Abstract
Objectives:
Apathy is a debilitating symptom of Huntington’s disease and manifests before motor diagnosis, making it an excellent therapeutic target in the preclinical phase of Huntington’s disease (HD). HD is a neurological genetic disorder characterized by cognitive and motor impairment, and psychiatric abnormalities. Apathy is not well characterized within the prodromal phase of HD. In previous literature, damage to the caudate and putamen has been correlated with increased apathy in other neurodegenerative and movement disorders. The objective of this study was to determine whether apathy severity in individuals with prodromal Huntington’s disease is related to striatum volumes and cognitive control. We hypothesized that, within prHD individuals, striatum volumes and cognitive control scores would be related to apathy.
Methods:
We constructed linear mixed models to analyze striatum volumes and cognitive control, a composite measure that includes tasks assessing with apathy scores from 797 prodromal HD participants. The outcome variable for each model was apathy, and the independent variables for the four separate models were caudate volume, putamen volume, cognitive control score, and motor symptom score. We also included depression as a covariate to ensure that our results were not solely related to mood.
Results:
Caudate and putamen volumes, as well as measures of cognitive control, were significantly related to apathy scores even after controlling for depression.
Conclusions:
The behavioral apathy expressed by these individuals was related to regions of the brain commonly associated with isolated apathy, and not a direct result of mood symptoms.
Keywords: Apathy, Huntington Disease, Executive function, Caudate, Putamen, Basal Ganglia diseases
INTRODUCTION
Huntington’s disease (HD) is a debilitating genetic disorder characterized by involuntary movements, cognitive decline, and eventual loss of bodily control. An extended tri-nucleotide cytosine-adenine-guanine (CAG) repetition causes the neurological atrophy responsible for these symptoms, and individuals with more repetitions of CAG will have an earlier onset of the disease (Chial, 2008). HD currently affects 12.3 to 17.2 people per 100,000 in the western world (Evans et al., 2013). Death usually occurs within 15-20 years after clinical diagnosis, and the disease typically develops around age 40 (Ross & Tabrizi, 2011).The prodromal HD population (prodromal HD) consists of anyone who tests positive for the genetic mutation (CAG repeat length > 35) but has not yet reached a full motor diagnosis (Paulsen et al., 2006; Paulsen et al., 2008a). Data analyzed for this study were collected as part of the Neurobiological Predictors of Huntington's Disease (PREDICT-HD) study, a national multi-site study with over 1,400 participants aiming to better specify factors contributing to HD onset in a prodromal (premanifest) sample (Paulsen, 2010; Paulsen, Long, Johnson, & Aylward, 2014).
Neurological and clinical changes are detectable in individuals in the prodromal phase of HD. Across studies, atrophy in the striatum, particularly in the caudate, is the most consistently reported imaging biomarker of prodromal and early HD and can be detected up to 15 years before diagnosis (Aylward et al., 2011; van den Bogaard et al., 2011; Wolf et al., 2013). Greater CAG repeat length is often correlated with smaller caudate volumes (Aylward et al., 2011). Caudate atrophy generates a variety of clinical symptoms detectable before manifest motor diagnosis (Halliday et al., 1998; Mendez, Adams, & Lewandowski, 1989; van den Bogaard et al., 2011).
Apathy is one of the most commonly reported prodromal HD symptoms, with a prevalence rate as high as 62% (K Duff et al., 2007; Epping & Paulsen, 2011; Julien et al., 2007). In prodromal HD literature, apathy has been characterized as a distinct mood symptom, separate from depression, and can exist independent of depressive symptoms (Alexopoulos et al., 2013; Marin, 1991). Apathy is related to CAG repeat length; longer CAG repeat lengths correlate with greater reported apathy (Baudic et al., 2006). Despite its high prevalence in the prodromal phase of HD, it is difficult to distinguish components of apathy from those of fatigue or depression (Bonnelle et al., 2015; Levy & Czernecki, 2006; Marin, 1991; Naarding, Janzing, Eling, van der Werf, & Kremer, 2009; Pagonabarraga, Kulisevsky, Strafella, & Krack, 2015).
Apathy is a multidimensional syndrome that has been variously defined for over two decades (Bonnelle et al., 2015; Marin, 1991; Naarding et al., 2009). Advances in our understanding of apathy and its neural mechanisms have emanated from preclinical models as well as neurodegenerative and neuropsychiatric diseases. Clinical assessment tools used to characterize the apathy phenotype have utilized descriptors such as indifference, avolition, lethargy, and diminished drive (e.g., in grooming/hygiene, impersistence in work or school, and physical anergia) (Grace, 2011). Levy and Czernecki (2006) define apathy as a reduction of voluntary, goal-directed behaviors.
It is well established in the literature that processes that precede goal-directed behavior are dependent upon a number of executive and cognitive control functions. The regions of the brain associated with these functions are the prefrontal cortex, the caudate, and the putamen, such that individuals with lesions or atrophy in these regions often experience impairment in planning and cognitive control (Bhatia & Marsden, 1994; Mendez et al., 1989; Monchi, Petrides, Strafella, Worsley, & Doyon, 2006; Pauli, O’Reilly, Yarkoni, & Wager, 2016; Robertson, Hiebert, Seergobin, Owen, & MacDonald, 2015). In a variety of neurodegenerative diseases and states of neuronal injury, such as Parkinson’s disease, stroke, and traumatic brain injuries, caudate and putamen atrophy correlate with apathy such that as striatum volume decreases, apathy increases (Carriere et al., 2014; Kos, van Tol, Marsman, Knegtering, & Aleman, 2016; Pagonabarraga, Kulisevsky, Strafella, & Krack, 2015; Worthington & Wood, 2018).
In the diagnosed HD population, apathy has been correlated with cognitive performance (Baudic et al., 2006; Naarding et al., 2009). Prodromal HD individuals exhibit impairment on cognitive control tasks and tasks that measure cognitive flexibility, on average, but this has not been linked to apathy measures (Papp et al., 2013; Snowden, Craufurd, Thompson, & Neary, 2002). We previously determined that striatum atrophy is related to more deficient cognitive performance in the prodromal HD population, but this analysis did not investigate apathy in conjunction with cognitive performance (Misiura et al., 2017). The cognitive control score is a composite measure that includes tasks assessing inhibition. Our test of cognitive control is a composite score taken from a variety of measurements that include Stroop tasks, Trail Making tests, and the Symbol-digit Modalities test (Misiura et al., 2017). Understanding the relationship between apathy, atrophy, and cognition can provide valuable clinical insight into the prodromal phase of HD.
The purpose of this study was to determine whether apathy severity in a prodromal HD population is related to striatum volumes and cognitive control. We hypothesized that apathy would be related to striatum volumes and cognitive control scores. We anticipated that higher apathy would be associated with reduced volumes and lower cognitive scores, reflective of atrophy in the striatum and related cognitive impairment (Jang & Kwon, 2017; Pagonabarraga et al., 2015; Paulsen, 2010; Paulsen et al., 2006; Worthington & Wood, 2018). To ensure that the relationships that we identified could not purely be explained by mood symptoms, we included depression as a covariate in our analyses.
METHODS
Participants
We extracted this legacy data from the PREDICT-HD study-wide dataset (Paulsen, Long, Johnson, & Aylward, 2014; Paulsen et al., 2008b), which includes data collected across 32 sites in the United States, Canada, Europe and Australia. Data were collected from over 1,400 prodromal HD and healthy control participants, and included demographic information such as sex, age, and years of education, HD specific genetic information, and data for approximately 30 different signs and symptoms of HD (i.e., measures of motor, cognitive, and psychiatric functioning). All participants were 18 years of age or older and not diagnosed with manifest HD at study entry. Exclusion criteria included unstable medical or psychiatric illness, active substance abuse, history of a significant developmental cognitive disorder, head trauma or other CNS disease, presence of a pacemaker or other metallic implant, use of antipsychotic medication in the 6 months before enrollment, or use of phenothiazine antiemetic medication in the 3 months before enrollment (Paulsen et al., 2008b). All participants provided informed consent as approved by their individual sites’ Institutional Review Boards. Participants provided their consent for their de-identified data to be shared and analyzed for further HD research. This study was approved at each data collection site, and all data was shared in accordance with the University of Iowa and Georgia State University Institutional Review Boards. Consistent with the genetic definition of HD, individuals with more than 35 CAG repeats who had not met motor criteria for clinical diagnosis were considered to be in the prodromal phase of HD. For this study, we included any participant who had corresponding clinical and neuroimaging data, for a total of 797 participants. A healthy control sample (N = 208) was used, consisting of individuals previously considered at-risk for HD due to family history but subsequently found to lack the causal mutation. With the exception of a calculated CAP score, data from the same variables measured in prodromal participants were also collected from members of the control group. Please see Paulsen, et al. (2014) for more detailed data collection information.
At the baseline visit, participants: provided a blood sample; underwent motor, cognitive, psychiatric, and functional assessments; and had a brain MRI scan. Information was obtained from participants regarding past medical and psychiatric histories, current medication use, and time since HD genetic testing. Motor exams were completed by certified motor raters using the Unified Huntington Disease Rating Scale (UHDRS), and all other assessments were obtained by trained research technicians (Huntington’s Disease Study Group, 1996). The CAG Age Product (CAP) score is used as a measure of disease burden, to capture the growing toxicity of mutant huntingtin protein as an individual ages. Created by the PREDICT-HD team, CAP score reflects the interaction between age and CAG repeat length (Zhang et al., 2011). Table 1 provides demographic information for our sample.
Table 1.
Participant characteristics
| prHD (N=797) Mean (SD) |
Controls (N =208) Mean (SD) |
prHD - controls t score (df) |
|
|---|---|---|---|
| Sex (M/F) | 271/526 | 75/133 | |
| Age | 41.87 (11.08) | 45.56 (12.02) | |
| Years of Education | 14.46 (2.6) | 14.88 (2.50) | |
| CAG Repeat Length | 42.49 (2.06) | 20.28 (3.48) | |
| Low/Medium/High | 28.5/ 35.5/ 36.1 | ------ | |
| Apathy | 12.4 (5.54) | 11.0 (4.29) | 3.39(1003)** |
| Motor Score | 5.99(6.32) | 2.91(3.65) | 6.74(1003)** |
| Depression | 53.91(15.15) | 48.67(9.45) | 4.75(1003)** |
Note. Low/Medium/High group were generated from the CAPd scores and represent the percentage of individuals in our sample in each of the disease severity groups. Categories denote disease burden as defined by Zhang et.al, 2012. Low indicates further from motor diagnosis of HD, and Hid indicates close to motor diagnosis of HD. Motor score was created based on motor symptom cluster scores such that a higher score indicates greater symptom severity (Misiura, et. al, 2017).
= p<.001.
Apathy Scores
The apathy scores used for this study were from a modified 24-item form of the Frontal Systems Behavioral (FrSBe) apathy subscale, which asks questions about recent behavior (Grace, 2011). This apathy subscale score is calculated as a summation of 8 apathy-related items (Duff et al., 2010). Example apathy items include “Has difficulty starting an activity, lacks iniative, motivation” and “Does things without being requested to do so”. We used companion-reported apathy scores for this analysis because previous research suggests that companion ratings are more consistently associated with disease progression. We used previously-developed z-scores standardized across the entire PREDICT-HD sample (Paulsen et al., 2008).
Depression Scores
Depression scores for this study were taken from the Symptom Checklist 90 (SCL90) (Derogatis & Unger, 2010) depression subscale. Companion reported scores were used to maintain consistency with apathy items.
HD Phenotype Cluster Scores
We used aggregated clinical and cognitive scores from a previous study designed to capture cognitive control and motor symptoms from clustering analyses (Misiura et al., 2017). Following clustering analyses, phenotype scores are averaged within a cluster to obtain a measure for each cognitive domain (Misiura et al., 2017). The cognitive control cluster scores are composed of measures that test executive function and inhibitory control: Stroop, Trail Making, and Symbol Digit Modalities tests. All assessments were re-coded such that a higher score indicates better performance. Motor symptom cluster scores are comprised of a variety of scales that each measure motor abnormality; a higher score indicates greater motor abnormality. Scores were created by z-scoring assessments in each cluster, adding them together, and then dividing by the number of assessments to create an average z-score for each cluster. Z-scores of the cluster scores were used in our analyses. For a full description, see Misiura et al. (2017). A full table describing the measures included in the cognitive control cluster, as well as other cluster scores, is included in the supplementary material. To ensure that the relationships that we identify are specific to apathy and not generalized cognitive impairment, we included a language and memory cluster score as a control measure (Stout et al., 2011). We did not anticipate a significant relationship between apathy and the language and memory cluster score.
Brain Volumes
Individuals considered for this analysis had high-quality T1 weighted images that were compatible with the BRAINSTools algorithm (Ghayoor, Vaidya, & Johnson, 2013; Kim, Magnotta, Liu, & Johnson, 2014). Because these regions are the most commonly associated with apathy, we used structural bilateral caudate and putamen volumes extracted using the BRAINSTools algorithm (Kim, Magnotta, Liu, & Johnson, 2014; Young Kim & Johnson, 2013). Thalamus volume was included as a control volume, as we did not anticipate any relationships between the thalamus and apathy. All brain volumes were calculated as a percentage of intracranial volumes and were then converted to standardized z-scores. To ensure that the relationships between brain volumes and apathy were specific to the basal ganglia, we included the thalamus as a control brain volume (Aylward et al., 2011)
Statistical Analyses
Prodromal HD
We constructed linear mixed models using the lme4 package in R (Verbeke & Molenberghs, 2000). Consistent with previous papers published with this dataset, data collection site was modeled as a random effect (Paulsen, Smith, Long, et al., 2013; Verbeke & Molenberghs, 2000). Sex was dummy coded and included as a fixed factor. Motor cluster scores, CAP score and years of education were modeled as continuous covariates, and years of education were included as a covariate in the cognitive control model. We also included depression as a covariate to ensure that the relationships we identified were unique to apathy, independent of general mood. The outcome variable for each model was either apathy or depression, and the independent variables for the separate models were caudate volume, putamen volume, thalamus volume, executive function cluster score, and language/memory cluster score. P values were corrected for multiple comparisons using false discovery rate (FDR) correction in R. The exclusion of outliers greater than three standard deviations did not significantly change the results, such that the nature of the relationships we identified and the significance of our regression coefficients did not change. We report the results with all data points.
Controls
All analyses were conducted in the same manner with controls except that there was no CAP score, which requires an expanded CAG repeat length and is not possible to calculate for controls. We did include age and CAG length as a covariate in this analysis. Controls and cases were analyzed separately.
RESULTS
Prodromal HD
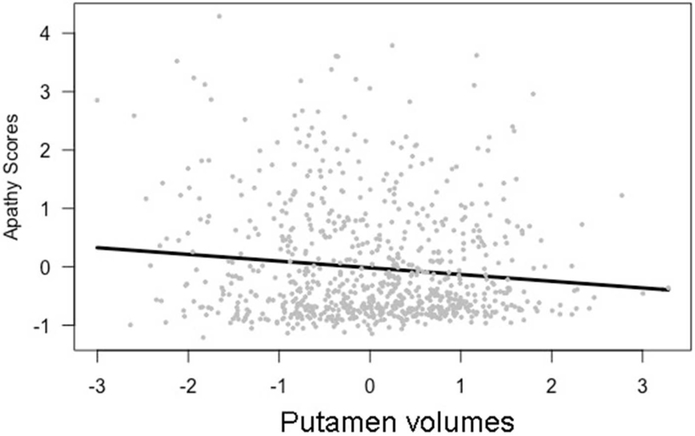
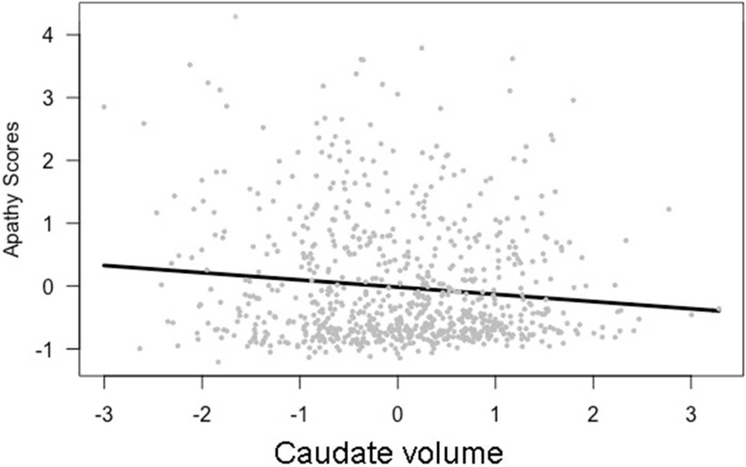
Apathy was significantly related to both putamen and caudate volumes. As putamen volumes decreased, apathy scores increased (β = −0.13, t[763] = −3.31, p < 0.05, model results in Table 2). A similar relationship was identified for caudate volumes: as caudate volumes decreased, apathy scores increased (β = −0.11, t[763] = −3.51, p < 0.05, model results in Table 3). These relationships are shown in Figures 1 and 2. Apathy was not significantly related to thalamus volumes.
Table 2.
Apathy as a function of Putamen volumes
| β Estimate+ | Std. Error | t value | Effect Size | |
|---|---|---|---|---|
| CAP score | −0.04 | 0.04 | −1.05 | −1 |
| Sex | 0.02 | 0.06 | 0.31 | 0.33 |
| Motor Score | 0.07 | 0.03 | 2.36* | 2.33 |
| Depression | 0.64 | 0.03 | 22.82** | 21.33 |
| Putamen volume | −0.13 | 0.05 | −2.78** | −2.6 |
Note.
p < 0.05 after False Discovery Rate correction
p < 0.001 after False Discovery Rate correction
all regression estimates are standardized. Effect size for regression coefficients were calculated as the ratio between the estimate and the standard error. Effect size for the whole model: Cohen’s f2= .03 (small effect).
Table 3.
Apathy as a function of Caudate volumes
| β Estimate+ | Std. Error | t value | Effect Size | |
|---|---|---|---|---|
| CAP score | 0.09 | 0.04 | 2.03 | 2.25 |
| Sex | 0.01 | 0.06 | 0.13 | 0.17 |
| Motor Score | 0.07 | 0.03 | 2.34* | 2.33 |
| Depression | 0.63 | 0.03 | 22.83** | 21 |
| Caudate volume | −0.11 | 0.04 | −2.56** | −2.75 |
Note.
p < 0.05 after False Discovery Rate correction
p < 0.001
all regression estimates are standardized. Effect size for regression coefficients were calculated as the ratio between the estimate and the standard error. Effect size for the whole model: Cohen’s f2 .04 (small effect).
Figure 1.
Apathy scores and putamen volumes, (β = −0.13, t(763) = −3.31, p <0.05),
Figure 2.
Apathy scores and caudate volumes, (β = −0.11, t(763) = −3.51, p < 0.05),
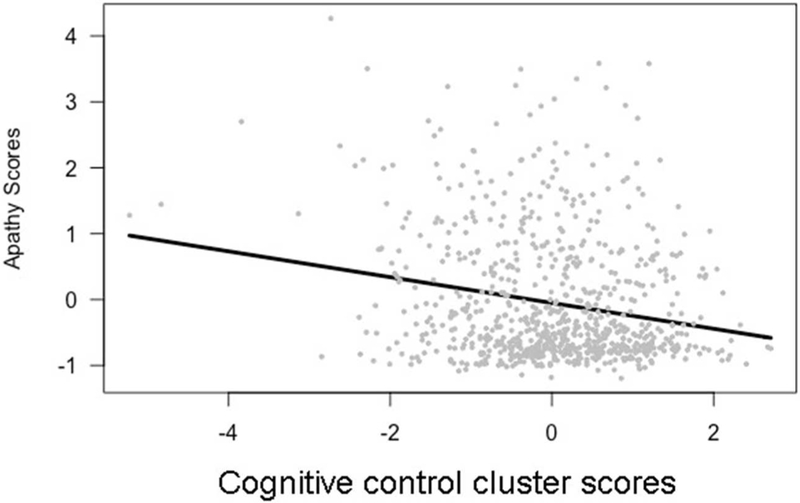
We found a significant relationship between apathy scores and both the cognitive control and motor cluster scores. Apathy scores were significantly negatively related to cognitive control cluster scores (β = −0.13, t[763] = −1.96, p < 0.01), as shown in Figure 3 and Table 4. As anticipated, apathy was not significantly related to language and memory cluster scores.
Figure 3.
Apathy scores and cognitive control scores, β = −0.20, t(763) = - −4.95, p < 0.001
Table 4.
Apathy as a function of cognitive control
| β Estimate+ | Std. Error | t value | Effect Size | |
|---|---|---|---|---|
| CAP score | −0.26 | 0.24 | −1.12 | −1.08 |
| Sex | −0.26 | 0.42 | 0.63 | −0.62 |
| Years of Education | −0.02 | 0.04 | −0.45 | −0.5 |
| Motor Score | 0.16 | 0.24 | 1.97* | 0.67 |
| Depression | 0.64 | 0.03 | 13.33** | 21.33 |
| Cog. Control | −0.13 | 0.25 | −1.96* | −0.52 |
Note.
p < 0.05 after False Discovery Rate correction
p < 0.001
all regression estimates are standardized. Effect size for regression coefficients were calculated as the ratio between the estimate and the standard error. Effect size for the whole model Cohen’s f2 .05 (small effect).
In all of our models, motor scores and depression were significantly related to apathy (see specific model results for estimates after FDR correction).
Controls
Within the control group, we did not identify significant relationships between cognitive control cluster scores and apathy, or between brain volumes and apathy. Depression was related to apathy scores (β = −0.45, t[235] = −20.17, p < 0.001).
DISCUSSION
Apathy is associated with caudate and putamen volume in prodromal HD, after accounting for age, CAG repeat length, motor scores, and depression. As anticipated, greater severity of apathy was related to smaller striatum volumes.
In the prodromal HD population, increased apathy appears to be related to reduced cognitive control. Findings of reduced cognitive control that correlates with increased apathy are consistent with previous literature regarding prodromal HD and related dysexecutive syndromes (Levy & Dubois, 2006; Martinez-Horta et al., 2016). The inability to plan for the participation in goal-directed behavior can be characterized as apathy, not exclusively because of an inability to recognize and respond to rewards. The inability to engage in the necessary cognitive preparation required to engage in goal-directed behavior may be a reason for apathetic behavior in this population. Psychomotor retardation is a common symptom of Huntington’s disease, and the argument could be made that the apathy experienced by this population is really just motor slowing (Epping & Paulsen, 2011; Fritz et al., 2018). Indeed cognitive control scores do capture motor retardation as well as executive processes, and was related to apathy(Misiura et al., 2017). However, apathy was also related to caudate volumes, and in a previous paper, caudate volumes were not related to cognitive control. If apathy was merely capturing psychomotor slowing, we would not expect it to be related to caudate volumes. Apathy has many facets, and unfortunately for this study, we could not measure them individually. It is possible that the type of apathy exhibited by individuals with prodromal HD is more behavioral, generating a lack of motivation that leads to lack of effort and poorer performance on cognitive tests. It could also be that the apathy exhibited is more cognitive, directly related to impairment in the cognitive abilities required to plan and execute goal directed behavior and thus to poorer performance on cognitive tests. However, the results of this study do indicate the need for better characterization of apathy within this population.
A plausible explanation for the relationships that we have identified is that as striatal degeneration occurs, impairment emerges in cognitive control processes that are essential to the "initiation" phase of goal-directed behavior. This impairment may lead to a decline in goal-directed behavior, which can be outwardly observed and defined as apathetic. While the relationships identified were significant, the strengths of the associations were not robust; the effect sizes for the relationships that we identified were not large, indicating that striatum atrophy and cognitive performance may only account for some of the apathy present in this population. Other factors, such as mental health issues or temporary neurochemical imbalances, likely also contribute to apathy. In models of apathy, striatum volumes and cognitive performance are related to early processes of goal directed behavior. There are other regions of the brain involved in later phases of goal-directed behavior, such as behavioral output and reward evaluation mechanisms, that may also be impaired. Future studies concerning apathy should include specific scales that probe apathy subtypes. Apathy is typically divided into three subtypes: cognitive, behavioral, and affective (Bonnelle et al., 2015; Kos, van Tol, Marsman, Knegtering, & Aleman, 2016). Different portions of the frontostriatal circuitry may be responsible for separate facets of apathy. For example, damage to the supplementary motor areas produces behavioral apathy, characterized by impairment in the ability to initiate desired motor programs necessary to engage in GDB, and damage to the caudate is likely to cause cognitive apathy, defined as an “impairment in the cognitive functions needed to elaborate the plan of actions” (Bonnelle et al., 2015; Levy & Dubois, 2006; Levy & Czernecki, 2006). Scales that include separate facets of apathy may allow us to delineate and characterize the apathy experienced by HD individuals, and can point to better markers of behavioral changes for caregivers and clinicians to identify.
Also, it is likely that the prodromal HD sample included persons who were very far from motor onset as well as those close to motor onset, and that the overall relationship may reflect the average of the group. The presence of significance is notable, however, considering that individuals more than 15 years from estimated diagnosis are included. There are likely several factors that contribute to the variance in apathy in prodromal HD, however our findings suggest that basal ganglia degeneration may be one of these factors. To shed more light on the caudate-apathy relationship, future fMRI studies could include a task paradigm that probes goal-directed behavior, and striatal involvement in the task could be studied. The non-significant findings that we identified using depression as an outcome, thalamus as a brain region, and language/memory as cognitive impairment, as well as the analyses using the control group suggest that the type of apathy manifested in HD may be more specific to executive function deficits and striatum atrophy rather than global volume loss, general cognitive impairment, or mood symptoms.
We identified a significant relationship between depressive symptoms and motor symptoms and this finding may be important to further explore in future research. There are many possible explanations for this finding. It is possible that conscious awareness of motor abnormalities contributes to increased depressed mood, or that impairment in mood and motor systems may be related within shared brain circuitry. The effect sizes of the depression parameter estimates were very large. On the surface, it may seem that much of the variability in apathy can be explained simply as an aspect of depressed mood. However, many of the questions on the depression inventory overlap with questions on the apathy scale, such as losing interest in activities, and “feeling blocked getting things done”. Conducting research in the future with apathy scales that include a mood or emotional subscale can help clarify this overlap.
The results of this study must be taken in the context of some limitations. Although we can hypothesize about the nature of the relationships among our variables, it is important to conduct a longitudinal analysis to establish a timeline of atrophy, presentation of mood symptoms, and cognitive decline to further determine whether atrophy predates apathy and executive dysfunction. In future analyses, we plan to analyze longitudinal clinical data in concert with imaging data to determine the progression of striatum atrophy and apathy. It may be possible to identify factors that make some individuals more resilient to psychiatric symptoms than others. CAP score was not significantly related to apathy, indicating that apathy may not be directly related to HD progression and onset, but alternatively may be more related to downstream effects such as striatum atrophy and associated cognitive impairment.
Because the apathy scale used in this study was measured by a reduction in observable behavior, findings offer no conclusion about internal affect. Because some prodromal HD individuals exhibit mild motor impairment, the observable behavior could reflect increased effort to carry out the behavior rather than increased apathy. In the future, it would be appropriate to look at apathy from emotional and behavioral perspectives to investigate whether participants experience apathy or just an outward manifestation of motor impairment.
Our results suggest that apathy is related to cognitive control and striatal atrophy. It can be difficult for individuals who are not trained clinicians to identify changes in general cognitive domains such as "cognitive flexibility" and "pre-planning." However, a decline in goal-directed behavior is routinely assessed by caretakers and companions and could signify neurological changes in the absence of an MRI scan. Although this analysis cannot reveal whether increased apathy is the cause of more unsatisfactory performance, it does suggest that apathy may be an essential disease-related marker that could signify other, more widespread changes. As methods for early HD interventions develop, initial onset of apathy may be a helpful indicator of necessary treatment initiation.
Supplementary Material
Acknowledgments
This project was supported by 1U01NS082074 (V.D. Calhoun and J.A. Turner, co-PIs) from the National Institutes of Health, National Institute of Neurological Disorders and Stroke. The PREDICT-HD study was supported by NIH/NINDS grants 5R01NS040068 and 5R01NS054893 awarded to Jane S. Paulsen and the CHDI Foundation, Inc., A3917 and 6266 awarded to Jane S. Paulsen. We thank the PREDICT-HD sites, the study participants, the National Research Roster for Huntington's Disease Patients and Families, the Huntington's Disease Society of America and the Huntington's Study Group.
Footnotes
We have read and understood the policy on declaration of interests and all authors declare that we have no competing interests.
References
- Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, Seirup J, Lim KO, & Gunning FM (2013). Functional Connectivity in Apathy of Late-life Depression: A Preliminary Study. Journal of Affective Disorders, 149(0), 398–405. 10.1016/j.jad.2012.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Liu D, Nopoulos PC, Ross CA, Pierson RK, Mills JA, … Paulsen JS (2011). Striatal Volume Contributes to the Prediction of Onset of Huntington Disease in Incident Cases. Biol Psychiatry, 71(9), 822–828. 10.1016/j.biopsych.2011.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Nopoulos PC, Ross CA, Langbehn DR, Pierson RK, Mills JA, … Coordinators of Huntington Study, G. (2011). Longitudinal change in regional brain volumes in prodromal Huntington disease. J Neurol Neurosurg Psychiatry, 82(4), 405–410. 10.1136/jnnp.2010.208264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward E, Mills J, Liu D, Nopoulos P, Ross CA, Pierson R, & Paulsen JS (2011). Association between age and striatal volume stratified by CAG repeat length in prodromal huntington disease. PLOS Currents Huntington Disease. Retrieved from http://currents.plos.org/hd/article/association-between-age-and-striatal-volume-stratified-by-cag-repeat-length-in-prodromal-huntington-disease/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudic S, Maison P, Dolbeau G, Boissé M-F, Bartolomeo P, Dalla Barba G, … Bachoud-Lévi A-C (2006). Cognitive Impairment Related to Apathy in Early Huntington’s Disease. Dementia and Geriatric Cognitive Disorders, 21(5–6), 316–321. [DOI] [PubMed] [Google Scholar]
- Bhatia KP, & Marsden CD (1994). The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain: A Journal of Neurology, 117(4), 859. [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Veromann K-R, Burnett Heyes S, Lo Sterzo E, Manohar S, & Husain M (2015). Characterization of reward and effort mechanisms in apathy. Journal of Physiology, Paris, 109(1–3), 16–26. 10.1016/j.jphysparis.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere N, Besson P, Dujardin K, Duhamel A, Defebvre L, Delmaire C, & Devos D (2014). Apathy in Parkinson’s disease is associated with nucleus accumbens atrophy: A magnetic resonance imaging shape analysis. Movement Disorders, 29(7), 897–903. 10.1002/mds.25904 [DOI] [PubMed] [Google Scholar]
- Derogatis LR, & Unger R (2010). Symptom Checklist-90-Revised. In The Corsini Encyclopedia of Psychology. John Wiley & Sons, Inc. Retrieved from 10.1002/9780470479216.corpsy0970 [DOI] [Google Scholar]
- Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC, & Predict, H. D. I. of the H. S. G. (2007). Psychiatric symptoms in Huntington’s disease before diagnosis: the predict-HD study. Biol Psychiatry, 62(12), 1341–1346. 10.1016/j.biopsych.2006.11.034 [DOI] [PubMed] [Google Scholar]
- Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Wang C, Stout JC, … Queller S (2010). “Frontal” behaviors before the diagnosis of Huntington’s disease and its relationship to markers of disease progression: Evidence of early lack of awareness. The Journal of Neuropsychiatry and Clinical Neurosciences, 22(2), 196–207. 10.1176/appi.neuropsych.22.2.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping EA, & Paulsen JS (2011). Depression in the early stages of Huntington disease. Neurodegener Dis Manag, 1(5), 407–414. 10.2217/nmt.11.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SJ, Douglas I, Rawlins MD, Wexler NS, Tabrizi SJ, & Smeeth L (2013). Prevalence of adult Huntington’s disease in the UK based on diagnoses recorded in general practice records. Journal of Neurology, Neurosurgery & Psychiatry, 84(10), 1156–1160. 10.1136/jnnp-2012-304636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz NE, Boileau NR, Stout JC, Ready R, Perlmutter JS, Paulsen JS, … Lai J-S (2018). Relationships Among Apathy, Health-Related Quality of Life, and Function in Huntington’s Disease. The Journal of Neuropsychiatry and Clinical Neurosciences, 30(3), 194–201. 10.1176/appi.neuropsych.17080173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghayoor A, Vaidya JG, & Johnson HJ (2013). Development of a novel constellation based landmark detection algorithm (Vol. 8669, p. 86693F – 86693F – 6). Retrieved from 10.1117/12.2006471 [DOI] [Google Scholar]
- Grace J (2011). Frontal Systems Behavior Scale In Kreutzer JS, DeLuca J, & Caplan B (Eds.), Encyclopedia of Clinical Neuropsychology (pp. 1090–1093). New York, NY: Springer New York; Retrieved from 10.1007/978-0-387-79948-3_1895 [DOI] [Google Scholar]
- Halliday GM, McRitchie DA, Macdonald V, Double KL, Trent RJ, & McCusker E (1998). Regional Specificity of Brain Atrophy in Huntington’s Disease. Experimental Neurology, 154(2), 663–672. 10.1006/exnr.1998.6919 [DOI] [PubMed] [Google Scholar]
- Huntington’s Disease Study Group. (1996). Unified Huntington’s Disease Rating Scale: Reliability and Consistency. Movement Disorders, 11(2), 136–142. [DOI] [PubMed] [Google Scholar]
- Jang SH, & Kwon HG (2017). Apathy Due to Injury of the Prefrontocaudate Tract Following Mild Traumatic Brain Injury. American Journal of Physical Medicine & Rehabilitation, 96(7). Retrieved from https://journals.lww.com/ajpmr/Fulltext/2017/07000/Apathy_Due_to_Injury_of_the_Prefrontocaudate_Tract.11.aspx [DOI] [PubMed] [Google Scholar]
- Julien CL, Thompson JC, Wild S, Yardumian P, Snowden JS, Turner G, & Craufurd D (2007). Psychiatric disorders in preclinical Huntington’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 78(9), 939–943. 10.1136/jnnp.2006.103309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Magnotta VA, Liu D, & Johnson HJ (2014a). Stable Atlas-based Mapped Prior (STAMP) machine-learning segmentation for multicenter large-scale MRI data. Magnetic Resonance Imaging, 32(7), 832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Magnotta VA, Liu D, & Johnson HJ (2014b). Stable Atlas-based Mapped Prior (STAMP) machine-learning segmentation for multicenter large-scale MRI data. Magnetic Resonance Imaging, 32(7), 832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos C, van Tol M-J, Marsman J-BC, Knegtering H, & Aleman A (2016). Neural correlates of apathy in patients with neurodegenerative disorders, acquired brain injury, and psychiatric disorders. Neuroscience & Biobehavioral Reviews, 69, 381–401. 10.1016/j.neubiorev.2016.08.012 [DOI] [PubMed] [Google Scholar]
- Levy R, & Czernecki V (2006). Apathy and the basal ganglia. Journal of Neurology, 253(7), vii54–vii61. 10.1007/s00415-006-7012-5 [DOI] [PubMed] [Google Scholar]
- Levy R, & Dubois B (2006). Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex, 16(7), 916–928. 10.1093/cercor/bhj043 [DOI] [PubMed] [Google Scholar]
- Marin RS (1991a). Apathy: A neuropsychiatric syndrome The Journal of Neuropsychiatry and Clinical Neurosciences. US: American Psychiatric Assn. [DOI] [PubMed] [Google Scholar]
- Marin RS (1991b). Apathy: A neuropsychiatric syndrome. (Vol. 3). US: American Psychiatric Assn. [DOI] [PubMed] [Google Scholar]
- Martinez-Horta S, Perez-Perez J, van Duijn E, Fernandez-Bobadilla R, Carceller M, Pagonabarraga J, … Kulisevsky J (2016). Neuropsychiatric symptoms are very common in premanifest and early stage Huntington’s Disease. Parkinsonism & Related Disorders, 25, 58–64. 10.1016/j.parkreldis.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Mendez MF, Adams NL, & Lewandowski KS (1989). Neurobehavioral changes associated with caudate lesions. Neurology, 39(3), 349–349. 10.1212/WNL.39.3.349 [DOI] [PubMed] [Google Scholar]
- Misiura MB, Lourens S, Calhoun VD, Long J, Bockholt J, Johnson H, … for the PREDICT-HD Investigators and Working Group. (2017). Cognitive Control, Learning, and Clinical Motor Ratings Are Most Highly Associated with Basal Ganglia Brain Volumes in the Premanifest Huntington’s Disease Phenotype. Journal of the International Neuropsychological Society, 23(02), 159–170. 10.1017/S1355617716001132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi Oury, Petrides Michael, Strafella Antonio P., Worsley Keith J., & Doyon Julien. (2006). Functional role of the basal ganglia in the planning and execution of actions. Annals of Neurology, 59(2), 257–264. 10.1002/ana.20742 [DOI] [PubMed] [Google Scholar]
- Naarding P, Janzing JGE, Eling P, van der Werf S, & Kremer B (2009). Apathy is not depression in Huntington’s disease. The Journal of Neuropsychiatry and Clinical Neurosciences, 21(3), 266–270. 10.1176/appi.neuropsych.21.3.266 [DOI] [PubMed] [Google Scholar]
- Pagonabarraga J, Kulisevsky J, Strafella AP, & Krack P (2015). Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. The Lancet Neurology, 14(5), 518–531. [DOI] [PubMed] [Google Scholar]
- Papp KV, Snyder PJ, Mills JA, Duff K, Westervelt HJ, Long JD, … Paulsen JS (2013). Measuring executive dysfunction longitudinally and in relation to genetic burden, brain volumetrics, and depression in prodromal Huntington disease. Arch Clin Neuropsychol, 28(2), 156–168. 10.1093/arclin/acs105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli WM, O’Reilly RC, Yarkoni T, & Wager TD (2016). Regional specialization within the human striatum for diverse psychological functions. Proceedings of the National Academy of Sciences, 113(7), 1907 10.1073/pnas.1507610113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS (2010). Early Detection of Huntington Disease. Future Neurology, 5(1), 10.2217/fnl.09.78 10.2217/fnl.09.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross C. a, Nance M, … Hayden M (2008a). Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. Journal of Neurology, Neurosurgery, and Psychiatry, 79(8), 874–880. 10.1136/jnnp.2007.128728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross C. a, Nance M, … Hayden M (2008b). Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. Journal of Neurology, Neurosurgery, and Psychiatry, 79(8), 874–880. 10.1136/jnnp.2007.128728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Long D, Johnson HJ, & Aylward EH (n.d.). Clinical and biomarker changes in premanifest Huntington disease show trial feasibility : a decade of the PREDICT-HD study, (Icv), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Magnotta V. a., Mikos AE, Paulson HL, Penziner E, Andreasen NC, & Nopoulos PC (2006). Brain structure in preclinical Huntington’s disease. Biological Psychiatry, 59(1), 57–63. 10.1016/j.biopsych.2005.06.003 [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Smith MM, Long JD, & PREDICT HD investigators and coordinators of the Huntington Study Group. (2013). Cognitive decline in prodromal Huntington Disease: Implications for Clinical Trials. Journal of Neurology, Neurosurgery, and Psychiatry, 84(11), 1233–1239. 10.1136/jnnp-2013-305114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson BD, Hiebert NM, Seergobin KN, Owen AM, & MacDonald PA (2015). Dorsal striatum mediates cognitive control, not cognitive effort per se, in decision-making: An event-related fMRI study. NeuroImage, 114, 170–184. 10.1016/j.neuroimage.2015.03.082 [DOI] [PubMed] [Google Scholar]
- Ross CA, & Tabrizi SJ (2011). Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurology, 10(1), 83–98. 10.1016/S1474-4422(10)70245-3 [DOI] [PubMed] [Google Scholar]
- Snowden JS, Craufurd D, Thompson J, & Neary D (2002). Psychomotor, Executive, and Memory Function in Preclinical Huntington’sDisease. Journal of Clinical & Experimental Neuropsychology, 24(2), 133. [DOI] [PubMed] [Google Scholar]
- Stout JC, Paulsen JS, Queller S, Solomon AC, Whitlock KB, Campbell JC, … Aylward EH (2011). Neurocognitive signs in prodromal Huntington disease. Neuropsychology, 25(1), 1–14. 10.1037/a0020937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaard SJA, Dumas EM, Acharya TP, Johnson H, Langbehn DR, Scahill RI, … Group, T.-H. I. (2011). Early atrophy of pallidum and accumbens nucleus in Huntington’s disease. Journal of Neurology, 258(3), 412–420. 10.1007/s00415-010-5768-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke G, & Molenberghs G (2000). Linear mixed models for longitudinal data. New York: Springer. [Google Scholar]
- Wolf RC, Thomann PA, Thomann AK, Vasic N, Wolf ND, Landwehrmeyer GB, & Orth M (2013). Brain Structure in Preclinical Huntington’s Disease: A Multi-Method Approach. Neurodegenerative Diseases, 12(1), 13–22. [DOI] [PubMed] [Google Scholar]
- Worthington A, & Wood RL (2018). Apathy following traumatic brain injury: A review. Neuropsychologia. 10.1016/j.neuropsychologia.2018.04.012 [DOI] [PubMed] [Google Scholar]
- Young Kim E, & Johnson HJ (2013). Robust multi-site MR data processing: iterative optimization of bias correction, tissue classification, and registration. Frontiers in Neuroinformatics, 7, 29 10.3389/fninf.2013.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Long JD, Mills JA, Warner JH, Lu W, Paulsen JS, … Study, C. of the H. (2011). Indexing Disease Progression at Study Entry with Individuals At-Risk for Huntington Disease. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics : The Official Publication of the International Society of Psychiatric Genetics, 156(7), 751–763. 10.1002/ajmg.b.31232 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.