Abstract
Adjuvants are components of vaccine that enhance the specific immune response against co-inoculated antigens. Recently, we reported the characterization of a synthetic sulfolipid named Sulfavant A (1) as a promising candidate of a novel class of molecular adjuvants based on the sulfoquinovosyl-diacylglycerol skeleton. Here, we report an improved synthesis of the sulfolipid scaffold, as well as the preparation of two analogs named Sulfavant-S (2) and Sulfavant-R (3) with enhanced property to modulate master immune targets such as human dendritic cells (DCs). According to the present approach, synthesis of 1 is reduced from 14 to 11 steps with nearly triplication of the overall yield (11%). The new members 2 and 3 elicit DC maturation at a concentration of 10 nM, which is 1000 times more potent than the parent molecule 1. Analysis of dynamic light scattering indicates self-assembly of Sulfavants and formation of colloidal particles with a small hydrodynamic radius (50 nm) for the epimers 2 and 3 and a larger radius (150 nm) for 1. The colloidal aggregates are responsible for the bell-shaped dose–response curve of these products. We conclude that the particle size also affects the equilibrium with free monomers, thus determining the effective concentration of the sulfolipid molecule at the cellular targets and the different immunological efficacy of 1–3. Sulfavants (1–3) do not show in vitro cytotoxicity at concentrations 105 higher than the dose that triggers maximal immune response, thus predicting a low level of toxicological risk in their formulation in vaccines.
Introduction
Adjuvants are aspecific components of vaccines that improve the capacity of the immune system to build a long standing and efficient response to antigens. Adjuvants achieve these effects through different mechanisms, including modulation of T helper subsets.1−4 In the last years, there has been a considerable effort to introduce a rational approach to the identification of novel adjuvants that increase safety of vaccines and reinforce the immune response in weakened immune patients.5−10
A major breakthrough of these studies has been the identification of pathogen-associated molecular patterns and the characterization of antigen-presenting cells (APCs) as convenient targets of novel molecules or delivery systems.11 Sulfavant A (1) is a synthetic sulfolipid that activates human dendritic cells (DCs), a specific type of APCs that operate as master regulators of the initiation of adaptive immune response. Exposure of cultured DC to Sulfavant A (1) triggers the transformation of DC to the “mature” stage with upregulation of T-cell co-stimulatory factors (HLA-DR, CD83, CD86) and expression of specific cytokine subsets (e.g., IL-12p40 and INF-γ).12 In mice, this process induced antigen-specific immunization with antibody titers that are comparable to traditional adjuvants (e.g., TiterMax). In agreement with these results, vaccination with hgp10 peptide antigen and Sulfavant A elicited a protective response with reduction of tumor growth and increase of survival in the murine B16 melanoma model.12
Although the
mechanism has not been fully elucidated, activity
of Sulfavant A (1) is independent of toll-like receptor
2 and 4.12 This marks a clear difference
with other glycolipid adjuvants (e.g., monophosphoryl lipid A) currently
under investigation13−18 and suggests that the sulfoquinovosyl-glycerol backbone may be a
distinctive trait of a novel family of immunomodulators.19 The aim of the present study was the additional
characterization of the chemical determinants that affect the biological
activity of this group of molecular adjuvants, as well as the preparation
of new analogs with a higher immune efficacy. Here, we report a new
synthetic strategy of the sulfolipid scaffold and synthesis of two
epimers, named Sulfavant-S (2) and Sulfavant-R (3), that induce maturation and cytokine gene expression of
DCs at nanomolar concentrations. We also show that these compounds
form colloidal nanoparticle aggregates that are first responsible
of the difference in the cellular response to 1–3.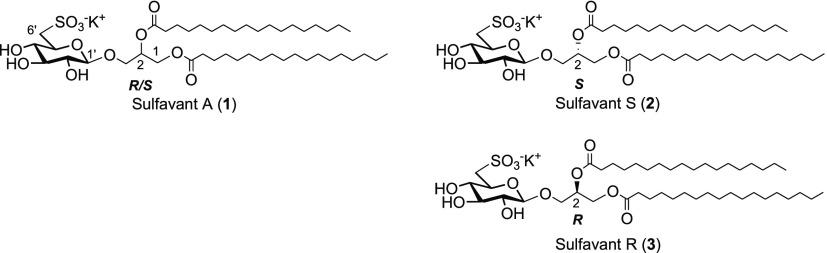
Results and Discussion
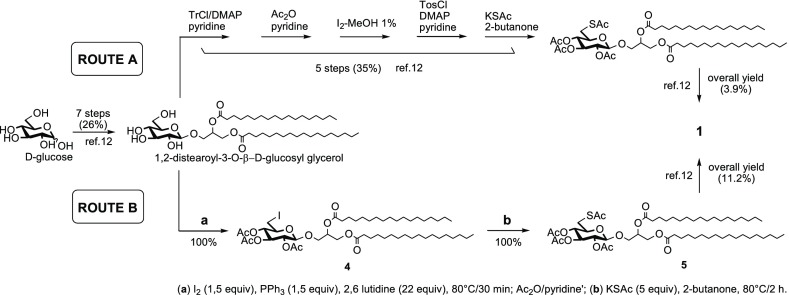
Sulfavant A (1) is an epimeric mixture at C-2 (R/S about 1.3:1) of β-sulfoquinovoside-distearoyl glycerol that is prepared starting with acetylation of d-glucose, followed by selective deacetylation of the anomeric hydroxyl group with benzylamine. Coupling with 1,2-O-isopropylidene glycerol by trichloroacetimidate methodology gave 3-O-(2′,3′,4′,6′-tetra-acetyl)-β-d-glucosyl-glycerol that was after derivatized by stearoyl groups to obtain the key intermediate 1,2-distearoyl-3-O-β-d-glucosyl glycerol.12,19 In the original work (route A of Scheme 1),12,19a sulfonation at carbon-6′ of glucose was achieved by multiple steps of protection and deprotection that affected negatively the overall synthetic yield. In order to overtake this issue, we tested the direct sulfonation of the 6′-carbon through an iodinate derivative in agreement with Traboni and co-workers.20
Scheme 1. Improved Synthesis of Sulfavant A (1).
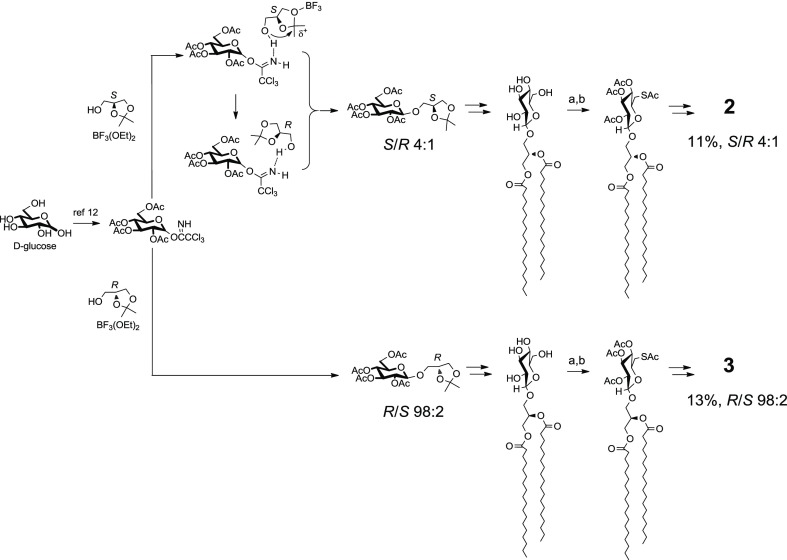
With Sulfavant A (1), iodination of 1,2-distearoyl-3-O-β-d-glucosyl glycerol followed by conversion to thioacetate reduced the number of steps from 14 to 11, as well as triplicated the overall yield from 4 to 11.2% (route B of Scheme 1). The new approach preserved the versatility of the original synthesis and was also tested on the preparation of the two epimers Sulfavant S (2) and Sulfavant R (3) from (S)- or (R)-1,2-O-isopropilidene glycerol, respectively (Scheme 2).
Scheme 2. Synthesis of Sulfavant S (2) and Sulfavant R (3); (a) I2 (1.5 equiv), PPh3 (1,5 equiv), 2,6-Lutidine (22 equiv), 80 °C/30 min; Ac2O/Pyridine; (b) KSAc (5 equiv), 2-Butanone, 80 °C/2 h; If Not Stated Otherwise, Steps Are Identical to Those Described in Ref (12).
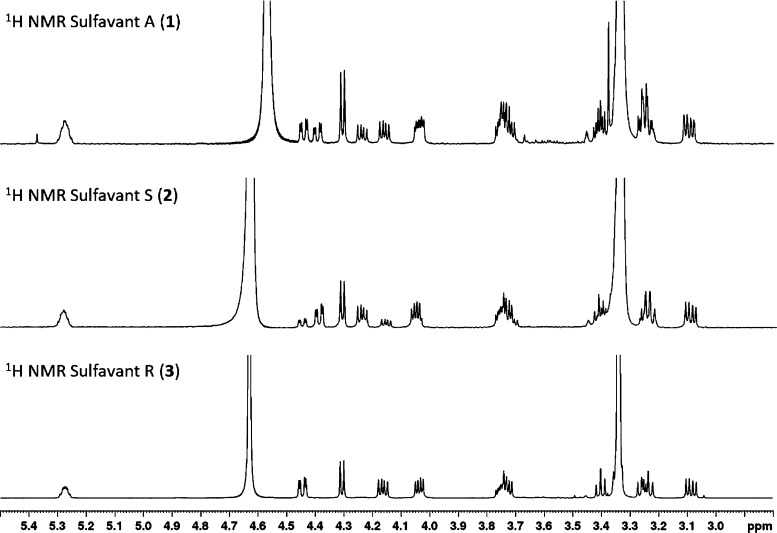
As depicted in Scheme 2, the single stereoisomers were both prepared with overall yield higher than 10%. MS and NMR data of the two new products were identical to Sulfavant A (1) in all aspects but for the signals of the protons H2-1 that fall at δ 4.40 (1H, dd, J = 2.7, 12.0 Hz, H-1a) and 4.24 (1H, dd, J = 6.9, 12.0 Hz, H-1b) in the S epimer (2) and at δ 4.45 (1H, dd, J = 2.6, 12.1 Hz, H-1a) and 4.17 (1H, dd, J = 6.7, 12.1 Hz, H-1b) in the R epimer (3) (Figure 1). While the stereochemistry of 3 is preserved throughout the sequence of reactions, synthesis of 2 showed a partial epimerization of C-2 due to the diastereoselective opening and subsequent closure of the acetonide during the coupling step. Therefore, Sulfavant S (2) was composed of a mixture of 4:1 S/R diastereomers at C-2 of glycerol (Scheme 2) and, in this respect, it was similar to Sulfavant A (1) but with a different diastereomeric ratio (S/R about 1:1.3).
Figure 1.
1H NMR (400 MHz, CD3OD/CDCl3 1/1) spectra of 1–3. Partial epimerization of Sulfavant S (2) is clearly detectable by the presence of the double doublets at 4.45 and 4.17 ppm due to the R epimer.
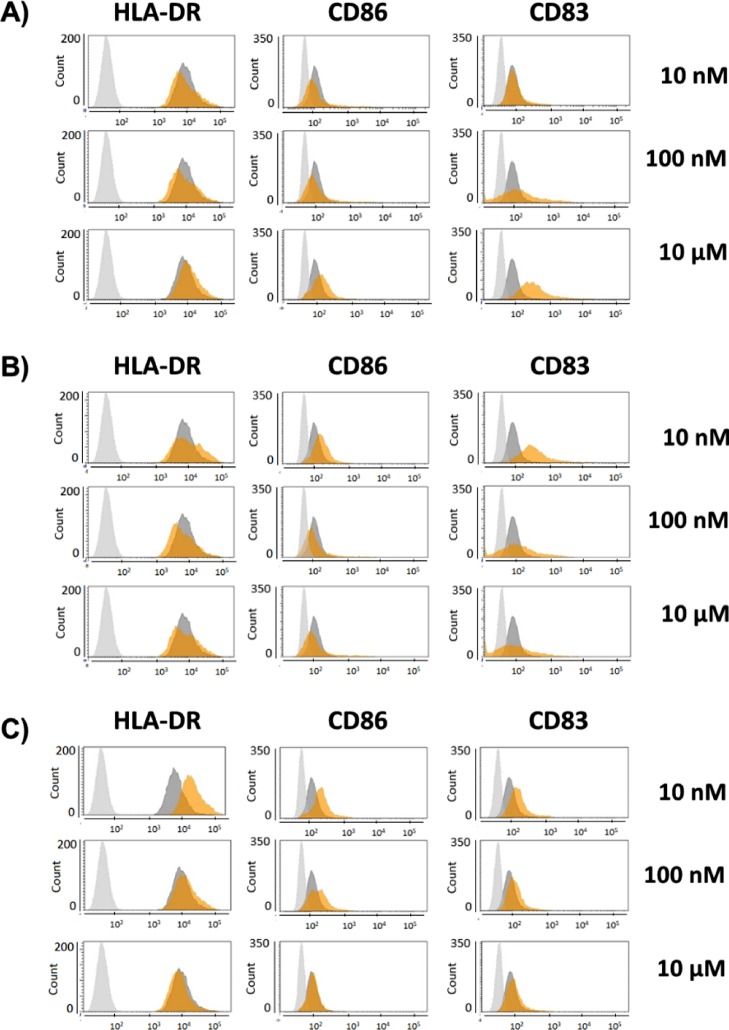
The new analogs up-regulated expression of the maturation markers HLA-DR, CD83, and CD86 at 10 nM (Figure 2). This was drastically different from the reported response to Sulfavant A (1) that triggers clear differentiation only of the CD83 + DC population at 10 μM.12 The activity of both epimers decreased at the higher concentrations even if Sulfavant S (2) stimulated residual overexpression of CD83 in the whole range. CD83 is highly expressed on mature DCs and is not detectable in other APCs that do not prime naive T cells. Thus, CD83 + DCs are considered a hallmark of the ability to prime a protective T cell response and have a profound clinical implication for vaccines against many widespread infectious diseases, including HIV-AIDS, malaria, and tuberculosis, or for therapeutic treatment of cancers.11
Figure 2.
Flow-cytometry analysis of maturation phenotyping markers (HLA-DR, CD86, CD83) in moDCs stimulated with (A) Sulfavant A (1), (B) Sulfavant S (2), and (C) Sulfavant R (3) at concentrations of 0.01, 0.1, and 10 μM; gray = isotype control; dark gray = unstimulated cells; orange = stimulated.
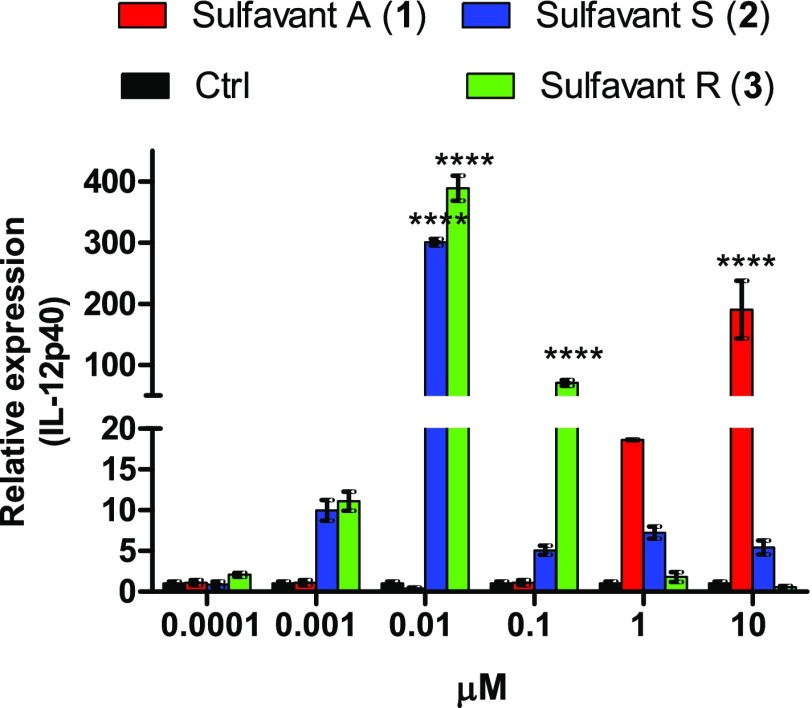
After 24 h, compounds 2 and the 3 also enhanced IL-12p40 gene expression (Figure 3) and no effect on expression of IL-10. IL-12 is a pro-inflammatory cytokine released by DC in response to infection of bacteria and virus,21,22 whereas IL-10 downregulates immune and inflammatory response and mediates many of the tolerogenic effects exerted by DCs.23,24 On the whole, the effect of 2 and 3 on these cytokines is qualitatively similar to the results previously reported with Sulfavant-A (1) even if the new analogs 2 and 3 gave a maximal activation at 10 nM, whereas 1 showed the strongest effect only at 10 μM.12 As previously noted for the surface markers, IL-12p40 expression decreased with the increase of the concentration of both epimers and only 2 conserved a residual activity at 10 μM. Compounds 1–3 did not show toxic activity on DCs and other primary cells at concentration up to 105 times higher than the effective dose (Supporting Information Figure S5).
Figure 3.
Gene expression analysis of IL-12p40 in DCs by stimulation with increasing dose of Sulfavants 1–3. Asterisks indicate significant differences from the control group at a 95% (P < 0.05) confidence level, as determined using two-way ANOVA analysis.
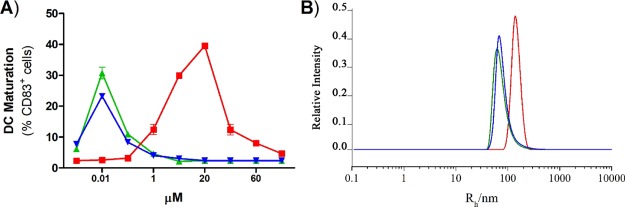
These tests indicate that 1–3 are all safe DC activators but also underline a divergent response that correlates with stereochemical aspects, as the diastereomixture 2 (S/R 4:1) and the diastereopure 3 are significantly more potent than their epimeric mixture 1 (S/R about 1:1.3). As reported by diagnostic expression of CD83 (Figure 4A), efficacy of single epimers 2 and 3 did not increase sigmoidally with concentration but followed a typical “bell-shaped” curve with decrease of activity above 10 nM. A similar response is also observable with Sulfavant A (1) but only at a concentration higher than 10 μM. Bell-shaped dose–response curves are not the rule but several drugs show this behavior. Recently, occurrence of colloidal species has been related to the biological response of these products.25 Colloidal properties are also reported to affect biological activity26−29 and chemical reactivity30 of sulfoquinovosides in aqueous or polar environment.
Figure 4.
Correlation of immunomodulatory activity vs colloidal behavior of Sulfavants 1–3. (A) Percentage of mature DCs after stimulation by 1–3. Data are expressed as mean and standard deviation from a duplicate of two independent experiments and compared to cells treated only with vehicle (Ctrl). ****P < 0.0001 vs control. (B) Hydrodynamic radius distribution of particles of 1–3 in aqueous suspension at 0.2 mM as measured by dynamic light scattering (DLS) on three independent measurements. Red = Sulfavant A (1); blue = Sulfavant S (2); green = Sulfavant R (3).
DLS is commonly used for the analysis of supramolecular lipid aggregation.31Figure 4B shows the hydrodynamic radius of the aggregates of 1–3 at 0.2 mM in Milli-Q water as measured by DLS. Sulfavant S (2) and R (3) have a smaller hydrodynamic radius (around 50 nm), whereas the self-aggregation of Sulfavant A (1) led to vesicles of 150 nm with higher size dispersity. Surface tension measurements performed with Sulfavant A, S, and R (1–3) further highlighted the marked difference between the aggregation behaviors of these molecules. Indeed, analysis of the surface tension as a function of concentrations of 1–3 showed a different slope in the premicellar region with indication of a minimum surface area per molecule (Amin) larger for Sulfavant A (1) than for Sulfavant S (2) and R (3).
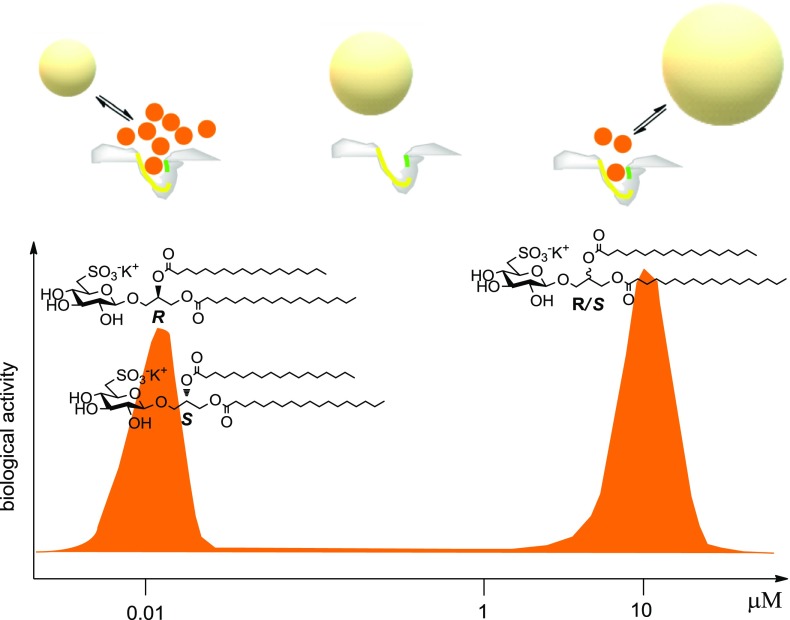
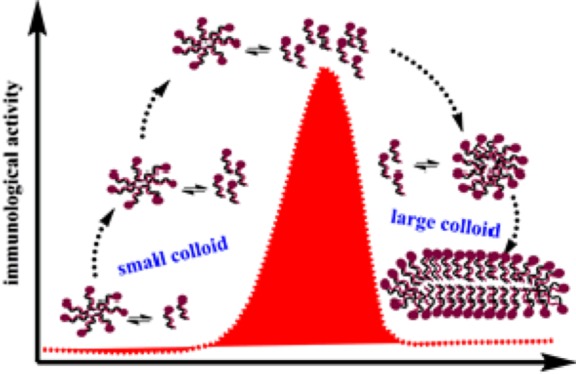
These data proved a different supramolecular organization among 1–3 in water, as well as a remarkable parallelism between self-aggregation behavior and biological response. In agreement with Shoichet and co-workers,25 we suggest that formation of colloidal particles of Sulfavants reduces the activity because it affects the effective concentration of the free sulfolipids at the target site. In line with this view, we suggest that the concentration of free monomers is higher with the small colloidal aggregates made by the epimers 2 and 3, whereas it is lower with the larger aggregates of Sulfavant A (1) (Figure 5). With the increase of the concentration, the size of both colloid particles change and the monomers are tied up, thus leading to the bell-shaped curves measured experimentally around 10 nM with 2 and 3 and around 10 μM with 1.
Figure 5.
Representation of the proposed colloidal mechanism of action of Sulfavants. Self-association of the sulfolipids into colloidal particles depends on diasteropurity of the organic molecules. With epimers 2 and 3 (left side), the concentration of free monomers in equilibrium with small aggregates is high and the products occupy effectively the receptor target at very low dose (EC50 10 nM). With the increase of the concentration, there is a gradual loss of the activity leading to a typical bell-shaped dose response curve that we attribute to occurrence of larger colloidal nanoparticles. The epimeric mixture 1 (right side) produces large aggregates that are able to hold the monomers, thus reducing the effective concentration of the monomers and the biological potency (EC50 10 μM). Increase of the concentration of 1 induces loss of activity for the formation of very large aggregates that cannot interact with the cell target in an effective manner. At the moment, we have no direct cue to explain the dependence of the size of the colloidal aggregates on the diastereomeric purity of the glycerol center. However, it is reasonable that the presence of both epimers can break the symmetry of the packing of the alkyl chains, thus leading to less dense and less tightly packed structures.
Conclusions
Modern vaccines are no longer made with inactivated or attenuated pathogens, indeed they use pathogen-related proteins obtained by molecular biology techniques. Therefore, all vaccines require adjuvants to stimulate innate immune cells or additional receptors on lymphocytes such as complement receptors.32 Here, we report an enhancement of the synthesis and activity of immunomodulatory compounds based on the sulfoquinovoside-glycerol skeleton. The two new analogs, Sulfavant S (2) and Sulfavant R (3), trigger maturation of the innate immune DCs at 10 nM, which is 3 orders of magnitude lower than the prototype molecule Sulfavant A (1). The increase of the biological potency correlates with the assembling of different colloidal particles that is dependent on diasteropurity of 1–3. We suggest that epimers 2 and 3 can form aggregates smaller and less “cohesive”, thus in equilibrium with a “more effective” fraction of monomers that can freely diffuse and interact with cell targets. The colloid hypothesis also well explains the bell-shaped dose–response curve that seems to be typical of this family of compounds. Notably, it has been already reported that stereochemical characteristics can determine the supramolecular organization of amphipathic substances and change the biological activity by interfering with interaction and binding affinity with protein and cellular structures.33−37 DCs are emerging as a critical cell type in controlling the immune response; therefore, the stimulation of DC by Sulfavants must be considered a promising feature to generate therapeutic vaccines and, in view of in vivo tests, the formation of stable self-aggregates and the absence of in vitro toxic effects are predictive of a low level of toxicological risk.
Experimental Section
General Experimental Procedures
NMR spectra were recorded on a Bruker AVANCE-400 (400.13 MHz). HR-MS spectra were acquired by a Q Exactive Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Scientific). TLC plates (Kieselgel 60 F254) and silica gel powder (Kieselgel 60, 0.063–0.200 mm) were from Merck.
All the reagents were purchased from Sigma-Aldrich and used without any further purification. DLS measurements were performed with a home-made instrument composed by a Photocor compact goniometer, an SMD 6000 Laser Quantum 50 mW light source operating at 5325 Å, a photomultiplier (PMT-120-OP/B), and a correlator (Flex02-01D, correlator.com). The surface tension of aqueous Sulfavant samples was measured with a Sigma 70 tensiometer (KSV, Stockholm, Sweden) using the Du Noüy ring method.
1,2-O-Isopropilidene-3-O-[(2′,3′,4′,6′-tetra-O-acetyl)-β-D-glucosyl]-S-glycerol
1H NMR (400 MHz, CDCl3): δ 5.19 (1H, bt, J = 9.4 Hz, H-3′), 5.06 (1H, bt, J = 9.9 Hz, H-4′), 4.99 (1H, bt, J = 8.4 Hz, H-2′), 4.60 (1H, d, J = 7.8 Hz, H-1′), 4.25 (1H, dd, J = 5.0, 12 Hz, H-1a), 4.14–3.69 (6H, overlapped, H-1b, H-2, H2-6′, H-5′, H-3a), 3.62 (1H, dd, J = 5.9, 10.7 Hz, H-3b), 2.08 (3H, s, OAc), 2.04 (3H, s, OAc), 2.01 (3H, s, OAc), 1.99 (3H, s, OAc), 1.40 (3H, s, CH3), 1.33 (3H, s, CH3); the spectrum 1H NMR showed traces of signals related to the epimer R; HRESIMS m/z: 485.1639 [M + Na]+ (calcd for C20H30O12Na, 485.1635).
1,2-Di-O-stearoyl-3-O-β-D-glucosyl-S-glycerol
1H NMR (400 MHz, CDCl3): δ 5.24 (1H, m, H-2), 4.38 (1H, dd, J = 2.5, 12.0 Hz, H-1a), 4.29 (1H, d, J = 7.5 Hz, H-1′), 4.16 (1H, dd, J = 6.7, 12.0 Hz, H-1b), 3.88 (1H, dd, J = 5.1, 10.6 Hz, H-3a), 3.81 (2H, m, H2-6′), 3.68 (1H, dd, J = 6.9, 10.6 Hz, H-3b), 3.55 (1H, bt, J = 9.1 Hz, H-3′), 3.49 (1H, bt, J = 9.1 Hz, H-4′), 3.34 (1H, bt, J = 8.0 Hz, H-2′), 3.29 (1H, m, H-5′), 2.28 (4H, overlapped, α-methylenes of acyl portions), 1.65–1.52 (4H, overlapped, β-methylenes of acyl portions), 1.33–1.19 (aliphatic methylenes), 0.86 (6H, bt, J = 6.3 Hz, 2CH3); the spectrum 1H NMR showed traces of signals related to the epimer R; HRESIMS m/z: 809.6116 [M + Na]+ (calcd for C45H86O10Na, 809.6119).
1,2-Di-O-stearoyl-3-O-[(2′,3′,4′-tri-O-acetyl-6′-thioacetyl)-β-D-glucosyl]-S-glycerol
1H NMR (400 MHz, CDCl3): δ 5.19–5.10 (2H, overlapped, H-2, H-3′), 4.97–4.88 (2H, overlapped, H-2′, H-4′), 4.47 (1H, d, J = 8.0 Hz, H-1′), 4.27 (1H, dd, J = 3.5, 10.0 Hz, H-1a), 4.09 (1H, dd, J = 6.4, 10.0 Hz, H-1b), 3.88 (1H, dd, J = 4.7, 11.0 Hz, H-3a), 3.64 (1H, dd, J = 5.3, 11.0 Hz, H-3b), 3.60 (1H, m, H-5′), 3.22 (1H, dd, J = 2.4, 14.3 Hz, H-6′a), 3.03 (1H, dd, J = 6.9, 14.3 Hz, H-6′b), 2.32 (3H, s, SAc), 2.27 (4H, bt, J = 7.0 Hz, α-methylenes of acyl portions), 2.05 (3H, s, OAc), 2.01 (3H, s, OAc), 1.96 (3H, s, OAc), 1.63–1.54 (4H, overlapped, β-methylenes of acyl portions), 1.32–1.19 (aliphatic methylenes), 0.85 (6H, bt, J = 6.2 Hz, 2CH3); the spectrum 1H NMR showed traces of signals related to the epimer R; HRESIMS m/z: 993.6302 [M + Na]+ (calcd for C53H94NaO13S, 993.6313).
1,2-O-Isopropilidene-3-O-[(2′,3′,4′,6′-tetra-O-acetyl)-β-D-glucosyl]-R-glycerol
1H NMR (400 MHz, CDCl3): δ 5.13 (1H, bt, J = 9.4 Hz, H-3′), 4.99 (1H, bt, J = 9.8 Hz, H-4′), 4.89 (1H, bt, J = 8.4 Hz, H-2′), 4.53 (1H, d, J = 7.8 Hz, H-1′), 4.18–3.95 (3H, overlapped, H2-1, H-2) 3.72–3.51 (5H, overlapped, H2-6′, H-5′, H2-3), 2.00 (3H, s, OAc), 1.97 (3H, s, OAc), 1.94 (3H, s, OAc), 1.92 (3H, s, OAc), 1.35 (3H, s, CH3), 1.30 (3H, s, CH3); HRESIMS m/z: 485.1641 [M + Na]+ (calcd for C20H30O12Na, 485.1635).
1,2-Di-O-stearoyl-3-O-β-D-glucosyl-R-glycerol
1H NMR (400 MHz, CDCl3): δ 5.25 (1H, m, H-2), 4.34 (1H, dd, J = 2.7, 12.0 Hz, H-1a), 4.26 (1H, d, J = 7.5 Hz, H-1′), 4.10 (1H, dd, J = 6.7, 12.0 Hz, H-1b), 3.87 (1H, dd, J = 5.9, 11.0 Hz, H-3a), 3.77 (2H, overlapped, H2-6′), 3.67 (1H, dd, J = 5.9, 11.0 Hz, H-3b), 3.47 (1H, bt, J = 8.9 Hz, H-3′), 3.42 (1H, bt, J = 8.9 Hz, H-4′), 3.25–3.20 (2H, overlapped, H-2′, H-5′), 2.26 (4H, overlapped, α-methylenes of acyl portions), 1.58–1.51 (4H, overlapped, β-methylenes of acyl portions), 1.27–1.18 (aliphatic methylenes), 0.83 (6H, bt, J = 6.4 Hz, 2CH3); HRESIMS m/z: 809.6112 [M + Na]+ (calcd for C45H86O10Na, 809.6119).
1,2-Di-O-stearoyl-3-O-[(2′,3′,4′-tri-O-acetyl-6′-thioacetyl)-β-D-glucosyl]-R-glycerol
1H NMR (400 MHz, CDCl3): δ 5.18–5.11 (2H, overlapped, H-2, H-3′), 4.98–4.91 (2H, overlapped, H-4′, H-2′), 4.47 (1H, d, J = 8.0 Hz, H-1′), 4.27 (1H, dd, J = 3.8, 12.0 Hz, H-1a), 4.07 (1H, dd, J = 6.3, 12.0 Hz, H-1b), 3.90 (1H, dd, J = 4.9, 11.0 Hz, H-3a), 3.65 (1H, dd, J = 5.6, 11.0 Hz, H-3b), 3.62 (1H, m, H-5′), 3.24 (1H, dd, J = 2.7, 14.0 Hz, H-6′a), 3.05 (1H, dd, J = 6.9, 14.0 Hz, H-6′b), 2.33 (3H, s, SAc), 2.32–2.26 (4H, overlapped, α-methylenes of acyl portions), 2.10–1.98 (9H, s, 3OAc), 1.63–1.56 (4H, m, β-methylenes of acyl portions), 1.32–1.20 (aliphatic methylenes), 0.87 (6H, bt, J = 6.5 Hz, 2CH3); HRESIMS m/z: 993.6321 [M + Na]+ (calcd for C53H94NaO13S, 993.6313).
1,2-Di-O-stearoyl-3-O-[(2′,3′,4′-tri-O-acetyl-6′-iodo)-β-D-quinovosyl]-R/S-glycerol (4)
Iodine (49 mg, 0.191 mmol) was added to a mixture of 1,2-distearoyl-3-O-β-d-glucosyl glycerol (100 mg, 0.127 mmol), triphenylphosphine (50 mg, 0.191 mmol), and 2,6-dimethylpyridine (450 mg, 4.2 mmol) at temperature of 80 °C; the mixture was stirred for 30 min at 80 °C and subsequently acetylated by addition of pyridine (0.5 mL) and acetic anhydride (0.5 mL); after evaporation of the solvent under a stream of nitrogen, the mixture was purified by silica gel chromatography using a gradient of petroleum ether/diethylether to give compound 4 (195 mg, 0.191 mmol, 100%) as a colorless oil; 1H NMR (400 MHz, CDCl3): δ 5.21–5.16 (2H, overlapped, H-2, H-3′), 4.99–4.94 (1H, m, H-2′), 4.87 (t, J = 8.03 Hz, H-4′), 4.56 and 4.55 (each 1H, d, J = 8.1 Hz, H-1′ of the two epimers), 4.33–428 (1H, m, H-1a), 4.17–4.09 (1H, m, H-1b), 4.02–3.97 (1H, m, H-3a), 3.78–3.71 (1H, m, H-3b), 3.52 (1H, m, H-5′), 3.28 (1H, dd, J = 3.06, 11.1 Hz, H-6′a), 3.13 (1H, dd, J = 8.4, 11.1 Hz, H-6′b), 2.35–2.26 (4H, overlapped, α-methylenes of stearoyl portions), 2.07–1.97 (9H, s, OAc), 1.64–1.58 (4H, overlapped, β-methylenes of stearoyl portions), 1.33–1.21 (60H, aliphatic methylenes), 0.91–0.84 (6H, overlapped, 2CH3); HRESIMS m/z: 1045.5460 [M + Na]+ (calcd for C51H91NaO12I, 1045.5453).
1,2-Distearoyl-3-O-[(2′,3′,4′-tri-acetyl-6′-thioacetyl)-β-D-glucosyl]-R/S-glycerol (5)
1,2-distearoyl-3-O-[(2′,3′,4′-tri-acetyl-6′-iodo)-β-d-glucosyl]-glycerol (4) (170 mg, 0.166 mmol) was dissolved in 2-butanone (15 mL) and potassium thioacetate (94 mg, 0.830 mmol). The reaction mixture was stirred at 80 °C for 2 h, and then, the solvent was evaporated under reduced pressure. The resulting material was purified by silica gel chromatography using a light petroleum ether/diethyl ether gradient to give 1,2-distearoyl-3-O-[(2′,3′,4′-tri-acetyl-6′-thioacetyl)-β-d-glucosyl]-glycerol (161 mg, 0.166 mmol, 100%) as a colorless oil; 1H NMR (400 MHz,CDCl3): δ 5.20–5.14 (2H, m, H-2, H-3′), 4.96–4.89 (2H, m, H-2′, H-4′), 4.50 (1H, d, J = 8.0 Hz, H-1′), 4.28 (1H, dd, J = 4.1, 11.8 Hz, H-1a), 4.09 (1H, dd, J = 5.7, 11.8 Hz, H-1b), 3.91 (1H, dd, J = 4.5, 11.1 Hz, H-3a), 3.65 (1H, dd, J = 5.4, 11.1 Hz, H-3b), 3.62 (1H, m, H-5′), 3.25 (1H, bd, J = 11.4 Hz, H-6′a), 3.06 (1H, dd, J = 2.4 Hz, 11.4 Hz), 2.35 (3H, s, SAc), 2.33–2.29 (4H, m, α-methylene of stearoyl portion), 2.13–1.99 (9H, s, 3OAc), 1.64–1.57 (4H, m, β-methylene of stearoyl portion), 1.32–1.23 (60H, aliphatic methylenes), 0.93–0.87 (6H, overlapped, 2CH3); HRESIMS m/z: 993.6329 [M + Na]+ (calcd for C53H94O13NaS, 993.6313).
Sulfavant S (2)
White solid; 1H NMR (400 MHz, CD3OD/CDCl3 1/1): δ values are referred to CHD2OD (3.34 and 49.0 ppm): 5.28 (1H, m, H-2), 4.40 (1H, dd, J = 2.7, 12.0 Hz, H-1a), 4.31 (1H, d, J = 7.6 H-1′), 4.24 (1H, dd, J = 6.9, 12.0 Hz, H-1b), 4.05 (1H, dd, J = 5.4, 11.0 Hz, H-3a), 3.79–3.71 (3H, H-3b, H-3′, H-4′), 3.41 (1H, bt, J = 8.9 Hz, H-2′), 3.26 (1H, H-6′a), 3.25 (1H, H-5′), 3.09 (1H, dd, J = 7.2, 15.7 Hz, H-6′b), 2.36–2.27 (4H, α-methylenes of stearoyl portions), 1.65–1.56 (4H, β-methylenes of stearoyl portions), 1.36–1.20 (60H, aliphatic methylenes), 0.89 (6H, bt, J = 6.0 Hz, 2CH3); 13C NMR (100 MHz, CD3OD/CDCl3 1/1): δ 174.1, 173.7 (C, acyl esters of stearoyl part), 103.2 (CH, C1′), 76.1 (CH, C2′), 73.8 (CH, C5′), 72.4 (CH, C3′), 72.3 (CH, C4′), 70.2 (CH, C2), 68.2 (CH2, C3), 63.2 (CH2, C1), 53.6 (CH2, C6′), 34.2 (CH2, α-methylene of stearoyl portion), 32.2–29.0 (CH2, methylenes of stearoyl portion), 24.9 (CH2, β-methylene of stearoyl portion), 13.8 (CH3, methyls of stearoyl portion); HRESIMS m/z: 849.5772 [M – K]− (calcd for C45H85O12S–, 849.5767).
Sulfavant R (3)
White solid; 1H NMR (400 MHz, CD3OD/CDCl3 1/1): δ values are referred to CHD2OD at 3.34 and 49.0 ppm): δ 5.28 (1H, m, H-2), 4.45 (1H, dd, J = 2.6, 12.1 Hz, H-1a), 4.31 (1H, d, J = 7.7 Hz, H-1′), 4.17 (1H, dd, J = 6.7, 12.1 Hz, H-1b), 4.05 (1H, dd, J = 5.2, 11.1 Hz, H-3a), 3.78–3.71 (3H, overlapped, H-3b, H-3′, H-4′), 3.40 (1H, bt, J = 8.7 Hz, H-2′), 3.26 (1H, H-5′), 3.24 (1H, H-6′a), 3.09 (1H, dd, J = 7.2, 15.7 Hz, H-6′b), 2.35–2.29 (4H, α-methylenes of stearoyl portions), 1.64–1.57 (4H, β-methylenes of stearoyl portions), 1.32–1.22 (60H, aliphatic methylenes), 0.88 (6H, bt, J = 6.9 Hz, 2CH3); 13C NMR (100 MHz, CD3OD/CDCl3 1/1): δ 174.0, 173.8 (C, acyl esters of stearoyl part), 103.1 (CH, C1′), 76.3 (CH, C2′), 73.5 (CH, C5′), 72.3 (CH, C3′), 72.2 (CH, C4′), 70.5 (CH, C2), 68.1 (CH2, C3), 63.1 (CH2, C1), 53.2 (CH2, C6′), 34.1 (CH2, α-methylene of stearoyl portion), 32.5–29.2 (CH2, methylenes of stearoyl portion), 24.8 (CH2, β-methylene of stearoyl portion), 13.7 (CH3, methyls of stearoyl portion); HRESIMS m/z: 849.5775 [M – K]− (calcd for C45H85O12S–, 849.5767).
Characterization of Colloidal Nanoparticles
After purification by high-performance liquid chromatography, samples were prepared in 1 mL of Millipore water at 0.2 mM (170 μg) of each compound. After sonication for 40 min at 35 °C, the solutions were maintained at room temperature (20 °C) for 24 h. The mean diffusion coefficient was obtained as an average of at least three measurements at 25 °C. Stability of the systems over time (1 week) was systematically controlled by the reproducibility of the diffusion coefficients.
Acknowledgments
A.F. thanks BioSEArch SRL for the generous support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b03304.
Experimental procedures including DLS, human monocytes–DC differentiation, cell staining and stimulation, real-time PCR analysis, cytotoxic assay on moDCs, and surface tension measures; flow-cytometry analysis of maturation phenotyping markers in moDCs stimulated with Sulfavants in different concentrations; and copies of 1H NMR spectra of compounds 2–5, as well as copies of 13C NMR spectra of compounds 2 and 3 (PDF)
Author Contributions
∥ E.M. and C.G. contributed equally.
This study was carried out in the frame of the project “Antigens and adjuvants for vaccines and immunotherapy” (PON01_00117) funded by the National Operational Program for Research and Competitiveness 2007–2013.
The authors declare no competing financial interest.
Supplementary Material
References
- Aguilar J. C.; Rodríguez E. G. Vaccine adjuvants revisited. Vaccine 2007, 25, 3752–3762. 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- Tritto E.; Mosca F.; De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine 2009, 27, 3331–3334. 10.1016/j.vaccine.2009.01.084. [DOI] [PubMed] [Google Scholar]
- Rappuoli R.; Mandl C. W.; Black S.; De Gregorio E. Vaccines for the twenty-first century society. Nat. Rev. Immunol. 2011, 11, 865–872. 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsdottir T.; Lindqvist M.; Harandi A. M. Molecular signatures of vaccine adjuvants. Vaccine 2015, 33, 5302–5307. 10.1016/j.vaccine.2015.04.099. [DOI] [PubMed] [Google Scholar]
- Xu F.; Valiante N. M.; Ulmer J. B.. Small molecule immunopotentiators as vaccine adjuvants. In Vaccine Adjuvants and Delivery Systems; Singh M., Ed.; John Wiley & Sons, Inc, 2007; pp 175–189. [Google Scholar]
- Egli A.; Santer D. M.; Barakat K.; Zand M.; Levin A.; Vollmer M.; Weisser M.; Khanna N.; Kumar D.; Tyrrell D. L.; Houghton M.; Battegay M.; O’Shea D. Vaccine adjuvants—Understanding molecular mechanisms to improve vaccines. Swiss Med. Wkly. 2014, 144, w13940. 10.4414/smw.2014.13940. [DOI] [PubMed] [Google Scholar]
- De Gregorio E.; D’Oro U.; Wack A. Immunology of TLR-independent vaccine adjuvants. Curr. Opin. Immunol. 2009, 21, 339–345. 10.1016/j.coi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Wu T. Y. H.; Singh M.; Miller A. T.; De Gregorio E.; Doro F.; D’Oro U.; Skibinski D. A. G.; Mbow M. L.; Bufali S.; Herman A. E.; Cortez A.; Li Y.; Nayak B. P.; Tritto E.; Filippi C. M.; Otten G. R.; Brito L. A.; Monaci E.; Li C.; Aprea S.; Valentini S.; Calabro S.; Laera D.; Brunelli B.; Caproni E.; Malyala P.; Panchal R. G.; Warren T. K.; Bavari S.; O’Hagan D. T.; Cooke M. P.; Valiante N. M. Rational design of small molecules as vaccine adjuvants. Sci. Transl. Med. 2014, 6, 263ra160. 10.1126/scitranslmed.3009980. [DOI] [PubMed] [Google Scholar]
- De Gregorio E.; Rappuoli R. From empiricism to rational design: a personal perspective of the evolution of vaccine development. Nat. Rev. Immunol. 2014, 14, 505–514. 10.1038/nri3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. A.; Baldridge J. R.. TLR4 Agonists as vaccine adjuvants; In Vaccine Adjuvants and Delivery Systems; Singh M., Ed.; John Wiley & Sons, Inc, 2007; pp 131–156. [Google Scholar]
- Coffman R. L.; Sher A.; Seder R. A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo E.; Cutignano A.; Pagano D.; Gallo C.; Barra G.; Nuzzo G.; Sansone C.; Ianora A.; Urbanek K.; Fenoglio D.; Ferrera F.; Bernardi C.; Parodi A.; Pasquale G.; Leonardi A.; Filaci G.; De Palma R.; Fontana A. A new marine-derived sulfoglycolipid triggers dendritic cell activation and immune adjuvant response. Sci. Rep. 2017, 7, 6286. 10.1038/s41598-017-05969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. T.; Cluff C. W.; Johnson D. A.; Lacy M. J.; Persing D. H.; Baldridge J. R. Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi.529. Expert Rev. Vaccines 2003, 2, 219–229. 10.1586/14760584.2.2.219. [DOI] [PubMed] [Google Scholar]
- Cluff C. W. Monophosphoryl lipid A (MPL) as an adjuvant for anti-cancer vaccines: clinical results. Adv Exp Med Biol 2010, 667, 111–123. 10.1007/978-1-4419-1603-7_10. [DOI] [PubMed] [Google Scholar]
- Baldrick P.; Richardson D.; Elliott G.; Wheeler A. W. Safety Evaluation of Monophosphoryl Lipid A (MPL): An Immunostimulatory Adjuvant. Regul. Toxicol. Pharmacol. 2002, 35, 398–413. 10.1006/rtph.2002.1541. [DOI] [PubMed] [Google Scholar]
- Kensil C. R.; Kammer R. QS-21: a water-soluble triterpene glycoside adjuvant. Expert Opin. Invest. Drugs 1998, 7, 1475–1482. 10.1517/13543784.7.9.1475. [DOI] [PubMed] [Google Scholar]
- Kensil C. R.; Patel U.; Lennick M.; Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 1991, 146, 431–437. [PubMed] [Google Scholar]
- Garçon N.; Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing Adjuvant Systems. Expert Rev. Vaccines 2011, 10, 471–486. 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- a Manzo E.; Fioretto L.; Pagano D.; Nuzzo G.; Gallo C.; De Palma R.; Fontana A. Chemical synthesis of marine-derived sulfoglycolipids, a new class of molecular adjuvants. Mar. Drugs 2017, 15, 288. 10.3390/md15090288. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Manzo E.; Ciavatta M. L.; Pagano D.; Fontana A. An efficient and versatile chemical synthesis of bioactive glyco-glycerolipids. Tetrahedron Lett. 2012, 53, 879–881. 10.1016/j.tetlet.2011.12.030. [DOI] [Google Scholar]
- Traboni S.; Bedini E.; Iadonisi A. Solvent-Free Conversion of Alcohols to Alkyl Iodides and One-Pot Elaborations Thereof. ChemistrySelect 2018, 3, 1616–1622. 10.1002/slct.201800130. [DOI] [Google Scholar]
- Teng M. W. L.; Bowman E. P.; McElwee J. J.; Smyth M. J.; Casanova J.-L.; Cooper A. M.; Cua D. J. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 2015, 21, 719–729. 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- Kaka A. S.; Foster A. E.; Weiss H. L.; Rooney C. M.; Leen A. M. Using Dendritic Cell Maturation and IL-12 Producing Capacity as Markers of Function: A Cautionary Tale. J. Immunother. 2008, 31, 359–369. 10.1097/cji.0b013e318165f5d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E. J.; Everts B. Dendritic cell metabolism. Nat. Rev. Immunol. 2015, 15, 18–29. 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper K. N.; Blount D. G.; Riley E. M. IL-10: The Master Regulator of Immunity to Infection. J. Immunol. 2008, 180, 5771–5777. 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Owen S. C.; Doak A. K.; Ganesh A. N.; Nedyalkova L.; McLaughlin C. K.; Shoichet B. K.; Shoichet M. S. Colloidal Drug Formulations Can Explain “Bell-Shaped” Concentration-Response Curves. ACS Chem. Biol. 2014, 9, 777–784. 10.1021/cb4007584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K.; Sakai H.; Takeuchi R.; Tsuchiya K.; Ohta K.; Sugawara F.; Abe M.; Sakaguchi K. Effective form of sulfoquinovosyldiacyglycerol (SQDG) vesicles for DNA polymerase inhibition. Colloids Surf., B 2005, 46, 175–181. 10.1016/j.colsurfb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y.; Sahara H.; Takenouchi M.; Matsumoto Y.; Imai A.; Fujita T.; Tamura Y.; Takahashi N.; Gasa S.; Matsumoto K.; Ohta K.; Sugawara F.; Sakaguchi K.; Jimbow K.; Sato N. Inhibition of CD62L+ T-cell response in vitro via a novel sulfo-glycolipid, β-SQAG9 liposome that binds to CD62L molecule on the cell surface. Cell. Immunol. 2004, 232, 105–115. 10.1016/j.cellimm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Aoki S.; Ohta K.; Matsumoto K.; Sakai H.; Abe M.; Miura M.; Sugawara F.; Sakaguchi K. An emulsion of sulfoquinovosylacylglycerol with long-chain alkanes increases its permeability to tumor cells. J. Membr. Biol. 2006, 213, 11–18. 10.1007/s00232-006-0054-x. [DOI] [PubMed] [Google Scholar]
- Matsumoto K.; Takenouchi M.; Ohta K.; Ohta Y.; Imura T.; Oshige M.; Yamamoto Y.; Sahara H.; Sakai H.; Abe M.; Sugawara F.; Sato N.; Sakaguchi K. Design of vesicles of 1,2-di-O-acyl-3-O-(β-d-sulfoquinovosyl)-glyceride bearing two stearic acids (β-SQDG-C18), a novel immunosuppressive drug. Biochem. Pharmacol. 2004, 68, 2379–2386. 10.1016/j.bcp.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Pagano D.; Cutignano A.; Manzo E.; Tinto F.; Fontana A. Glycolipids synthesis: improved hydrazinolysis conditions for preparation of 1,2-polyunsaturated fatty acyl-β-monogalactosyl-glycerols. Carbohydr. Res. 2016, 424, 21–23. 10.1016/j.carres.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Vaccaro M.; Mangiapia G.; Paduano L.; Gianolio E.; Accardo A.; Tesauro D.; Morelli G. Structural and Relaxometric Characterization of Peptide Aggregates Containing Gadolinium Complexes as Potential Selective Contrast Agents in MRI. ChemPhysChem 2007, 8, 2526–2538. 10.1002/cphc.200700505. [DOI] [PubMed] [Google Scholar]
- Bergmann-Leitner E.; Leitner W. Adjuvants in the Driver’s Seat: How Magnitude, Type, Fine Specificity and Longevity of Immune Responses Are Driven by Distinct Classes of Immune Potentiators. Vaccines 2014, 2, 252–296. 10.3390/vaccines2020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemet A.; Lemiègre L.; Lambert O.; Benvegnu T. How the stereochemistry of a central cyclopentyl ring influences the self-assembling properties of archaeal lipid analogues: Synthesis and cryoTEM observations. J. Org. Chem. 2011, 76, 9738–9747. 10.1021/jo201827h. [DOI] [PubMed] [Google Scholar]
- Jaeger D. A.; Kubicz-Loring E.; Price R. C.; Nakagawa H. Vesicular Properties of Stereoisomeric Surfactants. Langmuir 1996, 12, 5803–5808. 10.1021/la960482l. [DOI] [Google Scholar]
- Aleandri S.; Bonicelli M. G.; Bordi F.; Casciardi S.; Diociaiuti M.; Giansanti L.; Leonelli F.; Mancini G.; Perrone G.; Sennato S. How stereochemistry affects the physicochemical features of gemini surfactant based cationic liposomes. Soft Matter 2012, 8, 5904. 10.1039/c2sm25193k. [DOI] [Google Scholar]
- Bombelli C.; Stringaro A.; Borocci S.; Bozzuto G.; Colone M.; Giansanti L.; Sgambato R.; Toccaceli L.; Mancini G.; Molinari A. Efficiency of liposomes in the delivery of a photosensitizer controlled by the stereochemistry of a gemini surfactant component. Mol. Pharm. 2010, 7, 130–137. 10.1021/mp900173v. [DOI] [PubMed] [Google Scholar]
- Fuhrhop J. H.; Schnieder P.; Boekema E.; Helfrich W. Lipid bilayer fibers from diastereomeric and enantiomeric N-octylaldonamides. J. Am. Chem. Soc. 1988, 110, 2861–2867. 10.1021/ja00217a028s. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.