Abstract
Background
Background parenchymal enhancement (BPE) on dynamic contrast-enhanced (DCE)-MRI has been associated with breast cancer risk, both based on qualitative and quantitative assessments.
Purpose
To investigate whether BPE of the contralateral breast on preoperative DCE-MRI is associated with therapy outcome in ER-positive, HER2-negative, node-negative invasive breast cancer.
Study Type
Retrospective.
Population
In all, 289 patients with unilateral ER-positive, HER2-negative, node-negative breast cancer larger than 5 mm.
Field Strength/Sequence
3T, T1-weighted DCE sequence.
Assessment
BPE of the contralateral breast was assessed qualitatively by two dedicated radiologists and quantitatively (using region-of-interest and automatic breast segmentation).
Statistical Tests
Cox regression analysis was used to determine associations with recurrence-free survival (RFS) and distant metastasis-free survival (DFS). Interobserver variability for parenchymal enhancement was assessed using kappa statistics and intraclass correlation coefficient (ICC).
Results
The median follow-up time was 75.8 months. Multivariate analysis showed receipt of total mastectomy (hazard ratio [HR]: 5.497) and high Ki-67 expression level (HR: 5.956) were independent factors associated with worse RFS (P < 0.05). Only a high Ki-67 expression level was associated with worse DFS (HR: 3.571, P = 0.045). BPE assessments were not associated with outcome (RFS [qualitative BPE: P = 0.75, 0.92 for readers 1 and 2; quantitative BPE: P = 0.38–0.99], DFS, [qualitative BPE: P = 0.41, 0.16 for readers 1 and 2; quantitative BPE: P = 0.68–0.99]). For interobserver variability, there was good agreement between qualitative (k = 0.700) and good to perfect agreement for most quantitative parameters of BPE.
Data Conclusion
Contralateral BPE showed no association with survival outcome in patients with ER-positive, HER2-negative, node-negative invasive breast cancer. A high Ki-67 expression level was associated with both worse recurrence-free and distant metastasis-free survival.
Level of Evidence
3
Technical Efficacy
Stage 4
Breast cancer is a heterogeneous disease for which gene expression profiling can provide valuable classification according to different phenotypes.1 These subtypes can be approximated using immunohistochemical markers: estrogen receptor (ER)-positive (and human epidermal growth factor receptor 2 [HER2]-negative, independent of progesterone receptor status), HER2-positive (independent of ER and progesterone receptor [PR] status), and triple negative (ER negative, PR negative, and HER2 negative.2
Most patients with hormone receptor (HR)-positive, lymph node-negative breast cancers have a favorable prognosis, and therefore, at least 85% of patients would be over-treated if adjuvant chemotherapy was offered to everyone.3,4 Recently, several multigene markers have been developed for predicting prognosis and effectiveness of treatment in breast cancer.5 These include the 70-gene MammaPrint microarray assay, the 21-gene Oncotype DX assay, and the 50-gene PAM50 assay.6 The National Comprehensive Cancer Network (NCCN) guidelines recommend consideration of the 21-gene reverse-transcription polymerase chain reaction (RT-PCR) assay for planning chemotherapy in HR-positive, lymph node-negative breast cancers greater than 0.5 cm (T1b).7 Although such multigene assays can be used as decision aids for adjuvant chemotherapy, they are expensive and have limited accessibility.
Background parenchymal enhancement (BPE) on dynamic contrast-enhanced / magnetic resonance imaging (DCE-MRI) has been associated with breast cancer risk, both based on qualitative and quantitative assessments. Recently, one study reported that BPE of the contralateral breast was significantly associated with long-term outcome in patients with unilateral ER-positive, HER2-negative breast cancer who underwent adjuvant endocrine therapy and/or adjuvant chemotherapy.2 However, this association has not been investigated by other study groups and included patients with various axillary lymph node burden. Although patients with node-positive breast cancer are generally treated with adjuvant chemotherapy, treatment options for patients with HR-positive, lymph node-negative breast cancer vary. Therefore, this subgroup would benefit from risk stratification tools that can be obtained from preoperative imaging, which can aid in decision making for adjuvant therapy.
Thus, the purpose of our study was to investigate whether BPE of the contralateral breast on preoperative DCE-MRI is associated with therapy outcome in ER-positive, HER2-negative, node-negative invasive breast cancer.
Materials and Methods
Study Design and Patient Data
Our Institutional Review Board approved this retrospective study and requirement for informed consent was waived. Between November 2009 and July 2011, 1435 breast MR examinations were performed in 1287 patients. Among them, 371 patients were diagnosed with ER-positive, HER2-negative, lymph node-negative invasive breast cancer and underwent surgery during the study period. Of them, 22 patients who underwent neoadjuvant chemotherapy for preoperatively diagnosed axillary lymph node metastasis, two patients with bilateral breast cancer, nine patients previously treated for breast cancer at the contralateral breast, two patients who previously underwent excision for the contralateral breast, one patient with biopsy-proven atypical ductal hyperplasia at the contralateral breast, one patient who underwent bilateral interstitial mammoplasty, four patients with poor image quality, and seven patients who were lost to follow-up immediately after surgery were excluded. As our study focused on ER-positive, HER2-negative, lymph node-negative invasive breast cancers for which treatment options vary and which would benefit from additional risk stratification, 34 patients with a pathological invasive tumor size smaller than 5 mm were excluded.7 Finally, 289 consecutive patients with ER-positive, HER2-negative, lymph node-negative invasive breast cancer (median age, 49 years; range, 30–80 years) larger than 5 mm were included and comprised our study population.
Clinical-Pathologic Analysis
Patient age, information on menopausal status, menstrual cycle, clinical follow-up, and treatment methods (surgery type, radiation therapy, endocrine therapy, and adjuvant chemotherapy) were obtained from medical records. Pathological data including pathological tumor size, histologic grade, lymphovascular invasion, lymph node metastasis (LNM), and the expression status of ER, PR, and HER2 were obtained from pathology records. ER and PR positivity were defined as the presence of at least 10% positively stained nuclei. Tumors were classified as HER-2-positive when scored as at least 3 + at immunohistochemical staining or when gene amplification using fluorescence in situ hybridization demonstrated gene amplification more than ratios of 2.2.8
MRI Technique
MR examinations were performed using a 3T MR scanner (Trio-Tim; Siemens, Erlangen, Germany). Imaging was performed with a dedicated four-channel phased array breast coil with the patient in the prone position. After obtaining three-plane localizer images, axial T2-weighted (T2W) turbo spin-echo images, axial T2 STIR images, axial diffusion-weighted images with a 2D spin-echo echo-planar imaging (EPI) sequence, and T1-weighted (T1W) DCE-MRI including one precontrast acquisition and six postcontrast bilateral axial acquisitions were obtained. Acquisition time of each postcontrast series was 73 seconds. For T1W DCE-MRI, gadolinium-based contrast agent (Dotarem, Guerbet, Paris, France; Magnevist, Berlex Laboratories, Wayne, NJ) was given into an antecubital vein at a dose of 0.2 cc/kg of body weight and at a rate of 2 mL/s, using an automated injector and followed by a 20-mL saline flush. Postcontrast images were obtained 20 seconds after the start of contrast material injection. Detailed information on the MRI technique is presented in the Supplementary Material.
MRI Analysis
BPE was analyzed qualitatively and quantitatively. For qualitative analysis, two dedicated radiologists (G.W.S. and V.Y.P., with 2 and 4 years, respectively, of subspecialty experience in interpretation of breast MRI) independently reviewed all MR images. According to the 5th edition of the American College of Radiology (ACR) BI-RADS, the level of BPE was categorized as minimal, mild, moderate, or marked on the basis of both volume and intensity of enhancement by using a combination of precontrast and early postcontrast T1-weighted fat-suppressed images and subtraction images.9 The amount of fibroglandular tissue was categorized as almost entirely fat, scattered fibroglandular tissue, heterogeneous fibroglandular tissue, and extreme fibroglandular tissue, on the basis of a combination of T2-weighted fat-suppressed imaging and precontrast T1-weighted fat-suppressed imaging.10
Quantitative BPE Analysis: ROI-Based Approach
For quantitative analysis, BPE was assessed by two methods. First, a region of interest (ROI)-based approach was used for quantitative analysis. Three circular ROIs were manually placed in the contralateral breast for every MR study by the two readers, with the ROI size (mean, 0.6 cm2; range 0.3–3.4cm2) adjusted to the size of the parenchymal area to be evaluated, using a software program (nordi-cICE; Nordic Imaging Lab, Bergen, Norway). ROIs were placed at the area of the breast that showed the strongest enhancement (Fig. 1). Subsequently, values for early and delayed enhancement rate, late enhancement, and signal enhancement ratio (SER) were calculated from each ROI. The equation for each variable was as follows: the early enhancement rate was defined as (S1–S0)/S0 for a selected ROI in the contralateral breast parenchyma, where Si corresponds to the image intensity in the first postcontrast acquisition and S0 to the intensity in the precontrast acquisition.11,12 The delayed enhancement rate was defined as (S6–S0)/S0 for a selected ROI in the contralateral breast parenchyma, where S6 corresponds to the intensity in the sixth (last) postcontrast acquisition. Late enhancement was defined as (S6–S1)/S1.2 SER was defined as (S1–S0)/(S6–S0).12,13 The respective average values of the three ROIs from each reader were used for further analysis.
FIGURE 1:
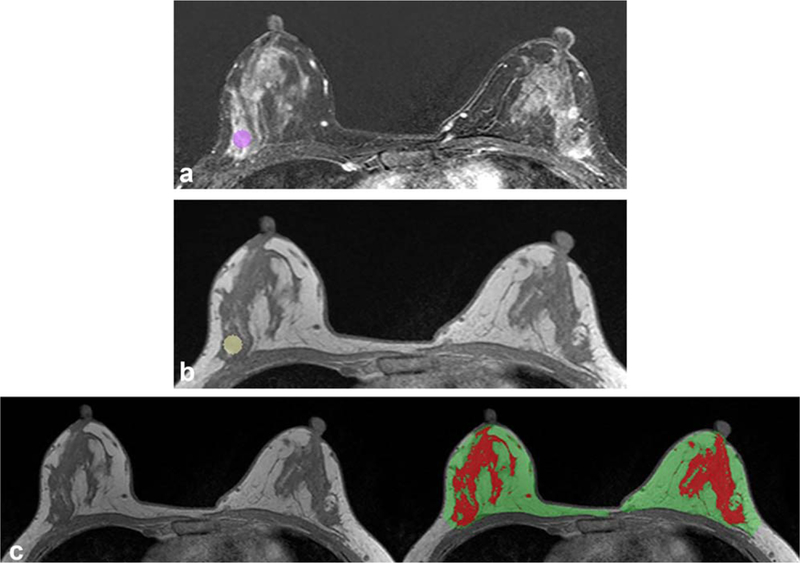
Example of quantitative assessment of background parenchymal enhancement using ROI and computer-based fibro-glandular tissue segmentation in a 39-year-old woman with invasive ductal carcinoma in the left breast. Circular ROI was placed at the area of the right breast that showed the strongest enhancement, which is shown on axial early subtraction MR image (A) and axial T1-weighted precontrast MR image without fat suppression (B). In every study examination, three ROIs were placed, and the respective average values were used for analysis. For calculation of late enhancement, a fully automatic segmentation of the contralateral breast fibroglandular tissue was also performed (C). T1-weighted precontrast MRI without fat suppression is shown without (left) and with (right) the breast parenchymal mask (area in green and red) and fibroglandular tissue segmentation (area in red). Subsequently, late enhancement was calculated for every voxel location in the fibroglandular tissue of the contralateral breast and the mean of the top 10% (LE90+) was used for further analysis.
Quantitative BPE Analysis: Computer-Based Fibroglandular Tissue Segmentation
Considering possible bias coming from arbitrary ROI selection and a recent study using parenchymal segmentation,2 we also used a fully automatic segmentation method for calculation of late enhancement. We used a fully automatic template-based segmentation method to segment the breast and the fibroglandular tissue on MRI. Detailed procedures were described in the study of Lin et al14,15 and the Supplementary Material. Late enhancement was calculated for every voxel location in the parenchyma of the contralateral breast. These values were then sorted from high to low values, and the mean of the top 10% was calculated and denoted as LE90.2
Statistical Analysis
Clinical-pathologic data and assessments for BPE were compared between patients with and without recurrence using the Mann–Whitney U-test, the chi-square test, or Fisher’s exact test.
For survival analysis, a Cox proportional hazard model was used to analyze the hazard ratio (HR) with 95% confidence intervals (CIs) for recurrence-free survival and distant metastasis-free survival with clinical-pathologic and BPE variables. Recurrence-free survival was defined as the difference between date of definitive surgery to date of the first documented local or distant recurrence. In the absence of recurrence, recurrence-free survival was calculated as the difference between date of surgery to the last clinical follow-up. Distant metastasis-free survival was defined as the difference between date of definite surgery to date of the first documented distant metastasis or last clinical follow-up. The level of significance was defined as P < 0.05 for all analyses.
To minimize possible hormonal effects on BPE, we performed a subgroup analysis by excluding 75 premenopausal women who underwent breast MRI during the 1st or 4th week of the menstrual cycle and 23 premenopausal women lacking information about their menstrual cycle on medical records.16–18 An additional Cox proportional hazards model was performed to analyze a possible association between survival outcome and BPE in this subgroup.
Interobserver variability for parenchymal enhancement was analyzed using kappa statistics for qualitative assessments and using the intraclass correlation coefficient (ICC) for quantitative assessments. Statistical analyses regarding BPE were performed independently for the values obtained by readers 1 and 2.
Statistical analysis was conducted using SAS v. 9.4 (SAS Institute, Cary, NC) and SPSS Statistics v. 23.0 (IBM, Armonk, NY).
Results
Patients and Survival Outcomes
Patient and tumor characteristics are shown in Table 1. Among the 289 patients, 127 patients (43.9%) were postmenopausal and 162 patients (56.1%) were premenopausal women. Among them, 78 (27.0%) received mastectomy. All patients underwent endocrine therapy, and 151 (52.2%) and 195 (67.5%) underwent adjuvant chemotherapy and radiation therapy, respectively. The mean invasive tumor size at surgical pathologic examination was 16 mm ± 7 (range, 6–60 mm).
TABLE 1.
Characteristics of the Study Population
| Recurrence-free group (n = 272) |
Recurrence group (n = 17) |
P value |
Distant metastasis-free group (n = 277) |
Distant metas- tasis group (n = 12) |
P | ||
|---|---|---|---|---|---|---|---|
| Characteristics value |
|||||||
| Age (years) | 49 (30–80) | 45 (37–63) | 0.14 | 49 (30–80) | 50 (37–63) | 0.80 | |
| Menopause | 0.12 | 0.44 | |||||
| Premenopausal | 149 (54.8) | 13 (76.5) | 154 (55.6) | 8 (66.7) | |||
| Postmenopausal | 123 (45.2) | 4 (23.5) | 123 (44.4) | 4 (33.3) | |||
| Pathology | 0.52 | 0.70 | |||||
| Invasive ductal carcinoma | 224 (82.4) | 13 (76.5) | 226 (81.6) | 11 (91.7) | |||
| Other invasive carcinoma | 48 (17.6) | 4 (23.5) | 51 (18.4) | 1 (8.3) | |||
| Invasive tumor size (mm) | 15 (6–60) | 20 (10–40) | 0.004 | 15 (6–60) | 21 (10–35) | 0.02 | |
| Histologic grade | 0.29 | 0.69 | |||||
| Grade 1/2 | 232 (85.6) | 13 (76.5) | 235 (85.1) | 10 (83.3) | |||
| Grade 3 | 39 (14.4) | 4 (23.5) | 41 (14.9) | 2 (16.7) | |||
| Lymphovascular invasion | 0.07 | 0.34 | |||||
| Yes | 25 (9.3) | 4 (23.5) | 27 (9.8) | 2 (16.7) | |||
| No | 245 (90.7) | 13 (76.5) | 248 (90.2) | 10 (83.3) | |||
| Ki-67 levela | <0.001 | 0.03 | |||||
| Low | 181 (66.8) | 4 (23.5) | 181 (65.6) | 4 (33.3) | |||
| High | 90 (33.2) | 13 (76.5) | 95 (34.4) | 8 (66.7) | |||
| Surgical method | 0.002 | 0.02 | |||||
| Breast conserving surgery | 204 (75%) | 7 (41.2%) | 206 (74.4) | 5 (41.7) | |||
| Total mastectomy | 68 (25%) | 10 (58.8%) | 71 (25.6) | 7 (58.3) | |||
| Radiation therapy | 0.18 | 0.06 | |||||
| Yes | 186 (68.4) | 9 (52.9) | 190 (68.6) | 5 (41.7) | |||
| No | 86 (31.6) | 8 (47.1) | 87 (31.4) | 7 (58.3) | |||
| Adjuvant endocrine therapy | N/A | N/A | |||||
| Yes | 272 (100) | 17 (100) | 277 (100) | 12 (100) | |||
| No | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Adjuvant chemotherapy | 0.11 | 0.10 | |||||
| Yes | 139 (51.1) | 12 (70.6) | 142 (51.3) | 9 (75.0) | |||
| No | 133 (48.9) | 5 (29.4) | 135 (48.7) | 3 (25.0) | |||
| FGT density | |||||||
| Almost entirely fat / scattered FGT | 84 (94.4) | 5 (5.6) | 0.89 | 84 (94.4) | 5 (5.6) | 0.52 | |
| Heterogeneous FGT / extreme FGT | 188 (94.0) | 12 (6.0) | 0.89 | 193 (96.5) | 7 (3.5) | 0.52 | |
| Qualitative BPE | |||||||
| Minimal / Mild | Reader 1 | 198 (93.8) | 13 (6.2) | >0.99 | 201 (95.3) | 10 (4.7) | 0.52 |
| Reader 2 | 172 (94.0) | 11 (6.0) | 0.90 | 173 (94.5) | 10 (5.5) | 0.22 | |
| Moderate / Marked | Reader 1 | 74 (94.9) | 4 (5.1) | >0.99 | 76 (97.4) | 2 (2.6) | 0.52 |
| Reader 2 | 100 (94.3) | 6 (5.7) | 0.90 | 104 (98.1) | 2 (1.9) | 0.22 | |
| Quantitative BPE | |||||||
| Early enhancement rate | Reader 1 | 38.10 (2.73–133.91) | 41.16 (9.53–116.58) | 0.81 | 38.45 (2.73–133.91) | 36.50 (9.53–116.58) | 0.66 |
| Reader 2 | 32.46 (3.33–146.04) | 41.20 (4.94–78.86) | 0.61 | 32.69 (3.33–146.04) | 37.98 (4.94–78.86) | 0.99 | |
| Delayed enhancement rate | Reader 1 | 57.44 (−5.26–212) | 64.28 (11.58–158.52) | 0.51 | 58.57 (−5.26–212.00) | 61.36 (11.58–158.52) | 0.86 |
| Reader 2 | 50.27 (−24.43–176.69) | 64.10 (10.16–104.75) | 0.69 | 50.41 (−24.43–176.69) | 66.04 (10.16–104.75) | 0.97 | |
| Late enhancement | Reader 1 | 0.28 (−0.27–1.03) | 0.30 (0.08–0.51) | 0.56 | 0.28 (−0.26–1.03) | 0.29 (0.08–0.51) | 0.92 |
| Reader 2 | 0.25 (−0.34–0.86) | 0.25 (0.09–0.49) | 0.91 | 0.25 (−0.34–0.86) | 0.29 (0.09–0.49) | 0.92 | |
| SER | Reader 1 | 68.25 (−1220.78–148.34) | 63.26 >(54.80–83.00) | 0.22 | 67.89 (−1220.76–148.34) | 63.65 (54.80–83.00) | 0.37 |
| Reader 2 | 0.65 (−1.54–1.14) | 0.65 (0.49–0.85) | 0.91 | 0.65 (−1.54–1.14) | 0.65 (0.49–0.76) | 0.67 | |
| LE90+ | 0.75 (0.22–172.66) | 0.81 (0.55–83.71) | 0.28 | 0.75 (0.22–172.66) | 0.82 (0.58–83.71) | 0.20 | |
Numeric data are presented as median with range in parentheses. Nonnumeric data are presented as numbers of patients with percentages in parentheses.
FGT, fibroglandular tissue; BPE, background parenchymal enhancement; SER, signal enhancement ratio; LE90+, mean of the top 10% of late enhancement.
Low; <14, High; ≥14.
The median recurrence-free survival was 75.1 months (range, 6–95 months). There were 17 recurrences (five locoregional recurrences and 12 distant metastases) (Fig. 2). There were no cases of newly developed contralateral breast cancer during the study period. Recurrence occurred at a median of 37 months (range, 11–77 months) and distant metastasis at a median of 47 months (range, 12–77 months). The remaining 272 patients did not experience events during a median follow-up period of 75.8 months (range, 6–95 months).
FIGURE 2:
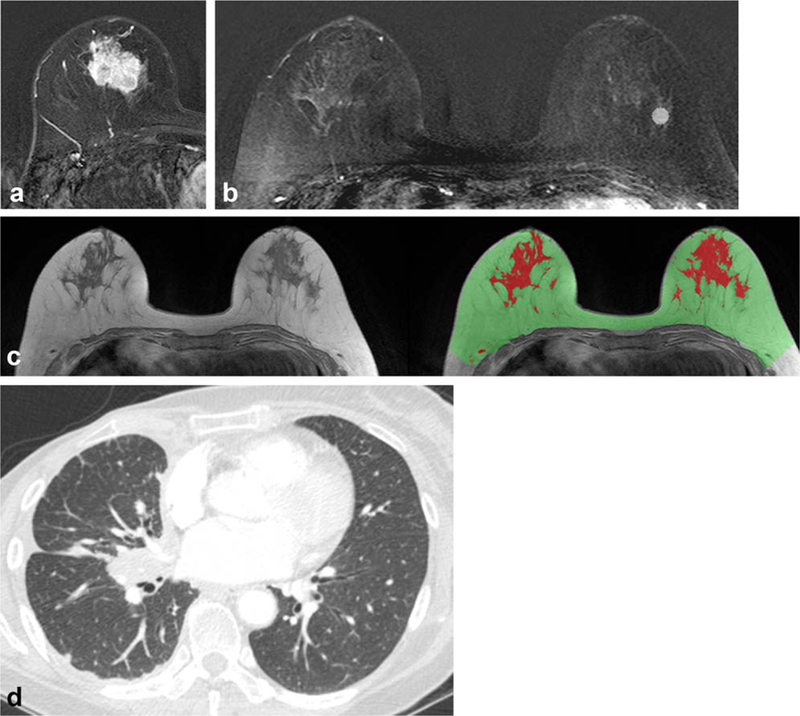
A 57-year-old woman with invasive ductal carcinoma in the right breast. (A) Axial T1-weighted contrast-enhanced early subtraction MR image shows a 25-mm size invasive breast cancer at the right breast, with a high Ki-67 level of 30%. (B) Axial T1-weighted contrast-enhanced early subtraction MR image shows the location of one of the three ROIs used for ROI-based quantitative BPE analysis. (C) T1-weighted precontrast MR imaging without fat suppression is shown with the breast parenchymal mask (area in green and red) and fibroglandular tissue segmentation (area in red). (D) Patient underwent right mastectomy, adjuvant chemotherapy, and endocrine therapy but developed lung metastasis 49.4 months after diagnosis. Chest CT image shows right pleural and interlobar lymph node metastasis.
Comparison of Clinical-Pathologic Variables and BPE According to Outcome
Patients with recurrence had a larger invasive tumor size (P = 0.004), a higher Ki-67 level (P = 0.001), and more often received total mastectomy (P = 0.003). Patients with distant metastasis also had a larger invasive tumor size (P = 0.038), higher Ki-67 level (P = 0.031), and more often received total mastectomy (P = 0.016) than patients without distant metastasis. Qualitative assessment and quantitative parameters of BPE did not differ according to the patient outcome (P > 0.05).
Recurrence-free Survival Analysis
Receipt of total mastectomy (HR: 4.230; CI: 1.610–11.116; P = 0.003), a high Ki-67 expression level (HR: 6.247; CI: 2.037–19.161; P = 0.001) and a larger pathologic invasive tumor size (HR: 1.057; CI: 1.018–1.097; P = 0.004) were significantly associated with worse recurrence-free survival at univariate analysis (Table 2). There was no significant association between BPE and recurrence-free survival, based on both qualitative and quantitative assessments of both readers (Table 2).
TABLE 2.
Univariate Cox Regression Analysis for Recurrence-Free Survival and Distant Metastasis-Free Survival Outcomes
| Variable | Recurrence-free survival | Distant metastasis-free survival |
|||
|---|---|---|---|---|---|
| HR (95% CI) |
P Value |
HR (95% CI) | P | ||
| Value | |||||
| Age | 0.96 (0.91, 1.01) | 0.16 | 0.98 (0.93, 1.04) | 0.69 | |
| Menopause | |||||
| Premenopausal | 1 | — | 1 | — | |
| Postmenopausal | 0.38 (0.12, 1.19) | 0.09 | 0.64 (0.19, 2.14) | 0.47 | |
| Surgery method | |||||
| Breast conserving surgery | 1 | — | 1 | — | |
| Total mastectomy | 4.23 (1.61, 11.11) | 0.003 | 4.09 (1.30, 12.90) | 0.01 | |
| Pathologic size of invasiveness | 1.05 (1.01, 1.09) | 0.004 | 1.05 (1.00, 1.10) | 0.03 | |
| Pathologic type | |||||
| IDC | 1 | — | 1 | — | |
| Non-IDC | 1.42 (0.46, 4.37) | 0.53 | 0.40 (0.05, 3.11) | 0.38 | |
| Histologic grade group | |||||
| Low | 1 | — | 1 | — | |
| High | 1.67 (0.54, 5.14) | 0.36 | 1.08 (0.23, 4.93) | 0.92 | |
| Lymphovascular invasion | |||||
| No | 1 | — | 1 | — | |
| Yes | 2.68 (0.87, 8.23) | 0.08 | 1.66 (0.36, 7.60) | 0.51 | |
| Ki-67 levela | |||||
| Low | 1 | — | 1 | — | |
| High | 6.24 (2.03, 19.16) | 0.001 | 3.73 (1.12, 12.39) | 0.03 | |
| Radiation therapy | |||||
| No | 1 | — | 1 | — | |
| Yes | 0.49 (0.19, 1.28) | 0.15 | 0.31 (0.09, 0.97) | 0.04 | |
| Adjuvant chemotherapy | |||||
| No | 1 | 1 | |||
| Yes | 2.20 (0.77, 6.25) | 0.13 | 2.75 (0.74, 10.16) | 0.12 | |
| Fibroglandular tissue | |||||
| Almost entirely fat/ Scattered FGT | 1 | — | 1 | — | |
| Heterogeneous FGT / Extreme FGT | 1.07 (0.37, 3.05) | 0.89 | 0.61 (0.19, 1.93) | 0.40 | |
| Qualitative BPE | |||||
| Reader 1 | Minimal / Mild | 1 | — | 1 | - |
| Moderate / Marked | 0.83 (0.27, 2.56) | 0.75 | 0.53 (0.11, 2.44) | 0.41 | |
| Reader 2 | Minimal / Mild | 1 | — | 1 | - |
| Moderate / Marked | 0.95 (0.35, 2.57) | 0.92 | 0.33 (0.07, 1.54) | 0.16 | |
| Quantitative BPE | |||||
| Early enhancement rate | Reader 1 | 1.00 (0.98, 1.01) | 0.96 | 0.99 (0.97, 1.01) | 0.68 |
| Reader 2 | 1.00 (0.98, 1.01) | 0.99 | 0.99 (0.97, 1.01) | 0.81 | |
| Delayed enhancement rate | Reader 1 | 1.00 (0.99, 1.01) | 0.57 | 0.99 (0.98, 1.01) | 0.89 |
| Reader 2 | 1.00 (0.98–1.01) | 0.93 | 0.99 (0.98, 1.01) | 0.94 | |
| Late enhancement | Reader 1 | 1.46 (0.07, 27.66) | 0.79 | 0.83 (0.02, 31.80) | 0.92 |
| Reader 2 | 0.61 (0.02, 16.50) | 0.77 | 0.98 (0.02, 46.74) | 0.99 | |
| SER | Reader 1 | 1.00 (0.99, 1.00) | 0.94 | 1.00 (0.99, 1.00) | 0.94 |
| Reader 2 | 1.19 (0.07, 19.79) | 0.90 | 0.70 (0.06, 8.27) | 0.77 | |
| LE90+ | 1.00 (0.99, 1.02) | 0.38 | 1.01 (0.99, 1.02) | 0.18 | |
FGT, fibroglandular tissue; BPE, background parenchymal enhancement; SER, signal enhancement ratio; LE90+, mean of the top 10% of late enhancement; HR, hazard ratio; CI, confidence interval.
Low; < 14, High; ≥ 14.
At multivariate analysis, receipt of total mastectomy (HR: 5.497; CI: 1.380–1.902; P = 0.016) and a high Ki-67 expression level (HR: 5.956; CI: 1.888–18.790; P = 0.002) were the only factors independently associated with worse recurrence-free survival (Table 3).
TABLE 3.
Multivariate Cox Analysis for Recurrence-Free Survival and Distant Metastasis-Free Survival Outcome
| Variable | Recurrence-free survival | Distant metastasis-free survival | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Pathologic size of invasiveness | 1.02 (0.98, 1.06) | 0.30 | 1.03 (0.97, 1.08) | 0.29 |
| Surgery method | 0.01 | 0.24 | ||
| Breast conserving surgery | 1 | 1 | ||
| Total mastectomy | 5.49 (1.38, 21.90) | 2.61 (0.52, 13.02) | ||
| Ki-67 levela | 0.002 | 0.04 | ||
| Low | 1 | 1 | ||
| High | 5.95 (1.88, 18.79) | 3.57 (1.02, 12.41) | ||
| Radiation therapy | 0.62 | 0.45 | ||
| No | 1 | 1 | ||
| Yes | 1.40 (0.36, 5.45) | 0.54 (0.11, 2.69) | ||
HR, hazard ratio; CI, confidence interval.
Low; < 14, High; ≥ 14.
Distant Metastasis-Free Survival Analysis
Receipt of total mastectomy (HR: 4.096; CI: 1.300–12.908; P = 0.016), a high Ki-67 expression level (HR: 3.733; CI: 1.124–12.398; P = 0.031), receipt of radiation therapy (HR: 0.310; CI: 0.098–0.977; P = 0.045), and a larger pathologic invasive tumor size (HR: 1.051; CI: 1.003–1.101; P = 0.038) were significantly associated with worse distant metastasis-free survival at univariate analysis (Table 2). There was no significant association between BPE and distant metastasis-free survival, based on both qualitative and quantitative assessments of both readers (Table 2).
At multivariate analysis, only a high Ki-67 expression level was associated with worse distant metastasis-free survival (HR: 3.571; CI: 1.027–12.417; P = 0.045) (Table 3).
Interobserver Variability of BPE
The kappa values between the two readers for qualitative classification of BPE was 0.700 (CI: 0.6139–0.7866), which indicates good agreement for qualitative parameters of BPE.19 The ICC values between the two readers for ROI-based quantitative assessments of BPE were 0.831 (CI: 0.7174–0.8910), 0.840 (CI: 0.7521–0.8911), and 0.793 (CI: 0.7314–0.8397) for early enhancement rate, delayed enhancement rate, and late enhancement, which indicate substantial to almost perfect agreement.20 Among ROI-based quantitative parameters of BPE, only SER showed fair agreement (ICC 0.340 [CI: 0.2342–0.4385]).
Result of Subgroup Analysis After Adjustment for the Menstrual Cycle
In subgroup analysis of 191 patients who either underwent breast MRI during the 2nd or 3rd week of the menstrual cycle (n = 64) or who were postmenopausal (n = 127), none of the qualitative or quantitative assessments of BPE were associated with survival outcome at univariate analysis (Supplementary Table 1). At the univariate and multivariate analysis, a high Ki-67 expression level was independently associated with worse recurrence-free survival (HR: 9.653; CI: 2.085–44.684; P = 0.004 at univariate analysis, HR: 6.611; CI: 1.348–32.416; P = 0.020 at multivariate analysis) in this subgroup (Supplementary Tables 1, 2).
Discussion
In our study consisting of 289 consecutive patients with unilateral node-negative invasive ER-positive/HER2-negative breast cancer, we found that a high Ki-67 expression level was the only independent factor associated with worse survival outcome, regardless of whether adjustment of the menstrual cycle was performed. Both qualitative and quantitative assessments of contralateral BPE did not show an association with survival outcome. Our results are consistent with previous literature regarding the prognostic value of Ki-67 in breast cancer patients, and recent studies emphasizing the important role of proliferation in breast cancer prognosis, especially in ER-positive breast cancer.21–24 Recently, an ultralow-risk classification based on the 70-gene signature was reported, of which 96% were Ki67-low and 89% were designated as luminal A.25,26 Our results also support that Ki-67 is an important prognostic parameter in ER-positive invasive breast cancer, whereas BPE may not provide prognostic information in node-negative tumors, which generally have a good outcome.27
Recently, van der Velden reported that contralateral BPE was significantly associated with long-term outcome, particularly in ER-positive, HER2-negative invasive breast cancer patients.2 However, subgroup analysis of node-negative ER-positive tumors was not performed and Ki-67 expression was not reported in their study.2 In a following study, contralateral BPE showed significant association with both overall survival and distant disease-free survival in high-risk patient groups classified by the Nottingham prognostic index or Dutch clinical guidelines, but showed an association with only overall survival in high-risk groups defined by molecular assays (70-gene signature and 21-gene recurrence score).28 The former clinicopathological guidelines incorporate tumor size, lymph node status, and tumor grade, whereas prognostic abilities of gene expression signatures are mostly due to the detection of proliferation activity.24,29 Although tumor grade is also a marker of proliferation, gene expression signatures have been reported to add prognostic information beyond that provided by tumor grade.23,24,30 In addition, a recent study reported that gene expression signatures failed to provide significant prognostic information beyond Ki-67 in ER+/LN− patients, whereas all signatures demonstrated prognostic strength beyond Ki-67 or immunohistochemical subtypes in ER+/LN+ patients.31 This may imply that contralateral BPE may provide less additional information after consideration of tumor proliferation activity, and further risk stratification beyond Ki-67 would be even more difficult in ER-positive, LN-negative patients, who already have a good prognosis.
Methods used for quantitative measurements of BPE vary across studies.32 Earlier studies on BPE have mainly used qualitative BPE assessments or an ROI-based quantitative method by including the most enhancing portion of the breast.11,12,33–35 Recently, several studies have applied quantitative BPE assessment using fibroglandular tissue segmentation.2,36–38 ROI-based quantitative assessment has the advantage of being easy to perform and excludes coexisting benign enhancing breast lesions, but interobserver variability is inevitable. In contrast, BPE assessment using fully automatic fibroglandular tissue segmentation may be more robust, but is more complex and not yet widely available. In our study, ROI-based BPE assessment was performed by two readers and all quantitative parameters but SER showed good to perfect agreement. Although we additionally performed fully automatic fibroglandular tissue segmentation and subsequent calculation for LE90+, none of the qualitative or quantitative assessments of BPE showed association with survival outcome at univariate analysis. Therefore, our results suggest that contralateral BPE cannot serve as a prognostic marker in node-negative ER-positive, HER2-negative invasive breast cancer patients.
Our study had several limitations. First, this was a retrospective study performed at a single institution. Second, MRI examinations were not scheduled according the women’s menstrual cycles. Although scheduling of screening MRI is routinely recommended in the second week of the menstrual cycle to minimize BPE, diagnostic MRI for breast cancer staging is usually performed regardless of the menstrual cycle to avoid delay in surgery.9 However, we performed subgroup analysis including only women who underwent breast MRI during the 2nd or 3rd week of the menstrual cycle or were postmenopausal, and there was also no association between BPE and treatment outcome. Third, the percentage of events was small (5.9%), but considering the favorable prognosis of this subgroup with a 5-year disease-free survival of 93.8%, we believe the number of included cases is not small compared with other studies.27
Finally, our study population underwent different treatment methods, including type of surgery, radiation therapy, and adjuvant chemotherapy, which could have impacted survival outcome. However, all of the patients received endocrine therapy and different treatment methods were considered by using a multivariate Cox proportional hazard model.
In conclusion, contralateral BPE was not associated with survival outcome in patients with unilateral ER-positive, HER2-negative, node-negative invasive breast cancer. A high Ki-67 expression level was the only independent factor associated with both worse recurrence-free and distant metastasis-free survival.
Supplementary Material
Acknowledgment
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2016 (6-2016-0112) and by a Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03035995).
Footnotes
Additional supporting information may be found in the online version of this article.
References
- 1.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–752. [DOI] [PubMed] [Google Scholar]
- 2.van der Velden BH, Dmitriev I, Loo CE, Pijnappel RM, Gilhuijs KG. Association between parenchymal enhancement of the contralateral breast in dynamic contrast-enhanced MR imaging and outcome of patients with unilateral invasive breast cancer. Radiology 2015;276:675–685. [DOI] [PubMed] [Google Scholar]
- 3.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817–2826. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node- negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet 2004;364:858–868. [DOI] [PubMed] [Google Scholar]
- 5.Dialani V, Gaur S, Mehta TS, et al. Prediction of low versus high recurrence scores in estrogen receptor-positive, lymph node-negative invasive breast cancer on the basis of radiologic-pathologic features: comparison with oncotype DX test recurrence scores. Radiology 2016: 151149. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Zhu Y, Burnside ES, et al. MR imaging radiomics signatures for predicting the risk of breast cancer recurrence as given by research versions of MammaPrint, Oncotype DX, and PAM50 gene assays. Radiology 2016:152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive Breast Cancer Version 1. 2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compreh Cancer Network JNCCN 2016;14:324–354. [DOI] [PubMed] [Google Scholar]
- 8.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- 9.ACR ACR BI-RADS ATLAS. Breast Imaging Reporting and Data System, 5th ed. 2013. [Google Scholar]
- 10.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011;260:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrading S, Schild H, Kuhr M, Kuhl C. Effects of tamoxifen and aromatase inhibitors on breast tissue enhancement in dynamic contrast-enhanced breast MR imaging: a longitudinal intraindividual cohort study. Radiology 2014;271:45–55. [DOI] [PubMed] [Google Scholar]
- 12.Jansen SA, Lin VC, Giger ML, Li H, Karczmar GS, Newstead GM. Normal parenchymal enhancement patterns in women undergoing MR screening of the breast. Eur Radiol 2011;21:1374–1382. [DOI] [PubMed] [Google Scholar]
- 13.Kim SA, Cho N, Ryu EB, et al. Background parenchymal signal enhancement ratio at preoperative MR imaging: association with subsequent local recurrence in patients with ductal carcinoma in situ after breast conservation surgery. Radiology 2014;270:699–707. [DOI] [PubMed] [Google Scholar]
- 14.Lin M, Chan S, Chen JH, et al. A new bias field correction method combining N3 and FCM for improved segmentation of breast density on MRI. Med Phys 2011;38:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin M, Chen JH, Wang X, Chan S, Chen S, Su MY. Template-based automatic breast segmentation on MRI by excluding the chest region. Med Phys 2013;40:122301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhl CK, Bieling HB, Gieseke J, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology 1997;203:137–144. [DOI] [PubMed] [Google Scholar]
- 17.Muller-Schimpfle M, Ohmenhauser K, Stoll P, Dietz K, Claussen CD. Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology 1997;203:145–149. [DOI] [PubMed] [Google Scholar]
- 18.Choi JS, Ko ES, Ko EY, Han BK, Nam SJ. Background parenchymal enhancement on preoperative magnetic resonance imaging: association with recurrence-free survival in breast cancer patients treated with neoadjuvant chemotherapy. Medicine 2016;95:e3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleiss JL. The measurement of interrater agreement In: Statistical Methods for Rates and Proportions, 2nd ed. John Wiley & Sons, New York; 1981;212–236. [Google Scholar]
- 20.Landis JR, Koch GG The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 21.Aleskandarany MA, Green AR, Benhasouna AA, et al. Prognostic value of proliferation assay in the luminal, HER2-positive, and triple-negative biologic classes of breast cancer. Breast Cancer Res 2012; 14:R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inwald EC, Klinkhammer-Schalke M, Hofstadter F, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat 2013;139:539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiesner FG, Magener A, Fasching PA, et al. Ki-67 as a prognostic molecular marker in routine clinical use in breast cancer patients. Breast (Edinburgh, Scotland) 2009;18:135–141. [DOI] [PubMed] [Google Scholar]
- 24.Wirapati P, Sotiriou C, Kunkel S, et al. Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res 2008;10:R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delahaye L, Drukker CA, Dreezen C, et al. A breast cancer gene signature for indolent disease. Breast Cancer Res Treat 2017;164:461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esserman LJ, Yau C, Thompson CK, et al. Use of molecular tools to identify patients with indolent breast cancers with ultralow risk over 2 decades. JAMA Oncol 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015;373: 2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Velden BHM, Elias SG, Bismeijer T, et al. Complementary value of contralateral parenchymal enhancement on DCE-MRI to prognostic models and molecular assays in high-risk ER+HER2- breast cancer. Clin Cancer Res 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29.Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat 1992;22:207–219. [DOI] [PubMed] [Google Scholar]
- 30.Fan C, Oh DS, Wessels L, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med 2006; 355:560–569. [DOI] [PubMed] [Google Scholar]
- 31.Lundberg A, Lindstrom LS, Harrell JC, et al. Gene expression signatures and immunohistochemical subtypes add prognostic value to each other in breast cancer cohorts. Clin Cancer Res 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bignotti B, Signori A, Valdora F, et al. Evaluation of background parenchymal enhancement on breast MRI: a systematic review. Br J Radiol 2017;90:20160542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dontchos BN, Rahbar H, Partridge SC, et al. Are qualitative assessments of background parenchymal enhancement, amount of fibroglandular tissue on MR images, and mammographic density associated with breast cancer risk? Radiology 2015;276:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King V, Gu Y, Kaplan JB, Brooks JD, Pike MC, Morris EA. Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur Radiol 2012;22:2641–2647. [DOI] [PubMed] [Google Scholar]
- 35.Schrading S, Kuhl CK. Breast cancer: influence of taxanes on response assessment with dynamic contrast-enhanced MR imaging. Radiology 2015;277:687–696. [DOI] [PubMed] [Google Scholar]
- 36.Chen JH, Yu H, Lin M, Mehta RS, Su MY. Background parenchymal enhancement in the contralateral normal breast of patients undergoing neoadjuvant chemotherapy measured by DCE-MRI. Magn Reson Imaging 2013;31:1465–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klifa C, Suzuki S, Aliu S, et al. Quantification of background enhancement in breast magnetic resonance imaging. J Magn Reson Imaging 2011;33:1229–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu S, Weinstein SP, DeLeo MJ, 3rd, et al. Quantitative assessment of background parenchymal enhancement in breast MRI predicts response to risk-reducing salpingo-oophorectomy: preliminary evaluation in a cohort of BRCA½ mutation carriers. Breast Cancer Res 2015;17:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


