In 2015, Pediatric Blood and Cancer published a series of systematic reviews justifying evidence-based standards of psychosocial care for children with cancer.1 The standard for integrating pediatric palliative care (PPC) concepts such as symptom assessment, patient-reported outcomes, effective communication, and shared decision-making into clinical pediatric oncology care was the journal’s 7th most downloaded manuscript in 2016.2,3
Meeting this standard is critically important. Although the majority of children with cancer will ultimately survive their disease, its treatment is associated with significant short- and long-term physical and psychosocial challenges.4 For the minority who do not experience long-term cure, the toll of relapsed or refractory disease may be even more devastating. Integrated PPC has the potential to alleviate these burdens of cancer and improve patient and family quality of life, independent of the outcome of the disease.2
Who is responsible for delivering PPC to children with cancer? Implementation of PPC principles for children with cancer and their families represents a shared commitment across disciplines. Subspecialty PPC is designed to provide expertise in addition to that of pediatric oncology teams regarding complex pain and symptom management, communication assistance, and delineation of goals of care. Both pediatric oncology and subspecialty PPCs teams are routinely interdisciplinary, including physicians, nurses, psychosocial clinicians, and many others who support the patient and his or her family.
Here, we focus on the role of pediatric oncologists who not only maintain long and meaningful relationships with their patients, but who also tend to direct a child’s care throughout the cancer experience, regardless of his or her potential for cure.5 Indeed, pediatric oncologists routinely practice “primary” PPC when they engage in conversations about prognosis, weigh treatment options and side effects, provide symptom management and anticipatory guidance, and integrate the psychosocial needs of families. It follows that they should be familiar, if not fluent, with PPC concepts.
Previously known barriers to implementing PPC in pediatric oncology include systems issues (e.g., limited access to established services), as well as provider discomfort (e.g., with “palliative care conversations”), and misconceptions (e.g., beliefs that PPC is applicable only when cure is no longer possible).2 An additional challenge for pediatric oncologists may be limited formal training. While the Accreditation Council for Graduate Medical Education (ACGME) recognizes the need for “structured educational experiences in psychosocial support of patients, families, and staff” in its requirements for U.S. Pediatric Hematology/Oncology training programs, it does not specify what such training should look like.6 To our knowledge, the prevalence of PPC training within pediatric hematology/oncology fellowships has not been described, nor have PPC concepts been added to board certification exams.
A recent survey of three national list-serves suggested three additional and important barriers to PPC integration at academic pediatric oncology centers: (1) oncologists believe they provide adequate palliative care themselves; (2) referrals to specialty PPC occur too late in the disease trajectory for patients to experience significant benefit; and, (3) oncologists are unaware of the potential benefits and scope of PPC services (FIGURE).7 In order to implement the PPC standard in pediatric oncology, we must overcome these barriers.
FIGURE 1.
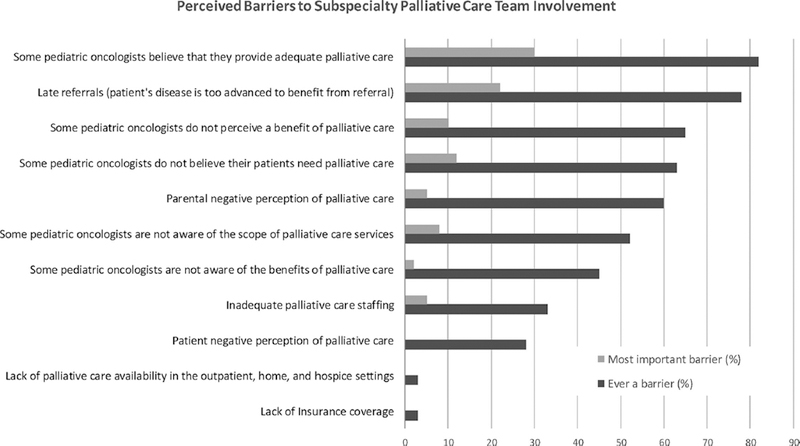
Barriers to subspecialty palliative care referral. Survey respondents with both pediatric palliative care services and pediatric hematology/ fellowship programs (n=58) asked first to select all relevant options and then to select the single most important barrier. Adapted from Ref.6.
Barrier #1: “Pediatric oncologists believe they provide adequate palliative care.”
PPC concepts may seem inseparable from standard pediatric oncology care, but their successful delivery is likely inconsistent. At the end-of-life, for example, more than 90% of pediatric oncologists learn how to provide care by trial and error.8 Half feel a sense of personal failure when considering the prospect of a patient’s death, translating to anxiety, offers of treatments that are unlikely to work, and avoidance of difficult conversations.8 Subsequent information delivery and shared decision-making styles may vary. Regarding symptom-assessment, most pediatric oncologists acknowledge they are less competent in treating psychosocial or other complex symptoms, although the vast majority believe they are “highly competent” in pain management.8 Most believe only a small minority or none of their patients die in pain;8 however, up to 80% of children experience pain and over half suffer from pain at the end of life.9,10 Taken together, the evidence suggests a disconnect between pediatric oncologist perceptions and the lived experiences of patients and families.
Barrier #2: “Patients’ disease is too advanced to benefit significantly from referral.”
Evidence suggests that patients and families welcome early partnerships between oncologists and PPC specialists.11 However, subspecialty PPC teams are particularly helpful at the end-of-life. Children who receive timely subspecialty PPC services at the end-of-life experience more comprehensive and successful pain and symptom assessment, fewer invasive procedures, and more documentation regarding advance care plans when compared to children who do not receive subspecialty PPC.12 In contrast, late PPC referrals translate to missed opportunities for patients and families to prepare for the end-of-life, in turn leading to increased child suffering and increased psychological distress among parents and surviving siblings.13 Involving subspecialty PPC neither negates nor replaces the role of pediatric oncologists. It simply provides an additional level of support for patients, families, as well as health care providers at a time when comprehensive patient-centered care is most important.
Barrier #3: “Pediatric oncologists are unaware of the potential benefits and scope of PPC.”
Although the evidence-base supporting the scope and potential benefit of PPC is well-established,13 pediatric oncologists may be unfamiliar with PPC research. Reasons include the overwhelming volume of medical literature with which they must keep up-to-date (their attention is directed to cutting edge biomedical studies), as well as an inherent bias to alternative types of science (the perception that PPC scientific findings are less compelling than classic laboratory-based experiments or drug-treatment clinical trials). But, evidence-based medicine requires attention to the literature and corresponding changes in clinical practice. Just as traditional pediatric oncology research informs optimal combinations and schedules of chemotherapy, PPC research informs patient- and family-centered care by targeting domains such as symptom-management, psychosocial support, and communication.
Overcoming these barriers need not be complicated. First, education in PPC concepts must begin in pediatric hematology/oncology fellowship.14 Prior studies suggest pediatric oncologists with formal training in PPC concepts report more confidence and success in treating complex end-of-life symptoms.15 In the afore-mentioned survey, only one-fifth of programs endorsed formal PPC training, suggesting a significant opportunity for improvement.7 At a minimum, fellows must become familiar with effective communication techniques, basic psychosocial assessment and support, and symptom assessment, including the evidence supporting each. They must also be exposed to the merits of subspecialty PPC services through clinical encounters and, potentially, formal rotations with PPC teams. Finally, the pediatric hematology/oncology board should consider including these crucial elements of care in its board certification specifications and exams.
Second, this education must be complemented with scholarship. Interestingly, there are ample opportunities to do so at academic pediatric hematology/oncology centers; 78% of the surveyed academic sites reported ongoing PPC research programs.7 Furthermore, because traditional palliative care fellowships are predominantly 1-year clinical programs, pediatric oncologists may be well-positioned to inform the science of palliative care for children with serious illnesses like cancer.
By recognizing existing barriers, integrating formal training in PPC concepts, and creating opportunities for ongoing PPC oncology research, we will ultimately take better care of children with cancer and their families. The question of “Who is responsible for delivering PPC to children with cancer?” is best answered with a resounding “us,” and this collective “us” benefits from early preparation, formal training, and empowered partnership.
Abbreviations:
- PPC
Pediatric Palliative Care
- ACGME
Accreditation Council for Graduate Medical Education
REFERENCES
- 1.Wiener L, Kazak AE, Noll RB, et al. Standards for the Psychosocial Care of Children With Cancer and Their Families: An Introduction to the Special Issue. Pediatr Blood Cancer. 2015;62 Suppl 5:S419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver MS, Heinze KE, Kelly KP, et al. Palliative Care as a Standard of Care in Pediatric Oncology. Pediatr Blood Cancer. 2015;62 Suppl 5:S829–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Personal Communication with Pediaric Blood and Cancer Editor-in-Chief, Peter Newburger, MD, September 25, 2017.
- 4.American Cancer Society. Cancer Treatment and Survivorship: Facts and Figures. 2015; https://old.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf. Accessed April 24, 2017.
- 5.Waldman E, Wolfe J. Palliative care for children with cancer. Nat Rev Clin Oncol. 2013;10(2):100–107. [DOI] [PubMed] [Google Scholar]
- 6.ACGME. Program requirements for graduate medical education in pediatric hematology-oncology. http://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013-PR-FAQ-PIF/327_hematology_oncology_peds_07012013.pdf. Accessed July 6, 2017.
- 7.Weaver MS, Rosenberg AR, Wiener L. A Summary of Pediatric Palliative Care Team Structure and Services as Reported by Centers Caring for Children with Cancer. J Pall Med. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilden JM, Emanuel EJ, Fairclough DL, et al. Attitudes and practices among pediatric oncologists regarding end-of-life care: results of the 1998 American Society of Clinical Oncology survey. J Clin Oncol. 2001;19(1):205–212. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. The New England Journal of Medicine. 2000;342(5):326–333. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe J, Orellana L, Ullrich C, et al. Symptoms and Distress in Children With Advanced Cancer: Prospective Patient-Reported Outcomes From the PediQUEST Study. J Clin Oncol. 2015;33(17):1928–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine DR, Mandrell BN, Sykes A, et al. Patients’ and Parents’ Needs, Attitudes, and Perceptions About Early Palliative Care Integration in Pediatric Oncology. JAMA Oncol. 2017;3(9):1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osenga K, Postier A, Dreyfus J, Foster L, Teeple W, Friedrichsdorf SJ. A Comparison of Circumstances at the End of Life in a Hospital Setting for Children With Palliative Care Involvement Versus Those Without. J Pain Symptom Manage. 2016;52(5):673–680. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg AR, Wolfe J. Approaching the 3rd Decade of Paediatric Palliative Oncology Investigation: historical progress and future directions. Lancet Child and Adolescent Health. 2017;1(1): 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiener L, Weaver MS, Bell CJ, Sansom-Daly UM. Threading the cloak: palliative care education for care providers of adolescents and young adults with cancer. Clin Oncol Adolesc Young Adults. 2015;5:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler K, Poehling K, Billheimer D, et al. Hospice referral practices for children with cancer: a survey of pediatric oncologists. J Clin Oncol. 2006;24(7):1099–1104. [DOI] [PubMed] [Google Scholar]


