Abstract
The majority of described hexapod species are holometabolous insects, undergoing an extreme form of metamorphosis with an intercalated pupal stage between the larva and adult, in which organs and tissues are extensively remodelled and in some cases completely rebuilt. Here, we review how and why this developmental strategy has evolved. While there are many theories explaining the evolution of metamorphosis, many of which fit under the hypothesis of decoupling of life stages, there are few clear adaptive hypotheses on why complete metamorphosis evolved. We propose that the main adaptive benefit of complete metamorphosis is decoupling between growth and differentiation. This facilitates the exploitation of ephemeral resources and enhances the probability of the metamorphic transition escaping developmental size thresholds. The evolution of complete metamorphosis comes at the cost of exposure to predators, parasites and pathogens during pupal life and requires specific adaptations of the immune system at this time. Moreover, metamorphosis poses a challenge for the maintenance of symbionts and the gut microbiota, although it may also offer the benefit of allowing an extensive change in microbiota between the larval and adult stages. The regulation of metamorphosis by two main players, ecdysone and juvenile hormone, and the related signalling cascades are now relatively well understood. The mechanics of metamorphosis have recently been studied in detail because of the advent of micro-CT and research into the role of cell death in remodelling tissues and organs. We support the argument that the adult stage must necessarily have preceded the larval form of the insect. We do not resolve the still contentious question of whether the larva of insects in general originated through the modification of existing preadult forms or through heterochrony as a modified embryonic stage (pronymph), nor whether the holometabolous pupa arose as a modified hemimetabolous final stage larva.
This article is part of the theme issue ‘The evolution of complete metamorphosis’.
Keywords: juvenile hormone, pupation, holometaboly, adaptation, insects
1. Introduction: what is complete metamorphosis?
The ecologist Henry Wilbur [1] noted that many organisms have complex life cycles that include ‘an abrupt ontogenetic change in an individual's morphology, physiology and behaviour, usually associated with a change in habitat’. Such an abrupt change has long been described as metamorphosis. Insects were particularly noted by the ancients as undergoing this kind of bodily transformation and change of habitat [2,3].
More than 80% of insect species [4], possibly representing around 60% of all animals [5], undergo a particularly marked form of metamorphosis in which an ecologically inactive life stage called the pupa is interposed between the larva and the adult, during which the insect's body is almost entirely rebuilt. This kind of transformation is called holometaboly or ‘complete metamorphosis', where the larval body is always markedly different in form from that of the adult. The papers in this special issue of Philosophical Transactions are devoted to understanding complete metamorphosis from three scientific standpoints: (a) development: the nature of the developmental programme of complete metamorphosis; (b) origins: the respective developmental origins of the larval and pupal stages and (c) adaptation: the evolution through natural selection of complete metamorphosis.
2. How complete metamorphosis is achieved
Feeding and growth in size cease at pupation. The pupal stage is a purely developmental component of the holometabolous life cycle; Aristotle and later William Harvey compared the pupa to an egg [3]. Despite the frequently encountered assertion that, during metamorphosis, the inside of the pupa is completely restructured, or turned into ‘soup’ [6], it has long been known that this is not so [7]. In the majority of holometabolous insects, most larval tissues and organs are actually re-specified; this includes the epidermis [8]; much of the nervous system [9] and many muscles [10]. Even where remodelling is extensive and involves cell death and replacement through stem cell proliferation such as in the gut [11], it is known that these organ systems persist and do not completely break down during complete metamorphosis. This is clearly shown in the paper by Hall & Martín-Vega [12], which concerns recent advances in the microcomputed tomography (micro-CT) X-ray technique that allow visualization in unprecedented detail of how internal anatomy changes during complete metamorphosis.
The work of Lockshin & Williams [13,14] showed that some cells, tissues and even whole organs are destroyed or drastically remodelled during the complete metamorphosis of some insects, a discovery that heralded the now widespread recognition that programmed cell death can be as important in the development of all eukaryotes as cell proliferation. Death comes to cells in two types, autophagy and apoptosis. The principal difference between them is that, while both are initiated within the cell that is destined to die, apoptosis requires the involvement of other phagocytic cells, while autophagy is a self-sufficient process, in which the materials of the dying cell are recycled and exported by the cell itself. Programmed cell death has two functions: to remodel the cellular content of tissues and organs and to make good use of the materials that were contained in them. Both autophagic and apoptotic cell death turn out to be important in holometabolous transformations, as is here reviewed by Tettamanti & Casartelli [15]. The extent and manner of cell death during metamorphosis differ considerably between different Endopterygota orders, and even different species. Moreover, there is often a complex interplay between autophagy and apoptosis even within different tissues within the same insect. Autophagy tends to occur earlier in metamorphosis than apoptosis, but the balance between these processes is dictated by the nature of the tissue being remodelled. A constant feature of cell death in complete metamorphosis, however, is that the process is regulated by the major insect developmental hormones, in very much the same way as metamorphosis generally.
3. Endocrine control of complete metamorphosis
Insect metamorphosis, whether complete or incomplete, is hormonally regulated. Juvenile hormone (JH), discovered by Wigglesworth [16], but not structurally elucidated until more than 30 years later [17], is the key to the transition to the adult stage. JH has diverse actions, but in metamorphosis it functions to maintain the immature condition, and its absence permits progression to the adult stage [18].
The action of JH is dependent on the simultaneous presence of the moult-initiating steroid hormone, 20-hydroxyecdysone. The modern consensus, the so-called status quo model, of how this control system works [19] is that a short-lived increase in the titre of ecdysteroid initiates moulting, and it is the accompanying level of JH that determines the developmental character of the structures produced at the next moult. Absence of JH at a crucial time at the end of larval life allows moulting to the pupal stage, and the hormone's continued absence in most of the pupal stage allows the formation of an adult at the next moult.
How are these hormonal signals transduced into the developmental responses that characterize larval, pupal and adult moults? The dimeric intracellular receptors that bind JH and transduce its actions are now known [20]. Once occupied, these receptors bind to DNA elements upstream of the genes that encode a small number of high-level stage-specific transcription factors, which act to promote or repress the expression of much larger numbers of lower-level target genes. The decision whether to develop as larva, pupa or adult is determined by the recruitment of successive and high-level cellular signalling pathways, each recruiting different downstream gene sets that differ mostly quantitatively rather than qualitatively in expression levels [21].
At least during larval life, it appears that JH has little effect between moults, its actions being limited to modulating the expression of genes in response to ecdysone. A large number of studies have shown that as long as the insect has reached a certain size, surgical removal of the source of JH from immature insects of both hemimetabolous insects and Holometabola results in premature metamorphosis at the next moult while administering exogenous JH results in an additional larval or nymphal instar [22]. The exact nature of the threshold for competence to respond to JH is not currently understood, but it is known that in the beetle Tribolium castaneum it requires the expression of the transcription factor E93 [23].
The consensus scheme of endocrine control described above paints a picture in which the downstream regulation of complete metamorphosis is now understood in unprecedented molecular detail. A number of papers in this issue make reference to this scheme. But there are plenty of actions remaining to be elucidated. Just as has been the case for other animal development processes including cell proliferation, differentiation, programmed cell death and reproduction [24,25], recent work has begun to reveal that an important part in regulating large sets of genes during metamorphic insect transformations is also played by non-coding RNAs such as microRNAs [26,27]. It is also possible that histone acetylation [28,29] is important in at least some aspects of complete metamorphosis. DNA methylation is also known to have a role in the environmentally influenced development of alternative phenotypes in insects [30,31], but there is so far no strong evidence supporting a role for this epigenetic process in metamorphosis. Ylla et al. [32] compared the hemimetabolous Blattella germanica and the holometabolan Drosophila melanogaster and found that methylation levels in Drosophila were much lower than in Blattella. The authors tentatively suggest that loss of embryonic DNA methylation may have played a role in the origin of holometaboly. On the other hand, Bewick et al. [33] examined DNA methylation levels in a number of species across the whole of the hexapod class (41 species from six orders) and found that methylation levels were very variable and that there was no obvious pattern, although the dipteran species they examined all had very low methylation levels. It is, however, at least evident that epigenetic mechanisms of transcriptional control are extensively used in many insects, and it is likely that further work of this type will open up new areas of metamorphosis-related research.
4. The evolutionary origin of the holometabolous larval stage
The developmental biologist Lewis Wolpert [34] asserted that it is self-evident that the larval stages of organisms with complex life histories evolved from pre-existing adult forms and not the other way around. He said [34, pp. 3–4]:
I … question whether indirect development could be the primitive condition on the grounds that it contradicts the principle of gradualism—any and every evolutionary scheme has to account for the adaptive advantage of each new stage. How then could one conceive of the selective advantage of set-aside cells and their later evolution? They would play no role until they had given rise to adult structures…… How could metamorphosis evolve? … … in the case of insects …, the larval stage was intercalated into the development of a direct developing organism.
Wolpert's brief original argument [34] has been developed and extended by Sly et al. [35] for a wide range of metamorphosing bilaterian metazoans. We suggest that the evolution of larval forms of insects from the adult condition is likely to have been achieved gradually and in stages. The possession of larval characters would have required a subsequent metamorphic developmental change from larva to adult, but it is not necessary that the transformation was rapid when it was first required, and early-evolving insect larvae may have transformed to the adult condition over several moults. Rapid metamorphosis would probably have been subsequently enabled by the convergent evolution of special developmental mechanisms, likely involving the heterochronous compression of metamorphic changes [36,37].
As noted by Sly et al. [35], the ‘adult first’ scheme is consistent with all stages of the evolution of the larval state being selectively advantageous. It also matches the observations that not all hemimetabolous insects display divergent larval and adult forms, but that all Holometabola do so, and that phylogenetic evidence conclusively shows that holometaboly evolved from a hemimetabolous ancestor [38].
But where does the larva come from? Both Wolpert [34] and Sly et al. [35] suggest that it would arise through the modification of an adultiform but preadult stage. In such a model, the nymphal stages of hemimetabolous insects would be homologous with the larval stages of Holometabola [39,40]. The larval traits that enabled such adaptation, which may have represented alternative developmental pathways used only under certain environmental conditions, must have been beneficial in themselves at the time that they were acquired.
The alternative explanation is that the larval stages of Holometabola are derived from a modified embryonic (pronymphal) form, with only the pupa being identified with the original ‘true’ larval or nymphal state as in hemimetabolous insects. This theory, which is prefigured by the comments of Aristotle and Harvey that the eggs of completely transforming insects are ‘born too soon’ [3], was introduced by Lubbock [41], further developed by Berlese [42], endorsed by Comstock [43] and Imms [44], and revived first by Williams, [45], and then again by Truman & Riddiford [46,47]. This model is contested by papers included in this issue, i.e. Jindra [48], Bellés [49] and Ishimaru et al. [50], all of whom contend that the evidence for homology between embryonic and larval stages is unsatisfactory, and that larval stages are better seen simply for what they are. As will be discussed below, this has knock-on implications for the evolution of the pupal stage. We do not consider that it is at present possible to distinguish between these contrasting hypotheses.
5. The evolutionary origin of complete metamorphosis
The evolution of holometaboly, however, requires not just the innovation of the larval form but also that of the pupa. A letter of 17 June 1868 from Fritz Müller to Charles Darwin (Darwin Correspondence project, 6248A_20488, [51]) expressed ‘the opinion that the ‘incomplete metamorphosis' of the Orthoptera is the primitive one, inherited from the original parents of all Insects, and the ‘complete metamorphosis’ of the Coleoptera, Diptera, & c., a subsequently acquired one.’
Müller's opinion of 150 years ago, that the ‘Hemimetabola’ is the ancestral, plesiomorphic group of insects, has since been confirmed by contemporary analysis of DNA sequences. The split probably took place almost 400 Ma [38] and gave rise to today's most speciose group of insects, the Holometabola. Although some other insect orders within the hemimetabolous insects (the so-called Paraneoptera) also have pupa-like stages, only members of the Holometabola display all of the characteristic features of complete metamorphosis [39,52], and the phylogenetic evidence clearly indicates that true holometaboly evolved only once and was quickly followed by sustained adaptive radiation involving an elevated rate of cladogenesis [38,53].
Is holometaboly developmentally irreversible? A small number of insects that unquestionably belong to the holometabolous clade have renounced metamorphosis; this is rare and has been accomplished entirely through acquiring the ability to reproduce neotenously, in some cases before the pupa is formed [54]. This group provides an interesting set of models with which to study complete metamorphosis. Since, in most of these cases, the insects in question have retained the capacity to resume under special conditions their ancestral holometabolous metamorphic life history (including a pupal stage), reversal of metamorphosis evidently has not involved the removal of the basic transcriptional controls over a stage-specific mechanism of developmental progression, but rather the premature activation of a separate system that promotes reproduction at a time before the normal adult body structure has been acquired. It is clear that this neotenous life history is a secondary adaptation, presumably to allow rapid population expansion when exploiting rich but transient resources [54]. Hodin [55] has suggested that such life-history adaptations are likely to involve specific modifications of the hormonal systems that control metamorphosis.
6. The developmental origin of the pupal stage
The nature of the evolutionary event that engendered the pupal stage has been a popular subject of speculation among entomologists for a long time, and the possibilities have over the years converged on two clearly different schemes: in the first, the pupa is considered to be a modified version of the final larval stage—this is referred to here as ‘Hinton 2’ [40]; and in the second, all holometabolous larval stages are supposed to be derived from a modified embryonic (‘pronymphal’) form, with the pupa being identified with the ‘true’ larval or nymphal state, the ‘Berlese’ model [41–47].
In this special issue, papers by Bellés [49], Ishimaru et al. [50], Jindra [48] and Truman & Riddiford [52] all address this evolutionarily important question. The papers naturally concentrate on ways in which the various theories differ. It is instructive, however, to look for common ground; interestingly the ‘Hinton 2’ and ‘Berlese’ models agree that the pupal stage must have arisen as a modified preadult (i.e. larval or nymphal) stage and is not a modified adult stage. The difference is that in the ‘Hinton 2’ scheme it is assumed that the evolution of the holometabolous larva predated the appearance of the pupa (which is thus a modified larva), and the preceding larval stages are considered homologous with the original larval or nymphal condition, while according to the ‘Berlese’ model, what are now designated as larval stages were originally the embryonic ‘pronymph’, and what is now the pupa is the survivor of the original prelarval stage. It is evident from the different conclusions reached by these authors that the matter is far from resolved.
We suppose that the relevant papers in this special issue will crystallize some of the questions that will need to be answered before textbook authors can decide how to present a single settled account of the matter to future entomologists. To those who are dismayed that no consensus about the origin of complete metamorphosis has yet been achieved, we point out that H. E. Hinton completely changed his mind about the evolution of the pupal stage between the first [39] and the second [40] of his two important contributions.
7. Adaptive benefits of complete metamorphosis
In the nineteenth century, an alternative ecological/evolutionary approach to understanding metamorphosis was initiated by Darwin, Lubbock and others to explain why metamorphic transformations evolved in the first place. Darwin pointed out that successive life stages may be differentially adapted to occupy particular niches [56, p. 530], a point also noted by Carroll Williams in 1952 [45].
We argue here that the big question ‘what is the adaptive benefit of complete metamorphosis?’ has hardly been addressed. Even so, there are a significant number of theories that address the evolution of metamorphosis. We will here briefly review them and discuss whether they provide any specific suggestions to understand the adaptive evolution of the pupal stage and complete metamorphosis and will formulate a hypothesis that has only been considered briefly before. We also discuss the nature of the possible costs of the pupal stage, including the problems caused by the significant re-organization of the insect's whole anatomy.
The idea that metamorphosis enables particular life stages to be adapted to particular niches is a long-standing one. Darwin [56, p. 530] credited Müller with the idea. It applies to a wide variety of organisms such as parasites with free-living stages [57], insects where the adult is specialized for dispersal [58], and many marine taxa where larvae are planktonic and adults are sessile [1]. While the idea that metamorphosis allows the occupation of multiple niches is clearly instructive, it does not give insight into the selective mechanisms underlying complete as opposed to incomplete metamorphosis.
Istock [59] provided a formal analysis for organisms with a two-stage complex life cycle, where the two stages experience their own environments imposing upon them challenges such as predator or physico-chemical pressures. The main idea is that an organism can use distinct resources throughout its life cycle, the switch between these resources being adaptive. This view has been elaborated by many authors [60,61]. It is difficult, however, to see how this resource-based approach can easily be extended to understand the evolution of an additional pupal stage, given that the pupa is a quiescent, purely developmental stage. Ecological models need not rely only on resource acquisition: Werner [62] proposed that ratios of mortality rates to growth rates determine the habitat switch enabled by metamorphosis.
Throughout ontogeny, because of the change in size, the given optimal body plan will change [62]. From this observation, a key evolutionary argument to explain the evolution of metamorphosis is the notion of decoupling life stages ([60,63] and references therein), which, for example, allows for stage-specifically different natural selection. Such decoupling could break genetic correlations and significantly increase the evolvability within each stage [60,63]. While this applies to all forms of metamorphosis, a pupal stage could be seen as an opportunity to generate a particularly radical remodelling of the body plan.
In his classic book on insect metamorphosis, Wigglesworth [64] proposed the idea of two genetic systems within one organism: one for the larval and one for the adult stage. One can go beyond this and consider the pupal stage to require a third such system. This kind of ‘partitioned genome’ model is now known to be simplistic (see, for example, [21]), but as noted above, the concept is still helpful in understanding what is occurring at the level of hormonally regulated transcription systems [49]. Wigglesworth's ‘multiple genetic systems’ idea can be interpreted in a modern context as a way of breaking the genetic correlations between the juvenile and adult stages. Few studies have investigated such decoupling in holometabolous insects. In Drosophila melanogaster, for example, heat resistance [65] and social behaviour and activity [66] are decoupled between the larval and the adult stage, while the expression of antimicrobial peptides is correlated between larvae and adults [67]. A general way to test the decoupling hypothesis and whether this effect is stronger in organisms with complete metamorphosis would be to compare the strengths of genetic correlations between larvae and adults in hemi- versus holometabolous insects.
Decoupling can also be tested at the level of the phenotype. Using the wood tiger moth, Galarza et al. [68] investigated the decoupling of a suite of traits including life-history traits, morphological traits and in gene expression between larva and adults. Depending on the traits, they found decoupling but also carry-over effects from the larval to the adult phenotype driven by the experimentally changed temperature regime. For example, adult size and wing melanization were decoupled from the larval environment, whereas body melanization and heating capacity showed clear signatures of carry-over effects. Similarly, Critchlow et al. [69] found evidence of both carry-over effects and decoupling in immune gene expression after parasite exposure in the flour beetle Tribolium castaneum. Exposure to gregarine parasites in the larval stage was associated with lasting downregulation of immune effectors and a recognition protein in the pupal and adult stages. There was also evidence of decoupling across life stages, with an absence of co-regulation among immune genes in the pupal stage.
We are aware of only two previously published hypotheses on the evolution of metamorphosis that specifically consider the adaptive value of complete metamorphosis. The first is the notion put forward by Hinton [39] that pupation would allow a winged insect to have a larval stage without external wing pads. The alleged selective advantage is that this makes it much easier for the larva to live beneath the surface of the substrate. There are, however, many examples of burrowing hemimetabolous insects, first and foremost cicadas. Also, if selection acts on just one trait (i.e. wing pads), it is difficult to understand how such a developmentally complex response as the evolution of the pupal stage results.
The second hypothesis, which we propose here, is that complete metamorphosis is an adaptation permitting the decoupling of growth and differentiation, a special case of the decoupling hypothesis. This is based on the observation by Arendt [70, p. 163] ‘that growth and differentiation may be partially decoupled in poikilotherms … For insects with complete metamorphosis…, growth is largely confined to the larval stage, while most development occurs in the pupal stage.’ This statement is supported by the many examples of fast growth within the insects, most of which are holometabolous species, such as house flies [71], lepidopteran caterpillars [72] or burying beetles [73]. In all of these cases, the growth rate during larval life is apparently maximized at the cost of food processing efficiency [72]. This is clearly adaptive where food is plentiful in the short term, but the window of opportunity in which to eat it may be limited. In the case of food plants, these resources may be transient because of a short growing season or the development of plant defences [74], or in the case of an animal carcass because of competition from other carnivores or saprophytes [75]. This time-limited ecological vulnerability is likely to be compounded in all these cases, because a specialized larval feeding stage is likely only to be lightly defended against predators and parasitoids, so that rapid growth will be advantageous in reducing the period in which the larva is vulnerable to complete loss of fitness through early death.
In directly developing organisms (e.g. hemimetabolous insects), studies of the compensatory growth that often occurs in response to a previous detrimental environmental condition show that such acceleration of growth comes at a substantial cost [76,77]. This implies that, in these organisms, growth rates are not normally maximized, but instead are optimized to achieve greater efficiency. Growth–differentiation trade-offs have been observed in starkly different organisms [77,78]. Taken together, these observations suggest that substantial benefits might have accrued to a previously directly developing insect that acquired the ability to separate growth and development by evolving completely separate preadult stages in which these processes can occur independently, i.e. growth in the larval stages and development of a complex adult body form in the pupal stage. We therefore propose that decoupling growth and differentiation could have been a fundamental selection mechanism that resulted in the evolution of a pupal stage.
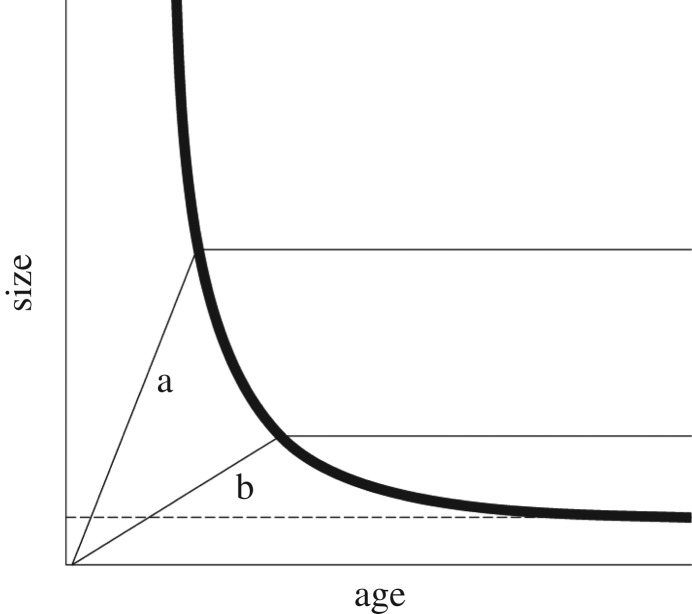
To further illustrate this point, we build on an argument put forward by Day & Rowe [79]. They investigated the trade-off between age and size at maturity. As depicted in figure 1, assuming a threshold size for metamorphosis, an L-shaped reaction norm arises. Such L-shaped reaction norms have been observed in mites [80], mosquitoes [81] and ladybirds [82]. The long tail, i.e. small size at maturity in individuals with very long development time, is explained by a developmental threshold [79]. Only organisms with a certain growth rate can attain a large size, and then become subject to the trade-off between age and size at transition. Under less favourable conditions, metamorphosis would result in longer development time without any size gain. It seems possible that if intrinsic growth rates are higher, as in some holometabolous insects, the chances of achieving a large size at maturity, given the L-shaped reaction norm, are increased. This would then constitute a selective scenario for fast growth that in certain situations might only be achievable by decoupling growth and differentiation. Alternatively, decoupling growth and differentiation could be beneficial in situations of intense competition for ephemeral resources.
Figure 1.
Growth trajectories under good (a) and bad (b) growth conditions (redrawn from [79], empirical examples in [80–82]). The horizontal dashed line depicts a threshold that needs to be reached before transition to maturity, or in the case of holometabolous insects the onset of pupation. The thick black line depicts the optimal point of switching from growth to reproduction. Faster growth, when the growth conditions are favourable, results in a larger size at maturity at a lower age. We propose that faster growth can also be achieved by decoupling growth and differentiation, which would allow holometabolous insects to grow faster.
Overall, given the evolutionary success of holometabolous insects, it is surprising that there are only a few studies or even hypotheses on the adaptive benefit of complete metamorphosis. As Hinton [39, p. 395] previously observed, ‘The preservation of such a stage (the pupa) in the life cycle is evidence that its compensating advantages must be enormous because of pupal mortality’.
8. Biotic interactions and metamorphosis
The evolution of the pupa, a single evolutionary event, may not only have solved problems of resource acquisition but also created other unrelated problems connected with biotic interactions with predators, pathogens and parasites. Pupae are basically sessile stages that face a number of challenges, including predation and infection. As discussed by Lindstedt et al. [83], predation on pupae is severe and caused by a variety of generalist predators such as shrews and birds. Moreover, specialists such as pupal parasitoids have evolved that also cause significant mortality [84]. These selective forces would have led to the evolution of specific defensive adaptations. Across insect species, pupae have evolved different anti-predator defence mechanisms, such as crypsis [83]. Such defences may be costly and their developmental regulation may have led to genetic correlations between metamorphosis and defence that have been hard to overcome.
Perhaps unsurprisingly, the degradation and replacement of the larval gut during metamorphosis can have drastic consequences for the gut microbiota. After early work on metamorphosis and gut microbes [85,86], interest has returned after a 100-year hiatus [87,88]. The internal re-organization required by complete metamorphosis poses an additional problem to the developing insect in the form of the possible escape of microbes from the inside of the gut into the insect's haemocoel [88]. In Lepidoptera, the host upregulates the expression of lysozymes and other immune effectors during pupation and before the adult moult, whereas in the hemimetabolous cricket, no such upregulation is found [89]. Previous work has shown that experimental reduction of this immune induction permits certain microbes to escape host control and survive into the adult stage [88]. Hammer & Moran [90] review the constraints imposed by metamorphosis on the maintenance of symbiosis across life stages, and how this might affect the evolution of vertically transmitted symbionts. Beyond constraints, they argue that metamorphosis enables restructuring of the microbiota across life stages, and as such may represent a special case of adaptive decoupling. Finally, they discuss the ways in which microbes can directly mediate metamorphosis, such as by providing cues for the initiation of metamorphosis, or by protecting vulnerable hosts.
9. Concluding remarks
Insects are of outstanding importance for biodiversity, for ecosystem services, as vectors of disease and as a source of protein. Complete metamorphosis is one of the key evolutionary innovations explaining the enormous and unique biodiversity of holometabolous insects. A unified view of complete metamorphosis, and especially a clear understanding of the adaptive evolution, has the potential to make important contributions to our understanding of the most speciose taxon, with implications for all the areas listed above. The contributions to this issue hopefully will motivate research on this fundamental question.
Acknowledgements
We are grateful to Sophie Armitage for feedback and to Helen Eaton for her excellent support.
Data accessibility
This article has no data.
Competing interests
We have no competing interests.
Funding
P.R.J. and J.R. were supported by DFG grant no. 389139730. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Wilbur HM. 1980. Complex life cycles. Annu. Rev. Ecol. Syst. 11, 67–93. ( 10.1146/annurev.es.11.110180.000435) [DOI] [Google Scholar]
- 2.Aristotle 1942. Generation of animals. (Transl. by A. L. Peck; Loeb Classical Library, no. 366.) Cambridge, MA: Harvard University Press.
- 3.Reynolds S. 2019. Cooking up the perfect insect: Aristotle's transformational idea about the complete metamorphosis of insects. Phil. Trans. R. Soc. B 374, 20190074 ( 10.1098/rstb.2019.0074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engel MS, Grimaldi D. 2005. Evolution of the insects. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B. 2011. How many species are there on Earth and in the ocean? PLoS Biol. 9, e1001127 ( 10.1371/journal.pbio.1001127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabr F. 2012. How does a caterpillar turn into a butterfly? Scient. Am. (10 August 2012, online only). See https://www.scientificamerican.com/article/caterpillar-butterfly-metamorphosis-explainer/. [Google Scholar]
- 7.Duncan PM. 1872. Insect metamorphosis. Part 1. Nature 14, 30–34. [Google Scholar]
- 8.Riddiford LM. 1978. Ecdysone-induced change in cellular commitment of the epidermis of the tobacco hornworm, Manduca sexta, at the initiation of metamorphosis. Gen. Comp. Endocrinol. 34, 438–446. ( 10.1016/0016-6480(78)90284-8) [DOI] [PubMed] [Google Scholar]
- 9.Weeks JC, Ernst-Utzschneider K. 1989. Respecification of larval proleg motoneurons during metamorphosis of the tobacco hornworm, Manduca sexta: segmental dependence and hormonal regulation. J. Neurobiol. 20, 569–592. ( 10.1002/neu.480200605) [DOI] [PubMed] [Google Scholar]
- 10.Lubischer JL, Verhegge LD, Weeks JC. 1999. Respecified larval proleg and body wall muscles circulate hemolymph in developing wings of Manduca sexta pupae. J. Exp. Biol. 202, 787–796. [DOI] [PubMed] [Google Scholar]
- 11.Tettamanti G, et al. 2007. Programmed cell death and stem cell differentiation are responsible for midgut replacement in Heliothis virescens during prepupal instar. Cell Tiss. Res. 330, 345–359. ( 10.1007/s00441-007-0449-8) [DOI] [PubMed] [Google Scholar]
- 12.Hall MJR, Martín-Vega D. 2019. Visualization of insect metamorphosis. Phil. Trans. R. Soc. B 374, 20190071 ( 10.1098/rstb.2019.0071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lockshin RA, Williams CM. 1964. Programmed cell death: 2. Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. J. Insect Physiol. 10, 643–649. ( 10.1016/0022-1910(64)90034-4) [DOI] [Google Scholar]
- 14.Lockshin RA, Williams CM. 1965. Programmed cell death: I. Cytology of degeneration in the intersegmental muscles of the Pernyi silkmoth. J. Insect Physiol. 11, 123–133. ( 10.1016/0022-1910(65)90099-5) [DOI] [PubMed] [Google Scholar]
- 15.Tettamanti G, Casartelli M.. 2019. Cell death during complete metamorphosis. Phil. Trans. R. Soc. B 374, 20190065 ( 10.1098/rstb.2019.0065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wigglesworth VB. 1934. The physiology of ecdysis in Rhodnius prolixus (Hemiptera). II. Factors controlling moulting and ‘metamorphosis’. J. Cell Sci. 77, 191–222. [Google Scholar]
- 17.Röller H, Dahm KH, Sweely CC, Trost BM. 1967. The structure of the juvenile hormone. Angew. Chem. Int. Edn Engl. 6, 179–180. ( 10.1002/anie.196701792) [DOI] [Google Scholar]
- 18.Li K, Jia QQ, Li S. 2019. Juvenile hormone signaling – a mini review. Insect Sci. 26, 600–606. ( 10.1111/1744-7917.12614) [DOI] [PubMed] [Google Scholar]
- 19.Jindra M, Palli SR, Riddiford LM. 2013. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 58, 181–204. ( 10.1146/annurev-ento-120811-153700) [DOI] [PubMed] [Google Scholar]
- 20.Jindra M, Bellés X, Shinoda T. 2015. Molecular basis of juvenile hormone signaling. Curr. Opin. Insect Sci. 11, 39–46. ( 10.1016/j.cois.2015.08.004) [DOI] [PubMed] [Google Scholar]
- 21.Willis JH. 2018. The evolution and metamorphosis of arthropod proteomics and genomics. Annu. Rev. Entomol. 63, 1–13. ( 10.1146/annurev-ento-020117-043447) [DOI] [PubMed] [Google Scholar]
- 22.Smykal V, Daimon T, Kayukawa T, Takaki K, Shinoda T, Jindra M. 2014. Importance of juvenile hormone signaling arises with competence of insect larvae to metamorphose. Dev. Biol. 390, 221–230. ( 10.1016/j.ydbio.2014.03.006) [DOI] [PubMed] [Google Scholar]
- 23.Chafino S, Ureña E, Casanova J, Casacuberta E, Franch-Marro X, Martín D. 2019. Upregulation of E93 gene expression acts as the trigger for metamorphosis independently of the threshold size in the beetle Tribolium castaneum. Cell Rep. 27, 1039–1049.e2. ( 10.1016/j.celrep.2019.03.094) [DOI] [PubMed] [Google Scholar]
- 24.Kloosterman WP, Plasterk RHA. 2006. The diverse functions of microRNAs in animal development and disease. Dev. Cell 11, 441–450. ( 10.1016/j.devcel.2006.09.009) [DOI] [PubMed] [Google Scholar]
- 25.Chawla G, Sokol NS. 2011. MicroRNAs in Drosophila development. Int. Rev. Cell Mol. Biol. 286, 1–65. ( 10.1016/B978-0-12-385859-7.00001-X) [DOI] [PubMed] [Google Scholar]
- 26.Asgari S. 2013. MicroRNA functions in insects. Insect Biochem. Mol. Biol. 43, 388–397. ( 10.1016/j.ibmb.2012.10.005) [DOI] [PubMed] [Google Scholar]
- 27.Ylla G, Piulachs M-D, Belles X. 2017. Comparative analysis of miRNA expression during the development of insects of different metamorphosis modes and germ-band types. BMC Genomics 18, 774 ( 10.1186/s12864-017-4177-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee K, Fischer R, Vilcinskas A. 2012. Histone acetylation mediates epigenetic regulation of transcriptional reprogramming in insects during metamorphosis, wounding and infection. Front. Zool. 9, 25 ( 10.1186/1742-9994-9-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy A, Palli SR. 2018. Epigenetic modifications acetylation and deacetylation play important roles in juvenile hormone action. BMC Genomics 19, 934 ( 10.1186/s12864-018-5323-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burggren WW. 2017. Epigenetics in insects: mechanisms, phenotypes and ecological and evolutionary implications. Adv. Insect Physiol. 53, 1–30. ( 10.1016/bs.aiip.2017.04.001) [DOI] [Google Scholar]
- 31.Glastad KM, Hunt BG, Goodisman MAD. 2019. Epigenetics in insects: genome regulation and the generation of phenotypic diversity. Annu. Rev. Entomol. 64, 185–203. ( 10.1146/annurev-ento-011118-111914) [DOI] [PubMed] [Google Scholar]
- 32.Ylla G, Piulachs M-D, Belles X. 2018. Comparative transcriptomics in two extreme neopterans reveals general trends in the evolution of modern insects. iScience 4, 164–179. ( 10.1016/j.isci.2018.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bewick K, Vogel KJ, Moore AJ, Schmitz RJ. 2017. Evolution of DNA methylation across insects. Mol. Biol. Evol. 34, 654–665. ( 10.1093/molbev/msw264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolpert L. 1999. From egg to adult to larva. Evol. Dev. 1, 3–4. ( 10.1046/j.1525-142x.1999.00111.x) [DOI] [PubMed] [Google Scholar]
- 35.Sly BJ, Snoke MS, Raff RA. 2003. Who came first–larvae or adults? Origins of bilaterian metazoan larvae. Int. J. Dev. Biol. 47, 623–632. [PubMed] [Google Scholar]
- 36.Alberch P, Gould SJ, Oster GF, Wake DB. 1979. Size and shape in ontogeny and phylogeny. Paleobiology 5, 296–317. ( 10.1017/S0094837300006588) [DOI] [Google Scholar]
- 37.Gould SJ. 1977. Ontogeny and phylogeny. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 38.Misof B, et al. 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767. ( 10.1126/science.1257570) [DOI] [PubMed] [Google Scholar]
- 39.Hinton HE. 1948. On the origin and function of the pupal stage. Trans. R. Entomol. Soc. Lond. 99, 395–409. ( 10.1111/j.1365-2311.1948.tb01227.x) [DOI] [Google Scholar]
- 40.Hinton HE. 1963. The origin and function of the pupal stage. Proc. R. Entomol. Soc. Lond. A Gen. Entomol. 38, 77–85. ( 10.1111/j.1365-3032.1963.tb00759.x) [DOI] [Google Scholar]
- 41.Lubbock J. 1890. On the origin and metamorphosis of insects. London, UK: Macmillan. [Google Scholar]
- 42.Berlese A. 1913. Intorno alle metamorfosi degli insetti. Redia 9, 121–136 (in Italian). [Google Scholar]
- 43.Comstock JH. 1920. An introduction to entomology, 2nd edn Ithaca, NY: Comstock Publishing Co. [Google Scholar]
- 44.Imms AD. 1931. Recent advances in entomology. London, UK: J. & A. Churchill. [Google Scholar]
- 45.Williams CM. 1952. Morphogenesis and the metamorphosis of insects. Harvey Lect. 47, 126–155. [PubMed] [Google Scholar]
- 46.Truman JW, Riddiford LM. 1999. The origins of insect metamorphosis. Nature 401, 447–452. ( 10.1038/46737) [DOI] [PubMed] [Google Scholar]
- 47.Truman JW, Riddiford LM. 2002. Endocrine insights into the evolution of metamorphosis in insects. Annu. Rev. Entomol. 47, 467–500. ( 10.1146/annurev.ento.47.091201.145230) [DOI] [PubMed] [Google Scholar]
- 48.Jindra M. 2019. Where did the pupa come from? The timing of juvenile hormone signalling supports homology between stages of hemimetabolous and holometabolous insects. Phil. Trans. R. Soc. B 374, 20190064 ( 10.1098/rstb.2019.0064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belles X. 2019. The innovation of the final moult and the origin of insect metamorphosis. Phil. Trans. R. Soc. B 374, 20180415 ( 10.1098/rstb.2018.0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishimaru Y, Tomonari S, Watanabe T, Noji S, Mito T. 2019. Regulatory mechanisms underlying the specification of the pupal-homologous stage in a hemimetabolous insect. Phil. Trans. R. Soc. B 374, 20190225 ( 10.1098/rstb.2019.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darwin Correspondence Project. 2019. Darwin Correspondence Project. Cambridge, UK: University of Cambridge; See https://www.darwinproject.ac.uk/ (accessed 25 June 2019). [Google Scholar]
- 52.Truman JW, Riddiford LM. 2019. The evolution of insect metamorphosis: a developmental and endocrine view. Phil. Trans. R. Soc. B 374, 20190070 ( 10.1098/rstb.2019.0070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rainford JL, Hofreiter M, Nicholson DB, Mayhew PJ. 2014. Phylogenetic distribution of extant richness suggests metamorphosis is a key innovation driving diversification in insects. PLoS ONE 9, e109085 ( 10.1371/journal.pone.0109085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMahon DP, Hayward A. 2016. Why grow up? A perspective on insect strategies to avoid metamorphosis. Ecol. Entomol. 41, 505–515. ( 10.1111/een.12313) [DOI] [Google Scholar]
- 55.Hodin J. 2006. Expanding networks: signaling components in and a hypothesis for the evolution of metamorphosis. Integr. Comp. Biol 46, 719–742. ( 10.1093/icb/icl038) [DOI] [PubMed] [Google Scholar]
- 56.Darwin CR. 1866. The origin of species by means of natural selection. London, UK: John Murray. [Google Scholar]
- 57.Poulin R. 2011. Evolutionary ecology of parasites. Princeton, NJ: Princeton University Press. [Google Scholar]
- 58.Dingle H. 2001. The evolution of migratory syndromes in insects. In Insect movements: mechanisms and consequences (eds Ian Woiwood, DR Reynolds, CD Thomas), pp. 159–181. Wallingford, UK: CABI.
- 59.Istock CA. 1966. The evolution of complex life cycle phenomena: an ecological perspective. Evolution 67, 595–605. ( 10.1111/j.1558-5646.1967.tb03414.x) [DOI] [PubMed] [Google Scholar]
- 60.Moran NA. 1994. Adaptation and constraint in the complex life cycles of animals. Annu. Rev. Ecol. Syst. 25, 573–600. ( 10.1146/annurev.es.25.110194.003041) [DOI] [Google Scholar]
- 61.ten Brink H, de Roos AM, Dieckmann U. 2019. The evolutionary ecology of metamorphosis. Am. Nat. 193, E116–E131. ( 10.1086/701779) [DOI] [PubMed] [Google Scholar]
- 62.Werner EE. 1986. Amphibian metamorphosis: growth rate, predation risk, and the optimal size at transformation. Am. Nat. 128, 319–341. ( 10.1086/284565) [DOI] [Google Scholar]
- 63.Ebenman B. 1992. Evolution in organisms that change their niches during the life cycle. Am. Nat. 139, 990–1021. ( 10.1086/285370) [DOI] [Google Scholar]
- 64.Wigglesworth VB. 1954. The physiology of insect metamorphosis. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 65.Loeschcke V, Krebs RA. 1996. Selection for heat-shock resistance in larval and in adult Drosophila buzzattii: comparing direct and indirect responses. Evolution 50, 2354–2359. ( 10.1111/j.1558-5646.1996.tb03623.x) [DOI] [PubMed] [Google Scholar]
- 66.Anderson BB, Scott A, Dukas R. 2016. Social behavior and activity are decoupled in larval and adult fruit flies. Behav. Ecol. 27, 820–828. ( 10.1093/beheco/arv225) [DOI] [Google Scholar]
- 67.Fellous S, Lazzaro BP. 2011. Potential for evolutionary coupling and decoupling of larval and adult immune gene expression. Mol. Ecol. 20, 1558–1567. ( 10.1111/j.1365-294X.2011.05006.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galarza JA, Dhaygude K, Ghaedi B, Suisto K, Valkonen J, Mappes J. 2019. Evaluating responses to temperature during pre-metamorphosis and carry-over effects at post-metamorphosis in the wood tiger moth (Arctia plantaginis). Phil. Trans. R. Soc. B 374, 20190295 ( 10.1098/rstb.2019.0295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Critchlow JT, Norris A, Tate AT. 2019. The legacy of larval infection on immunological dynamics over metamorphosis. Phil. Trans. R. Soc. B 374, 20190066 ( 10.1098/rstb.2019.0066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arendt JD. 1997. Adaptive intrinsic growth rates: an integration across taxa. Q. Rev. Biol. 72, 149–177. ( 10.1086/419764) [DOI] [Google Scholar]
- 71.Khan H, Shad S, Akram W. 2012. Effect of livestock manures on the fitness of house fly, Musca domestica L. (Diptera: Muscidae). Parasitol. Res. 111, 1165–1171. ( 10.1007/s00436-012-2947-1) [DOI] [PubMed] [Google Scholar]
- 72.Reynolds SE. 1990. Feeding in caterpillars: maximising or optimising food acquisition. In Animal nutrition and transport processes. 1: Nutrition in wild and domestic animals, vol. 5 (ed. Mellinger J.), pp. 106–118. Basel, Switzerland: Karger; ISBN: 3-8055-5157 [Google Scholar]
- 73.Smiseth PT, Darwell CT, Moore AJ.. 2003. Partial begging: an empirical model for the early evolution of offspring signalling. Proc. R. Soc. B, 270, 1773–1777. ( 10.1098/rspb.2003.2444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feeny P. 1970. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51, 565–581. ( 10.2307/1934037) [DOI] [Google Scholar]
- 75.Rozen D, Engelmoer D, Smiseth P. 2008. Antimicrobial strategies in burying beetles breeding on carrion. Proc. Natl Acad. Sci. USA 105, 17 890–17 895. ( 10.1073/pnas.0805403105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Metcalfe N, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260. ( 10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 77.Rolff J, Van de Meutter F, Stoks R. 2004. Time constraints decouple age and size at maturity and physiological traits. Am. Nat. 164, 559–565. ( 10.1086/423715) [DOI] [PubMed] [Google Scholar]
- 78.Zakrzewska A, van Eikenhorst G, Burggraaff JEC, Vis DJ, Hoefsloot H, Delneri D, Oliver SG, Brul S, Smits GJ. 2011. Genome-wide analysis of yeast stress survival and tolerance acquisition to analyze the central trade-off between growth rate and cellular robustness. Mol. Biol. Cell 22, 4435–4446. ( 10.1091/mbc.e10-08-0721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Day T, Rowe L. 2002. Developmental thresholds and the evolution of reaction norms for age and size at life-history transitions. Am. Nat. 159, 338–350. ( 10.1086/338989) [DOI] [PubMed] [Google Scholar]
- 80.Plaistow SJ, Lapsley CT, Beckerman AP, Benton TG. 2004. Age and size at maturity: sex, environmental variability and developmental thresholds. Proc. R. Soc. B 271, 919–924. ( 10.1098/rspb.2004.2682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phelan C, Roitberg B. 2015. An age–size reaction norm yields insight into environmental interactions affecting life-history traits: a factorial study of larval development in the malaria mosquito Anopheles gambiae sensu stricto. Ecol. Evol. 3, 1837–1847. ( 10.1002/ece3.589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agarwala BK, Bhowmik PJ. 2011. Effect of resource gradient on age and size at maturity and their influence on early-life fecundity in the predatory Asian lady beetle, Harmonia axyridis. Entomol. Exp. Appl. 141, 97–102. ( 10.1111/j.1570-7458.2011.01172.x) [DOI] [Google Scholar]
- 83.Lindstedt C, Murphy L, Mappes J. 2019. Antipredator strategies of pupae: how to avoid predation in an immobile life stage? Phil. Trans. R. Soc. B 374, 20190069 ( 10.1098/rstb.2019.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Godfray HCJ. 1994. Parasitoids: behavioral and evolutionary ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 85.Bacot AW. 1911. On the persistence of bacilli in the gut of an insect during metamorphosis. Trans. R. Entomol. Soc. Lond. 1911, 497–500. [Google Scholar]
- 86.Tebutt H. 1912. On the influence of the metamorphosis of Musca domestica upon bacteria administered during the larval stage. J. Hyg. 12, 516–526. ( 10.1017/s0022172400005179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hammer TJ, McMillan WO, Fierer N. 2014. Metamorphosis of a butterfly-associated bacterial community. PLoS ONE 9, e86995 ( 10.1371/journal.pone.0086995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnston PR, Rolff J. 2015. Host and symbiont jointly control gut microbiota during complete metamorphosis. PLoS Pathog. 11, e1005246 ( 10.1371/journal.ppat.1005246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnston PR, Paris V, Rolff J. 2019. Immune gene regulation in the gut during metamorphosis in a holo- versus a hemimetabolous insect. Phil. Trans. R. Soc. B 374, 20190073 ( 10.1098/rstb.2019.0073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hammer TJ, Moran NA. 2019. Links between metamorphosis and symbiosis in holometabolous insects. Phil. Trans. R. Soc. B 374, 20190068 ( 10.1098/rstb.2019.0068) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no data.