Abstract
Early detection of zoonotic diseases allows for the implementation of early response measures, reducing loss of human life and economic disruption. We implemented a surveillance system in hospitals in Bangladesh to screen acutely ill hospitalized patients with severe respiratory infection and meningoencephalitis for zoonotic exposures. Patients were screened for the risk of zoonotic exposures with five questions covering vocational exposures, sick domestic animal and wild animal contact, and date palm sap consumption in the three weeks preceding illness onset. Patients giving at least one positive response were considered a potential zoonotic exposure. From September 2013 to March 2017, a total of 11 429 hospitalized patients across 14 participating hospitals were screened for exposures. Overall, 2% of patients reported a potential zoonotic exposure in the three-week period prior to becoming ill. Sixteen per cent of hospitalized patients with reported exposures died. After routine surveillance diagnostic testing, 88% of patients admitted to the hospital after a potential zoonotic exposure did not have a laboratory diagnosed aetiology for their illness. Hospital-based surveillance systems such as the Bangladeshi example presented here could play an important future role in the early detection of zoonotic spillover diseases.
This article is part of the theme issue ‘Dynamic and integrative approaches to understanding pathogen spillover’.
Keywords: surveillance, emerging zoonotic viruses, humans, hospital-based
1. Introduction
Emerging and reemerging zoonotic infectious diseases represent a potential devastating threat to human health. Caused by pathogens originating in animals and spilling into human populations, most emerging human diseases are zoonotic and most originate in wildlife [1]. Spillover of zoonotic pathogens followed by person-to-person transmission in cases of severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) [2,3], Nipah [4,5], Lassa [6] and Ebola have caused loss of life, societal disruption and widespread economic losses [7,8].
The early detection of zoonotic diseases through surveillance allows for the implementation of early response measures, reducing loss of human life and economic disruption [9]. A One Health approach to surveillance for zoonotic pathogens involves the implementation and integration of both animal and human surveillance platforms [10]. While recent work has successfully focused on developing techniques for wildlife surveillance [11,12], additional work is needed to develop human surveillance platforms [13]. Ideally, a human-focused platform would allow for the detection of zoonotic exposures, identify cases of spillover disease and, for patients who go undiagnosed, prioritize samples for pathogen discovery.
Bangladesh is a high-risk location for zoonotic spillover, occupying a tropical latitude and with a high diversity of wildlife [14], dense human [15] and domestic animal [16] populations, and a high level of connectivity between people, domestic animals and wildlife. Western Bangladesh is a known site of frequent spillover events with outbreaks of Nipah virus (NiV), a zoonotic paramyxovirus harboured by Pteropus fruit bats, identified almost yearly since 2001 [17,18]. With over a 70% mortality and known human-to-human transmission, NiV has pandemic potential [19]. Identified risk factors for human infection have been the consumption of raw date palm sap [20,21], a delicacy that likely becomes contaminated by bat faeces, urine and saliva during sap collection [22], and contact with another human cases generally through caregiving [5].
In this article, we present our experience in Bangladesh enhancing pre-existing surveillance platforms to include human-focused, hospital-based zoonotic exposure surveillance. We describe the design of the system, present the basic epidemiologic and routine diagnostic data collected from September 2013 to March 2017 and review some of the difficulties in design and implementation.
2. Methods
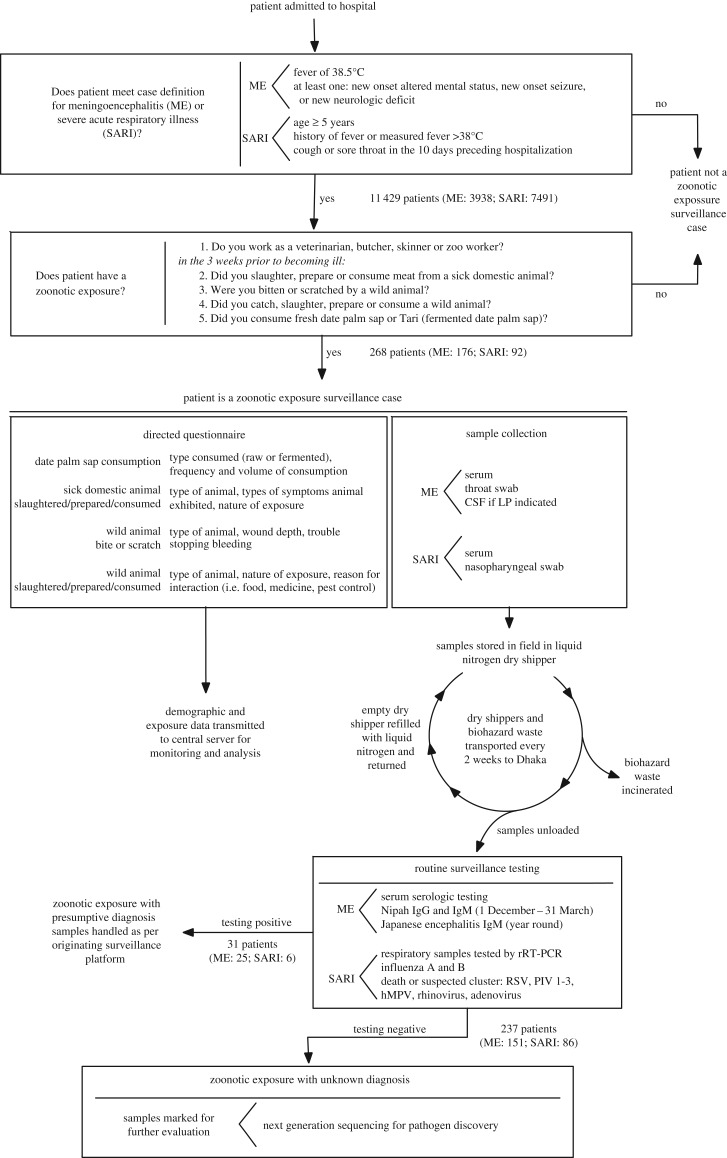
The zoonotic exposures surveillance system (figure 1) implemented in hospitals in Bangladesh screens acutely ill hospitalized patients presenting with severe acute respiratory infection or meningoencephalitis for potential zoonotic exposures.
Figure 1.
Design of the zoonotic exposure surveillance system in hospitalized patients. ME (meningoencephalitis) and SARI refer to the pre-existing surveillance platforms on which the zoonotic exposure surveillance system is built.
(a). Initial screening for meningoencephalitis and severe acute respiratory illnesses
The zoonotic exposures surveillance platform is layered on two existing surveillance systems maintained by International Centre for Diarrheal Disease Research, Bangladesh (icddr,b) and The Institute of Epidemiology, Disease Control and Research (IEDCR): meningoencephalitis surveillance targeting patients with neurologic presentations [18] and hospital-based respiratory illness surveillance [23].
Surveillance was conducted at a total of 14 hospital sites, with one hospital site (Rajshahi) participating in both meningoencephalitis and respiratory illness surveillance. The meningoencephalitis surveillance platform operates in three governmental tertiary care hospitals and defines meningoencephalitis cases as hospitalized patients with a history of self-reported fever or axillary temperature greater than 38.5° C combined with at least one of the following: new-onset altered mental status, new-onset seizures or new neurological deficit [18]. Respiratory illness surveillance operates in 12 districts (six governmental and six private tertiary care hospitals, representing all administrative divisions of the country) and defines severe acute respiratory illnesses (SARI) as patients aged 5 years and above with a history of fever or measured temperature of 38°C or above and cough or sore throat in the 10 days preceding hospital admission [23]. Both meningoencephalitis and respiratory illness surveillance systems collect basic demographic data and mortality data for patients meeting the case definitions and consenting to enrolment.
(b). Screening for potential zoonotic exposures
All patients meeting the case definition for meningoencephalitis or SARI were screened for the risk of zoonotic exposures with five brief questions. Patients giving at least one positive response were considered to have an animal exposure that was potentially infectious. The five screening questions addressed (i) vocational exposures and, in the three weeks prior to the onset of illness, any of the following: (ii) preparation or consumption of meat from a sick domestic animal; (iii) a history of being bitten or scratched by a wild animal; (iv) exposure to or consumption of meat from a wild animal; and (v) consumption of date palm sap. In cases of altered mental status, questions regarding the altered patient's behaviours and exposures were answered by accompanying family members.
If a case-patient reported exposure to a sick domestic animal, they were queried about the type of animal, symptoms that the animal exhibited and the extent of exposure. Patients reporting catching, killing, preparing or consuming wild animals were asked about the animal type and the nature of their exposure. Patients reporting a bite or wound from a wild animal were asked to report the type of animal encountered, the approximate wound depth and if they had trouble stopping the bleeding from the wound. Patients consuming date palm sap were asked to characterize the volume and frequency of their drinking habits and if date palm sap consumed was raw or fermented. Questionnaire data were collected on tablets and synchronized to a secure central server.
(c). Sample collection, transport and laboratory testing
In all patients meeting criteria for an animal exposure that was potentially infectious, surveillance staff arranged specimen collection with the help of hospital staff. Blood samples (approx. 10 ml) and throat or nasal swabs collected in viral transport medium were routinely obtained in all patients with zoonotic exposures. Serum was separated by centrifugation on site and divided into aliquots. In cases where the treating hospital doctor felt a lumbar puncture (LP) indicated, the procedure was performed per hospital protocol and an additional aliquot of cerebrospinal fluid (CSF, 1–3 ml) was collected.
Serum samples, respiratory specimens and CSF were stored in a dedicated liquid nitrogen dry shipper that was maintained at approximately −70° C. Dry shippers were returned to icddr,b in Dhaka every two weeks, where they were unloaded in a biosafety cabinet. The shippers were then refilled with liquid nitrogen and returned to the field. Each aliquot was checked for leakage and integrity before being transferred to a −80° C freezer.
Samples went through routine testing for either the meningoencephalitis or the respiratory disease surveillance platforms depending on the originating surveillance platform. Samples originating from the meningoencephalitis platform routinely undergo testing for NiV through detection of IgM and IgG by using IgM-capture and indirect IgG enzyme immunoassays [24,25] if the patient is hospitalized during the season when raw date palm sap is collected and consumed (1 December–31 March) and Japanese encephalitis using IgM-capture enzyme-linked immunosorbent assays [26] year-round.
Routine testing by real-time reverse transcription–polymerase chain reaction (rRT–PCR) [27] in the respiratory disease platform includes influenza A and B in all cases, and a respiratory viral panel including respiratory syncytial (RSV), parainfluenza virus (PIV) types 1–3, human metapneumovirus (hMPV), rhinovirus and adenovirus in cases involving a suspected respiratory disease cluster or death.
Samples that tested positive for a known pathogen through routine testing were not further evaluated as zoonotic exposure cases and were handled as per the protocol from the originating surveillance system. Samples that tested negative in routine surveillance testing were marked for pathogen discovery by next-generation sequencing [28]. This article does not cover results of sequencing and pathogen discovery, which will be reported in a future manuscript.
(d). Consent and ethics
Once identified as a potential zoonotic exposure but prior to answering the detailed questionnaire on their exposures and providing samples, adult patients (aged 18 or older) were consented for participation. Patients under the age of 18 years gave assent and their adult guardians also consented. If consent could not be obtained from the patient owing to altered mental status, consent was sought from the patient's medical decision maker. The zoonotic exposure screening questionnaires and sample collection protocols were reviewed and approved by the ethical review committee of icddr,b (PR-12093) as well as the Institutional Review Board of Stanford University (protocol 26513).
(e). Data analysis
We used descriptive statistics to characterize demographics, exposures, diagnostic laboratory results and case outcomes in patients meeting criteria for the zoonotic surveillance platform. We used bivariate logistic regression analysis both to evaluate associations between pathways of potential zoonotic pathogen exposure and demographics, presenting surveillance system and seasonality as well as factors associated with mortality in patients with reported zoonotic exposures.
3. Results
(a). Prevalence of animal exposures
From September 2013 to March 2017, surveillance hospital staff screened 3938 meningoencephalitis and 7491 respiratory illness patients for recent animal exposures that were potentially infectious (total of 11 429 hospitalized patients) across the 14 participating hospitals. Overall, 2% (268) of patients reported an animal exposure in the three-week period prior to becoming ill. The prevalence of patients reporting potential zoonotic exposures was higher in patients who met the case definition for meningoencephalitis at presentation than in patients meeting the case definition for respiratory disease (176 patients (4%) versus 92 patients (1%), OR 3.7, 95% CI 2.9–4.8).
(b). Demographics of patients reporting animal exposures
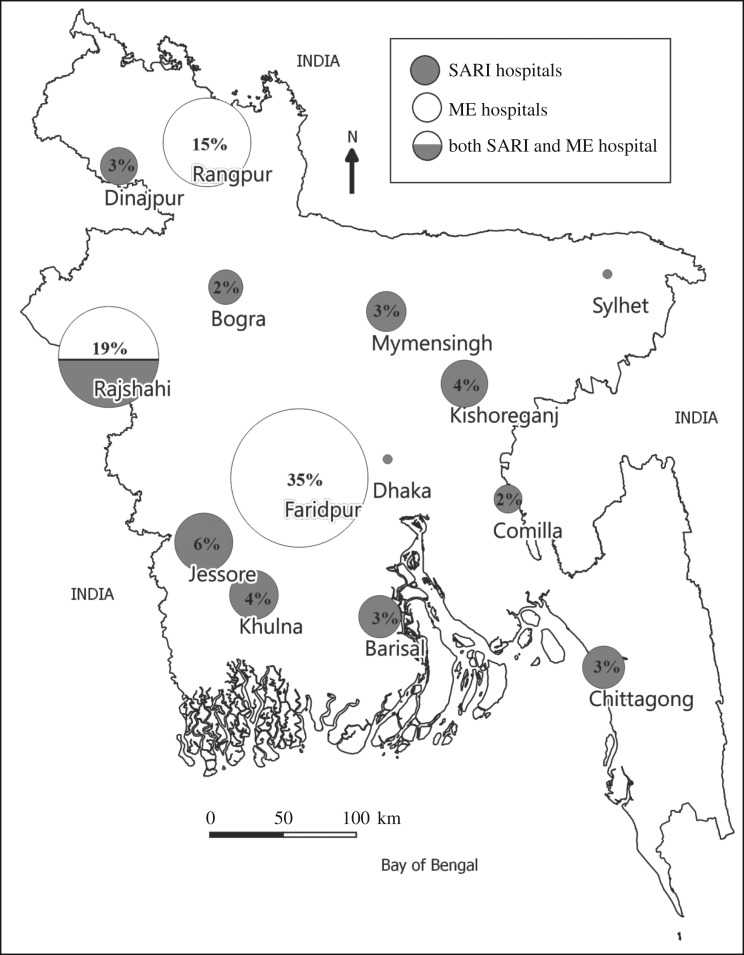
The age of patients with an animal exposure that was potentially infectious varied from 1 to 95 years, with a median age of 20 years. Patients were predominantly male (67%, 180/268). Thirty-four per cent (92/268) were enrolled students, 20% (54/268) were day labourers, 13% (35/268) were either minors or elderly dependents and not working, 12% (33/268) worked as farmers, 1 (less than 1%) worked as a butcher and the remaining 20% (53 patients) had other occupations. Sixty-six per cent (176/268) presented to three hospitals with meningoencephalitis surveillance: Rangpur, Rajshahi and Faridpur (figure 2).
Figure 2.
Location and proportion of patients presenting with zoonotic exposures at participating surveillance hospitals, Bangladesh, 2013–2017.
(c). Reported animal exposures and associated characteristics
Consumption of raw date palm sap was the most frequently reported exposure (54%, 144/268), followed by the slaughtering, preparing or consuming of a sick domestic animal (39%, 105/268). Only a minority of cases reported being bitten or scratched by a wild animal (4%, 11/268) or consuming a wild animal (2%, 5/268) (electronic supplementary material, table S1). Of the patients exposed to sick domestic animals, most reported exposure to sick chickens (87/105, 83%) followed by cows (9/105, 8%), ducks (8/105, 7%), pigeons (4/105, 4%) and a goat (1/105, 1%).
Among the patients reporting an animal exposure that was potentially infectious, patients meeting the case definition for respiratory illness were more likely to report contact with a sick domestic animal compared to patients with suspected meningoencephalitis (68% versus 24%, OR 0.14, 95% CI 0.08–0.3) (table 1). Meningoencephalitis patients reporting a potential zoonotic exposure were more likely to report consuming raw date palm sap (OR 6, 95% CI 3.4–10.5). Potential zoonotic exposures also varied seasonally, with date palm sap consumption being more common in the winter seasons and contact with both domestic and wild animals less common in the winter season (table 1).
Table 1.
Factors associated with different potential zoonotic exposures among patients admitted to surveillance hospitals in Bangladesh. OR, odds ratio; CI, confidence interval; ref, reference.
| factors | number (%) | date palm sap consumption OR (95% CI) | contact with sick domestic animal OR (95% CI) | bitten or scratched by wild animal OR (95% CI) | slaughtered or consumed wild animal OR (95% CI) |
|---|---|---|---|---|---|
| age | |||||
| age ≥ 18 years | 147 (55%) | ref | ref | ref | a |
| age < 18 years | 121 (45%) | 1.5 (0.95–2.50) | 0.6 (0.40–1.02) | 1.01 (0.30–3.40) | a |
| sex | |||||
| male | 180 (67%) | ref | ref | ref | ref |
| female | 88 (33%) | 1.05 (0.6–1.7) | 0.9 (0.5–1.5) | 0.8 (0.2–2.9) | 3.1 (0.5–19) |
| presenting surveillance platform | |||||
| respiratory illness | 92 (34%) | ref | ref | ref | ref |
| meningoencephalitis | 176 (66%) | 6 (3.4–10.5) | 0.14 (0.08–0.30) | 1.4 (0.4–5.4) | 0.12 (0.01–1.14) |
| season | |||||
| rainy (July–October) | 26 (10%) | ref | ref | ref | ref |
| summer (March–June) | 79 (29%) | 15 (1.8–112) | 0.7 (0.3–1.8) | 0.2 (0.04–1.04) | 0.15 (0.01–1.80) |
| winter (November–February) | 163 (61%) | 70 (9.2–530) | 0.14 (0.05–0.30) | 0.13 (0.03–0.60) | 0.15 (0.02–1.10) |
aAnalysis not done as all patients with wild animal contact were aged 18 years or over.
(d). Diagnostic testing
Routine surveillance diagnostic testing was able to identify a pathogen in 12% (31/268) of patients with potential zoonotic exposures. Eight per cent of patients (22/268) had serologic evidence of NiV, 1% (3/268) had serologic evidence of Japanese encephalitis, 1% (4/268) had influenza (two with influenza A, both H1N1 and two with influenza B). Among the patients presenting with respiratory infection who had reported a potential zoonotic exposure, testing for respiratory viruses other than influenza was only conducted in 15 patients who either died or were suspected to be part of a respiratory disease cluster. Of these, one patient had PCR evidence of RSV virus and one of adenovirus.
The majority of patients diagnosed with NiV reported consuming date palm sap (95%, 21/22); the single NiV patient without date palm sap consumption reported slaughtering a sick chicken prior to becoming ill. All three patients with Japanese encephalitis reported slaughtering sick chickens. The majority (75%) of patients with influenza A or B reported slaughtering sick chickens (electronic supplementary material, table S2). Consumption of palm sap was positively associated with the presence of antibodies to NiV (OR 19, 95% CI 2.5–143) (electronic supplementary material, table S3).
(e). Mortality in patients with animal exposures
Sixteen per cent (42/268) of hospitalized patients with recent animal exposures that were potentially infectious died (table 2). Among the patients reporting a potential zoonotic exposure, patients presenting with meningoencephalitis accounted for 95% of all deaths (40/42) and were more likely to die than patients with zoonotic exposures presenting with respiratory infection (OR 13, 95% CI: 3.1–56). Patients 18 years of age or younger were more likely to die than patients over 18 years of age (OR 2.1, 95% CI: 1.1–4.0). Patients who consumed date palm sap prior to becoming ill were more likely to die (OR 2.8, 95% CI: 1.4–5.6). Patients who had any diagnostic test return with a possible infectious aetiology were more likely to die than those who had no known diagnosis (OR 3, 95% CI 1.3–7). Patients with serologic evidence of NiV were more likely to die than patients who did not have a known diagnosis (OR 4.4, 95% CI 1.7–11.2) (electronic supplementary material, table S4). Even when patients with positive serologic testing for NiV were removed from the analysis, patients reporting date palm sap consumption prior to becoming ill were more likely to die (OR 2.6, 95% CI: 1.2–5.6).
Table 2.
Factors associated with mortality among hospitalized patients with recent potential zoonotic exposures. OR, odds ratio; CI, confidence interval; ref, reference.
| characteristics | deaths (%) (n = 42) | OR | (95% CI) |
|---|---|---|---|
| age | |||
| ≥18 years | 17 (40%) | ref | — |
| <18 years | 25 (60%) | 2.1 | 1.1–4.0 |
| gender | |||
| male | 27 (64%) | ref | — |
| female | 15 (36%) | 1.2 | 0.6–2.3 |
| presenting surveillance platform | |||
| respiratory illness | 2 (5%) | ref | — |
| meningoencephalitis | 40 (95%) | 13.2 | 3.1–56 |
| date palm sap consumption | |||
| no | 11 (26%) | ref | — |
| yes | 31 (74%) | 2.8 | 1.4–5.6 |
| volume of sap consumed per session | |||
| 0 ml (none) | 11 (26%) | ref | — |
| ∼125 ml (half glass) | 15 (36%) | 2.7 | 1.2–6.3 |
| 250 ml (one glass) | 15 (36%) | 3.2 | 1.4–7.5 |
| >250 ml (2 or more glasses) | 1 (2%) | 1.2 | 0.15–11.2 |
| frequency of sap consumption | |||
| none | 11 (26%) | ref | — |
| 1–5 sessions | 27 (64%) | 2.5 | 1.2–5.3 |
| >5 sessions | 4 (10%) | 13.7 | 2.7–69.2 |
| contact with sick domestic animal | |||
| no | 36 (86%) | ref | — |
| yes | 6 (14%) | 0.2 | 0.08–0.52 |
| bitten/scratched by wild animal | |||
| no | 38 (90%) | ref | — |
| yes | 4 (10%) | 3.3 | 0.9–11.8 |
4. Discussion
Only a minority of patients presenting to hospitals in Bangladesh with acute respiratory infection and meningoencephalitis report a potential zoonotic exposure in the three weeks prior to acute illness onset. Acutely ill patients reporting an animal exposure that was potentially infectious appear to represent a younger population with a male predominance compared to national averages [15].
Date palm sap consumption, a known risk factor for NiV and potentially a risk factor for other zoonotic infections, was the exposure reported in the majority of patients, followed by contact with sick domestic animals. While the majority of patients with serologic evidence of NiV reported date palm sap consumption, over half of patients reporting date palm sap consumption remained undiagnosed after routine surveillance diagnostic testing. While the high prevalence of date palm sap consumption may mean this finding is merely coincidental, it may also represent either the presence of undiagnosed NiV cases or an unknown zoonotic pathogen infecting date palm sap consumers. If this finding represents a novel pathogen being transmitted via date palm sap, this may further implicate bats, which are known to contaminate sap during collection [22,25], as a potential reservoir.
Overall mortality in the patients without laboratory diagnoses was lower than those patients who had a laboratory diagnosis, suggesting that severity of infection caused by unknown pathogens may be lower than known pathogens in our study area. However, in our study area, NiV, with its high mortality rate, leads to an overall high mortality rate in patients with diagnoses.
Although routine testing was conducted only for a limited number of pathogens, the majority of acutely ill patients with potential zoonotic exposures lacked a laboratory diagnosed aetiology for their illness. Samples from acutely ill patients without a known diagnosis and with high-risk exposures to animals would be good targets for detection of emerging pathogens by next-generation sequencing.
Our experience implementing human-focused surveillance for zoonotic exposures also brings to light the complexities in selecting surveillance sites, designing screening criteria and approaching diagnostics and pathogen identification and how the choices made in each of these areas affect the surveillance platform.
(a). Selection of surveillance sites
Hospitals serve as the surveillance sites in the zoonotic exposure surveillance system in Bangladesh. Hospital catchment areas are large, allowing for surveillance of a sizable population using a single accessible location where resources can be concentrated. Hospitals offer pre-existing resources: in this case, the overarching meningoencephalitis and respiratory disease surveillance systems, trained staff able to collect and handle human specimens and network connections required for uploading data.
Despite these benefits, the selection of hospitals as surveillance sites may not be the ideal solution for the detection of emerging zoonotic infectious diseases. Accessibility to healthcare is a major problem throughout South Asia, including Bangladesh [29]. The difficulties of poor populations reaching hospitals include limited geographical accessibility as well as substantial financial barriers [30]. Models using healthcare utilization data collected from the catchment areas of the same surveillance hospitals in Bangladesh presented here showed that case detection probabilities decrease steeply as distance from the hospital site increases [31] and that hospital-based surveillance misses nearly half of all Nipah outbreaks [32]. Finally, the selection of hospitals as surveillance sites and the requirement that patients be admitted to meet the case definition likely biases our system to detect more severe disease, as patients with more mild illnesses either do not come to hospitals or do not require admission.
Imbedding surveillance at a more local level, such as villages or popular market areas, may improve a surveillance system's ability to reach poor, rural populations at high risk for zoonotic infections. However, the use of more local sites would increase the surveillance costs by increasing the number of screening sites required to cover the same population, the number of samples collected, the number of required staff to lead case finding and to collect samples and the logistical complexity and costs of sample transport.
(b). Designing zoonotic exposure screening
The zoonotic screening questions implemented in Bangladeshi hospitals focus the system on the highest risk cases for spillover zoonotic pathogens, allocating the finite resources available for transportation and testing to, presumably, the highest yield samples. However, our methodology of initial screening for respiratory infection or meningoencephalitis followed by screening for risk of zoonotic exposures has an unknown sensitivity and specificity for identifying patients with high-risk zoonotic exposures.
Because of our selected screening criteria, the system in Bangladesh does not detect patients with potential zoonotic exposures who are not hospitalized or who present with initial symptoms that are not respiratory or neurologic. Our system, which focuses more on wild animal contact and pathways elucidated in NiV transmission, may miss zoonotic diseases like rabies and hantavirus that can be transmitted by more varied contact with domestic animals than those covered by our screening assessment.
The goal of maximizing the sensitivity and specificity of screening methodology for zoonotic spillover disease is hampered owing to the difficulty in collecting data to assess and improve the system. Zoonotic spillover is a rare event [33] and the failure of a system to detect cases does not necessarily mean that the surveillance system is faulty.
Despite this difficulty, we have been able to detect and fix potential shortcomings within the system. During the first weeks of piloting, for example, we noted that very few patients reported contact with rodents, which seemed incongruous with the village settings. Using qualitative interviews, we were able to identify that patients did not consider rodents wild animals and were not identifying these interactions. Because our platform is electronically administered, corrections and additions to screening questionnaires can easily be rolled out across the platform, and examples of wild animals, including rodents, were added to the screening questions. This oversight would have led the system to miss a major potential reservoir [34].
(c). Diagnostics and pathogen detection
Our hospital-based surveillance of potential zoonotic exposures uses logistic and testing pipelines already established for ongoing surveillance platforms. While this allows us to reduce costs, it does mean that our system is tied to initial diagnostic strategies that might not always be ideal for evaluating patients with potential zoonotic diseases. Patients in our system who remained without a diagnosis in initial testing may have received a diagnosis if our platform tested for diseases like rabies or tested all patients presenting with respiratory illness for a wider variety of respiratory pathogens, including bacterial infections.
In the absence of a known diagnosis, our plans for detection of potential unknown pathogens rely on next-generation sequencing. Our ability to detect pathogens will depend on the natural history of the zoonotic disease, the severity of the case, the presence of the pathogen in the samples collected and the window when the pathogen can be detected in the respective sample. Our ability to detect pathogens by next-generation sequencing will therefore vary for different pathogens: for example, while severe Ebola cases have a long duration of viraemia, the duration of viraemia varies based on disease severity [35], Zika viraemia weans comparatively quickly and at variable rates in different bodily fluids [36–38], and PCR evidence from a recent NiV outbreak suggests that virus is not detected in all samples and may be negative altogether in suspected cases [39].
Since sequencing was not readily available onsite during the surveillance period, laboratory evaluation for pathogen identification required transportation of samples and substantial lag times after case presentation. The availability of readily available and reliable sequencing would greatly reduce the barriers to testing samples, decrease the time between sample collection and testing and, if specific therapies were available, potentially allow results to direct clinical care. While there have been strides in portable sequencing devices that could allow for the implementation of sequencing at field screening locations in resource-poor locations [40,41], these solutions continue to be plagued by high input requirements, expense and error rates [10].
In the ideal setting, a human zoonotic detection platform would consist of collecting a wide variety of samples at varying times over the duration of illness, including early in the disease course. Testing would consist of cheap and rapid tests for the most common causes of illness, with follow-up testing on negative cases with portable sequencing technology deployed in the field. These solutions would maximize yield from diagnostic samples, simplify logistics and allow for more rapid detection.
(d). Financing zoonotic disease surveillance
Finally, and separate from the scientific questions, difficulties in determining responsibility for financing surveillance as well as sustaining funding are a substantial barrier to the deployment of zoonotic exposure surveillance. As a cornerstone of detecting and preventing potential pandemics, zoonotic disease surveillance serves a global benefit. While potential funding approaches have been suggested elsewhere [9], in the case of hospitals in Bangladesh, we were able to keep costs low by adding zoonotic screening to already running surveillance systems, using the same hospital sites, logistics, staff and initial diagnostic testing of pre-existing platforms. While this may not be feasible in all locations, our experience may offer a cost-effective roadmap to implementing zoonotic disease surveillance where functioning baseline surveillance already exists.
5. Conclusion
The example of the zoonotic exposure surveillance platform in Bangladesh demonstrates that hospital-based surveillance for zoonotic exposures in humans can be built on existing surveillance architecture. The integration of human surveillance of zoonotic exposures, along with animal-based surveillance, digital epidemiology and advances in real-time sequencing are part of a unified approach to detecting zoonotic spillover disease [10]. Implementation of human surveillance for zoonotic exposures could play an important future role in the early detection of zoonotic spillover diseases.
Supplementary Material
Ethics
The zoonotic exposure screening, questionnaires and sample collection protocols were reviewed and approved by the ethical review committee (ERC) of icddr,b (PR-12093) as well as the Institutional Review Board of Stanford University (protocol 26513).
Data accessibility
The data used in the analysis presented in this manuscript are available at doi:10.17605/OSF.IO/NAU94.
Authors' contributions
All authors assisted in development of protocols and implementation of the zoonotic surveillance platform, P.D. conducted data analysis presented in the manuscript, P.D. and J.J.O. drafted the manuscript, all authors reviewed the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research study was funded by Centers for Disease Control and Prevention, Atlanta, USA. icddr,b acknowledges with gratitude the commitment of US CDC to its research efforts. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden and the UK for providing core/unrestricted support. We are grateful to our study participants for their time and valuable information.
References
- 1.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–993. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman CM, Frieman MB. 2014. Coronaviruses: important emerging human pathogens. J. Virol. 88, 5209–5212. ( 10.1128/JVI.03488-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fehr AR, Channappanavar R, Perlman S. 2017. Middle East respiratory syndrome: emergence of a pathogenic human coronavirus. Annu. Rev. Med. 68, 387–399. ( 10.1146/annurev-med-051215-031152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arunkumar G, Chandni R, Mourya DT, Singh SK, Sadanandan R, Sudan P, Bhargava B, NIPAH: Nipah Investigators People And Health. 2018. Outbreak investigation of Nipah virus disease in Kerala, India, 2018. J. Infect. Dis. 12, 235 ( 10.1093/infdis/jiy612) [DOI] [PubMed] [Google Scholar]
- 5.Homaira N, et al. 2010. Nipah virus outbreak with person-to-person transmission in a district of Bangladesh, 2007. Epidemiol. Infect. 138, 1630–1636. ( 10.1017/S0950268810000695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyer O. 2019. Lassa outbreak: WHO warns of unusually rapid spread in Nigeria. BMJ 364, l781 ( 10.1136/bmj.l781) [DOI] [PubMed] [Google Scholar]
- 7.Drake JM, Kaul RB, Alexander LW, O'Regan SM, Kramer AM, Pulliam JT, Ferrari MJ, Park AW. 2015. Ebola cases and health system demand in Liberia. PLoS Biol. 13, e1002056 ( 10.1371/journal.pbio.1002056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber C, Finelli L, Stevens W. 2018. The economic and social burden of the 2014 Ebola Outbreak in West Africa. J. Infect. Dis. 218, S698–S704. ( 10.1093/infdis/jiy213) [DOI] [PubMed] [Google Scholar]
- 9.National Research Council (US) Committee on Achieving Sustainable Global Capacity for Surveillance and Response to Emerging Diseases of Zoonotic Origin, Keusch GT, Pappaioanou M, Gonzalez MC, Scott KA, Tsai P. 2009. Sustaining global surveillance and response to emerging zoonotic diseases Washington, DC: National Academies Press; ( 10.17226/12625) [DOI] [PubMed] [Google Scholar]
- 10.Gardy JL, Loman NJ. 2018. Towards a genomics-informed, real-time, global pathogen surveillance system. Nat. Rev. Genet. 19, 9–20. ( 10.1038/nrg.2017.88) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anthony SJ, et al. 2013. A strategy to estimate unknown viral diversity in mammals. MBio 4, e00598-13 ( 10.1128/mBio.00598-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly TR, Karesh WB, Johnson CK, Gilardi KVK, Anthony SJ, Goldstein T, Olson SH, Machalaba C, Mazet JAK. 2017. One Health proof of concept: bringing a transdisciplinary approach to surveillance for zoonotic viruses at the human–wild animal interface. Prev. Vet. Med. 137, 112–118. ( 10.1016/j.prevetmed.2016.11.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jephcott FL, Wood JLN, Cunningham AA. 2017. Facility-based surveillance for emerging infectious diseases; diagnostic practices in rural West African hospital settings: observations from Ghana. Phil. Trans. R. Soc. B 372, 20160544 ( 10.1098/rstb.2016.0544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster-Turley P, Das R, Hasan M, Hossain PR. 2016. Bangladesh tropical forests and biodiversity assessment. USAID; See https://www.usaid.gov/documents/1865/bangladesh-tropical-forests-and-biodiversity-assessment. [Google Scholar]
- 15.US Central Intelligence Agency. 2019 World Factbook: Bangladesh. Washington, DC: Central Intelligence Agency. See https://www.cia.gov/library/publications/the-world-factbook/geos/bg.html.
- 16.Department of Livestock Services, Government of Bangladesh. 2017 Livestock economy at a glance. See http://dls.portal.gov.bd/sites/default/files/files/dls.portal.gov.bd/page/5f7daa39_d71f_4546_aeaf_55b72ee868f2/Updated%20Livestock%20Economy%20(2015-2016).pdf.
- 17.Luby SP, et al. 2009. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001–2007. Emerg. Infect. Dis. 15, 1229–1235. ( 10.3201/eid1508.081237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sazzad HMS, et al. 2013. Nipah virus infection outbreak with nosocomial and corpse-to-human transmission, Bangladesh. Emerg. Infect. Dis. 19, 210–217. ( 10.3201/eid1902.120971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luby SP. 2013. The pandemic potential of Nipah virus. Antiviral Res. 100, 38–43. ( 10.1016/j.antiviral.2013.07.011) [DOI] [PubMed] [Google Scholar]
- 20.Luby SP, et al. 2006. Foodborne transmission of Nipah virus, Bangladesh. Emerg. Infect. Dis. 12, 1888–1894. ( 10.3201/eid1212.060732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegde ST, et al. 2016. Investigating rare risk factors for Nipah virus in Bangladesh: 2001–2012. Ecohealth 13, 720–728. ( 10.1007/s10393-016-1166-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan MSU, Hossain J, Gurley ES, Nahar N, Sultana R, Luby SP. 2010. Use of infrared camera to understand bats' access to date palm sap: implications for preventing Nipah virus transmission. Ecohealth 7, 517–525. ( 10.1007/s10393-010-0366-2) [DOI] [PubMed] [Google Scholar]
- 23.Ahmed M, et al. 2018. Estimates of seasonal influenza-associated mortality in Bangladesh, 2010–2012. Influenza Other Respir. Viruses 12, 65–71. ( 10.1111/irv.12490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniels P, Ksiazek T, Eaton BT. 2001. Laboratory diagnosis of Nipah and Hendra virus infections. Microbes Infect. 3, 289–295. ( 10.1016/S1286-4579(01)01382-X) [DOI] [PubMed] [Google Scholar]
- 25.Islam MS, et al. 2016. Nipah virus transmission from bats to humans associated with drinking traditional liquor made from date palm sap, Bangladesh, 2011–2014. Emerg. Infect. Dis. 22, 664–670. ( 10.3201/eid2204.151747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hossain MJ, et al. 2010. Hospital-based surveillance for Japanese encephalitis at four sites in Bangladesh, 2003–2005. Am. J. Trop. Med. Hyg. 82, 344–349. ( 10.4269/ajtmh.2010.09-0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Homaira N, et al. 2016. Respiratory viruses associated hospitalization among children aged <5 years in Bangladesh: 2010–2014. PLoS ONE 11, e0147982 ( 10.1371/journal.pone.0147982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipkin WI, Anthony SJ. 2015. Virus hunting. Virology 479–480, 194–199. ( 10.1016/j.virol.2015.02.006) [DOI] [PubMed] [Google Scholar]
- 29.Zaidi S, Saligram P, Ahmed S, Sonderp E, Sheikh K. 2017. Expanding access to healthcare in South Asia. BMJ 357, j1645 ( 10.1136/bmj.j1645) [DOI] [PubMed] [Google Scholar]
- 30.Peters DH, Garg A, Bloom G, Walker DG, Brieger WR, Rahman MH. 2008. Poverty and access to health care in developing countries. Ann. NY Acad. Sci. 1136, 161–171. ( 10.1196/annals.1425.011) [DOI] [PubMed] [Google Scholar]
- 31.Nikolay B, et al. 2017. Evaluating hospital-based surveillance for outbreak detection in Bangladesh: analysis of healthcare utilization data. PLoS Med. 14, e1002218 ( 10.1371/journal.pmed.1002218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hegde ST, et al. 2019. Using healthcare-seeking behaviour to estimate the number of Nipah outbreaks missed by hospital-based surveillance in Bangladesh. Int. J. Epidemiol. 14, 1001 ( 10.1093/ije/dyz057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, Lloyd-Smith JO. 2017. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 15, 502–510. ( 10.1038/nrmicro.2017.45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luis AD, et al. 2013. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. R. Soc. B 280, 20122753 ( 10.1098/rspb.2012.2753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanini S, et al. 2015. Blood kinetics of Ebola virus in survivors and nonsurvivors. J. Clin. Invest. 125, 4692–4698. ( 10.1172/JCI83111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorchakov R, Berry RM, Patel SM, El Sahly HM, Ronca SE, Murray KO. 2019. Optimizing PCR detection of Zika virus from various body fluids. Am. J. Trop. Med. Hyg. 100, 427–433. ( 10.4269/ajtmh.18-0755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barzon L, Percivalle E, Pacenti M, Rovida F, Zavattoni M, Del Bravo P, Cattelan AM, Palù G, Baldanti F. 2018. Virus and antibody dynamics in travelers with acute Zika virus infection. Clin. Infect. Dis. 66, 1173–1180. ( 10.1093/cid/cix967) [DOI] [PubMed] [Google Scholar]
- 38.Rossini G, Gaibani P, Vocale C, Cagarelli R, Landini MP. 2017. Comparison of Zika virus (ZIKV) RNA detection in plasma, whole blood and urine—case series of travel-associated ZIKV infection imported to Italy, 2016. J. Infect. 75, 242–245. ( 10.1016/j.jinf.2017.05.021) [DOI] [PubMed] [Google Scholar]
- 39.Yadav PD, et al. 2019. Nipah virus sequences from humans and bats during Nipah outbreak, Kerala, India, 2018. Emerg. Infect. Dis. 25, 1003–1006. ( 10.3201/eid2505.181076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoenen T, et al. 2016. Nanopore sequencing as a rapidly deployable Ebola outbreak tool. Emerg. Infect. Dis. 22, 331–334. ( 10.3201/eid2202.151796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quick J, et al. 2016. Real-time, portable genome sequencing for Ebola surveillance. Nature 530, 228–232. ( 10.1038/nature16996) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in the analysis presented in this manuscript are available at doi:10.17605/OSF.IO/NAU94.