Abstract
Epinephrine HFA (Primatene® Mist) is a newly formulated asthma metered dose inhaler developed to replace the previous Primatene® Mist CFC. The formulation of Epinephrine HFA contains thymol, a substance recognized to be safe by the FDA. Although the content of thymol contained in Epinephrine HFA is much lower compared to many common foods and medications available, there are no known nonclinical data about the chronic toxicity of thymol through inhalation. Two sequential 6‐month studies of identical design were conducted to assess the chronic toxicity of inhaled thymol in mice. Four treatment groups, (a) Air; (b) vehicle control; (c) Article‐1 (thymol 0.1%); and (d) Article‐2 (thymol 0.5%) were assessed in 128 mice for 26 weeks. The mice were sacrificed at the end of the treatment period and a histopathologic evaluation was performed with respect to lungs, bronchial lymph nodes, nasal passages/nasopharynx, and trachea. Forty‐five pathologic assessment parameters (PAPs) were evaluated. In total, 5591 data points from 487 mouse organs were assessed. Chronic toxicity index was calculated for 16 PAPs that had multiple histopathologic abnormal observations. The t tests were conducted for these 16 PAPs (Articles‐1 and 2 versus Air and vehicle control, respectively), and all P‐values were greater than .05 indicating no significant differences between all treatment groups. An evaluation was also conducted for 25 PAPs that had only a very small number of pathologic abnormalities. No significant differences for chronic toxicity were found when comparing mice under long‐term repeated exposure of high doses of inhaled thymol and mice that inhaled no thymol.
Keywords: epinephrine HFA, inhalation, mice, respiratory toxicology, thymol
1. BACKGROUND
Epinephrine HFA is a proposed replacement for Epinephrine chlorofluorocarbon (CFC) (Primatene® Mist CFC), an OTC asthma metered dose inhaler (MDI) that was removed from the market due to the environmental effects of its CFC propellant.1 Compared to the previous Epinephrine CFC, the newly formulated Epinephrine MDI contains hydrofluoroalkane (HFA) propellant along with other ingredients such as polysorbate 80, ethanol, and very low amount of thymol (2‐isopropyl‐5‐methylphenol), which is used as an antioxidant.
Previous findings demonstrated the safety of orally administered thymol through acute and subacute toxicity tests.2, 3 Furthermore, according to the Code of Federal Regulations: “Thymol is an essential oil that is extracted from thyme, mandarin and tangerine oils and is FDA approved when used as a synthetic flavoring (21 CFR 172.515), a preservative and indirect food additive of adhesives (21 CFR 175.105). The source plant (thyme), from which thymol is extracted, is generally recognized as a safe substance (GRASS) by FDA (21 CFR 182.10, 21 CFR 182.2)”.4
Historic findings from as early as 1964 have demonstrated the safety of thymol.2, 3 In an acute toxicity study, the oral LD50 of thymol in rats was found to be 980 mg/kg.2 By comparison, common products such as vitamin D and caffeine have an LD50 of 10 mg/kg and 200 mg/kg respectively in rats.5 In another study, no adverse effects were observed on gross pathology, hematology, body weight, food intake, and organ weight from a thymol amount of 10 000 ppm (300 mg kg−1 d−1) given to rats in their diet for 19 weeks.3 As an inter‐model comparison, the sub‐chronic relative thymol exposure of 300 mg kg−1 d−1 3 corresponds to a thymol exposure of 300 000 times greater than that contained in Epinephrine HFA (<0.001 mg kg−1 d−1). These studies also suggest that thymol, even at relatively high amounts, is considered a safe substance.
In addition, thymol is a common ingredient in many products such as perfumes, food flavorings, mouthwashes, pharmaceutical preparations, and cosmetics.6 As set forth in Appendix‐1 of the Federal Register dated 18 January 2006, “Inhalation exposure to thymol already occurs from contact with foodstuffs and seasonings containing thymol as it is FDA approved when used as a direct food additive and is generally recognized as safe by FDA as a spice, natural oil, oleoresin, or natural extract…” 4
Compared to various food and medication products including Halothane, a thymol containing inhalation medication that was available for over 40 years, the thymol content contained in Epinephrine HFA is substantially less than in many food products7 and medications that have been proven to be safe (Table S1). Therefore, the low exposure to thymol in the Epinephrine HFA formulation is expected to be minimal and is not expected to add any significant safety risk.
Although thymol is used in consumer food and hygienic products, and there have been significant preclinical and human studies on the effects of thymol in a broad range of applications, there are no known nonclinical data about the chronic toxicity of thymol through inhalation. Therefore, this paper assesses the chronic effects of inhaled thymol in lungs and respiratory tracts using a mouse model.
2. MATERIALS AND METHODS
Two sequential 6‐month studies of identical design were conducted to assess the chronic toxicity of thymol through inhalation in mice. Both chronic toxicity studies were conducted in accordance with 21 CFR §58, Good Laboratory Practice for Nonclinical Laboratory Studies. The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies.8
2.1. Test articles
There are four treatment groups in each study: (a) Group‐1 is negative control containing only ambient air; (b) Group‐2 is Vehicle Control that also served as negative control. This control article contained the same ingredient as Epinephrine HFA, but without the active ingredient (epinephrine) and the study substance thymol; (c) Group‐3 is test Article‐1 containing 0.1% of thymol; and (d) Group‐4 is test Article‐2 containing 0.5% of thymol. The two test articles were prepared using the same materials and ingredients as currently used to produce Epinephrine HFA, but without the active ingredient of epinephrine. The amount of thymol in the two test articles is 10 and 50 times the amount of thymol in the Epinephrine HFA formulation.
All three articles (Vehicle, Article‐1, and Article‐2) were prepared by Armstrong Pharmaceuticals, Inc under cGMP. The thymol amounts were tested and met the specification before they were released by Armstrong. At the end of the 6‐month studies, these articles were retested and confirmed to have good stability of thymol with all specifications met.
2.2. Test animals
Male and female CD‐1 mice with weights of approximately 30 g and age of 7 weeks were purchased from Harlan, Indianapolis, IN. All animals were free of pathogens and housed in a clean environment with ad libitum access to water and food, and kept in a temperature (64‐79°F) controlled environment maintained on a 12‐hour light/dark cycle. The routine diet for mice was 2016 Teklad Global 16% Protein Rodent Diet, purchased from Harlan Laboratories. No dietary contaminants were expected to interfere with the outcome of this study. All standard animal housing and handling procedures including sacrifice of the animals conformed to the lab facility standard operating procedures (SOPs), NIH Guide for the Care and Use of Laboratory Animals, and GLP guidelines. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC).
2.3. Chronic toxicity study design
All treatment procedures were identical in the two sequential 6‐month studies. Within each study, animals were randomly assigned to one of the four treatment groups, with each group consisting of eight male and eight female CD‐1 mice. Unique IDs were given to the animals. Each treatment group was subject to only one article. When combined, the total number of animals in each treatment group was 32, with a 1:1 ratio of male to female mice. In total, 128 (=2 × 4 × 16, two sets of study, four treatment groups and 16 animals per group) mice were studied as summarized in Table 1.
Table 1.
Animal information and treatments in the studies
| Study sets | Study 1 | Study 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Group No. | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Article and treatments | ||||||||
| Article name | Air | Vehicle | 0.1% Thymol | 0.5% Thymol | Air | Vehicle | 0.1% Thymol | 0.5% Thymol |
| Article lot No. | — | PL000114 | PL000314 | PL00414 | — | PL000114 | PL000314 | PL00414 |
| # of weeks for treatment | 26 | 26 | 26 | 26 | 26 | 26 | 26 | 26 |
| # of treatments per week | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Total # of treatments | 78 | 78 | 78 | 78 | 78 | 78 | 78 | 78 |
| Mice information | ||||||||
| # of Male Mice | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| # of Female Mice | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Subtotal # of Mice | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
In the studies, the test article was sprayed into a specially designed stainless steel 21.5‐L breathing tank as shown in Figure 1. The tank size is designed such that the total breath volume of all eight mice in the 10‐minute study time (1.8 L) is less than 10% of the tank size (21.5 L). The internal wall of the tank is electrically polished so that the thymol adsorption will be reduced to minimal. Eight mice were mounted to the tank with four mice on each side using small animal restraints. At the start of each treatment session, 15 sprays of test article were sprayed into the precleaned tank following the same spray procedures described in the Epinephrine HFA insert. In order to ensure consistent concentration of test article, a stirring fan installed inside the tank was set at 400 RPM and was started before the first spray of the test articles. Thirty seconds after the last spray (t = 0 minute), eight mice were mounted to the inhalation chamber to breathe the air from inside the breathing tank for 10 minutes in each session.
Figure 1.
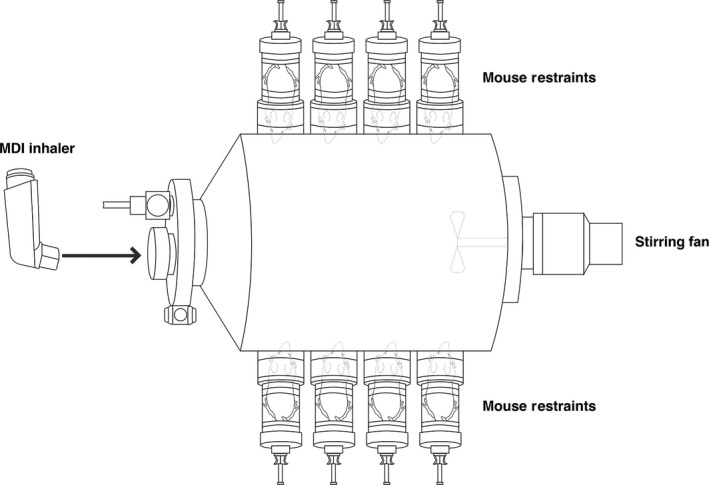
Eight mice are mounted to the tank during treatment
There were three treatment sessions per week for 6 months (26 weeks). In clinical practice, Epinephrine HFA should ideally be used no more than two times per week for patients with mild symptoms of intermittent asthma. At the end of the study, there were a total of 78, 10‐minute treatment sessions performed for each treatment group of mice. After each session, the breathing tank was washed and dried.
2.4. Determination of the amount of thymol in the breathing tank
After spraying the study article into the tank, the actual concentration of thymol in the air of the breathing tank was determined by a validated LC‐MS method. First, the air sampling pump (PCXR8 Universal Sample Pump, Catalog No. 224‐PCXR8, SKC Inc) was connected to the Sorbent Sample Tube (Anasorb CSC, Catalog No. 226‐01, SKC Inc) using Tygon tubing. The pump was preadjusted to 100 mL/min. The stirring fan was set at 400 RPM and started before the first spray of the test articles. The air sampling unit was then inserted into the tank. Thirty seconds after the last spray, the air sampling pump was turned on for 10 minutes. This drew 1000 mL of air through the sampling tube and thymol was captured on a coconut charcoal sorbent. Both the front and back ends of charcoal were desorbed into 1 mL ethyl acetate. Then, 0.1 mL of the ethyl acetate was transferred to 0.9 mL of the HPLC mobile phase (50/50 v/v Methanol/H2O with 0.1% formic acid) to analyze thymol concentration by LC‐MS.
For each of the two test articles (Article‐1 0.1% thymol, and Article‐2 0.5% thymol), three replicates of determinations were performed. Between any two tests of these three replicates, a method blank was run to assure the data quality. Tank air was also sampled and tested before the sprays of thymol, after the sprays of thymol, as well as after washing/cleaning of the tank between any two treatment sessions in order to ensure removal of residual thymol.
2.5. Determination of the thymol doses for tested Article‐1 and Article‐2
The amount of thymol inhaled by the mice was calculated based on: (a) the actual concentration of thymol in the air of the tank; (b) the mouse breathing time (10 minutes treatment session); and (c) the mouse breathing volume per minute, 22.5 mL/min, where the breath rates and tidal volume1 in mice are cited from previous literature.9
The mouse weekly thymol dose for each of the four treatment groups was calculated based on Equation S1 and information from Table S2. The relative weekly thymol dose used in a mouse versus that of a human was also determined per Equation S2.
2.6. General health examination of the mice
General health evaluation, including body weight, food intake, survival, general appearance, functional behaviors, etc was performed on the mice weekly. The mouse body weight data points were measured for: (a) four treatment groups; (b) 32 mice per group; and (c) 27 (0‐26 weeks) times. The general health conditions of the four test groups were evaluated and compared.
2.7. Histopathologic evaluation of the chronic toxicity study
After the last treatment, each mouse was sacrificed and four organs: (a) lungs, (b) bronchial lymph nodes, (c) nasal passages/nasopharynx, and (d) trachea were taken out and preserved in a labeled histology container prefilled with 10% Neutral Buffered Formalin. The organs were sent to Experimental Pathology Laboratories, Inc (EPL) for histopathologic evaluation. Forty‐five pathologic assessment parameters (PAP) were assessed by EPL.
Hematoxylin and eosin stained (H&E) slides of lung lobes, trachea, four levels of the nasal turbinates, and bronchial lymph nodes were prepared by EPL for microscopic evaluation by a board‐certified veterinary pathologist. The EPL rated the pathologic assessment parameters (PAPs) as Grade‐0 to 5, where
GRADE‐0 = “Nothing Abnormal Discovered” (NAD);
GRADE‐1 = minimal/very few/very small;
GRADE‐2 = slight/mild/few/small;
GRADE‐3 = moderate/moderate number/moderate size;
GRADE‐4 = marked/many/large/moderately severe; and
GRADE‐5 = massive/extensive number/extensive size/severe
To provide an overall picture or profile for pathologic result assessments, the total abnormal occurrences for a given “Grade‐j” (j = 0–5) for a specific PAP, denoted as , is defined as follows:
| (1) |
where is the number of abnormal occurrences of “Grade‐j” for a specific PAP in the entire treatment Group‐X.
Based on the number of abnormal histopathologic occurrences, each of the 45 PAPs was classified into two categories: If a PAP for all combined four test groups had more than three Grade‐1 occurrences, or more than one Grade‐2 occurrence, or any occurrences for Grades‐3 to 5, it was classified as a Category‐1 PAP. Otherwise, it was classified as a Category‐2 PAP.
In other words, Category‐1 PAPs have more frequent and/or more severe pathologic abnormalities than Category‐2 PAPs.
2.8. Statistical analysis for PAPs with more and/or severe pathologic abnormal occurrences
For each Category‐1 PAP, the chronic toxicity of inhaled thymol for a given Treatment Group‐X is described as the average chronic toxicity index (“CTI”) of Treatment Group‐X based on a given PAP, denoted as , which is defined as follows:
| (2) |
where is the CTI of the Treatment Group‐X for the PAP with observation Grade‐j; j runs over all possible grades used by EPL report, from “nothing abnormal discovered” (NAD), that is, Grade‐0, to Grade‐5 as specified by EPL report; is the number of observations with Grade‐j of the PAP in the Treatment Group‐X; NX is the total number of mouse organs that were assessed for the PAP for the Treatment Group‐X.
The CTI for a given PAP can be statistically analyzed among different treatment groups. A one‐sided t test was conducted for the following hypothesis:
| (3) |
where is the CTI for any Category‐1 PAPs for Treatment X, X is Treatment Group‐3 (Article‐1, 0.1% thymol), or Group‐4 (Article‐2, 0.5% thymol); is the CTI for any one of Category‐1 PAPs for Treatment Y, and Y is Treatment Group‐1 (Air), or Group‐2 (Vehicle);
Namely, per Hypothesis given in Equation (3), four types of t test were conducted for each CTI of Category‐1 PAP:
Group‐3 (Article‐1) vs Group‐1 (Air);
Group‐3 (Article‐1) vs Group‐2 (Vehicle);
Group‐4 (Article‐2) vs Group‐1 (Air);
Group‐4 (Article‐2) vs Group‐2 (Vehicle);
The study would demonstrate significant difference for chronic toxicity between the treatment groups if the P‐value of the t test was less than .05.
2.9. Statistical analysis for PAPs with minor and mild pathologic abnormal occurrences
To assess Category‐2 PAPs, the number of occurrences for a PAP group of a given Treatment X, denoted by gX, was defined as follows:
| (4) |
where k runs over all PAPs included in the PAP group; and is the number of occurrence of PAP‐k for the Treatment X.
To conclude that inhalation of thymol results in detectable chronic toxicity for a Category‐2 PAP‐group, the following conditions must be simultaneously satisfied:
the for Treatment Group‐3 (Article‐1, 0.1% thymol) and Group‐4 (Article‐2, 0.5% thymol) must be greater than the for Treatment Group‐1 (Air) and Group‐2 (Vehicle);
| (5a,5b) |
| (5c,5d) |
the for Treatment Group‐4 (Article‐2, 0.5% thymol) must be not less than the for Treatment Group‐3 (Article‐1, 0.1% thymol).
| (5e) |
Fisher’s Exact Test (FET) was performed to test the following hypotheses:
| (6a) |
| (6b) |
| (6c) |
| (6d) |
| (6e) |
In order to confirm that inhaled thymol is chronically toxic for a Category‐2 PAP‐group, all five P‐values of the hypotheses (6a) to (6e) must all be less than .05.
3. RESULTS
3.1. The actual thymol concentration in the breathing tank
The sprayed amounts of thymol delivered in 15 sprays were 687 and 3,434 mcg, respectively, for Article‐1 and Article‐2 (summarized in Table S3). The actual concentration of thymol in the tank sampled during 0‐10 minutes after 15 sprays of test articles is the average thymol concentration in the breathing tank during the 10 minutes of study period for each treatment (Table S3). It was found that: (a) after 15 sprays of Article‐1, 71% of thymol was absorbed on the wall and inner surface of the tank, while 29% remained at a free gaseous state in the air; (b) after 15 sprays of Article‐2, 57% of thymol was adsorbed on the wall and inner surface of the tank, whereas 43% remained in its free gaseous state in the air (Table S3).
3.2. The amount of thymol inhaled by the mice
The calculated results for the actual thymol doses for Article‐1 and Article‐2 inhaled by the mice were 57.2 and 420 mcg/kg for each treatment, or weekly accumulated inhaled thymol exposure of 171 and 1261 mcg kg−1 wk−1, respectively, as summarized in Table 2. Therefore, the weekly dose of thymol inhaled by mice was 117 to 858 times of that of the calculated maximum weekly thymol exposed in humans based on the Epinephrine HFA labeling, per Equation S2.
Table 2.
Mouse weekly thymol dose in each treatment group
| Group No. | Treated by | # of Sprays in the Tank | Actual thymol in tanka, c mcg/L | Mouse breathing parameters | Mouse thymol dose | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time, t Minutes | Volume, v mL/minb | Volume mL per Treatment | mcg per Treatment | Mean Mouse Body weight, w, gramsc | mcg/kg per Treatment | Mcg kg−1 wk−1 | * | ||||
| 1 | Room air | — | 0 | 10 | 22.5 | 225 | 0.0 | 38.1 ± 5.9 | 0.0 | 0.0 | 0 |
| 2 | Vehicle | 15 | 0 | 10 | 22.5 | 225 | 0.0 | 36.6 ± 5.6 | 0.0 | 0.0 | 0 |
| 3 | Article‐1 (0.1%) | 15 | 9.27 | 10 | 22.5 | 225 | 2.1 | 36.5 ± 4.6 | 57.2 | 171 | 117 |
| 4 | Article‐2 (0.5%) | 15 | 69.0 | 10 | 22.5 | 225 | 15.5 | 36.9 ± 6.3 | 420.3 | 1261 | 858 |
*Is defined as the mouse weekly thymol dose relative to the human maximum weekly thymol dose from Epinephrine HFA, which is 1.47 mcg/kg/week, refer to Table S2.
Actual concentrations measured by experiments, given in Table S3.
Refers to calculated mouse breath volume Table S2.
Mean body weight is an average determined from 32 mice over 26 weeks, in total 832 body weight data for each treatment group.
3.3. General health examination of the mice
No abnormal findings in mouse body weight change were observed. In total, 3456 mouse body weight data points were measured. There was no correlation between the mean body weight curves and thymol dose inhaled by mice (Figure S1). The mean mouse body weight change profile (as a function of time) for the treatment groups Group‐2, Group‐3, and Group‐4 has the same trend. This indicates that even at very high doses of thymol (117‐858 times of the human thymol dose due to use of Epinephrine HFA), there was no impact on the mouse body weight growth over the time period of 6 months.
No abnormal findings in food consumption were observed. The mice in all treatment groups appeared to be healthy and no mice died during the studies. In fact, there were no abnormalities in appearance or functional behavior observed in mice throughout the study period. Some male mice had the appearance of minor scratches on their skin, which were likely the result of fighting within the same cage.
3.4. Histopathologic evaluation
EPL assessed 119, 121, 126, and 121 mouse organs from Groups‐1 to 4, respectively. In total, 487 mouse organs from 128 mice were sent to EPL for assessment. The organs were assessed for 45 PAPs as follows: eight PAPs were assessed for lungs; seven for bronchial lymph nodes; one PAP for trachea; and 4, 12, 8, and 5 PAPs for four different sections of mouse nasal turbinates, Level I, II, III and IV, respectively.
EPL reported 1377, 1391, 1426, and 1397 PAP data points for Groups‐1 to 4, respectively. For the entire study, there was a total of 5,591 pathologic data of 45 PAPs. These were assessed by EPL as summarized in Table S4.
Based on the pathologic data, the 45 PAPs were initially classified as 14 Category‐1 parameters and 31 Category‐2 parameters. However, for pathologic assessments of Nasal turbinate Level II and III, there were three very similar Category‐2 PAPs:
Dilation; Bowman's Gland;
Dilation; Bowman's Gland; Lateral Wall/Turbinate, and
Dilation; Bowman's Gland; Septum.
Since these were all parameters for dilation of Bowman's Gland, a combined assessment may provide more meaningful information. Therefore, these three PAPs were recategorized into one Category‐1 PAP. After the combination/reassignment, there were 16 Category‐1 and 25 Category‐2 PAPs as summarized in Table 3.
Table 3.
Classification of 45 pathologic assessment parameters (PAPs)
| Mouse organs | All | Direct classification | After combination | |||
|---|---|---|---|---|---|---|
| Category‐1 | Category‐2 | Category‐1 | Category‐2 | Reduced # due to the combination | ||
| A. Lung | 8 | 3 | 5 | 3 | 5 | — |
| B. Lymph node, bronchi | 7 | 3 | 4 | 3 | 4 | — |
| C1. Nasal turbinate level I | 4 | 1 | 3 | 1 | 3 | — |
| C2. Nasal turbinate level II | 12 | 3 | 9 | 4* | 6** | 2 |
| C3. Nasal turbinate level III | 8 | 1 | 7 | 2* | 4** | 2 |
| C4. Nasal turbinate level IV | 5 | 2 | 3 | 2 | 3 | — |
| D. Trachea | 1 | 1 | 0 | 1 | 0 | |
| All organs | 45 | 14 | 31 | 16 | 25 | 4 |
| 45 | 45 | |||||
Combined into Category‐1 pathologic parameters.
Three very similar Category‐2 PAPs: (a) Dilatation; Bowman's Gland; Duct, (b) Dilatation; Bowman's Gland; Lateral Wall/Turbinate, and (c) Dilatation; Bowman's Gland; Septum; are combined as one Category‐1 PAP.
3.5. Statistical analysis for results of PAPs with more and/or severe pathologic abnormal occurrences
Using the above discussed statistical analysis method, 64 t tests were conducted for the 16 Category‐1 pathologic parameters in this study, and all P‐values were greater than .05 as summarized in Table 4. These analyses suggest that there are no statistically significant differences in the CTIs for the 16 Category‐1 PAPs between the mice that inhaled high dose of thymol (Groups‐3 and 4, d = 117 and 858, respectively), and those with no thymol inhaled (Groups‐1 and 2, d = 0).
Table 4.
P‐Values of chronic toxicity evaluation for inhaled thymol based on the 16 Category‐1 PAPs with t test
| # | Treatment group pairs | Group‐3 vs Group‐1 | Group‐3 vs Group‐2 | Group‐4 vs Group‐1 | Group‐4 vs Group‐2 | |
|---|---|---|---|---|---|---|
| Treatment dose pairs (weekly mouse thymol dose relative to human dose due to use of epinephrine HFA) | 117 vs 0 | 117 vs 0 | 858 vs 0 | 858 vs 0 | ||
| A. Lung | # Examined | 32 | 32 | 32 | 32 | |
| 1 | • Congestion | P‐value | .91 | .93 | .50 | .62 |
| 2 | • Hemorrhage; acute | P‐value | .89 | .72 | .79 | .57 |
| 3 | • Hyperplasia; bronchioloalveolar; focal | P‐value | .21 | .14 | .84 | .84 |
| B. Lymph node, bronchi | # Examined | 23 | 25 | 30 | 26 | |
| 4 | • Focus; mast cells; sinus | P‐value | .64 | .45 | .87 | .69 |
| 5 | • Hemorrhage; acute | P‐value | .97 | .95 | .76 | .63 |
| 6 | • Pigment; brown macrophages | P‐value | .21 | .59 | .28 | .70 |
| C1. Nasal turbinate level I | # Examined | 32 | 32 | 32 | 32 | |
| 7 | • Eosinophilic substance; Septum | P‐value | .58 | .79 | .58 | .77 |
| C2. Nasal turbinate level II | # Examined | 32 | 32 | 32 | 32 | |
| 8 | • Dilatation; Bowman's Gland; Duct, or Lateral Wall/Turbinate, or Septum | P‐value | .16 | .29 | .25 | .39 |
| 9 | • Eosinophilic Substance; Septum | P‐value | .64 | .65 | .64 | .64 |
| 10 | • Hemorrhage; Acute; Nasolacrimal Duct | P‐value | .37 | .50 | .50 | .66 |
| 11 | • Hyperplasia; Bowman's Gland; Septum | P‐value | .50 | .65 | .35 | .50 |
| C3. Nasal turbinate leveL III | # Examined | 32 | 32 | 32 | 32 | |
| 12 | • Dilatation; Bowman's Gland; Duct, or Lateral Wall/Turbinate, or Septum | P‐value | .50 | .50 | .63 | .63 |
| 13 | • Eosinophilic Substance; Septum | P‐value | .37 | .56 | .31 | .50 |
| C4. Nasal turbinate level IV | # Examined | 32 | 32 | 32 | 32 | |
| 14 | • Dilatation; Bowman's Gland; Septum | P‐value | .98 | .84 | .92 | .50 |
| 15 | • Hyperplasia; Bowman's Gland; Septum | P‐value | .98 | .84 | .79 | .27 |
| D. Trachea | # Examined | 32 | 32 | 32 | 31 | |
| 16 | • Dilation; Gland with Cell Debris | P‐value | .95 | .80 | .83 | .48 |
3.6. Statistical analysis for results of PAPs with minor and mild pathologic abnormal occurrences
There were a very small number of occurrences of pathologic abnormalities observed for the 25 Category‐2 PAPs, as summarized in Table 5. These 25 PAPs are grouped into six mouse organ PAP groups (ie, lung, bronchial lymph nodes, and nasal turbinate levels I, II, III, and IV). There was no Category‐2 PAP for Trachea. In total, 24 Fisher Exact Tests (FET) were conducted for the four treatments of the six Category‐2 PAP groups. The FET results demonstrate no significant difference in chronic toxicity when comparing mice under long‐term, repeated exposure of high doses of inhaled thymol (Groups‐3 and 4) versus those with no thymol inhaled (Groups‐1 and 2).
Table 5.
P‐values of chronic toxicity for inhaled thymol based on 25 Category‐2 PAPs with Fisher exact test
| Mouse organs and PAP Groups (Category‐2 PAPs) | Items | # of Occurrence, gx, |
FET P ‐values per hypotheses (8a) to (8e) |
Is thymol toxic? (meets all inequalities (6a‐6e) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | G1 | G2 | G3 | G4 | g3 > g1 | g3 > g2 | g4 > g1 | g4 > g2 | g4 ≥ g3 | ||
| Dose, d | 0 | 0 | 117 | 858 | 117 vs 0 | 117 vs 0 | 858 vs 0 | 858 vs 0 | 858 vs 117 | ||
| # of Mice | 32 | 32 | 32 | 32 | 32/32 | 32/32 | 32/32 | 32/32 | 32/32 | ||
|
A. Lung Adenoma; Bronchiolealveolar Inflammation; Chronic Macrophages; Alveolar; Focal; Subpleural Metaplasia; Osseous Mineralization; Vessel |
5 PAPs | 3 | 0 | 0 | 4 | 1.000 | n/a* | .500 | .057 | .057 | No |
|
B. Lymph Node, Bronchi Hyalinized Material; Medullary Cords; Increased High Endothelial Venules; Increased Lymphocytes; Cortex; Infiltrate; Neutrophils Focal. |
4 PAPs | 1 | 1 | 2 | 4 | .500 | .500 | .177 | .177 | .336 | No |
|
C1. Nasal Turbinate Level I Eosinophilic Globules (Droplets); Nasal Cavity; Exudate; Neutrophils; Hemorrhage; Acute; Nasolacrimal Duct |
3 PAPs | 1 | 3 | 1 | 1 | .754 | .943 | .754 | .943 | .754 | No |
|
C2. Nasal Turbinate Level II Eosinophilic Globules (Droplets); Nasal Cavity; Hyperplasia; Bowman's Gland; Lateral Wall/Turbinate; Hyperplasia; Squamous Epithelium; Nasolacrimal Duct; Increased Infiltrate; Mononuclear Cell; Dorsal Turbinate; Inflammation; Acute; Lateral Wall/Turbinate; Inflammation; Chronic; Septum. |
6 PAPs | 1 | 1 | 7 | 1 | .027 | .027 | .754 | .754 | .998 | No |
|
C3. Nasal Turbinate Level III Eosinophilic Globules (Droplets); Nasal Cavity; Hyperplasia; Bowman's Gland; Lateral Wall/Turbinate; Hyperplasia; Bowman's Gland; Septum Inflammation; Chronic; Septum |
4 PAPs | 2 | 0 | 2 | 2 | .694 | .246 | .694 | .246 | .694 | No |
|
C4. Nasal Turbinate Level IV Eosinophilic Globules (Droplets); Nasal Cavity; Eosinophilic Substance; Septum Exudate; Mucus/Hemorrhage |
3 PAPs | 0 | 1 | 0 | 2 | n/a* | 1.000 | .245 | .500 | .246 | No |
No observation from both groups, therefore no P‐values were obtained.
4. DISCUSSION
In the 6‐month chronic toxicity studies in mice, there were no test article‐related findings observed. According to the EPL evaluation, the findings in the lung were all considered incidental background findings common to this species and age or a consequence of euthanasia procedures. A benign bronchiole‐alveolar adenoma was found in the lung from a Group‐1 air control female mouse. Minimal to mild bronchiole‐alveolar hyperplasia was seen in two males, one from the vehicle group and one from the low‐dose 0.1% thymol HFA group. In the lung of one high‐dose female, the white raised area was correlated microscopically with minimal alveolar macrophage accumulation, another background finding common to this species.
For the bronchial lymph node, the findings were generally minimal and considered not to reflect toxicity of the administered test article. In one high‐dose male, minimal numbers of neutrophils were observed in the medullary cords, with no evidence of concurrent infection. In two of the high‐dose females, the high endothelial venules in the cortex of the bronchial lymph node were more prominent than observed in other lymph nodes. High endothelial venules are specialized postcapillary venous swellings that enable lymphocytes circulating in the blood to directly enter in the lymph node. High endothelial venules are a normal feature of the lymph node.
For the nasal turbinates, presence of minimal to mild eosinophilic globules (droplets) in the epithelium of the nasal cavity was observed. However, this finding occurred in only two of a total of 32 animals from the high‐dose (0.5% thymol HFA) and in one of 32 animals at the low‐dose (0.1% thymol HFA). With so few animals affected, this finding is likely related to the aging of the animal than to treatment with inhaled thymol HFA.
There were no test article‐related findings seen in the trachea in this study. Overall, it is evident that thymol inhaled by the mice during the 6‐month treatment, even in the highest study dose group (858 times of the maximum human dose from use of Epinephrine HFA) did not produce any chronic toxic effects on the lung or respiratory tracts.
The absence of histopathological evidence of any chronic toxic effects in the lung or respiratory tract from the doses of inhaled thymol administered in the 6‐month studies is likely due to the fact that the bioavailability was far below the toxic levels.
It is also worth noting that the very rapid breathing rate of the mouse 9 suggests that most of the thymol inhaled probably deposited in the nose and upper airway with relatively little actually reaching the lung. Lung scintigraphy studies in humans have shown that the faster the inspiratory flow, the less deposition of the respirable fraction of an orally administered therapeutic aerosol (MMAD 3‐5 µm) in the lower respiratory tract.10, 11, 12 Therefore, most of the dose delivered to the mice was probably deposited in the nose (with nasal breathing) and, to a lesser extent, in the oral cavity and nasopharynx and then swallowed.
The results of this chronic toxicity study are consistent with the fact that thymol is classified as Generally Recognized as Safe (GRAS) by the FDA.4
5. CONCLUSION
Overall, neither data from literature nor results from chronic toxicity studies presented herein provide any evidence for chronic toxicity of inhaled thymol. There were no nonclinical findings that would preclude the safe administration of thymol via inhalation to humans.
Based on this chronic toxicity study of mouse model, the no‐observed‐adverse‐effect level (NOAEL) of thymol by inhalation is 0.42 mg/kg for a single treatment and 1.26 mg kg−1 wk−1 for a long‐term treatment.
CONFLICT OF INTEREST
This study was financially supported by Amphastar Pharmaceuticals, Inc Dr Tashkin reports personal fees from Amphastar Pharmaceuticals, outside the submitted work. Kevin Xie, Dr Mary Z. Luo, and Dr Jack Y. Zhang are employees of Amphastar Pharmaceuticals, Inc at the time of the study and manuscript preparation.
AUTHOR CONTRIBUTIONS
Dr Jack Y. Zhang conceived the study and was in charge of overall direction and planning. Dr Jack Y. Zhang and Dr Mary Z. Luo designed the method and the computational framework and analyzed the data. Dr Jack Y. Zhang and Dr Kevin Xie carried out the implementation. Dr Mary Z. Luo wrote the manuscript with input from all authors especially from Dr Donald P. Tashkin. Dr Donald P. Tashkin provided critical feedback and helped in shaping the research, analysis, and manuscript. All authors discussed the results and contributed to the final manuscript.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank Lucy Pang of Non‐Clinical Study Group Amphastar NDRC for her Support of this work. The authors would also like to thank Pao Hean and Eric Wong for their preparation of this manuscript.
Xie K, Tashkin DP, Luo MZ, Zhang JY. Chronic toxicity of inhaled thymol in lungs and respiratory tracts in mouse model. Pharmacol Res Perspect. 2019;e00516 10.1002/prp2.516
ENDNOTE
Tidal volume is the lung volume representing the normal volume of air displaced between normal inhalation and exhalation when extra effort is not applied.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. The Montreal Protocol on Substances that Deplete the Ozone Layer. http://ozone.unep.org/new_site/en/montreal_protocol.php. (Accessed April 29, 2015).
- 2. Jenner PM, Hagan EC, Taylor JM, Cook EL, Fitzhuge OG; Research Section . Food flavourings and compounds of related structure. I. Acute oral toxicity. Food Cosmet Toxicol. 1964;2:327‐343. [DOI] [PubMed] [Google Scholar]
- 3. Hagan EC, Hansen WH, Fitzhuge OG, et al. Research Section . Food flavourings and compounds of related structure. II. Subacute and chronic toxicity. Food Cosmet Toxicol. 1967;5:141‐157. [DOI] [PubMed] [Google Scholar]
- 4. Federal Register . January 18, 2006. Thymol; Exemption from the Requirement of a Tolerance. Rules and Regulations. 71(11):2891. [Google Scholar]
- 5. Trautmann NM, Carlsen WC, Krasny ME, Cunningham CM. Assessing Toxic Risk: Student Edition. Arlington, VA: National Science Teachers Association; 2001. [Google Scholar]
- 6. EPA . 1993. RED facts: Thymol. EPA-738-F-93–011. Washington (DC): Office of Prevention, Pesticides, and Toxic Substances, United States Environmental Protection Agency. https://archive.epa.gov/pesticides/reregistration/web/pdf/3143fact.pdf. (Accessed June 8, 2015).
- 7. USEPA; Biopesticide Registration Action Document, THYMOL, 5-methyl-2-isopropyl-1-phenol (PC Code080402), March 23, 2006. https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/decision_PC-080402_23-Mar-06.pdf. (Accessed August 9, 2019).
- 8. Tveden‐Nyborg P, Bergmann TK, Lykkesfeldt J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol. 2018;123:233‐235. [DOI] [PubMed] [Google Scholar]
- 9. Gad SC, Chengelis CP. Animal Models in Toxicology. New York, NY: Marcel Dekker; 1992. [Google Scholar]
- 10. Labiris NR, Dolovich MB. Pulmonary drug delivery. Part II: The role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):600‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta2‐agonist particle size. Am J Respir Crit Care Med. 2005;172(12):1497‐1504. [DOI] [PubMed] [Google Scholar]
- 12. Lavorini F, Fontana GA, Usmani OS. New inhaler devices ‐ the good, the bad and the ugly. Respiration. 2014;88(1):3‐15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


