Abstract
Objectives:
To assess the prevalence of atherosclerotic cardiovascular disease (ASCVD) and its individual phenotypes of coronary artery disease (CAD), peripheral artery disease (PAD), and cerebrovascular disease (CVD) by age and sex in a large cohort of patients with SLE hospitalized in the United States.
Methods:
A nested case-control study of adults with and without SLE was conducted from the January 1, 2008-December 31, 2014 National Inpatient Sample. Patients hospitalized with a diagnosis of SLE were matched (1:3) by age, sex, race, and calendar year to patients hospitalized without a diagnosis of SLE. The prevalence of CAD, PAD, and CVD were evaluated and associations with SLE were determined after adjustment for common cardiovascular risk factors.
Results:
Among the 252,676 patients with SLE and 758,034 matched patients without SLE, the mean age was 51, 89% were women, and 49% were white. Patients with SLE had a higher prevalence of ASCVD compared to those without SLE (25.6% vs. 19.2%; OR 1.45, 95% CI 1.44 to 1.47, P<.001). After multivariable adjustment, SLE was associated with a greater odds of ASCVD (aOR 1.46, 95% CI 1.41–1.51). The association between SLE and ASCVD was observed in women and men and was attenuated with increasing age. SLE was also associated with increased odds of CAD (aOR 1.42, 95% CI 1.40–1.44), PAD (aOR 1.25, 95% CI 1.22–1.28), and CVD (aOR 1.68, 95% CI 1.65–1.71).
Conclusions:
Among patients hospitalized in the United States, SLE is associated with increased prevalence of ASCVD. The increased prevalence of ASCVD in SLE was observed in both sexes and was greatest in younger patients.
Keywords: Coronary Artery Disease, Lupus, Peripheral Artery Disease, Stroke, Systemic lupus erythematosus, Thrombosis
Introduction
Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune disorder that affects 20–150 per 100,000 individuals, with a majority of cases (70–90%) occurring in women.1 In the United States, individuals of African, Hispanic, or Asian ancestry have an increased incidence and prevalence of SLE compared with individuals of European ancestry.2,3 Infection, nephritis, and atherosclerotic cardiovascular disease (ASCVD), including myocardial infarction, stroke, and peripheral artery disease, are common complications of SLE that result in significant morbidity and mortality.4,5 Although causal mechanisms have not been firmly established, ASCVD in SLE is believed to be related to chronic inflammation,6,7 increased platelet activity,8,9 increased oxidative stress,10,11 a hypercoagulable state,12 endothelial dysfunction,13,14 chronic kidney disease,23 and dyslipidemia.15,16
ASCVD occurs more frequently in SLE patients compared with age and gender matched controls,17–20 but the magnitude of this increased risk is uncertain and associations between age, sex, and the prevalence of ASCVD in SLE are imprecisely characterized.5,21–24 Although prior literature demonstrates a 50-fold higher risk of myocardial infarction in SLE women 35–44 years of age, these data are based upon small sample sizes with low event rates.5 Existing data do not quantify the prevalence of ASCVD and its subtypes in SLE by age and sex compared with subjects without SLE. Therefore, the aim of the present study was to assess the prevalence of coronary artery disease (CAD), peripheral artery disease (PAD), and cerebrovascular disease (CVD) by age and sex in a large cohort of SLE patients hospitalized in the United States.
Methods
Study Population
Adults age ≥18 hospitalized between January 2008 and December 2014 were identified using the Healthcare Cost and Utilization Project’s (HCUP) National Inpatient Sample (NIS), an administrative database of discharge level data from a 20% stratified sample of all hospitals in the United States.25 The NIS is the largest publicly available all-payer inpatient healthcare database in the United States, containing unweighted data from approximately 8 million hospital stays annually. Each hospitalization is considered separately, and individual patients are not tracked across NIS hospitalizations.
Patients hospitalized with SLE were identified based on the presence of the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 710.0 for SLE, organ or system involvement unspecified, in any position.29 All hospitalizations with a diagnosis code 710.0 were included in the analysis. Each hospitalization with SLE was matched by age, sex, race, and calendar year of hospitalization to 3 hospitalizations without a diagnosis code for SLE.
Outcomes and Statistical Analysis
The primary outcome was the prevalence of ASCVD identified using ICD-9 diagnosis codes for CAD, PAD, and CVD as described in the supplemental appendix. Patients were subdivided by sex and decade of age (ages 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80–89, and 90+ years) for analysis. In order to determine independent associations between SLE diagnoses and ASCVD prevalence, multivariable logistic regression models were used to estimate odds ratios adjusted for clinical covariates (aOR). Hypertension, hyperlipidemia, diabetes, tobacco use, obesity, and chronic kidney disease (CKD) were included as covariates in all models. ICD-9 codes for CKD are listed in the supplemental appendix. Two-sided P-values <.05 were considered to be statistically significant. Statistical analyses were performed using SAS 9 (SAS Institute, Carey NC) and SPSS 23 (IBM SPSS Statistics, Armonk NY). The NIS is a publicly available dataset that does not contain identifiable protected health information, and the study was exempt from Institutional Review Board review.
Results
Study Population
From January1, 2008 to December 31, 2014, 252,676 hospitalizations with SLE and 758,034 matched hospitalizations without SLE were identified. Demographics and baseline characteristics of patients with and without SLE are shown in Table 1. The mean age of patients with and without SLE was 51, 89% were women, and 49% were white. Patients with SLE were more likely to have hypertension and renal disease, but less likely to have hyperlipidemia, diabetes, obesity, or active tobacco use than patients without SLE.
Table 1.
Clinical characteristics of adults with and without Systemic Lupus Erythematosus hospitalized between 2008 and 2012, matched by age, sex, race, and year of hospitalization.
| Adults with SLE (n = 252,676) | Adults without SLE (n = 758,034) | P Value | |
|---|---|---|---|
| Age (Mean ± SD) | 51.4 ± 16.9 | 51.4 ± 16.9 | .99 |
| Age Groups | >.99 | ||
| 20–29 | 27185 (10.9%) | 81552 (10.9%) | |
| 30–39 | 37754 (15.1%) | 113259 (15.1%) | |
| 40–49 | 48039 (19.2%) | 144132 (19.2%) | |
| 50–59 | 54154 (21.6%) | 162456 (21.6%) | |
| 60–69 | 43479 (17.4%) | 130434 (17.4%) | |
| 70–79 | 26049 (10.4%) | 78150 (10.4%) | |
| 80–89 | 12038 (4.8%) | 36114 (4.8%) | |
| 90+ | 1473 (0.6%) | 4492 (0.6%) | |
| Female Sex | 225061 (89.1%) | 675180 (89.1%) | .99 |
| Race / Ethnicity | >.99 | ||
| White NH | 123157 (48.7%) | 369471 (48.7%) | |
| Black NH | 68827 (27.2%) | 206478 (27.2%) | |
| Hispanic | 27287 (10.8%) | 81867 (10.8%) | |
| Other | 12539 (5.0%) | 37620 (5.0%) | |
| Unknown | 20866 (8.3%) | 62598 (8.3%) | |
| Hypertension | 147124 (58.2%) | 343996 (45.4%) | <.001 |
| Hyperlipidemia | 52644 (20.8%) | 171496 (22.6%) | <.001 |
| Diabetes | 49651 (19.7%) | 175917 (23.2%) | <.001 |
| Obesity | 31350 (12.4%) | 108143 (14.3%) | <.001 |
| Current or former smoking | 55808 (22.1%) | 170196 (22.5%) | <.001 |
| CKD | 58486 (23.1%) | 71701 (9.5%) | <.001 |
| ESRD | 27156 (10.7%) | 24627 (3.2%) | <.001 |
Prevalence of ASCVD
The prevalence of ASCVD is shown in Figure 1. Patients with SLE had a higher prevalence of ASCVD compared with control patients (25.6% vs. 19.2%, OR 1.45, 95% CI 1.44 to 1.47, P<.001), which was observed in both women (OR 1.47, 95% CI 1.45 to 1.48) and men (OR 1.40, 95% CI 1.36–1.44). Unadjusted odds ratios of ASCVD in patients with SLE and controls are noted in the Supplemental Table. The association between SLE and ASCVD was most apparent in younger individuals. Men (OR 4.05, 95% CI 3.33–4.93) and women (OR 12.44, 95% CI 11.13–13.91) age 20–29 with SLE had the greatest odds of ASCVD in comparison to age and sex matched controls. In contrast, men (OR 1.25, 95% CI 1.18–1.34) and women (OR 1.29 95% CI 1.26–1.32) age 60–69 with SLE had only a modest increased odds of ASCVD in comparison to age and sex matched individuals without SLE. The absolute increase in ASCVD prevalence in patients with SLE peaked in patients 30 to 49 years (Figure 2). Based on these data, 1 additional diagnosis of ASCVD would be anticipated for every 13 women and 9 men age 30–49 with SLE in comparison to individuals without SLE.
Figure 1.
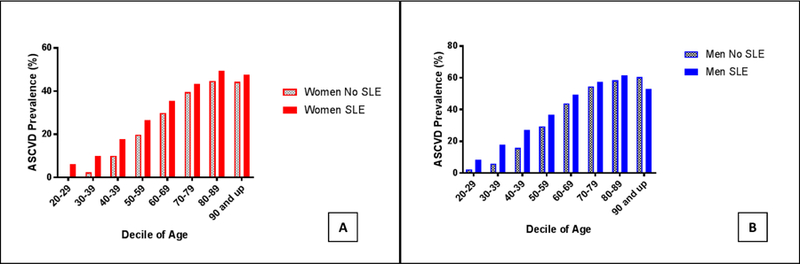
Prevalence of atherosclerotic cardiovascular disease (ASCVD) by age decile in A) women and B) men with and without Systemic Lupus Erythematosus hospitalized in the United States between 2008–2014
Figure 2.
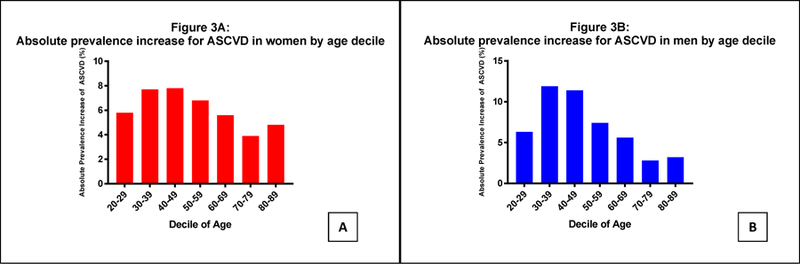
Absolute increase in prevalence of ASCVD in (A) women and (B) men with and without Systemic Lupus Erythematosus hospitalized in the United States between 2008–2014.
After multivariable adjustment for traditional cardiovascular risk factors, SLE was independently associated with greater odds of ASCVD (aOR 1.46, 95% CI 1.41–1.51; Table 2). The increased odds of ASCVD in SLE varied by sex after multivariable adjustment (P for interaction <.0001). Younger women with SLE had the greatest adjusted odds of ASCVD in comparison to matched control patients (Figure 3 A, B).
Table 2.
Multivariable regression model of the odds ratio for ASCVD in patients with SLE compared with control
| Adjusted Odds Ratio (95% CI) | |||
|---|---|---|---|
| Risk Factor | All Patients* | Women | Men |
| SLE | 1.46 (1.41–1.51) | 1.59 (1.57 – 1.61) | 1.53 (1.48 – 1.58) |
| Age (per year) | 1.048 (1.048–1.049) | 1.05 (1.05 – 1.05) | 1.05 (1.05 – 1.05) |
| Female Sex | 0.61 (0.59–0.62) | -- | -- |
| Hypertension | 1.82 (1.80–1.84) | 1.84 (1.82 – 1.87) | 1.72 (1.67 – 1.78) |
| Diabetes | 1.76 (1.74–1.78) | 1.79 (1.76 – 1.81) | 1.57 (1.52 – 1.62) |
| Hyperlipidemia | 2.33 (2.30–2.36) | 2.25 (2.23 – 2.28) | 2.87 (2.78 – 2.96) |
| Obesity | 0.98 (0.96–0.99) | 0.97 (0.95 – 0.98) | 1.06 (1.01 – 1.11) |
| Chronic kidney disease | 1.70 (1.67–1.72) | 1.75 (1.72 – 1.78) | 1.43 (1.37 – 1.48) |
| Tobacco use | 1.71 (1.68–1.73) | 1.74 (1.72 – 1.76) | 1.51 (1.47 – 1.56) |
Includes interaction term for Lupus*Sex (P<.001).
Figure 3.
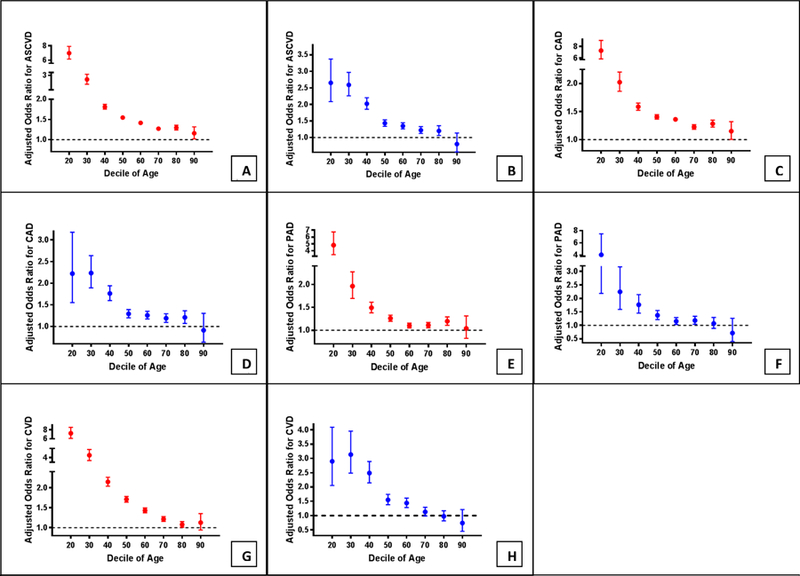
Adjusted odds ratio for ASCVD in woman (A) and men (B), coronary artery disease (CAD or AMI) in women (C) and men (D), peripheral artery disease in women (E) and men (F), and cerebrovascular disease (CVA or TIA) in women (G) and men (H) by age decile and sex in adults with and without Systemic Lupus Erythematosus hospitalized in the United States between 2008–2014 when adjusted for hypertension, hyperlipidemia, diabetes, obesity, tobacco use, and chronic kidney disease. Error bars indicate 95% confidence intervals.
Prevalence of ASCVD Subtypes
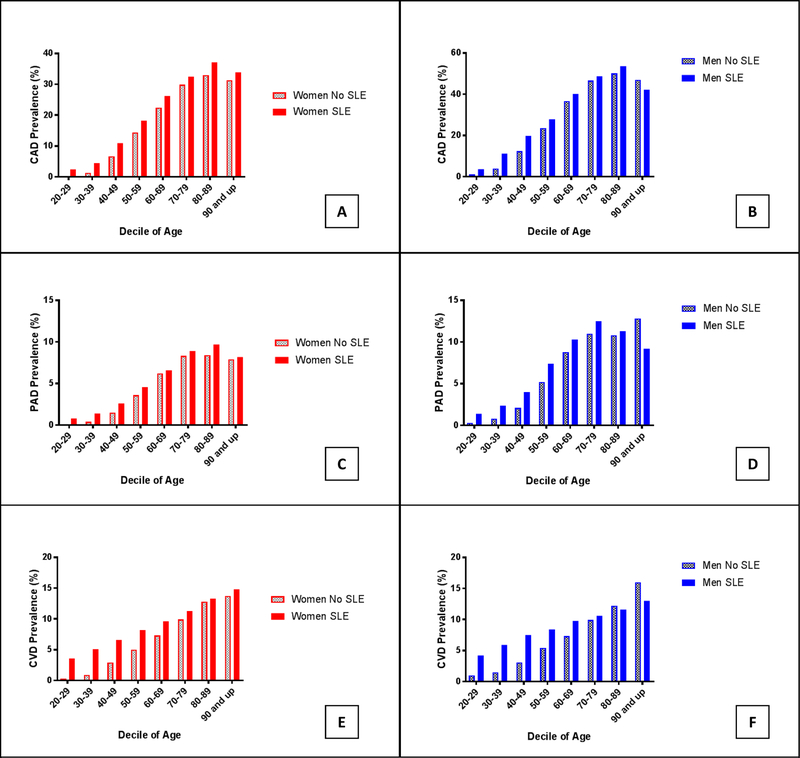
The prevalence of ASCVD subtypes is shown in Figure 4. The absolute increase in the prevalence of ASCVD subtypes in patients with SLE are shown in the Supplemental Figure. In all but the oldest individuals, SLE patients of both sexes had a higher prevalence of CAD (17.8% vs. 14.2%, OR 1.31, 95% CI 1.29 to 1.32, P<.001), PAD (4.6% vs. 3.7%, OR 1.26, 95% CI 1.23 to 1.29, P<.001), and CVD (7.8% vs. 4.8%, OR 1.67, 95% CI 1.64 to 1.70, P<.001) in comparison to matched patients without SLE. After multivariable adjustment for traditional ASCVD risk factors, SLE was independently associated with increased odds of CAD (aOR 1.42, 95% CI 1.40–1.44), PAD (aOR 1.25, 95% CI 1.22–1.28), and CVD (aOR 1.68, 95% CI 1.65–1.71). The increased odds of each vascular phenotype were attenuated with age (Figure 3 C-H).
Figure 4.
Prevalence of coronary artery disease (CAD or MI) in (A) women and (B) men; peripheral artery disease (PAD) in (C) women and (D) men; and cerebrovascular disease (TIA or CVA) in (E) women and (F) men by age decile in adults with and without Systemic Lupus Erythematosus hospitalized in the United States between 2008–2014.
Discussion
The present analysis is the largest study to evaluate the prevalence of ASCVD in SLE by age and sex in the contemporary era. The majority of prior studies have examined cohorts ranging from several dozen to several hundred patients and have not included a sample sufficiently large to provide precise estimates with narrow confidence intervals of ASCVD prevalence in SLE by age and sex.4,5,17–24 In this study, the relative magnitude of increased cardiovascular risk associated with SLE was greatest in women and younger patients and was attenuated with increasing age. These data corroborate prior reports of a >10-fold increased prevalence of ASCVD in young patients with SLE,5 although the magnitude of increase was less than previously described.5 Among individuals younger than 70, absolute increases in ASCVD prevalence associated with SLE were 5–12% in men and 5–8% in woman. These data provide useful clinical quantification for risk stratification in patients with SLE. Based on the present data, this corresponds to an additional diagnosis of ASCVD observed for every 13 woman age <50 with SLE and every 8 men age <50 with SLE. These findings are also consistent with previous vascular imaging studies that demonstrate the relationship between SLE and subclinical ASCVD, including coronary calcium,22 carotid intimal media thickness,21 and significant epicardial coronary artery stenoses.26
Although treatment for both lupus and ASCVD has improved since the increased prevalence of ASCVD was first described in SLE patients, it is unclear whether current management strategies in SLE optimally reduce the burden of ASCVD. Treatment of individuals with SLE is particularly challenging, as SLE appears to confer increased risk beyond that predicted by traditional cardiovascular risk factors, and atherosclerosis may not necessarily correlate with validated measures of SLE disease activity.21,27 Although the mechanisms that underlie this increased risk are complex and not fully understood, increased oxidative stress, platelet activation, endothelial dysfunction, CKD, and hypercoagulability are theorized to play a role. Due to the increasing prevalence of SLE,2 identification of subpopulations at high risk for ASCVD that warrant cardiovascular prevention may be beneficial. Given the observed increased prevalence of ASCVD in women and men and across different age groups, careful risk stratification and evaluation for ASCVD should be considered for many patients with SLE.
Study Limitations
The present study has a number of limitations. First, analyses based on administrative coding data are subject to reporting bias or coding errors. This is of particular importance with regard to ICD-9 diagnoses of SLE; in some cohorts, as few as 16% of patients who received ICD-9 codes for SLE truly had this diagnosis.28 Incorrect coding may also underestimate the true increased prevalence of ASCVD in patients with SLE. Second, since both renal dysfunction and hypertension can be direct manifestations of SLE disease activity, adjustment for these variables may lead to underestimation of the magnitude of cardiovascular risk associated with SLE. However, both adjusted and unadjusted differences in ASCVD risk demonstrated similar magnitude and directionality, suggesting the validity of the observed associations. Third, use of a hospital admission dataset may not provide reliable data on ASCVD prevalence in the overall SLE population, given the potential for differential associations between ASCVD and inflammatory disease in stable outpatients with SLE. Furthermore, since patients are not linked from one hospitalization to another in the NIS, our results are described by patient hospitalizations rather than unique patients. Fourth, hypercoagulable states associated with SLE, such as antiphospholipid syndrome, may be responsible for ischemic events in the study population. Unfortunately, administrative data cannot accurately identify antiphospholipid syndrome or the presence of antiphospholipid antibodies. Thus, the impact of antiphospholipid antibodies on the progression of ASCVD in this cohort is uncertain. Similarly, we are unable to distinguish atherosclerotic disease from de novo thrombotic events in otherwise healthy vessels. Fifth, our analysis does not have the ability to assess disease duration, control or treatment in SLE, so we are unable to determine associations between SLE disease parameters with prevalence of ASCVD. These limitations notwithstanding, this is a very large, contemporary analysis to quantify the magnitude of increased ASCVD prevalence in patients with SLE by age and sex. The data are derived from a national cohort of adults hospitalized in the United States, with adequate representation of men and women of all ages. Finally, in the present study we report associations between SLE and the prevalence of clinically relevant ASCVD, both overall and by the specific vascular distribution affected.
Conclusions:
In this large, cross-sectional analysis of patients with and without SLE hospitalized in the United States, SLE was associated with a substantial prevalence of ASCVD. The increased odds of ASCVD was observed in both women and men and was greatest in younger patients. Provider recognition of the increased risk of ASCVD associated with SLE is essential, since current cardiovascular disease guidelines do not address risk in this high-risk group. Studies investigating optimal screening strategies and prevention and treatment strategies of cardiovascular disease in this high-risk SLE group of patients are essential.
Supplementary Material
Acknowledgments
Funding: This work was supported, in part, by National Institutes of Health grants NIH R21AR071103 (JPB, RMC, JSB) and T32HL098129 (NRS).
Abbreviations:
- ASCVD
Atherosclerotic cardiovascular disease
- CAD
Coronary artery disease
- CVD
Cerebrovascular disease
- HCUP
Healthcare Cost and Utilization Project’s
- ICD-9
International Classification of Diseases, Ninth Revision
- NIS
National Inpatient Sample
- OR
Odds ratio
- SLE
Systemic lupus erythematosus
- PAD
Peripheral artery disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Understanding the Epidemiology and Progression of Systemic Lupus Erythematosus. Semin Arthritis Rheum 2010;39(4):257–268. doi: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsokos GC. Systemic Lupus Erythematosus. N Engl J Med 2011;365(22):2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 3.Izmirly PM, Wan I, Sahl S, et al. The Incidence and Prevalence of Systemic Lupus Erythematosus in New York County (Manhattan), New York: The Manhattan Lupus Surveillance Program. Arthritis Rheumatol 2017;69(10):2006–2017. doi: 10.1002/art.40192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum 1999;42(2):338–346. doi:. [DOI] [PubMed] [Google Scholar]
- 5.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 1997;145(5):408–415. http://www.ncbi.nlm.nih.gov/pubmed/9048514. Accessed January 17, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol 2001;21(12):1876–1890. http://www.ncbi.nlm.nih.gov/pubmed/11742859. Accessed May 2, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Rho YH, Chung CP, Oeser A, et al. Novel cardiovascular risk factors in premature coronary atherosclerosis associated with systemic lupus erythematosus. J Rheumatol 2008;35(9):1789–1794. http://www.ncbi.nlm.nih.gov/pubmed/18634156. Accessed May 2, 2017. [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph JE, Harrison P, Mackie IJ, Isenberg DA, Machin SJ. Increased circulating platelet-leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br J Haematol 2001;115(2):451–459. doi: 10.1046/j.1365-2141.2001.03101.x. [DOI] [PubMed] [Google Scholar]
- 9.Nhek S, Clancy R, Lee KA, et al. Activated Platelets Induce Endothelial Cell Activation via an Interleukin-1β Pathway in Systemic Lupus Erythematosus. Arterioscler Thromb Vasc Biol 2017. http://atvb.ahajournals.org/content/early/2017/02/02/ATVBAHA.116.308126. Accessed April 28, 2017. [DOI] [PMC free article] [PubMed]
- 10.Gurtner GH, Burke-Wolin T. Interactions of oxidant stress and vascular reactivity. Am J Physiol - Lung Cell Mol Physiol 1991;260(4). http://ajplung.physiology.org/content/260/4/L207.short. Accessed August 10, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Nuttall SL, Heaton S, Piper MK, Martin U, Gordon C. Cardiovascular risk in systemic lupus erythematosus--evidence of increased oxidative stress and dyslipidaemia. Rheumatology 2003;42(6):758–762. doi: 10.1093/rheumatology/keg212. [DOI] [PubMed] [Google Scholar]
- 12.Bruce IN, Clark-Soloninka CA, Spitzer KA, Gladman DD, Urowitz MB, Laskin CA. Prevalence of antibodies to beta2-glycoprotein I in systemic lupus erythematosus and their association with antiphospholipid antibody syndrome criteria: a single center study and literature review. J Rheumatol 2000;27(12):2833–2837. http://www.ncbi.nlm.nih.gov/pubmed/11128672. Accessed August 10, 2017. [PubMed] [Google Scholar]
- 13.El-Magadmi M, Bodill H, Ahmad Y, et al. Systemic Lupus Erythematosus: An Independent Risk Factor for Endothelial Dysfunction in Women. Circulation 2004;110(4):399–404. doi: 10.1161/01.CIR.0000136807.78534.50. [DOI] [PubMed] [Google Scholar]
- 14.Johnson S, Harvey P, Floras J, et al. Impaired brachial artery endothelium dependent flow mediated dilation in systemic lupus erythematosus: preliminary observations. Lupus 2004;13(8):590–593. doi: 10.1191/0961203304lu1072oa. [DOI] [PubMed] [Google Scholar]
- 15.Borba EF, Santos RD, Bonfa E, et al. Lipoprotein(a) levels in systemic lupus erythematosus. J Rheumatol 1994;21(2):220–223. http://www.ncbi.nlm.nih.gov/pubmed/8182628. Accessed August 9, 2017. [PubMed] [Google Scholar]
- 16.Nicholls SJ, Zheng L, Hazen SL. Formation of Dysfunctional High-Density Lipoprotein by Myeloperoxidase. Trends Cardiovasc Med 2005;15(6):212–219. doi: 10.1016/j.tcm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Bengtsson C, Ohman M-L, Nived O, Rantapää Dahlqvist S. Cardiovascular event in systemic lupus erythematosus in northern Sweden: incidence and predictors in a 7-year follow-up study. Lupus 2012;21(4):452–459. doi: 10.1177/0961203311425524. [DOI] [PubMed] [Google Scholar]
- 18.Hak AE, Karlson EW, Feskanich D, Stampfer MJ, Costenbader KH. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses’ health study. Arthritis Rheum 2009;61(10):1396–1402. doi: 10.1002/art.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urowitz MB, Gladman DD, Anderson NM, et al. Cardiovascular events prior to or early after diagnosis of systemic lupus erythematosus in the systemic lupus international collaborating clinics cohort. Lupus Sci Med 2016;3(1):e000143. doi: 10.1136/lupus-2015-000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mok CC, Ho LY, To CH. Annual incidence and standardized incidence ratio of cerebrovascular accidents in patients with systemic lupus erythematosus. Scand J Rheumatol 2009;38(5):362–368. doi: 10.1080/03009740902776927. [DOI] [PubMed] [Google Scholar]
- 21.Roman MJ, Shanker B-A, Davis A, et al. Prevalence and Correlates of Accelerated Atherosclerosis in Systemic Lupus Erythematosus. N Engl J Med 2003;349(25):2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 22.Asanuma Y, Oeser A, Shintani AK, et al. Premature Coronary-Artery Atherosclerosis in Systemic Lupus Erythematosus. n engl j med Rheumatol (ET N Engl J Med 2003;34925349:2407–2415. [DOI] [PubMed] [Google Scholar]
- 23.Gustafsson J, Gunnarsson I, Börjesson O, et al. Predictors of the first cardiovascular event in patients with systemic lupus erythematosus - a prospective cohort study. Arthritis Res Ther 2009;11(6):R186. doi: 10.1186/ar2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin LA, Urowitz MB, Gladman DD. Mortality in systemic lupus erythematosus: the bimodal pattern revisited. Q J Med 1985;55(216):87–98. http://www.ncbi.nlm.nih.gov/pubmed/4011845. Accessed March 22, 2017. [PubMed] [Google Scholar]
- 25.Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract 5(3):143–151. http://www.ncbi.nlm.nih.gov/pubmed/12088294. Accessed July 23, 2017. [PubMed] [Google Scholar]
- 26.Bulkley BH, Roberts WC. The heart in systemic lupus erythematosus and the changes induced in it by corticosteroid therapy. A study of 36 necropsy patients. Am J Med 1975;58(2):243–264. http://www.ncbi.nlm.nih.gov/pubmed/1115070. Accessed January 17, 2017. [DOI] [PubMed] [Google Scholar]
- 27.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 2001;44(10):2331–2337. http://www.ncbi.nlm.nih.gov/pubmed/11665973. Accessed March 22, 2017. [DOI] [PubMed] [Google Scholar]
- 28.Ugarte-Gil MF, Alarcón GS. Incomplete Systemic Lupus Erythematosus: Early Diagnosis or Overdiagnosis? Arthritis Care Res (Hoboken) 2016;68(3):285–287. doi: 10.1002/acr.22663. [DOI] [PubMed] [Google Scholar]
- 29.Knight AM, Weiss PF, Morales KH, Keren R. National trends in pediatric systemic lupus erythematosus hospitalization in the United States: 2000–2009. J Rheumatol 2014. March;41(3):539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.