Abstract
Background:
Ebstein anomaly is a rare congenital heart defect (CHD) that, when severe, requires corrective surgery or other catheter-based intervention in the first year of life. Due to its rarity, risk factors for Ebstein anomaly remain largely unknown. Using national data, we examined 18 potential risk factors for Ebstein anomaly.
Methods:
Using 1997–2011 data from the National Birth Defects Prevention Study, a population-based case-control study, we calculated crude and adjusted odds ratios and 95% confidence intervals for paternal age, maternal socio-demographics, reproductive history, and modifiable risk factors, and infant characteristics reported by mothers of 135 Ebstein anomaly cases and 11,829 controls.
Results:
Mothers of Ebstein anomaly cases had 4.1 (95% confidence interval: 1.8, 9.5) times the odds of reporting a family history of CHD compared with mothers of controls. Ebstein anomaly was associated with maternal second-hand cigarette smoke exposure at home (odds ratio = 2.2 [95% confidence interval: 1.1,4.4]), but not maternal cigarette smoking (odds ratio = 1.3 [95% confidence interval: 0.8, 2.1]). Odds were elevated, but the 95% confidence interval included 1.0, for maternal marijuana use (odds ratio = 1.8 [95% confidence interval: 0.9, 3.8]) and paternal age ≥40 years at delivery (odds ratio = 1.9 [95% confidence interval: 1.0, 3.5]).
Conclusions:
Maternal exposure to second-hand cigarette smoke at home and a family history of CHD were associated with elevated odds of Ebstein anomaly. Genetic analyses could clarify the potential heritability of Ebstein anomaly.
Keywords: Ebstein anomaly, congenital heart defect, risk factors, case–control study, prenatal exposure
Ebstein anomaly is a rare congenital heart defect (CHD) involving abnormal formation and position of the tricuspid valve, ranging from minimally symptomatic to critical right heart obstruction.1 When severe, Ebstein anomaly is considered a critical CHD requiring corrective surgery or other catheter-based intervention in the first year of life to improve systemic oxygenation; prognosis for these cases may be poor.2–5 With a prevalence of about 7 per 100,000 live births, the rarity of Ebstein anomaly has been an obstacle to examining potential risk factors.6,7 Several population-based cohorts and case-control studies have explored possible risk factors for occurrence of Ebstein anomaly: the Baltimore-Washington Infant Study,3,4 Hawaii Birth Defects Program,8 Texas Birth Defects Registry,6 EUROCAT multi-registry9 and National Birth Defects Prevention Study (1997–2011).10–12 Results from published reports have varied, but one or more have found maternal race, age, pre-pregnancy body mass index, lithium use during pregnancy, gastrointestinal medication use during pregnancy, anti-hypertensive medication use, marijuana use during pregnancy, proximity of residence to the Mexico border, and season of conception to be associated with Ebstein anomaly.3,6–8,10–13
While published reports have shed light on some potential risk factors for Ebstein anomaly, the small sample size of Ebstein anomaly cases in many studies hinders consistent risk estimates. Furthermore, the emphasis of some previous analyses has been on socio-demographic characteristics, maternal reproductive history (e.g. previous miscarriages), and infant-specific characteristics (e.g. gestational age), with less information published on potentially modifiable risk factors such as fertility treatments, maternal cigarette smoke exposure, maternal alcohol use, prenatal folic acid use, and maternal fever during pregnancy. Therefore, using data from the National Birth Defects Prevention Study, the objective of the present study was to examine a spectrum of potential risk factors for Ebstein anomaly, including modifiable exposures, many of which have not been previously examined.
Materials and methods
Data sources and population
The National Birth Defects Prevention Study is a population-based case-control study that examines risk factors for major birth defects. The National Birth Defects Prevention Study is a collaborative effort between 10 Centers for Birth Defects Research and Prevention, located in Arkansas, California, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, Utah, and the Centers for Disease Control and Prevention, which also serves as the Georgia Center for Birth Defects Research and Prevention. All sites collected data on live births with select major birth defects, and most sites additionally collected data on fetal deaths after 20 weeks gestation (all except New Jersey and New York prior to 2000) and elective terminations of pregnancy (all except New Jersey and Georgia before 1999 and Massachusetts before 2011). Infants with known genetic syndromes and chromosomal malformations were ineligible for National Birth Defects Prevention Study. Diagnoses, selected demographics, and pregnancy-and birth-related information on cases were ascertained from existing population-based active birth defects surveillance systems. Controls were live born infants with no major birth defects, who were randomly selected from the same source population as cases using either vital records or records from hospitals of birth. Case and control infants were born on or after October 1, 1997, and had an estimated date of delivery on or before December 31, 2011.
Between 6 weeks and 24 months after the estimated delivery date, mothers of case and control infants were invited to participate in a standardised computer-assisted telephone interview in either English or Spanish. The interview covered a range of modifiable risk factors that may be associated with the risk for birth defects, including infectious, chemical, physical, nutritional, and behavioural exposures. From 1997 to 2011, the National Birth Defects Prevention Study interview participation rate overall was 67% for cases and 65% for controls.14 Each study site and the Centers for Disease Control and Prevention obtained Institutional Review Board approval for the study and patients provided informed consent. A more detailed description of the National Birth Defects Prevention Study sampling and design can be found elsewhere.14,15
Case definition
Abstracted medical information of all cases was reviewed by a clinical geneticist and a clinician with expertise in paediatric cardiology to confirm Ebstein anomaly case eligibility.16 There were a total of 183 cases of Ebstein anomaly included in the National Birth Defects Prevention Study. For this investigation, we restricted our analysis to 135 Ebstein anomaly cases who had no major non-cardiac defects and no other cardiac defects aside from anomalies that are commonly co-occurring with Ebstein anomaly: pulmonary stenosis, ventricular septal defects, and atrial septal defects.
Potential risk factors
We identified 18 maternal, paternal, and infant characteristics to examine as potential risk factors for Ebstein anomaly. Maternal factors included age at delivery (<20, 20–34, 35–39, ≥40 years), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), pre-pregnancy body mass index (<25, 25–29, ≥30 kg/m2), and gravidity (0, ≥1); exposures reported during the first trimester of pregnancy such as fever (yes, no), folic acid use (yes, no), gastrointestinal medication use (yes, no; defined as maternal use of any antacids, anti-diarrheal agents, anti-emetics, anti-flatulents, and anti-ulcer agents), alcohol use (yes, no), marijuana use (yes, no), and cigarette smoke (analysed using three categorisation schemes described in more detail later); and any fertility treatment within 2 months prior to conception (yes, no; defined as use of any medications, procedures, or surgeries to help become pregnant) and maternal use of assisted reproductive technology within 2 months prior to conception (yes, no; defined as use of in vitro fertilisation or intra-cytoplasmic sperm injection). Paternal factors included age at delivery (<20, 20–34, 35–39, ≥40 years). Infant characteristics included 1st degree family history of CHDs (i.e. mother, father, or sibling of case with CHD; yes, no), season of conception (spring: March-May; summer: June-August; fall: September-November; winter: December-February), sex (male, female), plurality (singleton, multiple), and year of birth (1997–2001, 2002–2006, 2007–2011). Maternal lithium exposure during pregnancy was also examined as a potential risk factor, but the sample size of exposed cases (n = 1) and controls (n = 8) was insufficient for further analysis.
Similar to a previous National Birth Defects Prevention Study analysis,17 maternal cigarette smoke exposure was assessed using the following three categorisation schemes: any cigarette smoke exposure (maternal smoking regardless of second-hand smoke exposure; second-hand smoke exposure at home, work or school; no cigarette smoke exposure); second-hand cigarette smoke exposure at home among non-smokers (yes, no); and second-hand cigarette smoke exposure at work or school among non-smokers (yes, no). For maternal exposures reported during pregnancy, mothers were considered exposed if they reported the exposure from the month prior to conception through the end of the 1st trimester of pregnancy (hereafter referred to as 1st trimester) and unexposed if they did not report the exposure from 3 months before the date of conception to the estimated date of delivery (hereafter referred to as pregnancy). Mothers exposed outside of the exposure window of interest only were excluded. However, in two sensitivity analyses, we included mothers exposed outside the original exposure window (i.e. those excluded from the original analyses) and categorised them as either exposed (broadening the exposure window to all of pregnancy) or unexposed.
Potential confounders
Potential confounders for each of the 18 risk factor analyses were selected based on reported associations from previous literature and theoretical associations using directed acyclic graphs. Each risk factor model was considered separately, and thus potential con-founders varied across the 18 models. All multi-variable models included maternal age at delivery, maternal race/ethnicity, maternal pre-pregnancy body mass index, family history of CHDs, season of conception, maternal marijuana use, paternal age at delivery, and birth year. In addition to the variables listed earlier, anti-hypertensive medication use was included in the model examining maternal body mass index, and gastrointestinal medication use was included in the model examining maternal fever. Report of anti-hypertensive medication use was not assessed as an independent risk factor of interest in this analysis because two recent National Birth Defects Prevention Study analyses have already reported an association between Ebstein anomaly and anti-hypertensive medication use.10,11
Analysis
Descriptive statistics among Ebstein anomaly cases and controls were examined using chi-square tests, with a p value of <0.05 considered statistically significant. To assess the association between each of the 18 potential risk factors of interest and Ebstein anomaly, we used logistic regression to estimate crude odds ratios, adjusted odds ratios, and corresponding 95% confidence intervals. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
There were 135 Ebstein anomaly cases that met our case definition and 11,829 controls in National Birth Defects Prevention Study between 1997 and 2011 (Fig 1). Maternal and infant characteristics of Ebstein anomaly cases were similar to those of controls: about 76% of mothers were between 20 and 34 years of age at the time of delivery, about 60% were non-Hispanic white, and about 50% of infants were male (Table 1). However, more case mothers than control mothers reported a 1st degree family history of CHD (5% and 1%, respectively, p < 0.01) and exposure to anti-hypertensive medications during pregnancy (5% and 2%, respectively, p < 0.01). No other differences between cases and controls were statistically significant.
Figure 1.
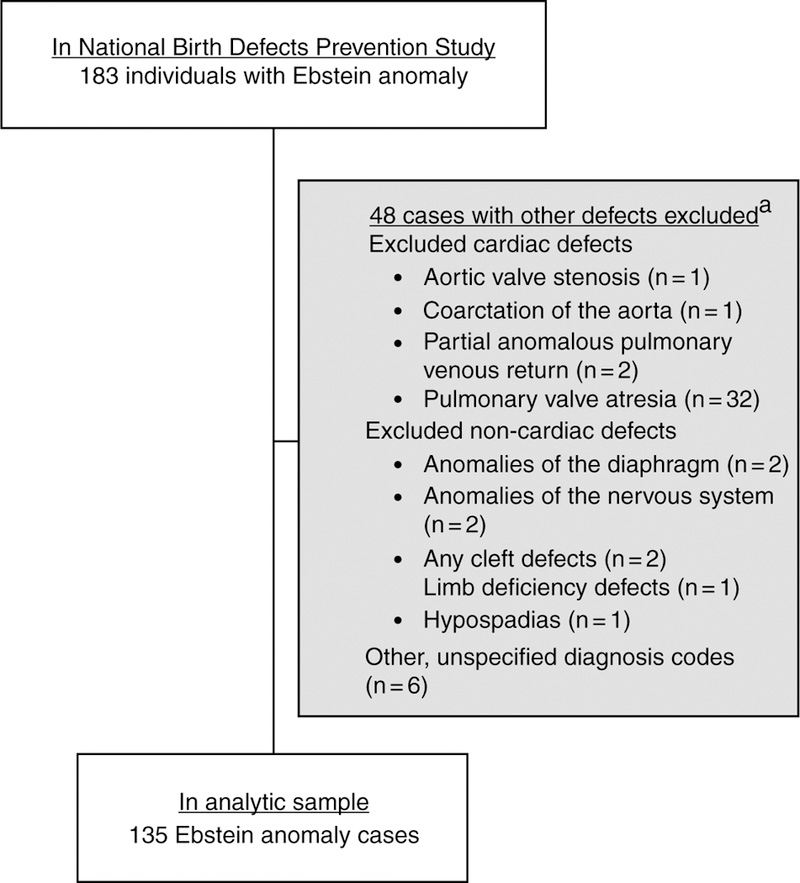
Exclusion criteria and final sample of Ebstein anomaly cases, National Birth Defects Prevention Study, 1997–2011.
Table 1.
Description of Ebstein anomaly cases and controls, National Birth Defects Prevention Study, 1997–2011.
| Characteristic | Ebstein Anomaly Cases (N = 135) |
Controls (N = 11,829) | p Value* | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Maternal age at delivery (years) | 0.86 | ||||
| <20 | 11 | 8.2 | 1177 | 10.0 | |
| 20–34 | 103 | 76.3 | 8988 | 76.0 | |
| 35–39 | 17 | 12.6 | 1388 | 11.7 | |
| ≥40 | 4 | 3.0 | 276 | 2.3 | |
| Maternal race/ethnicity | 0.39 | ||||
| Non-Hispanic white | 85 | 63.0 | 6836 | 57.8 | |
| Non-Hispanic black | 9 | 6.7 | 1308 | 11.1 | |
| Hispanic | 33 | 24.4 | 2908 | 24.6 | |
| Other | 8 | 5.9 | 770 | 6.5 | |
| Maternal pre-pregnancy BMI (kg/m2) | 0.49 | ||||
| <25 | 71 | 52.6 | 6644 | 56.2 | |
| 25–29 | 35 | 25.9 | 2557 | 21.6 | |
| ≥30 | 24 | 17.8 | 2074 | 17.5 | |
| Gravidity | 0.28 | ||||
| 0 | 34 | 25.2 | 3471 | 29.3 | |
| ≥1 | 101 | 74.8 | 8307 | 70.2 | |
| Maternal anti-hypertensive medication use | <0.01 | ||||
| Yes | 7 | 5.2 | 228 | 1.9 | |
| No | 121 | 89.6 | 11,256 | 95.2 | |
| Maternal fever | 0.44 | ||||
| Any 1st trimester exposure | 15 | 11.1 | 1127 | 9.5 | |
| Other exposure during pregnancy** | 21 | 15.6 | 1492 | 12.6 | |
| No exposure during pregnancy | 87 | 64.4 | 8147 | 69.0 | |
| Maternal folic acid use | 0.35 | ||||
| Any 1st trimester exposure | 111 | 82.2 | 10,113 | 85.5 | |
| Other exposure during pregnancy** | 12 | 8.9 | 844 | 7.1 | |
| No exposure during pregnancy | 9 | 6.7 | 535 | 4.5 | |
| Maternal gastrointestinal medication use | 0.69 | ||||
| Any 1st trimester exposure | 28 | 20.7 | 2796 | 23.6 | |
| Other exposure during pregnancy** | 6 | 4.4 | 605 | 5.1 | |
| No exposure during pregnancy | 98 | 72.6 | 8241 | 69.7 | |
| Maternal alcohol use | 0.18 | ||||
| Any 1st trimester exposure | 46 | 34.1 | 4280 | 36.2 | |
| Other exposure during pregnancy** | 6 | 4.4 | 994 | 8.4 | |
| No exposure during pregnancy | 78 | 57.8 | 6217 | 52.6 | |
| Maternal marijuana use | 0.24 | ||||
| Any 1st trimester exposure | 8 | 5.9 | 454 | 3.8 | |
| Other exposure during pregnancy** | 0 | 0.0 | 117 | 1.0 | |
| No exposure during pregnancy | 123 | 91.1 | 10,940 | 92.5 | |
| Maternal smoking/second-hand cigarette smoke exposure | 0.17 | ||||
| Any smoking or second-hand smoke exposure in 1st trimester | 26 | 19.3 | 2075 | 17.5 | |
| Second-hand smoke exposure only in 1st trimester | 23 | 17.0 | 1390 | 11.8 | |
| Other smoke exposure during pregnancy** | 1 | 0.7 | 137 | 1.2 | |
| No smoke exposure during pregnancy | 78 | 57.8 | 7795 | 65.9 | |
| Any maternal fertility treatment | 0.57 | ||||
| Yes | 8 | 5.9 | 573 | 4.8 | |
| No | 127 | 94.1 | 11,191 | 94.6 | |
| Maternal use of ART | 0.47 | ||||
| Yes | 3 | 2.2 | 130 | 1.1 | |
| Use of non-ART fertility treatment only | 5 | 3.7 | 442 | 3.7 | |
| No use of any fertility treatment | 127 | 94.1 | 11,191 | 94.6 | |
| Paternal age at delivery (years) | 0.18 | ||||
| <20 | 6 | 4.4 | 538 | 4.6 | |
| 20–34 | 83 | 61.5 | 7988 | 67.5 | |
| 35–39 | 24 | 17.8 | 1917 | 16.2 | |
| ≥40 | 18 | 13.3 | 987 | 8.3 | |
| 1st degree family history of CHDs | <0.01 | ||||
| Yes | 7 | 5.2 | 139 | 1.2 | |
| No | 128 | 94.8 | 11,690 | 98.8 | |
| Season of conception | 0.68 | ||||
| Spring | 29 | 21.5 | 2897 | 24.5 | |
| Summer | 34 | 25.2 | 2944 | 24.9 | |
| Fall | 33 | 24.4 | 3057 | 25.8 | |
| Winter | 39 | 28.9 | 2931 | 24.8 | |
| Infant sex | 0.36 | ||||
| Male | 63 | 46.7 | 6024 | 50.9 | |
| Female | 71 | 52.6 | 5793 | 49.0 | |
| Birth plurality | 0.32 | ||||
| Singleton | 129 | 95.6 | 11,452 | 96.8 | |
| Multiple | 6 | 4.4 | 351 | 3.0 | |
| Year of birth | 0.52 | ||||
| 1997–2001 | 38 | 28.1 | 3448 | 29.2 | |
| 2002–2006 | 44 | 32.6 | 4274 | 36.1 | |
| 2007–2011 | 53 | 39.3 | 4107 | 34.7 | |
BMI =Body mass index; CHDs = Congenital heart defects; ART = Assisted reproductive technology.
Assessed by chi-square tests, excluding patients with missing data
Exposed either 2–3 months prior to conception or during the 2nd or 3rd trimester
Only family history of CHD and maternal second-hand cigarette smoke exposure were significantly associated with Ebstein anomaly (Table 2). Ebstein anomaly case mothers had 4.1 (95% confidence interval: 1.8, 9.5) times the odds of reporting a 1st degree family history of CHD compared with control mothers. After excluding women who smoked cigarettes, case mothers had 2.0 (95% confidence interval: 1.2, 3.2) times the odds of second-hand smoke exposure compared with control mothers. However, maternal smoking during the first trimester, regardless of second-hand smoke exposure, was not associated with Ebstein anomaly (adjusted odds ratio = 1.3 [95% confidence interval: 0.8, 2.1]). After excluding smokers, case mothers had elevated odds of reporting second-hand smoke exposure at home (adjusted odds ratio = 2.2 [95% confidence interval: 1.1, 4.4]) and at work (adjusted odds ratio =1.8 [95% confidence interval: 0.9, 3.6]); however, the latter did not reach statistical significance. We also observed borderline statistically significant elevated odds of Ebstein anomaly for fathers aged 40 years and older (adjusted odds ratio = 1.9 [95% confidence interval: 1.0, 3.5]). The odds of case mothers reporting marijuana use was elevated, but not statistically significant (adjusted odds ratio =1.8 [95% confidence interval: 0.9, 3.8]). The results of both sets of sensitivity analyses did not substantially alter results.
Table 2.
Odds ratios for associations between various factors and risk for Ebstein anomaly, National Birth Defects Prevention Study, 1997–2011.
| Risk Factor | Crude OR | 95% CI | Adjusted OR* | 95% CI |
|---|---|---|---|---|
| Maternal age at delivery (years) | ||||
| <20 | 0.8 | 0.4, 1.5 | 0.8 | 0.3, 1.8 |
| 20–34 | Reference | Reference | ||
| 35–39 | 1.1 | 0.6, 1.8 | 0.7 | 0.4, 1.4 |
| ≥40 | 1.3 | 0.5, 3.5 | 0.9 | 0.3, 2.6 |
| Maternal race/ethnicity | ||||
| Non-Hispanic white | Reference | Reference | ||
| Non-Hispanic black | 0.6 | 0.3, 1.1 | 0.6 | 0.3, 1.2 |
| Hispanic | 0.9 | 0.6, 1.4 | 0.9 | 0.6, 1.4 |
| Other | 0.8 | 0.4, 1.7 | 0.9 | 0.4, 1.9 |
| Maternal pre-pregnancy BMI** (kg/m2) | ||||
| ≤25 | Reference | Reference | ||
| 25–29 | 1.2 | 0.8, 1.7 | 1.2 | 0.8, 1.9 |
| ≥30 | 1.1 | 0.5, 2.4 | 0.9 | 0.6, 1.6 |
| Gravidity | ||||
| 0 | 0.8 | 0.5, 1.2 | 0.9 | 0.6, 1.4 |
| 1 or more | Reference | Reference | ||
| Maternal fever*** | ||||
| Any 1st trimester exposure | 1.2 | 0.7, 2.2 | 1.0 | 0.5, 2.0 |
| No exposure during pregnancy | Reference | Reference | ||
| Maternal folic acid use | ||||
| Any 1st trimester exposure | Reference | Reference | ||
| No exposure during pregnancy | 1.5 | 0.7, 3.0 | 1.5 | 0.7, 3.2 |
| Maternal gastrointestinal medication use | ||||
| Any 1st trimester exposure | 0.8 | 0.5, 1.2 | 0.8 | 0.5, 1.2 |
| No exposure during pregnancy | Reference | Reference | ||
| Maternal alcohol use | ||||
| Any 1st trimester exposure | 0.8 | 0.5, 1.2 | 0.8 | 0.5, 1.2 |
| No exposure during pregnancy | Reference | Reference | ||
| Maternal marijuana use | ||||
| Any 1st trimester exposure | 1.6 | 0.8, 3.2 | 1.8 | 0.9, 3.8 |
| No exposure during pregnancy | Reference | Reference | ||
| Maternal smoking/second-hand cigarette smoke exposure | ||||
| Any smoking or second-hand smoke exposure during 1st trimester | 1.3 | 0.8, 2.0 | 1.3 | 0.8, 2.1 |
| Second-hand exposure only during 1st trimester | 1.7 | 1.0, 2.6 | 2.0 | 1.2, 3.2 |
| No smoke exposure during pregnancy | Reference | Reference | ||
| Second-hand cigarette smoke exposure at home**** | ||||
| Any 1st trimester exposure | 2.1 | 1.2, 3.9 | 2.2 | 1.1, 4.4 |
| No exposure during pregnancy | Reference | Reference | ||
| Second-hand cigarette smoke exposure at work or school***** | ||||
| Any 1st trimester exposure | 1.6 | 0.8, 3.1 | 1.8 | 0.9, 3.6 |
| No exposure during pregnancy | Reference | Reference | ||
| Any fertility treatment | ||||
| Yes | 1.2 | 0.6, 2.5 | 0.9 | 0.4, 2.0 |
| No | Reference | Reference | ||
| Maternal use of ART | ||||
| Yes | 2.0 | 0.6, 6.5 | 1.2 | 0.3, 4.9 |
| No use of any fertility treatment | Reference | Reference | ||
| Paternal age at delivery (years) | ||||
| <20 | 1.0 | 0.4, 2.3 | 1.6 | 0.6, 4.3 |
| 20–34 | Reference | Reference | ||
| 35–39 | 1.2 | 0.8, 1.9 | 1.4 | 0.8, 2.3 |
| ≥40 | 1.8 | 1.1, 2.9 | 1.9 | 1.0, 3.5 |
| 1st Degree family history of CHDs | ||||
| Yes | 4.6 | 2.1, 10.0 | 4.1 | 1.8, 9.5 |
| No | Reference | Reference | ||
| Season of conception | ||||
| Spring | 0.8 | 0.5, 1.4 | 1.0 | 0.6, 1.6 |
| Summer | Reference | Reference | ||
| Fall | 1.0 | 0.6, 1.5 | 1.0 | 0.6, 1.7 |
| Winter | 1.2 | 0.7, 1.8 | 1.2 | 0.7, 1.9 |
| Infant sex | ||||
| Male | Reference | Reference | ||
| Female | 1.2 | 0.8, 1.6 | 1.2 | 0.9, 1.8 |
| Birth plurality | ||||
| Singleton | Reference | Reference | ||
| Multiple | 1.5 | 0.7, 3.5 | 1.6 | 0.7, 3.6 |
| Year of birth | ||||
| 1997–2001 | 0.9 | 0.6, 1.3 | 0.9 | 0.6, 1.4 |
| 2002–2006 | 0.8 | 0.5, 1.2 | 0.8 | 0.5, 1.2 |
| 2007–2011 | Reference | Reference |
OR = odds ratio; CI = confidence interval; BMI = body mass index; CHDs = congenital heart defects; ART = assisted reproductive technology
All adjusted models include the following variables: maternal age at delivery, maternal race/ethnicity, maternal pre-pregnancy BMI, family history of CHDs, season of conception, maternal marijuana use, paternal age at delivery, and birth year
Also adjusted for anti-hypertensive medication use during pregnancy
Also adjusted for gastrointestinal medication use during pregnancy
Excluding women reporting smoking during pregnancy, second-hand smoke exposure at work, or second-hand smoke exposure at home outside of 1st trimester
Excluding women reporting smoking during pregnancy, working from home, second-hand smoke exposure at home, or second-hand smoke exposure at work outside of 1st trimester.
Discussion
In this risk factor analysis with the largest sample of Ebstein anomaly cases published thus far, we observed higher odds of Ebstein anomaly among cases with family histories of CHD and maternal second-hand cigarette smoke exposure at home. We also found elevated but not statistically significant odds of Ebstein anomaly among infants born to fathers 40 years of age and older, mothers with second-hand cigarette smoke exposure at work, and mothers reporting marijuana use. There may be a genetic component with Ebstein anomaly as evidenced by the four times higher odds observed among those with a family history of CHD in this analysis. Although there does not appear to be any prior study that has analysed a family history of CHD and Ebstein anomaly specifically, several studies point to a genetic component in the heritability of non-syndromic CHD and right ventricular outflow defects, a group in which Ebstein anomaly is often included.18–20 The recurrence rate of CHD overall was about 4% in the offspring of 1,483 women reporting a family history of CHD who participated in a clinical study in Italy.19 In a Danish national cohort study, the recurrence risk ratio of right ventricular outflow defects was 48.6 (95% confidence interval: 27.5, 85.6) among infants with 1st degree relatives that also had right ventricular outflow defects.18 Familial incidence of any cardiovascular malformation was 11% among 1st degree relatives of hypoplastic left heart syndrome cases in a United States-based clinical study.20 However, in our study, which excludes infants with known genetic syndromes and chromosomal malformations, only 5% of Ebstein anomaly cases reported a 1st degree family history of CHD, suggesting that genetics may not explain the large majority of isolated Ebstein anomaly cases, though further study is required.
We also found that maternal second-hand cigarette smoke exposure, specifically at home, but not maternal cigarette smoking itself was associated with increased odds of Ebstein anomaly in the infant. Other National Birth Defects Prevention Study analyses have found similar associations between maternal second-hand smoke exposure and congenital limb deficiencies, anorectal atresia, neural tube defects, and orofacial clefts.21–24 Maternal second-hand cigarette smoke exposure at home may be a proxy for paternal cigarette smoking or unmeasured socio-demographic or biological factors associated with both second-hand smoke exposure and Ebstein anomaly. A meta-analysis of 125 studies found an elevated risk of CHDs associated with maternal passive smoking as well as paternal active smoking.25 The increasing body of evidence observing associations between maternal second-hand cigarette smoke exposure during pregnancy and birth defects is supported by findings that second-hand smoke introduces higher concentrations of some toxic constituents than maternal smoking.26
The evidence supporting an association between paternal age and CHDs is varied. We observed non-significant but elevated odds of Ebstein anomaly among infants born to fathers over 40 years of age. Using data from the Texas Birth Defects Registry, Lupo et al. found no association between paternal age and Ebstein anomaly.6 An analysis using national registry data in Denmark assessing the association between paternal age and all CHDs combined as well as a few specific CHD sub-types other than Ebstein anomaly reported that older paternal age was associated with elevated risk of patent ductus arteriosus only.27 A previous National Birth Defects Prevention Study analysis using data from 1997 to 2004 reported an elevated odds of right ventricular outflow tract obstruction and pulmonary valve stenosis for each year increase in paternal age.28
Our analyses could not confirm several associations with Ebstein anomaly reported in previously published literature. Correa-Villasenor et al. identified mothers 30 years and older at delivery and Lupo et al. identified women older than 39 years at delivery to be more likely than younger women to deliver an infant with Ebstein anomaly.3,6 Our results showed no association between Ebstein anomaly and maternal age at delivery. In previous literature on race and ethnicity, offspring of mothers who were non-Hispanic white tended to be at elevated risk for Ebstein anomaly compared with non-white mothers.3,7 Likewise, in our analysis, the odds of Ebstein anomaly was lower among non-Hispanic blacks (adjusted odds ratio = 0.6 [95% confidence interval: 0.3, 1.2]) compared with non-Hispanic whites; however, the confidence interval was wide and included 1.0. Unlike a previous National Birth Defects Prevention Study analysis using data from 1997 to 2004,12 we did not observe an association between Ebstein anomaly and maternal pre-pregnancy overweight or obesity (data not shown). In a previous study, infants conceived in the fall or winter had higher odds of Ebstein anomaly than those conceived in the summer6; however, we observed no association between season of conception and Ebstein anomaly. While maternal marijuana use was not significantly associated with Ebstein anomaly in this analysis, we observed an elevated odds ratio in both crude and adjusted analyses. Correa-Villasenor et al. identified maternal marijuana use to be significantly associated with Ebstein anomaly in crude analyses; however, they found the odds ratio was elevated but no longer statistically significant after adjustment for confounders.3 In the same study, infants with Ebstein anomaly were found to be three times more likely to have a mother who used gastrointestinal medications during pregnancy, whereas we found no association.3 These differences between our findings and others may be the consequence of low exposure prevalence in our sample or a small case sample size, despite having the largest sample size of any risk factor analysis on Ebstein anomaly thus far. The present study adds to the body of evidence on risk factors associated with Ebstein anomaly, which will help identify true associations as opposed to spurious findings.
There are several limitations to the present study. All exposures are self-reported by the mother and misclassification may occur if the mother cannot remember the timing of her exposure or is reluctant to disclose socially undesirable behaviours (e.g. prenatal alcohol use). Additionally, maternal smoke exposure is self-reported and smokers may report only second-hand smoke exposure rather than their own smoking behaviours because of social desirability, leading to misclassification of smoking exposure.29 Differential recall bias may occur if mothers of cases were better able to recall details of exposures that they believed to be related to the outcome than mothers of controls. We note that 30% of eligible mothers did not complete the computer-assisted telephone interview; however, the 70% response rate is relatively high for a large case-control study, and the control patients are representative of their base populations.30
The multiple testing we performed with 18 potential risk factors increases the likelihood that some of our findings may be due to chance. However, selection of exposures was based on previously published literature and theoretically plausible etiologies; therefore, multiple inference procedures are not advised.31 Previous literature considers maternal lithium exposure to be an important risk factor for Ebstein anomaly,13 but we did not have sufficient sample size of exposed mothers (only 1 exposed case mother and 8 exposed control mothers) to examine lithium in this analysis. We suspect that paternal smoking may be a potential risk factor given the association between Ebstein anomaly and second-hand smoke exposure at home, but we could not include paternal tobacco smoking in our analysis. Even with these limitations, this is the largest population-based analysis of risk factors for Ebstein anomaly. Because of both sample size and the variety of questions included in the maternal interview, we were able to consider a wide spectrum of potential risk factors and associations, including some not previously examined. Additionally, the present study had the advantage of a refined case classification involving expert review of medical records.
Conclusion
Compared with mothers of control infants, mothers of Ebstein anomaly cases had higher odds of maternal second-hand cigarette smoke exposure at home and a family history of CHD; additional research is needed to further investigate these associations. Future studies could assess how paternal tobacco exposure and other factors associated with maternal second-hand smoke exposure affect the risk for Ebstein anomaly. Additionally, genetic analyses could evaluate the potential heritability of isolated Ebstein anomaly.
Acknowledgements.
The authors thank Shannon Pruitt for replicating the results. We thank the families who participated in the National Birth Defects Prevention Study, which made this research possible. The authors also thank each site’s clinical geneticist, abstractors, study coordinators, and study investigators. This project was supported in part by an appointment to the Internship/Research Participation Program at the Centers for Disease Control, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and EPA.
Financial Support. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Footnotes
Conflicts of Interest. The authors have no conflicts of interest to disclose.
Disclaimer. The findings and conclusion in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Ethical Standards. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the Institutional Review Boards for the Centers for Disease Control and Prevention and the Arkansas, California, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah Centers for Birth Defects Research and Prevention.
References
- 1.Cetta F, Dearani JA, O’Leary PW, Driscoll DJ. Chapter 38: tricuspid valve disorders: atresia, dysplasia, and Ebstein anomaly In: Moss & Adams’ Heart Disease in Infants, Children, and Adolescents, Including the Fetus and Young Adult. Vol 2, 9th edn Lippincott Williams & Wilkins, Philadelphia, PA, 2016. [Google Scholar]
- 2.Attenhofer Jost CH, Connolly HM, Edwards WD, Hayes D, Warnes CA, Danielson GK. Ebstein’s anomaly-review of a multifaceted congenital cardiac condition. Swiss Med Wkly 2005; 135: 269–281. [DOI] [PubMed] [Google Scholar]
- 3.Correa-Villasenor A, Ferencz C, Neill CA, Wilson PD, Boughman JA. Ebstein’s malformation of the tricuspid valve: genetic and environmental factors. The Baltimore-Washington Infant Study Group. Teratology 1994; 50: 137–147. [DOI] [PubMed] [Google Scholar]
- 4.Roberson DA, Silverman NH. Ebstein’s anomaly: echocardiographic and clinical features in the fetus and neonate. J Am Coll Cardiol 1989; 14: 1300–1307. [DOI] [PubMed] [Google Scholar]
- 5.Zeng Z, Zhang H, Liu F, Zhang N. Current diagnosis and treatments for critical congenital heart defects. Exp Ther Med 2016; 11: 1550–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lupo PJ, Langlois PH, Mitchell LE. Epidemiology of Ebstein anomaly: prevalence and patterns in Texas, 1999–2005. Am J Med Genet A 2011; 155a: 1007–1014. [DOI] [PubMed] [Google Scholar]
- 7.Correa-Villasenor A, McCarter R, Downing J, Ferencz C. White-black differences in cardiovascular malformations in infancy and socioeconomic factors. The Baltimore-Washington Infant Study Group. Am J Epidemiol 1991; 134: 393–402. [DOI] [PubMed] [Google Scholar]
- 8.Forrester MB, Merz RD. Descriptive epidemiology of selected congenital heart defects, Hawaii, 1986–1999. Paediatr Perinat Epidemiol 2004; 18: 415–424. [DOI] [PubMed] [Google Scholar]
- 9.Knudsen TM, Hansen AV, Garne E, Andersen AM. Increased risk ofsevere congenital heart defects in offspring exposed to selective serotonin-reup-take inhibitors in early pregnancy - an epidemiological study using validated EUROCAT data. BMC Pregnancy Childbirth 2014; 14: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caton AR, Bell EM, Druschel CM, et al. Antihypertensive medication use during pregnancy and the risk of cardiovascular malformations. Hypertension 2009; 54: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher SC, Van Zutphen AR, Werler MM, et al. Maternal antihypertensive medication use and congenital heart defects: updated results from the National Birth Defects Prevention Study. Hypertension 2017; 69: 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilboa SM, Correa A, Botto LD, et al. Association between prepregnancy body mass index and congenital heart defects. Am J Obstet Gynecol 2010; 202: 51.e1–e10. [DOI] [PubMed] [Google Scholar]
- 13.Patorno E, Huybrechts KF, Hernandez-Diaz S. Lithium use in pregnancy and the risk of cardiac malformations. New Engl J Med 2017; 377: 893–894. [DOI] [PubMed] [Google Scholar]
- 14.Reefhuis J, Gilboa SM, Anderka M, et al. The National Birth Defects Prevention Study: a review of the methods. Birth Defects Res A Clin Mol Teratol 2015; 103: 656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon PW, Rasmussen SA, Lynberg MC, et al. The National Birth Defects Prevention Study. Public Health Rep 2001; 116 Suppl 1: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botto LD, Lin AE, Riehle-Colarusso T, Malik S, Correa A. Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol 2007; 79: 714–727. [DOI] [PubMed] [Google Scholar]
- 17.Caspers KM, Oltean C, Romitti PA, et al. Maternal periconceptional exposure to cigarette smoking and alcohol consumption and congenital diaphragmatic hernia. Birth Defects Res A Clin Mol Teratol 2010; 88: 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyen N, Poulsen G, Boyd HA, Wohlfahrt J, Jensen PK, Melbye M. Recurrence of congenital heart defects in families. Circulation 2009; 120: 295–301. [DOI] [PubMed] [Google Scholar]
- 19.Fesslova V, Brankovic J, Lalatta F, et al. Recurrence of congenital heart disease in cases with familial risk screened prenatally by echocardiography. J Pregnancy 2011; 2011: 368067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelle AM, Qureshi MY, Olson TM, Eidem BW, O’Leary PW. Familial incidence of cardiovascular malformations in hypoplastic left heart syndrome. Am J Cardiol 2015; 116: 1762–1766. [DOI] [PubMed] [Google Scholar]
- 21.Miller EA, Manning SE, Rasmussen SA, Reefhuis J, Honein MA. Maternal exposure to tobacco smoke, alcohol and caffeine, and risk of anorectal atresia: National Birth Defects Prevention Study 1997–2003. Paediatr Perinat Epidemiol 2009; 23: 9–17. [DOI] [PubMed] [Google Scholar]
- 22.Suarez L, Ramadhani T, Felkner M, et al. Maternal smoking, passive tobacco smoke, and neural tube defects. Birth Defects Res A Clin Mol Teratol 2011;91:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caspers KM, Romitti PA, Lin S, Olney RS, Holmes LB, Werler MM. Maternal periconceptional exposure to cigarette smoking and congenital limb deficiencies. Paediatr Perinat Epidemiol 2013; 27: 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoyt AT, Canfield MA, Romitti PA, et al. Associations between maternal periconceptional exposure to secondhand tobacco smoke and major birth defects. Am J Obstet Gynecol 2016; 215: 613.e1–e11. [DOI] [PubMed] [Google Scholar]
- 25.Zhao L, Chen L, Yang T, et al. Parental smoking and the risk of congenital heart defects in offspring: an updated meta-analysis of observational studies. Eur J Prev Cardiol 2019; 0: 1–10. [DOI] [PubMed] [Google Scholar]
- 26.Feldkamp ML, Srisukhumbowornchai S, Romitti PA, Olney RS, Richardson SD, Botto LD. Self-reported maternal cigarette smoke exposure during the periconceptional period and the risk for omphalocoele. Paediatr Perinat Epidemiol 2014; 28: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su XJ, Yuan W, Huang GY, Olsen J, Li J. Paternal age and offspring congenital heart defects: a national cohort study. PloS One 2015; 10: e0121030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green RF, Devine O, Crider KS, et al. Association of paternal age and risk for major congenital anomalies from the National Birth Defects Prevention Study, 1997 to 2004. Ann Epidemiol 2010; 20: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietz PM, Homa D, England LJ, et al. Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol 2011; 173: 355–359. [DOI] [PubMed] [Google Scholar]
- 30.Cogswell ME, Bitsko RH, Anderka M, et al. Control selection and participation in an ongoing, population-based, case-control study of birth defects: the National Birth Defects Prevention Study. Am J Epidemiol 2009; 170: 975–985. [DOI] [PubMed] [Google Scholar]
- 31.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd edn Lippincott Williams & Wilkins, Philadelphia, 2008. [Google Scholar]


