Abstract
Spillover of a pathogen from a wildlife reservoir into a human or livestock host requires the pathogen to overcome a hierarchical series of barriers. Interventions aimed at one or more of these barriers may be able to prevent the occurrence of spillover. Here, we demonstrate how interventions that target the ecological context in which spillover occurs (i.e. ecological interventions) can complement conventional approaches like vaccination, treatment, disinfection and chemical control. Accelerating spillover owing to environmental change requires effective, affordable, durable and scalable solutions that fully harness the complex processes involved in cross-species pathogen spillover.
This article is part of the theme issue ‘Dynamic and integrative approaches to understanding pathogen spillover’.
Keywords: ecological interventions, cross-species transmission, management, spillover, zoonotic diseases
1. Introduction
Pathogen spillover, or the transmission of infections among species, can occur from animals to humans (zoonoses), from humans to animals (reverse zoonoses), or even from abiotic environmental reservoirs into vertebrates (sapronoses). Environmental change—including deforestation, habitat fragmentation or climate change—can create new opportunities for pathogens that were previously circulating only in wildlife or environmental reservoirs to spill over into people or livestock hosts [1]. The ecological drivers of pathogen spillover have become a focus of attention after a series of high-profile spillover events, including avian influenza, Ebola and Hendra viruses. Spillover to humans can be common for some disease agents, as in the case of Lyme disease, where every human case is a spillover event from a wildlife reservoir; or rare, as with HIV, which emerged after a handful of spillover events of simian immunodeficiency virus mutated into HIV [2]. While it would be ideal to prevent spillover, especially in cases like Ebola virus and HIV, where onward transmission leads to many human cases, data on the best way to mitigate risk at specific points along the spillover process are lacking.
Here, we focus on ecological interventions: actions that target the ecological context in which the spillover process occurs. We distinguish between ecological interventions and conventional interventions. We make this distinction as a practical way to focus our attention on novel (ecological) interventions and distinguish them from more conventional approaches in the medical and veterinary literature that have been well-treated previously, although we acknowledge that the designation of ‘ecological’ versus ‘conventional’ can be context-specific and not mutually exclusive. We define conventional interventions as medical and veterinary approaches, like disinfection, vaccination and treatment, that have been used widely by public health communities and focus primarily on the medical or chemical management of risk in human or domestic animals, or their immediate environments, without regard to more complex ecological interactions. While acknowledging successful conventional interventions, here, we focus on systems-based approaches that target spillover by harnessing a better understanding of a system's ecology. For example, although culling the reservoir and mass vaccinating the spillover hosts indeed change the ecology of pathogen transmission, here we expand to a diverse set of additional interventions that target the natural interactions or ecosystem services that occur upstream or downstream in the spillover process. If we can better understand the disease ecology, including the interactions among disease-carrying organisms, or between organisms and their complex environments contributing to spillover, we may be able to devise novel, actionable solutions to manage or reduce spillover (for example, augmentation of natural enemies, habitat modification or restoration of ecosystem services such as water purification provided by wetlands, etc.; table 1). Drawing on real-world examples of well-studied spillover systems, we outline some important collective insights and general concepts about successfully using ecological interventions to manage spillover.
Table 1.
Spillover barriers and associated conventional and ecological interventions that target each barrier layer.
| location | spillover barrier | conventional intervention | ecological intervention | examples of ecological interventions | status | intervention no. (figure) |
|---|---|---|---|---|---|---|
| zoonotic reservoir | reservoir density or distribution | fences, culling | habitat modification | altered food distribution on elk feeding grounds to reduce brucellosis [3]. | demonstrated, with correlational/observational support | 1 |
| natural enemies | maintenance of leopard populations to limit rabid feral dog populations [4]. See also [5]. | hypothesized | 2 | |||
| pathogen prevalence (in reservoir) | chemotherapy, vaccination of reservoir, test and remove | dilution hosts | increased diversity of host community for Ixodes ticks (e.g. by increasing size of forest fragments) may increase abundance of incompetent hosts for Borrelia burgdorferi, reducing Lyme disease spillover [6]. | demonstrated, but generality of dilution effect of increased biodiversity is debated [7–9] | 3 | |
| genetic management | reducing population size and stay-time of poultry in markets minimizes prevalence and genome reassortment of influenza viruses [10]. | demonstrated | 4 | |||
| infection intensity or pathogen shedding | reservoir nutrition and susceptibility | supplementing key flowering tree food resources for flying foxes (via habitat conservation/restoration) to boost nutrition and immunity in bats and decrease viral shedding rates of Hendra by bats [11], or similarly preserving native prey communities for vampire bats (rabies) via habitat conservation/restoration, which also encourages bats to feed on wildlife rather than humans or livestock. See also [12]. | hypothesized | 5 | ||
| environment | pathogen survival and spread | insecticides, disinfection | habitat modification | Anopheles (malaria) [13] and Culex (West Nile virus, Japanese encephalitis, St Louis encephalitis, also filariasis) mosquito reductions by fish additions to rice fields, while simultaneously increasing rice yields [14]. | hypothesized | 6 |
| gene management | gene drive in Anopheles gambiae to control spread of Plasmodium spp. causing malaria [15]. | demonstrated | 7 | |||
| natural enemies | maintaining the scavenger community (e.g. eagles and coyotes in the USA, vultures in India [16]) as an important consumer of carcasses that harbour Brucella, anthrax, and other pathogens. See also [17]. | demonstrated, with correlational/observational support | 8 | |||
| spillover host | spillover host exposure | chemical repellents, biosecurity | human behaviour modification | bamboo skirts over date palm sap collection pots to reduce bat contamination of sap with Nipah virus in Bangladesh [18,19]. See also [20]. | demonstrated | 9 |
| spillover host susceptibility and infection | chemotherapy, vaccination | managing coinfections or microbiome, genetic management | the use of faecal transplant procedures to treat Clostridium difficile with microbial competitors [21]. | demonstrated | no corresponding number in figure |
2. Ecological interventions targeting different spillover barriers
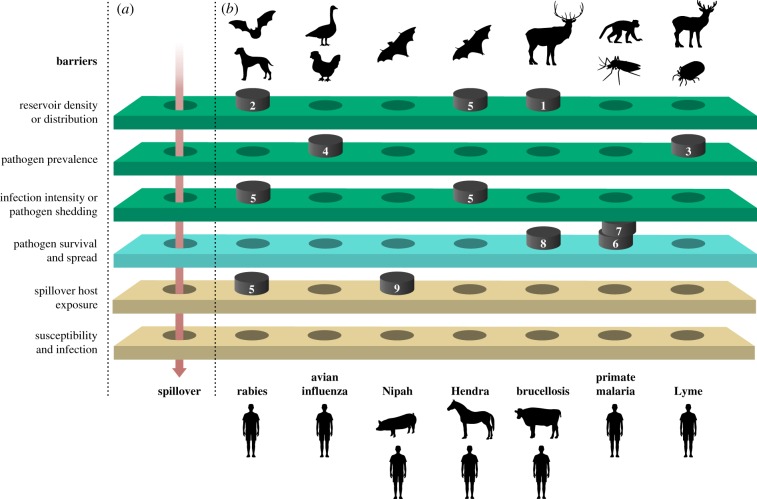
The ecological processes governing spillover can be described as a series of barriers that a pathogen must overcome to eventually traverse from the vertebrate reservoir to the final spillover host at a particular place and moment in time [22]. While our understanding of disease ecology is improving, options to manage or control spillover in wildlife hosts remain limited. Conventional solutions like culling, vaccination and chemical control (e.g. drugs, insecticides and disinfectants) can have adverse consequences such as environmental damage, the evolution of resistance or non-target effects, and are often logistically challenging to implement. In general, we can prevent or limit spillover by reducing or preventing the flow of pathogens across one or more of the potential barriers (e.g. managing population size or prevalence in reservoir hosts, pathogen persistence in the environment, or vector abundance, or by changing reservoir distribution or contact between reservoir and spillover hosts to prevent the pathogens and hosts from aligning in space and time). An ecological intervention may also target pathogen flow in several layers and systems, not just one (figure 1).
Figure 1.
Ecological interventions to manage spillover. Ecological interventions may offer creative solutions to reduce or prevent spillover at various barrier layers of the spillover process. The barriers occur in reservoir hosts (green), the environment and vectors (cyan) and spillover hosts (beige). For spillover to occur, holes in the barriers need to line up in space and time (a). To prevent this, interventions can be applied to reduce the sizes of the holes, or prevent the holes from aligning in space and time. The black numbered plugs blocking the holes represent some example ecological interventions (numbers refer to interventions in table 1) that could be implemented to manage spillover (b).
A major difference between many ecological and conventional interventions is in how these actions alter the pathogen transmission process. Many conventional management actions directly—and often temporarily—change the numbers of susceptible, infectious and recovered individuals. This is true, for instance, of vaccination (which reduces the number of susceptible individuals), culling (which temporarily reduces reservoir host density) and test-and-slaughter (which temporarily reduces diseased individuals but also reduces herd immunity). However, without sustained management effort, the effects of these actions can wane. This was observed in human measles, for instance, whereby measles risk increased following vaccination disruption after the 2014 Ebola epidemic in Sierra Leone, Liberia and Guinea [23]; we would expect the same pattern to emerge in many vaccination scenarios aimed at managing spillover. Ecological interventions, on the other hand, try to manage the underlying transmission processes, based on ecological understanding. For instance, introduction or restoration of a natural enemy through conservation of its habitat could impose a longer-term change on host mortality rates than a single reduction in host density owing to culling (table 1 and figure 1) and increasing host genetic diversity might provide a lasting reduction in susceptibility [24].
Here, we begin to explore some of the complexities involved in designing effective solutions that target ecological processes involved in zoonotic spillover. We focus on case studies that demonstrate logical ecological interventions that can (or have been proposed to) control the density, distribution or infectiousness of vertebrate reservoir hosts; survival or spread of pathogens in the environment; or contact risk, susceptibility or treatment success in the focal spillover host (table 1 and figure 1).
(a). Targeting reservoir hosts: moving beyond culling towards alternative non-lethal approaches
Throughout history, culling the reservoir host has been a common intervention for reducing spillover risk from wild or domestic vertebrates, but culling often incurs unacceptable economic or ecological costs, or unintended negative consequences [25,26], such as potential increases in pathogen transmission or virulence [25,27]. For example, Nipah virus was first discovered after it caused encephalitis outbreaks in Malaysia and Singapore among people involved in raising or slaughtering commercial pigs [28]. Culling pigs was effective at managing disease risk for people; however, there was substantial economic fallout, including production losses and the loss of approximately 36 000 jobs from farms that were not re-opened after the pigs were culled [29] (table 1). It was soon discovered that the natural reservoirs of the virus included several species of flying fox (i.e. Pteropus spp. bats). An ecological intervention to reduce transmission from bats to pigs was devised as a more sustainable solution to manage spillover: policies were put in place that required fruit trees, which attract bats and were implicated as the pathway for multiple spillover events on the outbreak's index farm, to be planted a minimum distance from pig sties [12] (table 1). Because the pig farming communities were heavily affected by the outbreak and incurred minimal cost from adopting this practice, this relatively simple ecological intervention has prevented further outbreaks of Nipah virus in Malaysia since 1998 [12].
Rabies control has also relied on culling at the level of the bat reservoir host. In Latin America, bats account for more cases of rabies than canines or other carnivores [30,31], and control efforts focus on the main reservoir host, the common vampire bat (Desmodus rotundus) [32,33]. Control efforts have included destruction of roosts, which indiscriminately kill other bat species in addition to vampire bats [34], alongside the application of a lethal anticoagulant paste applied to captured bats that spreads through colonies via allogrooming at the roost [33,35]. Recent studies suggest that rabies seroprevalence in vampire bats was highest in bat colonies with a history of culling, and that culling might inadvertently increase viral transmission by altering vampire bat movement [25,36].
Culling can alter host movement dynamics, in tandem with host densities, leading to unexpected disease consequences, as shown in several well-studied systems (e.g. Mycobacterium bovis in badgers; Mycoplasma aggasizi in desert tortoises). Culling can also alter pathogen dynamics in the reservoir host through increased population turnover. For instance, a theoretical analysis of classical swine fever in wild boars showed that culling led to the counterintuitive result that both disease prevalence and absolute number of infectious individuals increased as a consequence of host population reduction [27,37]. Studies of test-and-cull in bison and elk in an effort to control brucellosis have produced similar counterintuitive results, whereby herd immunity was reduced, resulting in subsequent outbreaks [38]. Thus, (often reactive) culling practices can be an effective intervention in controlling wildlife diseases, or can be ineffective, especially where efforts are not spatially coordinated and do not account for important nonlinearities and heterogeneities in disease transmission and host demography.
Beyond culling, there are many non-lethal interventions that can be employed to reduce spillover at the level of the reservoir host, including reservoir-host vaccination, treating infections or co-infections and ecological interventions such as contact or connectivity manipulations (e.g. fences and translocation), or fertility control. Oral vaccination of vampire bats has been proposed to reduce rabies spillover by capitalizing on the same social behaviour that facilitates anticoagulant-based bat culling efforts (table 1). Yet, while vaccination of reservoir hosts has been a successful alternative to culling for terrestrial rabies control in North America and Europe, no commercial vaccine is available for rabies control in vampire bats [39].
Complications associated with widespread vaccination campaigns are not limited to rabies. For many wildlife pathogens, vaccines are unavailable, costly to develop and deploy, and logistically challenging to implement at appropriate spatial and temporal scales [40,41]. Even where vaccines for reservoir hosts are available, vaccination is sometimes not socially acceptable. For example, after the recent development of a highly effective Hendra virus vaccine for horses, social factors including spread of anti-vaccination information by some members of the community, cost of the vaccine and export implications for vaccinated horses has meant that vaccine uptake is relatively low [42]. Similarly, in the case of avian influenza, decreasing spillover risk at the wild bird–poultry interface through vaccination may not always be effective against newly (rapidly) evolving strains, and vaccination of poultry is sometimes not affordable owing to the large number and high turnover of poultry, relative to the rare frequency of spillover of highly pathogenic avian influenza strains [43].
Employing natural enemies to control disease may sometimes be more effective and less costly than culling and can have additional benefits for the environment, like restoring threatened or endangered predators [44]. Predators are likely to affect the diseases of their prey through several mechanisms: killing sick individuals [43,44], lowering prey population size and altering aggregation patterns. For these reasons, wolf management has been proposed as a potential intervention for reducing chronic wasting disease and brucellosis in elk [5,45], but this ecological intervention has not been fully implemented owing to potential societal costs associated with larger wolf populations.
Ecological interventions to control reservoir host movement, connectivity or distribution have sometimes been employed, with variable success owing to opposing impacts on multiple layers of the spillover process. For example, food distribution to keep elk away from cattle during winter months and to reduce risk of brucellosis spillover has been ongoing for many decades in the Yellowstone area [3]. However, while supplemental feeding helps to separate elk and cattle, it also concentrates elk on feed grounds during winter, potentially elevating brucellosis prevalence within the wildlife reservoir and increasing the spillover risk associated with contacts that do occur [46,47]. Research continues to flesh out the multiple interacting effects of supplemental feeding, but to date, the effects are equivocal [48,49] and it is hard to detect any benefit in this highly variable system with many environmental drivers.
Ultimately, ecological approaches targeting the reservoir require a sophisticated understanding of the structure of, and processes involved with, the various components of the reservoir community [50]. Gaps in our understanding of the complex ecology of reservoirs have hindered progress in managing spillover of Ebola virus and rabies virus [51,52], among other zoonotic pathogens. Interventions can offer important clues to disentangle which reservoir components are most important to spillover [53]. For example, in Zimbabwe, sylvatic canids may play a role in the maintenance and spillover of human rabies in some areas. If domestic dogs are the main reservoir and source of spillover cases in people, then a campaign vaccinating domestic dogs within a region should lead to strong reductions in human infection, but if jackals are a secondary component of spillover risk (which some studies suggest) then oral baiting of jackals with rabies vaccine may be additionally required to reduce human rabies [50]. This illustrates a broader theme in spillover management, namely, that one strategy does not fit all cases owing to differences in reservoir ecologies.
(b). Targeting the environment: habitat, vector control and ecosystem management
Understanding pathogen persistence in abiotic environmental reservoirs sometimes leads to simple interventions that operate on many interacting levels to manage spillover risk. For example, spillover transmission of avian influenza can be managed in live-bird market systems by ‘rest days’ (during which no birds are brought to market) and lessening stay-time in markets; if birds are removed before the virus can infect and become infectious in a new host, then outbreaks can be avoided [54]. Limiting stay-time also serves to strongly reduce viral genome reassortment (gene shuffling that can result in novel strains that may have expanded host range or higher virulence in donor hosts) of avian influenza in retail markets by limiting co-infection and thus reducing the probability of generating novel spillover strains [10]. For Hendra virus spillover, blocking horses' overnight access to trees in pastures has been proposed as a solution to prevent viral transmission from bats to horses, since this intervention would delay horses’ access to grass contaminated by bat urine (if bats happen to roost in those trees), thereby reducing the probability that a horse would come into contact with recently secreted, live Hendra virus [55].
Targeting the environmental components in the ecology of disease transmission has a long history in vector management. For example, vector control using chemical pesticides has been a primary method of defence in reducing vector-borne disease risk, but this conventional intervention is prone to limitations, such as resistance evolution, non-target effects and environmental damage [56]. Chemical control can be replaced or enhanced by stocking mosquito predators in mosquito breeding habitats and this strategy has been used in diverse habitats to control disease-carrying mosquito vectors, including ponds, cisterns, irrigation canals and rice fields, with mixed success [57,58]. Similarly, control of blacklegged ticks (Ixodes scapularis, a Lyme disease vector) by spraying entomopathogenic fungi (e.g. Beauveria bassiana or Metarhizium anisopliae) on pastures has shown promise [17].
Natural habitat manipulation to reduce environmental persistence of pathogens has been used less, but holds promise. For example, scavengers like vultures compete with spillover pathogens for host tissue (a form of intra-guild predation). In India and Pakistan, declines in vulture populations owing to lethal effects of an anti-inflammatory drug, diclofenac, have resulted in increased volumes of uneaten carcasses, which act as environmental breeding grounds for diverse zoonotic spillover pathogens including anthrax, brucellosis and bovine tuberculosis [59–61]. Feral dog populations have also grown owing to increased access to carcasses, and although a causal association has not been definitively established, correlative evidence suggests that loss of vultures indirectly led to an increase in dogs and human rabies spillover [62]. Recent policy reform in India and Pakistan, banning diclofenac, may allow wild vulture restoration and lead to both conservation and public health benefits.
(c). Targeting the interface between reservoir and spillover hosts
Spillover can increase when landscape modification—like habitat encroachment, agricultural expansion and road building—increase contact rates between reservoir and spillover hosts [63]. Targeting this interface can sometimes offer the most effective interventions for reducing spillover, but interface controls could operate at a variety of scales. For instance, the use of bed nets to curb malaria is a classic example of controlling the interface between mosquitos and people. In addition, a combination of ecological and conventional interventions have helped reduce Hendra virus spillover risk in Australia by preventing contact at the interface between horses and flying fox urine (e.g. covering food and water, keeping horses away from fruiting and flowering trees [55]) and preventing exposure at the horse–human interface through use of personal protective equipment for veterinarians and owners dealing with sick horses [20].
Biosecurity is another example of a conventional intervention to reduce spillover along the wildlife–domestic animal interface. For example, biosecurity efforts to reduce rates of contact appropriate for avian influenza virus transmission between wild birds and poultry have been an important component of avian influenza risk management. But identifying biosecurity measures that prevent exposure can be challenging [64]: prior to 2014, no highly pathogenic avian influenza had been detected in the USA but then, after three different highly pathogenic reassortants were detected almost simultaneously in wild birds, these strains soon caused at least 18 independent emergence events in US commercial poultry operations, despite biosecurity measures [64,65].
Ecological interventions aimed at the interface between donor and recipient hosts have sometimes targeted shared food resources [48]. For example, Nipah virus in Bangladesh can be transmitted to people through drinking uncooked date palm sap contaminated by excreta from infected fruit bats [66,67]. By limiting bat access to sap that is drip-collected in clay pots overnight, viral contamination by bats can be reduced [19,68]. In principle, this should be an effective and acceptable ecological intervention because it only needs to be implemented on trees from which sap will be collected for drinking. However, wholesale adoption of this approach, relying on modifications to human behaviour, has been difficult to achieve across Bangladesh [18,69].
There can be ecological interventions that act at the scale of habitat modification to alter the contact rate of reservoir and spillover hosts. For example, forest fragmentation, wildlife population declines and the proliferation of cattle rearing have prompted shifts in vampire bat feeding from wildlife to human and livestock prey [70]. It has been proposed that rabies vaccination of livestock might be a viable conventional intervention [71]. Yet, if vaccination coverage is low, and livestock density continues to increase, then growing bat populations reliant on cattle near human settlements might still worsen rabies spillover risk to humans, despite a livestock vaccine [72,73]. Also, because vampire bats preferentially feed on livestock, even when wildlife are available [74], rapid withdrawal of livestock has been associated with prey switching to humans by vampire bats, with consequent increases in human rabies [75,76]. There might be more durable, conservation-based approaches to mitigate bat–human contact, or reduce forest-to-agricultural edge habitat where bats are exposed to cattle, but more research about how shifting prey distributions could impact vampire bat feeding ecology is needed to disentangle many complex and interacting factors [71].
Similarly, the movement ecology of traditionally nomadic flying foxes (Pteropus spp.) in Australia has shifted owing to the loss of critical nectar resources after land clearing for agriculture and urban development [11]. Flying foxes, in turn, experience acute episodes of nutritional stress [77]. To decrease the energetic costs of foraging, colonies split into many smaller populations that remain close to consistent but poor-quality urban food resources [78]. Nutritional stress and urban habituation likely drive shedding of Hendra virus from these reservoir hosts as well as more contact with equine recipient hosts [79]. One proposed habitat solution to this problem has been to restore native winter nectar habitat patches to draw flying foxes out of urban areas, away from horses and people and towards their preferred resource [79].
(d). Targeting susceptibility and infection in spillover hosts
Conventional biomedical approaches remain an important tool in managing spillover and may be synergistic with ecological interventions applied at processes that are upstream in the spillover chain. Treating human or livestock cases, treating co-infections (to reduce susceptibility) and vaccinating recipient hosts are classic examples and remain necessary tools to preserve public health. But, particularly where treatments or vaccines are not available or not affordable, such as for understudied pathogens and in resource-poor settings, taking advantage of synergies with ecological approaches along the spillover hierarchy may be beneficial.
3. Modelling and measuring disease systems to find potential interventions to reduce spillover
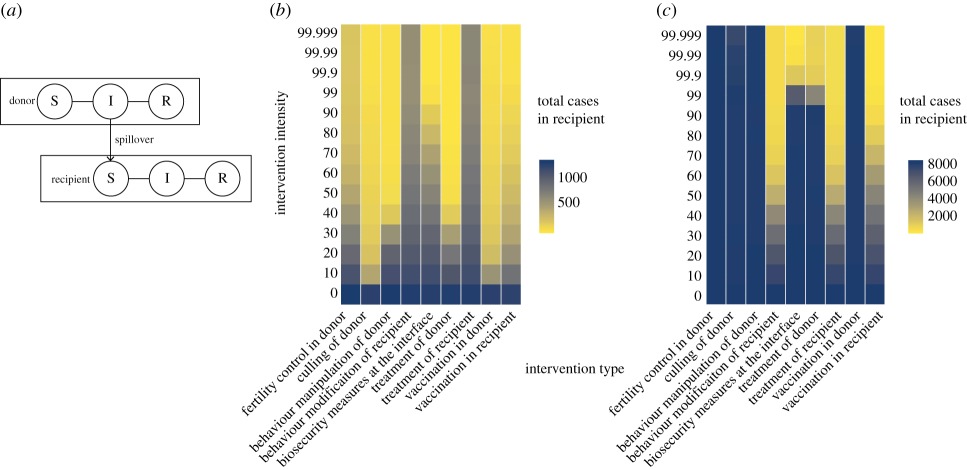
Modelling a system to explore sensitivity to various interventions (as well as costs and benefits) can help determine which interventions are most important, which ones are not viable and which ones require more monitoring data for better decisions. Even simple model systems can demonstrate nonlinearities in outcomes that make straightforward comparisons of interventions difficult (box 1). Sometimes thresholds emerge that can be advantageous for control (e.g. cost-effective interventions that disproportionately reduce spillover in the recipient host with relatively little effort). Other times, nonlinearities introduce challenges when an intervention results in a large reduction in a particular parameter (or set of parameters) but may have little effect on spillover rates to recipient populations if a particular disease-transmission threshold is not surpassed. For example, box 1c shows a threshold effect in a simple simulation of a hypothetical spillover disease system (parameterized to resemble bat–human spillover of viruses with high human-to-human transmission, like Ebola): treatment of the donor (bats) almost eliminates disease in the recipient when the coverage is greater than 99%, but has little to no effect at lower intervention intensities. Our toy model also illustrates that understanding the dynamics in both host species is critical because some interventions could inadvertently increase spillover rates to recipient hosts (i.e. negative ecological feedback). For example, in box 1c, representing a disease with high human-to-human onward transmission after spillover (like Ebola), behaviour modification of the recipient (humans) to reduce contact with other sick people and rapid treatment of human cases were the most two most sensitive interventions in reducing total human disease; however, these measures also resulted in an increase in the total number of spillover transmissions (even while reducing the total number of human cases; see electronic supplementary material) because of a consequent build-up of susceptible people in the system. A simulation approach like the one we present here can enable visualization of complex outcomes, including unintended consequences, which could be useful for designing formal tests of ecological interventions for managing spillover. The exercise we present here is intended to be illustrative, not prescriptive, because tailoring models of this sort to specific systems in order to guide real management decisions would require a better parameterization effort, including deep understanding of the ecological dynamics of donor and recipient hosts and the potential for density-dependent processes (e.g. the possibility of compensatory population growth in response to culling activities); see, for example, [80] in this issue.
Box 1. A simple model system simulating stochastic Susceptible–Infectious–Recovered disease dynamics, involving transmission among donor (reservoir) and recipient (focal) hosts, coupled by spillover. (a) Model schematic (for details, see electronic supplementary material). (b,c) Heat maps simulating cumulative cases in the recipient, given a set of interventions applied to varying degrees (ecological and conventional interventions targeting different model parameters). An ‘intervention intensity’ of 0 represents the base case scenario, with no intervention, and all other intervention intensities can be compared to the base case in each column. This exercise demonstrates the nonlinearities that emerge when comparing potential interventions in a relatively simple, but qualitatively flexible, spillover system. This model is flexible enough to qualitatively represent several types of spillover diseases, including those where onward transmission from human to human is limited (as in b), like (but not parameterized exactly as) Nipah virus, and those where onward transmission is high (as in c), like (but not parameterized exactly as) Ebola virus. See electronic supplementary material for more details on model structure, parameterization and results of the simulations.
4. Economic, social and political considerations can determine success or failure in managing spillover
How can we integrate ecology, public health, stakeholder perspectives and economics into the recommendations for managing ecological interventions to reduce spillover risk? It is not straightforward for a manager to decide which intervention to invest in, and whether it should be ecological, conventional, or both. In general, successful implementation of an ecological intervention requires: knowledge (we must be aware of and understand the intervention), means (both financial and logistical), mandate (jurisdiction) and motivation (benefits outweigh costs and those incurring costs also realize the value of the benefits) [81]. When the benefits of an action (e.g. reduced spillover) do not align with where (and by whom) the costs are incurred (e.g. one particular sector), social and political attention to aligning or subsidizing those costs and benefits across sectors may be necessary, and this is difficult.
In particular, ecological interventions that target habitats and natural populations are likely to fall under the jurisdiction of government agencies that have mandates other than human or livestock health. So, wildlife and land management agencies may have the means and mandate, but do not necessarily have the motivation. Not all ecological interventions will be win–win for all interested parties. In some cases, reducing wildlife densities or manipulating habitats to improve human or livestock health may not be a priority for hunter or conservationist communities. In this case, more collaboration among sectors and the sharing of costs and benefits will be essential, and yet difficult to implement.
Just as for new biomedical tools (e.g. drugs or vaccines), new potential ecological interventions should not be rolled out wholesale, everywhere, until their safety and effectiveness have been evaluated. Or, if this is not possible owing to the urgency of a situation, interventions could be implemented in an adaptive management framework, with attention to monitoring both the effectiveness of the intervention and comparable controls, wherever possible [82].
Conversely, sometimes potentially effective tools still fail because of social, economic or political constraints. For example, decreasing wolf hunts and removals has not been implemented as an intervention to reduce brucellosis, owing to the potential predation risk to livestock as well as the interests of some in the hunting community to maintain large populations of elk. Also, the anti-vaccination movement highlights how even conventional interventions like vaccination, although relatively safe and effective, are not without controversy.
5. Conclusion
Spillover involves cross-species pathogen transmission across a highly complex landscape of ecological processes, which calls for ecological solutions. In this piece, we introduce the notion of an ecological intervention as a potentially underused approach to find effective, long-lasting and creative solutions to reduce spillover, with minimal environmental damage. Moreover, ecological interventions can be complementary, not antagonistic, to conventional approaches, which often target different barriers in the spillover process. However, conventional interventions such as culling and medical treatment are often reactive, short-lived, and can introduce further complications: culling can sometimes inadvertently enhance disease transmission, drugs can enhance virulence and/or alter resistance, and for many spillover diseases, vaccines and effective treatments are not yet available. In these cases, managing upstream risks using ecological interventions may be the best option. Ecological interventions, like many conventional ones, are not without their caveats and controversies. Social, political and economic considerations can limit broad changes to ecosystems that are sometimes needed to implement ecological interventions. Here, we have explored some of the next steps towards identifying and implementing effective interventions to manage or reduce spillover. Examples of ecological interventions provided here target reservoir hosts (i.e. preventing wildlife–livestock contact), the environment (i.e. ecosystem management) and the whole spectrum of the interface between ecological reservoirs and people (or other focal hosts like livestock). Finally, we demonstrate a simple modelling framework for visualizing the complex and nonlinear effects of various interventions for simple disease spillover systems. By better understanding and harnessing our understanding of complex ecological systems, ecological interventions might offer new ways to design cost-effective, socially acceptable, sustainable interventions that can reduce spillover risk.
Supplementary Material
Acknowledgements
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government. This software has been approved for release by the US Geological Survey (USGS). Although the software has been subjected to rigorous review, the USGS reserves the right to update the software as needed pursuant to further analysis and review. No warranty, expressed or implied, is made by the USGS or the US Government as to the functionality of the software and related material nor shall the fact of release constitute any such warranty. Furthermore, the software is released on condition that neither the USGS nor the US Government shall be held liable for any damages resulting from its authorized or unauthorized use. We thank Alex Washburne for useful discussions on the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
S.H.S. and H.M. conceived of the manuscript. S.H.S., K.M.P., J.R.C.P. and G.A.D.L. developed, coded and analysed the model results. S.H.S., N.N., K.M.P., A.J.P., K.M., P.C.C., D.J.B., R.K.P., J.R.C.P., H.M. and G.A.D.L. wrote the manuscript. N.N. produced the artwork.
Competing interests
We declare we have no competing interests.
Funding
G.A.D.L. and S.H.S. were supported by a grant from the Bill and Melinda Gates Foundation no. OPP1114050, a 2018 Environmental Venture Program grant from the Woods Institute for the Environment (no. 1226707-10-WTABP), a GDP SEED grant from the Freeman Spogli Institute at Stanford University, a National Institutes of Health grant no. R01TW010286 and the National Science Foundation grant no. 1414102. N.N. was funded by The Bing Fellowship in Honor of Paul Ehrlich. J.R.C.P. is supported by SACEMA, which is a DST-NRF Centre of Excellence in South Africa. K.M.P. was supported by the United States Department of Agriculture, Animal and Plant Health Inspection Services. H.M., A.J.P. and R.K.P. were funded by the US National Science Foundation (grant no. DEB-1716698) and the DARPA PREEMPT program Cooperative Agreement no. D18AC00031. R.K.P. was also funded by DARPA D16AP00113, US National Institute of General Medical Sciences of the US National Institutes of Health (grant nos P20GM103474 and P30GM110732) and the USDA National Institute of Food and Agriculture (grant no. Hatch project 1015891). A.J.P. was also supported by a Queensland Government Accelerate Postdoctoral Research Fellowship.
References
- 1.Borremans B, Faust C, Manlove KR, Sokolow SH, Lloyd-Smith JO. 2019. Cross-species pathogen spillover across ecosystem boundaries: mechanisms and theory. Phil. Trans. R. Soc. B 374, 20180344 ( 10.1098/rstb.2018.0344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharp PM, Hahn BH. 2010. The evolution of HIV-1 and the origin of AIDS. Phil. Trans. R. Soc. B 365, 2487–2494. ( 10.1098/rstb.2010.0031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotterill GG, Cross PC, Cole EK, Fuda RK, Rogerson JD, Scurlock BM, Du Toit JT. 2018. Winter feeding of elk in the Greater Yellowstone Ecosystem and its effects on disease dynamics. Phil. Trans. R. Soc. B 373, 20170093 ( 10.1098/rstb.2017.0093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braczkowski AR, O'Bryan CJ, Stringer MJ, Watson JEM, Possingham HP, Beyer HL. 2018. Leopards provide public health benefits in Mumbai, India. Front. Ecol. Environ. 16, 176–182. ( 10.1002/fee.1776) [DOI] [Google Scholar]
- 5.Wild MA, Hobbs NT, Graham MS, Miller MW. 2011. The role of predation in disease control: a comparison of selective and nonselective removal on prion disease dynamics in deer. J. Wildl. Dis. 47, 78–93. ( 10.7589/0090-3558-47.1.78) [DOI] [PubMed] [Google Scholar]
- 6.Ostfeld RS, Keesing F. 2000. Biodiversity and disease risk: the case of Lyme disease. Conserv. Biol. 14, 722–728. ( 10.1046/j.1523-1739.2000.99014.x) [DOI] [Google Scholar]
- 7.Civitello DJ, et al. 2015. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl Acad. Sci. USA 112, 8667–8671. ( 10.1073/pnas.1506279112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood CL, Lafferty KD, Deleo G, Young HS, Hudson PJ, Kuris AM. 2014. Does biodiversity protect humans against infectious disease? Ecology 95, 817–832. ( 10.1890/13-1041.1) [DOI] [PubMed] [Google Scholar]
- 9.Randolph SE, Dobson ADM. 2012. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology 139, 847–863. ( 10.1017/S0031182012000200) [DOI] [PubMed] [Google Scholar]
- 10.Pinsent A Pepin KM, Zhu H, Guan Y, White MT, Riley S. 2017. The persistence of multiple strains of avian influenza in live bird markets. Proc. R. Soc. B 284, 20170715 ( 10.1098/rspb.2017.0715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessler MK, et al. 2018. Changing resource landscapes and spillover of henipaviruses. Ann. NY Acad. Sci., 1429, 78–99. ( 10.1111/nyas.13910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulliam JR, et al. 2012. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J. R. Soc. Interface 9, 89–101. ( 10.1098/rsif.2011.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukhari T, Takken W, Koenraadt CJM. 2013. Biological tools for control of larval stages of malaria vectors – a review. Biocontrol Sci. Technol. 23, 987–1023. ( 10.1080/09583157.2013.810706) [DOI] [Google Scholar]
- 14.Huancahuari M, Pensati F, Rongoni A, Carrieri M, Bellini R. 2016. The introduction of Gambusia holbrooki in rice field for mosquito control can positively affect rice production. Bull. Insectol. 69, 131–141. [Google Scholar]
- 15.Windbichler N, et al. 2011. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature 473, 212–215. ( 10.1038/nature09937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz-Pedreros A, Gil C, Yáñez J, Rau JR. 2010. Raptor habitat management and its implication on the biological control of the Hantavirus. Eur. J. Wildl. Res. 56, 703–715. ( 10.1007/s10344-010-0364-2) [DOI] [Google Scholar]
- 17.Kaaya GP. 2000. Laboratory and field evaluation of entomogenous fungi for tick control. Ann. N Y Acad. Sci. 916, 559–564. ( 10.1111/j.1749-6632.2000.tb05336.x) [DOI] [PubMed] [Google Scholar]
- 18.Nahar N, Mondal UK, Hossain MJ, Khan MSU, Sultana R, Gurley ES, Luby SP. 2014. Piloting the promotion of bamboo skirt barriers to prevent Nipah virus transmission through date palm sap in Bangladesh. Global Health Promot. 21, 7–15. ( 10.1177/1757975914528249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan SU, Gurley ES, Hossain MJ, Nahar N, Sharker MAY, Luby SP. 2012. A randomized controlled trial of interventions to impede date palm sap contamination by bats to prevent Nipah virus transmission in Bangladesh. PLoS ONE 7, e42689 ( 10.1371/journal.pone.0042689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kung N, Mclaughlin A, Taylor M, Moloney B, Wright T, Field H. 2013. Hendra virus and horse owners—risk perception and management. PLoS ONE 8, e80897 ( 10.1371/journal.pone.0080897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandt LJ, Reddy SS. 2011. Fecal microbiota transplantation for recurrent Clostridium difficile infection. J. Clin. Gastroenterol. 45, S159–S167. ( 10.1097/MCG.0b013e318222e603) [DOI] [PubMed] [Google Scholar]
- 22.Plowright RK, Parrish CR, Mccallum H, Hudson PJ, Ko AI, Graham AL, Lloyd-Smith JO. 2017. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 15, 502–510. ( 10.1038/nrmicro.2017.45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi S, Metcalf CJE, Ferrari MJ, Moss WJ, Truelove SA, Tatem AJ, Grenfell BT, Lessler J. 2015. Reduced vaccination and the risk of measles and other childhood infections post-Ebola. Science 347, 1240–1242. ( 10.1126/science.aaa3438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luikart G, Pilgrim K, Visty J, Ezenwa VO, Schwartz MK. 2008. Candidate gene microsatellite variation is associated with parasitism in wild bighorn sheep. Biol. Lett. 4, 228–231. ( 10.1098/rsbl.2007.0633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streicker DG, et al. 2012. Ecological and anthropogenic drivers of rabies exposure in vampire bats: implications for transmission and control. Proc. R. Soc. B 279, 3384–3392. ( 10.1098/rspb.2012.0538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bielby J, Donnelly CA, Pope LC, Burke T, Woodroffe R. 2014. Badger responses to small-scale culling may compromise targeted control of bovine tuberculosis. Proc. Natl Acad. Sci. USA 111, 9193–9198. ( 10.1073/pnas.1401503111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolzoni L, De Leo GA. 2013. Unexpected consequences of culling on the eradication of wildlife diseases: the role of virulence evolution. Am. Nat. 181, 301–313. ( 10.1086/669154) [DOI] [PubMed] [Google Scholar]
- 28.Chua KB, et al. 2000. Nipah virus: a recently emergent deadly paramyxovirus. Science 288, 1432–1435. ( 10.1126/science.288.5470.1432) [DOI] [PubMed] [Google Scholar]
- 29.Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife–threats to biodiversity and human health. Science 287, 443–449. ( 10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 30.Belotto A, Leanes LF, Schneider MC, Tamayo H, Correa E. 2005. Overview of rabies in the Americas. Virus Res. 111, 5–12. ( 10.1016/j.virusres.2005.03.006) [DOI] [PubMed] [Google Scholar]
- 31.Schneider MC, Belotto A, Adé MP, Hendrickx S, Leanes LF, Rodrigues MJ, Medina G, Correa E. 2007. Current status of human rabies transmitted by dogs in Latin America. Cad. Saude Publica 23, 2049–2063. ( 10.1590/S0102-311X2007000900013) [DOI] [PubMed] [Google Scholar]
- 32.Condori-Condori RE, Streicker DG, Cabezas-Sanchez C, Velasco-Villa A. 2013. Enzootic and epizootic rabies associated with vampire bats, Peru. Emerg. Infect. Dis. 19, 1463–1469. ( 10.3201/eid1909.130083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson N, Arechiga-Ceballos N, Aguilar-Setien A. 2014. Vampire bat rabies: ecology, epidemiology and control. Viruses 6, 1911–1928. ( 10.3390/v6051911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Shea TJ, Cryan PM, Hayman DTS, Plowright RK, Streicker DG. 2016. Multiple mortality events in bats: a global review. Mamm. Rev. 46, 175–190. ( 10.1111/mam.12064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linhart SB, Flores Crespo R, Mitchell GC. 1972. Control of vampire bats by means of an anticoagulant. Bol Oficina Sanit Panam 73, 100–109. [PubMed] [Google Scholar]
- 36.Blackwood JC, Streicker DG, Altizer S, Rohani P. 2013. Resolving the roles of immunity, pathogenesis, and immigration for rabies persistence in vampire bats. Proc. Natl Acad. Sci. USA 110, 20 837–20 842. ( 10.1073/pnas.1308817110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choisy M, Rohani P. 2006. Harvesting can increase severity of wildlife disease epidemics. Proc. R. Soc. B 273, 2025–2034. ( 10.1098/rspb.2006.3554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehinger MR Cross P, Wallen R, White PJ, Treanor J. 2011. Simulating sterilization, vaccination, and test-and-remove as brucellosis control measures in bison. Ecol. Appl. 21, 2944–2959. ( 10.1890/10-2239.1) [DOI] [Google Scholar]
- 39.Fisher CR, Streicker DG, Schnell MJ. 2018. The spread and evolution of rabies virus: conquering new frontiers. Nat. Rev. Microbiol. 16, 241–255. ( 10.1038/nrmicro.2018.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cross ML, Buddle BM, Aldwell FE. 2007. The potential of oral vaccines for disease control in wildlife species. Vet. J. 174, 472–480. ( 10.1016/j.tvjl.2006.10.005) [DOI] [PubMed] [Google Scholar]
- 41.Gortazar C, Diez-Delgado I, Barasona JA, Vicente J, De La Fuente J, Boadella M. 2014. The wild side of disease control at the wildlife–livestock–human interface: a review. Front. Vet. Sci. 1, 27 ( 10.3389/fvets.2014.00027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manyweathers J, Field H, Longnecker N, Agho K, Smith C, Taylor M. 2017. Why won't they just vaccinate?’ Horse owner risk perception and uptake of the Hendra virus vaccine. BMC Vet. Res. 13, 103 ( 10.1186/s12917-017-1006-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Domenech J, Dauphin G, Rushton J, Mcgrane J, Lubroth J, Tripodi A, Gilbert J, Sims L. 2009. Experiences with vaccination in countries endemically infected with highly pathogenic avian influenza: the Food and Agriculture Organization perspective. Rev. Sci. Tech. 28, 293–305. ( 10.20506/rst.28.1.1865) [DOI] [PubMed] [Google Scholar]
- 44.Packer C, Holt RD, Hudson PJ, Lafferty KD, Dobson AP. 2003. Keeping the herds healthy and alert: implications of predator control for infectious disease. Ecol. Lett. 6, 797–802. ( 10.1046/j.1461-0248.2003.00500.x) [DOI] [Google Scholar]
- 45.Anon. 2017. Revisiting brucellosis in the greater Yellowstone area, in the national academies of sciences, engineering, and medicine. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 46.Cross PC, Cole EK, Dobson AP, Edwards WH, Hamlin KL, Luikart G, Middleton AD, Scurlock BM, White PJ. 2010. Probable causes of increasing brucellosis in free-ranging elk of the Greater Yellowstone Ecosystem. Ecol. Appl. 20, 278–288. ( 10.1890/08-2062.1) [DOI] [PubMed] [Google Scholar]
- 47.Cross PC, Edwards WH, Scurlock BM, Maichak EJ, Rogerson JD, 2007. Effects of management and climate on elk brucellosis in the Greater Yellowstone Ecosystem. Ecol. Appl. 17, 957–964. ( 10.1890/06-1603) [DOI] [PubMed] [Google Scholar]
- 48.Altizer S, et al. 2018. Food for contagion: synthesis and future directions for studying host–parasite responses to resource shifts in anthropogenic environments. Phil. Trans. R. Soc. B 373, 20170102 ( 10.1098/rstb.2017.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becker DJ, Streicker DG, Altizer S. 2015. Linking anthropogenic resources to wildlife–pathogen dynamics: a review and meta-analysis. Ecol. Lett. 18, 483–495. ( 10.1111/ele.12428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haydon DT, Cleaveland S, Taylor LH, Laurenson MK. 2002. Identifying reservoirs of infection: a conceptual and practical challenge. Emerg. Infect. Dis. 8, 1468–1473. ( 10.3201/eid0812.010317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zulu GC, Sabeta CT, Nel LH. 2009. Molecular epidemiology of rabies: focus on domestic dogs (Canis familiaris) and black-backed jackals (Canis mesomelas) from northern South Africa. Virus Res. 140, 71–78. ( 10.1016/j.virusres.2008.11.004) [DOI] [PubMed] [Google Scholar]
- 52.Biek R, Walsh PD, Leroy EM, Real LA. 2006. Recent common ancestry of Ebola Zaire virus found in a bat reservoir. PLoS Pathog. 2, e90 ( 10.1371/journal.ppat.0020090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viana M, Mancy R, Biek R, Cleaveland S, Cross PC, Lloyd-Smith JO, Haydon DT. 2014. Assembling evidence for identifying reservoirs of infection. Trends Ecol. Evol. 29, 270–279. ( 10.1016/j.tree.2014.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pepin KM, Lloyd-Smith JO, Webb CT, Holcomb K, Zhu H, Guan Y, Riley S. 2013. Minimizing the threat of pandemic emergence from avian influenza in poultry systems. BMC Infect. Dis. 13, 592 ( 10.1186/1471-2334-13-592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin G Plowright R, Kault D, Skerratt LF, Martin G, Selleck P, 2015. Hendra virus survival does not explain spillover patterns and implicates relatively direct transmission routes from flying foxes to horses. J. Gen. Virol. 96, 1229–1237. ( 10.1099/vir.0.000073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas MB, Read AF. 2016. The threat (or not) of insecticide resistance for malaria control. Proc. Natl Acad. Sci. USA 113, 8900–8902. ( 10.1073/pnas.1609889113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghosh SK, Dash AP. 2007. Larvivorous fish against malaria vectors: a new outlook. Trans. R. Soc. Trop. Med. Hyg. 101, 1063–1064. ( 10.1016/j.trstmh.2007.07.008) [DOI] [PubMed] [Google Scholar]
- 58.Walshe DP, Garner P, Adeel AA, Pyke GH, Burkot TR. 2017. Larvivorous fish for preventing malaria transmission. Cochrane Database Syst. Rev. 12, CD008090 ( 10.1002/14651858.CD008090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shultz S, et al. 2004. Diclofenac poisoning is widespread in declining vulture populations across the Indian subcontinent. Proc. R. Soc. B 271(Suppl. 6), S458–S460. ( 10.1098/rsbl.2004.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogada DL, Torchin ME, Kinnaird MF, Ezenwa VO. 2012. Effects of vulture declines on facultative scavengers and potential implications for mammalian disease transmission. Conserv. Biol. 26, 453–460. ( 10.1111/j.1523-1739.2012.01827.x) [DOI] [PubMed] [Google Scholar]
- 61.Ogada DL, Keesing F, Virani MZ. 2012. Dropping dead: causes and consequences of vulture population declines worldwide. Ann. NY Acad. Sci. 1249, 57–71. ( 10.1111/j.1749-6632.2011.06293.x) [DOI] [PubMed] [Google Scholar]
- 62.Markandya A, Taylor T, Longo A, Murty MN, Murty S, Dhavala K. 2009. Counting the cost of vulture decline—an appraisal of the human health and other benefits of vultures in India. Ecol. Econ. 67, 194–204. ( 10.1016/j.ecolecon.2008.04.020) [DOI] [Google Scholar]
- 63.Faust CL, McCallum HI, Bloomfield LSP, Gottdenker NL, Gillespie TR, Torney CJ, Dobson AP, Plowright RK. 2018. Pathogen spillover during land conversion. Ecol. Lett. 21, 471–483. ( 10.1111/ele.12904) [DOI] [PubMed] [Google Scholar]
- 64.Shriner SA, et al. 2016. Surveillance for highly pathogenic H5 avian influenza virus in synanthropic wildlife associated with poultry farms during an acute outbreak. Sci. Rep. 6, 36237 ( 10.1038/srep36237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L, et al. 2018. Genetic evidence supports sporadic and independent introductions of subtype H5 low-pathogenic avian influenza A viruses from wild birds to domestic poultry in North America. J. Virol. 92, e00913-18 ( 10.1128/JVI.00913-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luby SP, et al. 2006. Foodborne transmission of Nipah virus, Bangladesh. Emerg. Infect. Dis. 12, 1888–1894. ( 10.3201/eid1212.060732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rahman MA, et al. 2012. Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector Borne Zoonotic Dis. 12, 65–72. ( 10.1089/vbz.2011.0656) [DOI] [PubMed] [Google Scholar]
- 68.Khan MS, Hossain J, Gurley ES, Nahar N, Sultana R, Luby SP. 2010. Use of infrared camera to understand bats' access to date palm sap: implications for preventing Nipah virus transmission. Ecohealth 7, 517–525. ( 10.1007/s10393-010-0366-2) [DOI] [PubMed] [Google Scholar]
- 69.Nahar N, Sultana R, Gurley ES, Hossain MJ, Luby SP. 2010. Date palm sap collection: exploring opportunities to prevent Nipah transmission. Ecohealth 7, 196–203. ( 10.1007/s10393-010-0320-3) [DOI] [PubMed] [Google Scholar]
- 70.Streicker DG, Allgeier JE. 2016. Foraging choices of vampire bats in diverse landscapes: potential implications for land-use change and disease transmission. J. Appl. Ecol. 53, 1280–1288. ( 10.1111/1365-2664.12690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stoner-Duncan B, Streicker DG, Tedeschi CM. 2014. Vampire bats and rabies: toward an ecological solution to a public health problem. PLoS Negl. Trop. Dis. 8, e2867 ( 10.1371/journal.pntd.0002867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Becker DJ, et al. 2018. Livestock abundance predicts vampire bat demography, immune profiles and bacterial infection risk. Phil. Trans. R. Soc. B 373, 20170089 ( 10.1098/rstb.2017.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sota T, Mogi M. 1989. Effectiveness of zooprophylaxis in malaria control: a theoretical inquiry, with a model for mosquito populations with two bloodmeal hosts. Med. Vet. Entomol. 3, 337–345. ( 10.1111/j.1365-2915.1989.tb00240.x) [DOI] [PubMed] [Google Scholar]
- 74.Voigt CC, Kelm DH. 2006. Host preference of the common vampire bat (Desmodus rotundusera) assessed by stable isotopes. J. Mammalogy 87, 1–6. ( 10.1644/05-MAMM-F-276R1.1) [DOI] [Google Scholar]
- 75.Lopez A, Percy Miranda P, Edgar Tejada V, Fishbein DB, Fishbein DB. 1992. Outbreak of human rabies in the Peruvian jungle. Lancet 339, 408–411. ( 10.1016/0140-6736(92)90088-K) [DOI] [PubMed] [Google Scholar]
- 76.McCarthy TJ. 1989. Human depredation by vampire bats (Desmodus rotundus) following a hog cholera campaign. Am. J. Trop. Med. Hyg. 40, 320–322. ( 10.4269/ajtmh.1989.40.320) [DOI] [PubMed] [Google Scholar]
- 77.Plowright RK, Field HE, Smith C, Divljan A, Palmer C, Tabor G, Daszak P, Foley JE. 2008. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proc. R. Soc. B 275, 861–869. ( 10.1098/rspb.2007.1260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paez DJ, Restif O, Eby P, Plowright RK. 2018. Optimal foraging in seasonal environments: implications for residency of Australian flying foxes in food-subsidized urban landscapes. Phil. Trans. R. Soc. B 373, 20170097 ( 10.1098/rstb.2017.0097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plowright RK, Peel AJ, Streicker DG, Gilbert AT, Mccallum H, Wood J, Baker ML, Restif O. 2016. Transmission or within-host dynamics driving pulses of zoonotic viruses in reservoir-host populations. PLoS Negl. Trop. Dis. 10, e0004796 ( 10.1371/journal.pntd.0004796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manlove KR, Sampson LM, Borremans B, Cassirer EF, Miller RS, Pepin KM, Besser TE, Cross PC. 2019. Epidemic growth rates and host movement patterns shape management performance for pathogen spillover at the wildlife–livestock interface. Phil. Trans. R. Soc. B 374, 20180343 ( 10.1098/rstb.2018.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castillo O, et al. 2006. Casting for conservation actors: people partnerships and wildlife. New York, NY: Wildlife Conservation Society. [Google Scholar]
- 82.Shea K, Tildesley MJ, Runge MC, Fonnesbeck CJ, Ferrari MJ. 2014. Adaptive management and the value of information: learning via intervention in epidemiology. PLoS Biol. 12, e1001970 ( 10.1371/journal.pbio.1001970) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.