pyrrhocoris. I further thank Petr Švácha for discussion, reading the manuscript, and for providing specimens of snakeflies. Martina Hajdušková (www.biographix.cz) prepared artwork for figure 2.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
This study was supported by the Czech Science Foundation (15-23681S).
References
- 1.Sehnal F, Svacha P, Zrzavy J. 1996. Evolution of insect metamorphosis. In Metamorphosis. Postembryonic reprogramming of gene expression in amphibian and insect cells (eds Gilbert LI, Tata JR, Atkinson BG), pp. 3–58. San Diego, CA: Academic Press. [Google Scholar]
- 2.Bishop CD, et al. 2006. What is metamorphosis? Integr. Comp. Biol. 46, 655–661. ( 10.1093/icb/icl004) [DOI] [PubMed] [Google Scholar]
- 3.Kristensen N. 1999. Phylogeny of endopterygote insects, the most successful lineage of living organisms. Eur. J. Entomol. 96, 237–253. [Google Scholar]
- 4.Peters RS, et al. 2014. The evolutionary history of holometabolous insects inferred from transcriptome-based phylogeny and comprehensive morphological data. BMC Evol. Biol. 14, 52 ( 10.1186/1471-2148-14-52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heming BS. 2003. Insect development and evolution. Ithaca, NY: Cornell University Press. [Google Scholar]
- 6.Erezyilmaz DF. 2006. Imperfect eggs and oviform nymphs: a history of ideas about the origins of insect metamorphosis. Integr. Comp. Biol. 46, 795–807. ( 10.1093/icb/icl033) [DOI] [PubMed] [Google Scholar]
- 7.Belles X. 2011. Origin and evolution of insect metamorphosis. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 8.Svacha P. 1992. What are and what are not imaginal discs: reevaluation of some basic concepts (Insecta, Holometabola). Dev. Biol. 154, 101–117. ( 10.1016/0012-1606(92)90052-I) [DOI] [PubMed] [Google Scholar]
- 9.Bate M, Arias AM. 1991. The embryonic origin of imaginal discs in Drosophila. Development 112, 755–761. [DOI] [PubMed] [Google Scholar]
- 10.Hinton H. 1963. The origin and function of the pupal stage. Proc. R. Ent. Soc. Lond. A 38, 77–85. [Google Scholar]
- 11.Bocak L, Bocakova M, Hunt T, Vogler AP. 2008. Multiple ancient origins of neoteny in Lycidae (Coleoptera): consequences for ecology and macroevolution. Proc. R. Soc. B 275, 2015–2023. ( 10.1098/rspb.2008.0476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Besuchet C. 1956. Biologie, morphologie et systematique des Rhipidius (Col. Rhipiphoridae). Mitt. Schweiz. Entomol. Ges. 29, 73–144. [Google Scholar]
- 13.Truman JW, Riddiford LM. 1999. The origins of insect metamorphosis. Nature 401, 447–452. ( 10.1038/46737) [DOI] [PubMed] [Google Scholar]
- 14.Truman JW, Riddiford LM. 2002. Endocrine insights into the evolution of metamorphosis in insects. Annu. Rev. Entomol. 47, 467–500. ( 10.1146/annurev.ento.47.091201.145230) [DOI] [PubMed] [Google Scholar]
- 15.Truman JW, Riddiford LM. 2019. The evolution of insect metamorphosis: a developmental and endocrine view. Phil. Trans. R. Soc. B 374, 20190070 ( 10.1098/rstb.2019.0070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rédei D, Štys P. 2016. Larva, nymph and naiad—for accuracy's sake. Syst. Entomol. 41, 505–510. ( 10.1111/syen.12177) [DOI] [Google Scholar]
- 17.Wigglesworth V. 1934. The physiology of ecdysis in Rhodnius prolixus (Hemiptera). II. Factors controlling moulting and ‘metamorphosis’. Quart. J. Micr. Sci. 77, 191–222. [Google Scholar]
- 18.Wigglesworth V. 1954. The physiology of insect metamorphosis. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 19.Jindra M, Palli SR, Riddiford LM. 2013. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 58, 181–204. ( 10.1146/annurev-ento-120811-153700) [DOI] [PubMed] [Google Scholar]
- 20.Jindra M, Bellés X, Shinoda T. 2015. Molecular basis of juvenile hormone signaling. Curr. Opin. Insect Sci. 11, 39–46. ( 10.1016/j.cois.2015.08.004) [DOI] [PubMed] [Google Scholar]
- 21.Konopova B, Smykal V, Jindra M. 2011. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS ONE 6, e28728 ( 10.1371/journal.pone.0028728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smykal V, Daimon T, Kayukawa T, Takaki K, Shinoda T, Jindra M. 2014. Importance of juvenile hormone signaling arises with competence of insect larvae to metamorphose. Dev. Biol. 390, 221–230. ( 10.1016/j.ydbio.2014.03.006) [DOI] [PubMed] [Google Scholar]
- 23.Ureña E, Manjón C, Franch-Marro X, Martín D. 2014. Transcription factor E93 specifies adult metamorphosis in hemimetabolous and holometabolous insects. Proc. Natl Acad. Sci. USA 111, 7024–7029. ( 10.1073/pnas.1401478111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellés X, Santos CG. 2014. The MEKRE93 (Methoprene tolerant-Krüppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect Biochem. Mol. Biol . 52C, 60–68. ( 10.1016/j.ibmb.2014.06.009) [DOI] [PubMed] [Google Scholar]
- 25.Ureña E, Chafino S, Manjón C, Franch-Marro X, Martín D. 2016. The occurrence of the holometabolous pupal stage requires the interaction between E93, Krüppel-homolog 1 and Broad-Complex. PLoS Genet. 12, e1006020 ( 10.1371/journal.pgen.1006020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Truman JW, Riddiford LM. 2007. The morphostatic actions of juvenile hormone. Insect Biochem. Mol. Biol. 37, 761–770. ( 10.1016/j.ibmb.2007.05.011) [DOI] [PubMed] [Google Scholar]
- 27.Novák VJ. 1969. Morphogenetic analysis of the effects of juvenile hormone analogues and other morphogenetically active substances on embryos of Schistocerca gregaria (Forskål). J. Embryol. Exp. Morphol. 21, 1–21. [PubMed] [Google Scholar]
- 28.Erezyilmaz DF, Riddiford LM, Truman JW. 2004. Juvenile hormone acts at embryonic molts and induces the nymphal cuticle in the direct-developing cricket. Dev. Genes Evol. 214, 313–323. ( 10.1007/s00427-004-0408-2) [DOI] [PubMed] [Google Scholar]
- 29.Bergot BJ, Baker FC, Cerf DC, Jamieson G, Schooley DA. 1981. Qualitative and quantitative aspects of juvenile hormone titers in developing embryos of several insect species: discovery of a new JH-like substance extracted from eggs of Manduca sexta. In Juvenile hormone biochemistry (eds Pratt GE, Brooks GT), pp. 33–45. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 30.Grossniklaus-Bürgin C, Lanzrein B. 1990. Qualitative and quantitative analyses of juvenile hormone and ecdysteroids from the egg to the pupal molt in Trichoplusia ni. Arch. Insect Biochem. Physiol. 14, 13–30. [DOI] [PubMed] [Google Scholar]
- 31.Polivanova EN. 1979. Embryonization of ontogenesis, origin of embryonic moults and types of development in insects. Zool. Zh. 58, 1269–1280. [Google Scholar]
- 32.Konopova B, Zrzavý J. 2005. Ultrastructure, development, and homology of insect embryonic cuticles. J. Morphol. 264, 339–362. ( 10.1002/jmor.10338) [DOI] [PubMed] [Google Scholar]
- 33.Maestro JL, Pascual N, Treiblmayr K, Lozano J, Bellés X. 2010. Juvenile hormone and allatostatins in the German cockroach embryo. Insect Biochem. Mol. Biol. 40, 660–665. ( 10.1016/j.ibmb.2010.06.006) [DOI] [PubMed] [Google Scholar]
- 34.Daimon T, Uchibori M, Nakao H, Sezutsu H, Shinoda T. 2015. Knockout silkworms reveal a dispensable role for juvenile hormones in holometabolous life cycle. Proc. Natl Acad. Sci. USA 112, E4226–E4235. ( 10.1073/pnas.1506645112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Nicolas A, Bellés X. 2017. Juvenile hormone signaling in short germ-band hemimetabolan embryos. Development 144, 4637–4644. ( 10.1242/dev.152827) [DOI] [PubMed] [Google Scholar]
- 36.Beck Y, Pecasse F, Richards G. 2004. Krüppel-homolog is essential for the coordination of regulatory gene hierarchies in early Drosophila development. Dev. Biol. 268, 64–75. ( 10.1016/j.ydbio.2003.12.017) [DOI] [PubMed] [Google Scholar]
- 37.Minakuchi C, Zhou X, Riddiford LM. 2008. Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech. Dev. 125, 91–105. ( 10.1016/j.mod.2007.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minakuchi C, Namiki T, Shinoda T. 2009. Krüppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev. Biol. 325, 341–350. ( 10.1016/j.ydbio.2008.10.016) [DOI] [PubMed] [Google Scholar]
- 39.Lozano J, Bellés X. 2011. Conserved repressive function of Krüppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci. Rep. 1, 163 ( 10.1038/srep00163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charles J-P, Iwema T, Epa VC, Takaki K, Rynes J, Jindra M. 2011. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl Acad. Sci. USA 108, 21 128–21 133. ( 10.1073/pnas.1116123109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li M, Mead EA, Zhu J. 2011. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl Acad. Sci. USA 108, 638–643. ( 10.1073/pnas.1013914108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kayukawa T, et al. 2012. Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc. Natl Acad. Sci. USA 109, 11 729–11 734. ( 10.1073/pnas.1204951109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdou MA, et al. 2011. Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem. Mol. Biol. 41, 938–945. ( 10.1016/j.ibmb.2011.09.003) [DOI] [PubMed] [Google Scholar]
- 44.Jindra M, Uhlirova M, Charles J-P, Smykal V, Hill RJ. 2015. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet. 11, e1005394 ( 10.1371/journal.pgen.1005394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aboulafia-Baginsky N, Pener MP, Staal GB. 1984. Chemical allatectomy of late Locusta embryos by a synthetic precocene and its effect on hopper morphogenesis. J. Insect Physiol. 30, 839–852. ( 10.1016/0022-1910(84)90057-X) [DOI] [Google Scholar]
- 46.Brüning E, Saxer A, Lanzrein B. 1985. Methyl farnesoate and juvenile hormone III in normal and precocene treated embryos of the ovoviviparous cockroach, Nauphoeta cinerea. Int. J. Invertebr. Reprod. Dev. 8, 269–278. ( 10.1080/01688170.1985.10510155) [DOI] [Google Scholar]
- 47.Brüning E, Lanzrein B. 1987. Function of juvenile hormone III in embryonic development of the cockroach Nauphoeta cinerea. Int. J. Invertebr. Reprod. Dev. 12, 29–44. ( 10.1080/01688170.1987.10510300) [DOI] [Google Scholar]
- 48.Treiblmayr K, Pascual N, Piulachs M-D, Keller T, Bellés X. 2006. Juvenile hormone titer versus juvenile hormone synthesis in female nymphs and adults of the German cockroach, Blattella germanica. J. Insect Sci. 6, 1–7. ( 10.1673/031.006.4301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riddiford L. 1994. Cellular and molecular actions of juvenile hormone. I. General considerations and premetamorphic actions. Adv. Insect Physiol. 24, 213–274. ( 10.1016/S0065-2806(08)60084-3) [DOI] [Google Scholar]
- 50.Huang J-H, Lozano J, Bellés X. 2013. Broad-complex functions in postembryonic development of the cockroach Blattella germanica shed new light on the evolution of insect metamorphosis. Biochim. Biophys. Acta 1830, 2178–2187. ( 10.1016/j.bbagen.2012.09.025) [DOI] [PubMed] [Google Scholar]
- 51.Gujar H, Palli SR. 2016. Krüppel homolog 1 and E93 mediate Juvenile hormone regulation of metamorphosis in the common bed bug, Cimex lectularius. Sci. Rep. 6, 26092 ( 10.1038/srep26092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishimaru Y, Tomonari S, Watanabe T, Noji S, Mito T.. 2019. Regulatory mechanisms underlying the specification of the pupal-homologous stage in a hemimetabolous insect. Phil. Trans. R. Soc. B 374, 20190225 ( 10.1098/rstb.2019.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konopova B, Jindra M. 2007. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc. Natl Acad. Sci. USA 104, 10 488–10 493. ( 10.1073/pnas.0703719104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nijhout H. 1994. Insect hormones. Princeton, NJ: Princeton University Press. [Google Scholar]
- 55.Kayukawa T, et al. 2014. Hormonal regulation and developmental role of Krüppel homolog 1, a repressor of metamorphosis, in the silkworm Bombyx mori. Dev. Biol. 388, 48–56. ( 10.1016/j.ydbio.2014.01.022) [DOI] [PubMed] [Google Scholar]
- 56.Pecasse F, Beck Y, Ruiz C, Richards G. 2000. Krüppel-homolog, a stage-specific modulator of the prepupal ecdysone response, is essential for Drosophila metamorphosis. Dev. Biol. 221, 53–67. ( 10.1006/dbio.2000.9687) [DOI] [PubMed] [Google Scholar]
- 57.Riddiford LM, Ashburner M. 1991. Effects of juvenile hormone mimics on larval development and metamorphosis of Drosophila melanogaster. Gen. Comp. Endocrinol. 82, 172–183. ( 10.1016/0016-6480(91)90181-5) [DOI] [PubMed] [Google Scholar]
- 58.Zhou X, Riddiford LM. 2002. Broad specifies pupal development and mediates the ‘status quo’ action of juvenile hormone on the pupal-adult transformation in Drosophila and Manduca. Development 129, 2259–2269. [DOI] [PubMed] [Google Scholar]
- 59.Bittova L, Jedlicka P, Dracinsky M, Kirubakaran P, Vondrasek J, Hanus R, Jindra M. 2019. Exquisite ligand stereoselectivity of a Drosophila juvenile hormone receptor contrasts with its broad agonist repertoire. J. Biol. Chem. 294, 410–423. ( 10.1074/jbc.RA118.005992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inui T, Daimon T. 2017. Implantation assays using the integument of early stage Bombyx larvae: insights into the mechanisms underlying the acquisition of competence for metamorphosis. J. Insect Physiol. 100, 35–42. ( 10.1016/j.jinsphys.2017.05.002) [DOI] [PubMed] [Google Scholar]
- 61.Konopova B, Jindra M. 2008. Broad-Complex acts downstream of Met in juvenile hormone signaling to coordinate primitive holometabolan metamorphosis. Development 135, 559–568. ( 10.1242/dev.016097) [DOI] [PubMed] [Google Scholar]
- 62.Suzuki Y, Truman JW, Riddiford LM. 2008. The role of Broad in the development of Tribolium castaneum: implications for the evolution of the holometabolous insect pupa. Development 135, 569–577. ( 10.1242/dev.015263) [DOI] [PubMed] [Google Scholar]
- 63.Parthasarathy R, Tan A, Bai H, Palli SR. 2008. Transcription factor broad suppresses precocious development of adult structures during larval-pupal metamorphosis in the red flour beetle, Tribolium castaneum. Mech. Dev. 125, 299–313. ( 10.1016/j.mod.2007.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiss I, Beaton AH, Tardiff J, Fristrom D, Fristrom JW. 1988. Interactions and developmental effects of mutations in the Broad-Complex of Drosophila melanogaster. Genetics 118, 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou B, Hiruma K, Shinoda T, Riddiford LM. 1998. Juvenile hormone prevents ecdysteroid-induced expression of broad complex RNAs in the epidermis of the tobacco hornworm, Manduca sexta. Dev. Biol. 203, 233–244. ( 10.1006/dbio.1998.9059) [DOI] [PubMed] [Google Scholar]
- 66.Uhlirova M, Foy BD, Beaty BJ, Olson KE, Riddiford LM, Jindra M. 2003. Use of Sindbis virus-mediated RNA interference to demonstrate a conserved role of Broad-Complex in insect metamorphosis. Proc. Natl Acad. Sci. USA 100, 15 607–15 612. ( 10.1073/pnas.2136837100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erezyilmaz DF, Riddiford LM, Truman JW. 2006. The pupal specifier broad directs progressive morphogenesis in a direct-developing insect. Proc. Natl Acad. Sci. USA 103, 6925–6930. ( 10.1073/pnas.0509983103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erezyilmaz DF, Rynerson MR, Truman JW, Riddiford LM. 2010. The role of the pupal determinant broad during embryonic development of a direct-developing insect. Dev. Genes Evol. 219, 535–544. ( 10.1007/s00427-009-0315-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piulachs M-D, Pagone V, Bellés X. 2010. Key roles of the Broad-Complex gene in insect embryogenesis. Insect Biochem. Mol. Biol. 40, 468–475. ( 10.1016/j.ibmb.2010.04.006) [DOI] [PubMed] [Google Scholar]
- 70.Baehrecke EH, Thummel CS. 1995. The Drosophila E93 gene from the 93F early puff displays stage- and tissue-specific regulation by 20- hydroxyecdysone. Dev. Biol. 171, 85–97. ( 10.1006/dbio.1995.1262) [DOI] [PubMed] [Google Scholar]
- 71.Chafino S, Ureña E, Casanova J, Casacuberta E, Franch-Marro X, Martín D. 2019. Upregulation of E93 gene expression acts as the trigger for metamorphosis independently of the threshold size in the beetle Tribolium castaneum. Cell Rep. 27, 1039–1049.e2. ( 10.1016/j.celrep.2019.03.094) [DOI] [PubMed] [Google Scholar]
- 72.Belles X. 2019. The innovation of the final moult and the origin of insect metamorphosis. Phil. Trans. R. Soc. B 374, 20180415 ( 10.1098/rstb.2018.0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kayukawa T, Nagamine K, Ito Y, Nishita Y, Ishikawa Y, Shinoda T. 2016. Krüppel homolog 1 inhibits insect metamorphosis via direct transcriptional repression of Broad-Complex, a pupal specifier gene. J. Biol. Chem. 291, 1751–1762. ( 10.1074/jbc.M115.686121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kayukawa T, Jouraku A, Ito Y, Shinoda T. 2017. Molecular mechanism underlying juvenile hormone-mediated repression of precocious larval-adult metamorphosis. Proc. Natl Acad. Sci. USA 114, 1057–1062. ( 10.1073/pnas.1615423114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohde T, Takehana Y, Shiotsuki T, Niimi T. 2018. CRISPR/Cas9-based heritable targeted mutagenesis in Thermobia domestica: a genetic tool in an apterygote development model of wing evolution. Arthropod Struct. Dev. 47, 362–369. ( 10.1016/j.asd.2018.06.003) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.