Abstract
Objective:
Alcohol Use Disorder (AUD) and chronic pain are widespread conditions with extensive public health burden. This review seeks to describe neuroanatomical links and major mediating influences between AUD and chronic pain, in the service of identifying factors that predict the risk of chronic pain in precipitating or facilitating AUD.
Method:
We review the neural bases of pain and the influence of AUD on processes involved in pain perception. We propose potential mechanisms involved in the development of chronic pain in AUD, and we consider implications for pain management in recovery from AUD.
Results:
Pain is a multidimensional and subjective experience that in its acute form is essential for survival, but in chronic form, pain is a disorder that negatively impacts quality of life. Neural substrates involved in initiating and maintaining chronic pain include dysfunction in descending pain pathways and reward network circuitry. AUD involves preoccupation or craving, intoxication, withdrawal, and negative affect. Neural substrates of AUD involve widespread mesocorticolimbic and cerebro-cerebellar networks. Both conditions involve dysfunction of extended reward and oversight circuitry, and particularly prefrontal cortex.
Conclusions:
The interrelationship between chronic pain and AUD resides in the intersection of etiological influences, mental experiences, and neurobiological processes. Characterization of the connection between brain and behavioral abnormalities in AUD’s precipitation of chronic pain — and vice versa — allows for early detection and treatment of patients at risk for developing either or both of these conditions, and for preemptive interventional approaches to reduce the risk of consequent vulnerabilities and harm.
Keywords: Alcohol Use Disorder, pain, mesocorticolimbic system, prefrontal cortex
Introduction
Approximately 15 million Americans suffer from alcohol abuse or dependence (National Survey on Drug Use and Health 2015 (“National survey on drug use and health - SAMHSA,” 2015), and an estimated 116 million American adults suffer from chronic pain (Egli, Koob, & Edwards, 2012; Grant et al., 2004). Bidirectional associations between alcohol use disorder (AUD) and chronic pain syndromes also have been reported (Apkarian, Bushnell, Treede, & Zubieta, 2005; Apkarian et al., 2013; Brennan, Schutte, & Moos, 2005; Egli et al., 2012; Zale, Maisto, & Ditre, 2015). The prevalence of AUD is increased in adult patients suffering from chronic pain conditions, partly due to its analgesic properties (Hoffmann, Olofsson, Salen, & Wickstrom, 1995), which may be heightened among individuals with alcohol dependence (Cutter, Maloof, Kurtz, & Jones, 1976). Egli and colleagues (Egli et al., 2012) have even proposed that alcohol dependence itself may stem from aberrant neurobiological substrates of pain, and have conceptualized alcohol dependence as a chronic pain disorder.
Recurrent pain is highly prevalent among treatment seeking problem drinkers (Boissoneault, Lewis, & Nixon, 2018; Sheu et al., 2008), and alcoholism is considered a risk factor, both for the development of chronic pain in patients who suffer from AUD, and for relapse in those attempting to remain abstinent. Alcohol misuse also is commonly reported in association with drug abuse. According to the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) (Hasin & Grant, 2015), non-medical use of prescription medications, including analgesics, is reported highest among patients with a history of binge drinking or AUD (McCabe, Cranford, & Boyd, 2006), with a positive association between pain and the rates of non-medical use of prescription medications (Novak, Herman-Stahl, Flannery, & Zimmerman, 2009). AUD patients with pain also are likely to report current opioid use (Witkiewitz & Vowles, 2018). But despite numerous reports on the associations between chronic pain and AUD, the underlying mechanisms involved in linking them remain elusive. AUD may share common neural pathways with chronic pain, which may facilitate pain affecting alcohol use patterns, or facilitate modulatory effects of alcohol on pain processing, thereby precipitating the risk of chronic pain development. The relationship between pain and AUD is multifactorial. It is influenced by a host of familial, biological, environmental, and socioeconomic mediators that affect drinking behavior and susceptibility to pain disorders.
Because pain can be a significant risk factor for relapse in those recovering from AUD, there is an urgent need to understand the links between AUD and development of chronic pain. As mainly central rather than peripheral mechanisms are thought to be involved in the chronification of pain, identifying structural and functional differences in the brain in relation to AUD is key to recognizing links between the two conditions. Herein, we begin with a review of the neural bases of pain, and we discuss the influence of alcohol on processes involved in pain perception. We then proceed by proposing some potential mechanisms involved in the development of chronic pain in AUD. Finally, we consider implications for pain management in recovery from AUD.
Neural Bases of Pain
Pain is an essential and protective mechanism in recognizing and avoiding harmful stimuli and is a key to survival. According to the International Association for the Study of Pain definition, pain is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (“IASP Terminology,” ; Merskey, Bogduk, & International Association for the Study of Pain. Task Force on Taxonomy. 1994). In this context, the neural processes involved in encoding painful or noxious stimuli are referred to as nociception, which may not necessarily be associated with pain sensation, but may instead be associated with behavioral, autonomic, or other physiological changes (Kyle & McNeil, 2014). The most common pain is nociceptive pain. It arises from noxious or potentially harmful mechanical, chemical, or thermal stimuli that activate nociceptors (sensory receptors of the somatosensory system) in the intact and normally functioning somatosensory nervous system around the body, and it serves to alert the body to possible harm. But if pain arises from damage to somatosensory nerves following an infection, inflammation, or the acute injury, then it is referred to as pathological pain or neuropathic pain. Neuropathic pain has been described a shooting pain, which can be constant or intermittent, as it travels along peripheral nerves; it also can involve activation of the immune system (Woolf, 2010). Another category of pain conditions has been referred to as nociplastic or algopathic pain. It involves dysfunction of the nervous system, rather than a direct link to actual or threatened damage to the peripheral somatosensory system that cannot be established. This category of pain describes conditions such as fibromyalgia and irritable bowel syndrome.
As a multifaceted experience that is not exclusively driven by the noxious input, pain involves much more than sensory activities. It also involves emotion and cognition. In fact, much of the complexity of pain arises from the involvement of higher centers in the brain rather than periphery, thereby making pain a uniquely experienced phenomenon by each individual and, as such, a subjective experience. Understanding how (and why) different regions and networks in the brain interact and interface to shape experiences of pain, and mapping that to neurobiological mechanisms, including structural and functional architecture of the brain in health and disease, have been a focus of numerous studies in the pain field (Apkarian et al., 2005; Davis & Moayedi, 2013; Duerden & Albanese, 2013). Such studies have revealed that functional activity in the primary and secondary somatosensory cortices are linked to the sensory-discriminative processing aspect of pain, such as sensing the intensity of pain or discriminating the site of pain (Bushnell et al., 1999; Hofbauer, Rainville, Duncan, & Bushnell, 2001). Anterior cingulate cortex, insular cortex, and prefrontal cortex are linked to affective-motivational processing aspects of pain, such as finding it to be unpleasant and bothersome even though sensory-wise it may be considered to have low intensity (Apkarian et al., 2005; Auvray, Myin, & Spence, 2010; Gu et al., 2012). Attention, expectation, and reappraisal are thought to be the most important contributing factors for the cognitive modulation of pain (Porro et al., 2002; Wiech, Ploner, & Tracey, 2008). Notably, recent studies have highlighted a primary link to activity in prefrontal cortex (Seminowicz & Moayedi, 2017) and to prefrontal volumetric differences in response to cognitive behavioral therapy in patients with chronic pain (Seminowicz et al., 2013).
If the pain persists beyond three months (Loeser & Bonica, 2001), it is considered to be chronic pain. Chronic pain implicates central nervous system involvement as evidenced by the association between chronic pain conditions and central nervous system pain mechanisms such as loss of descending analgesic activity, or the presence of central sensitization characteristics such as allodynia (pain response to a non-noxious stimulus) or hyperalgesia (pain hypersensitivity or larger pain response to a noxious stimulus) (Woolf, 2011). Chronic pain is linked to regions other than those usually associated with acute pain in healthy individuals (Tracey & Bushnell, 2009). Moreover, different pain conditions are associated with their own distinct patterns of brain-region involvement (Apkarian, 2011; Apkarian, Hashmi, & Baliki, 2011). Regions within the descending pain modulation system, as well as in prefrontal, limbic, and paralimbic brain regions, are particularly implicated in the pathology of chronic pain (Apkarian, 2011; Apkarian et al., 2005; Ossipov, Morimura, & Porreca, 2014; Seminowicz & Moayedi, 2017), and they facilitate cognitive, regulatory, motivational, and emotional influences on pain (Apkarian, Baliki, & Geha, 2009; Tracey & Bushnell, 2009).
Influences of Alcohol on Processes Involved in Pain Perception
The analgesic effects of alcohol on pain perception have been measured in a variety of ways, including examining pain threshold, tolerance, and pain ratings (e.g., intensity). Early studies on experimentally induced pain showed that alcohol intake significantly increased pain threshold up to 45% (Witkiewitz et al., 2015), which is consistent with current reviews showing lower ratings or higher thresholds of experimentally-induced pain on measures of intensity/discomfort and tolerance following alcohol administration (Horn-Hofmann, Buscher, Lautenbacher, & Wolstein, 2015; Witkiewitz et al., 2015). Regarding ratings of discomfort versus intensity of pain, alcohol alleviates discomfort at lower doses and to a greater extent than intensity, suggesting the effect of alcohol may vary across components of pain. In addition, pain is influenced by alcohol dose and blood alcohol concentration (BAC), with the magnitude of the analgesic effects increasing at higher BACs (Cutter et al., 1976; Gustafson & Kallmen, 1988; Horn-Hofmann et al., 2015; Stewart, Finn, & Pihl, 1995; Thompson, Oram, Correll, Tsermentseli, & Stubbs, 2017). Studies also have shown that alcohol has less of an impact on pain as the BAC drops, due to metabolism, excretion, or evaporation (Duarte, McNeill, Drummond, & Tiplady, 2008; Horn-Hofmann et al., 2015; Zacny, Camarillo, Sadeghi, & Black, 1998). In other words, the analgesic effects of alcohol decrease over the time since the last drink.
Although the literature shows a clear impact of alcohol on pain reduction, this picture becomes more complicated when looking at longer-term alcohol use among patient populations exhibiting pain. For example, in a study of adults reporting onset of acute lower back pain within the past two weeks (Klyne, Moseley, Sterling, Barbe, & Hodges, 2018), greater or more frequent alcohol consumption was associated with lower pain thresholds and insensitivity to the effects of “conditioned pain modulation” techniques (paradigms commonly used to assess the function of endogenous pain inhibitory pathways). Additionally, alcohol dependent individuals who were abstaining also were more sensitive to pain than controls (Brown & Cutter, 1977). This finding was associated with changes in pain perception during withdrawal. Men in acute alcohol withdrawal, for example, exhibited increased sensitivity to pain up to two weeks after withdrawal (Jochum, Boettger, Burkhardt, Juckel, & Bar, 2010). These findings have been supported in nonhuman animal literature as well. Bergeson et al. (2016) showed that binge drinking in mice led to an increased pain response and sensitivity over time. Others have reported increased sensitivity to pain in rats during withdrawal, as indicated by a decrease in time between noxious stimulation and the retraction of the paw or tail from the site of the noxious stimulus (Gatch & Lal, 1999; Shumilla, Liron, Mochly-Rosen, Kendig, & Sweitzer, 2005).
In general, the experience of pain is related to affective processes. For example, anxiety has been shown to interfere with analgesic effects of alcohol on pain (Horn-Hofmann et al., 2015), by mediating the association between alcohol use and pain: higher negative affect is associated positively with pain ratings and increases in the experience of pain (Witkiewitz et al., 2015). Additionally, while negative affect can lead to an enhanced experience of pain, positive affect has been shown to act as a buffer to pain, both in controls and in substance dependent individuals (Jochum et al., 2010; Rhudy, Dubbert, Parker, Burke, & Williams, 2006; Wagner, Koschke, Leuf, Schlosser, & Bar, 2009). Pain perception during periods of heavy drinking, as well as during periods of alcohol withdrawal, has been explained in part by emotional state. Patients in treatment for alcohol or substance use disorders, who also experienced significant pain, have reported anxiety and mood impairments (Potter, Prather, & Weiss, 2008; Trafton, Oliva, Horst, Minkel, & Humphreys, 2004), and these individuals are at greater risk for suicide (Jakubczyk, Ilgen, et al., 2016).
Anxiety is one of the main comorbidities associated with AUD, and alcohol consumption is a primary coping mechanism in individuals suffering from anxiety (Chavarria et al., 2015). Even the expectation that alcohol reduces anxiety is motivation for alcohol consumption, independently of alcohol’s actual physiological effects (Abrams & Kushner, 2004). But whether alcohol consumption actually reduces anxiety is a matter of debate (Carrigan & Randall, 2003), and the findings from investigations on if-and-how alcohol reduces anxiety are for the most part inconclusive (J. P. Smith & Randall, 2012). However, In the context of a cognitive behavioral framework (Larimer, Palmer, & Marlatt, 1999; Morley, Eccleston, & Williams, 1999), out of which has come efficacious psychotherapeutic treatments for both alcohol abuse and anxiety disorders, drinking alcohol is considered an avoidance behavior that may serve to reduce anxiety in the short-term but prevent improvement, or even exacerbate, anxiety symptoms long-term (Kushner, Abrams, & Borchardt, 2000; Larimer et al., 1999). For example, feeling numb and with no anxiety when drinking heavily, often is followed later when experiencing a hangover and a heightened level of anxiety, which may then lead to more drinking. In general, the nature of the bidirectional relationship between AUD and anxiety is largely dependent on which came first (anxiety or alcohol abuse), or whether the two presented concurrently (DeMartini & Carey, 2011; Kushner et al., 2000).
Chronic Pain and Alcohol Use Disorder
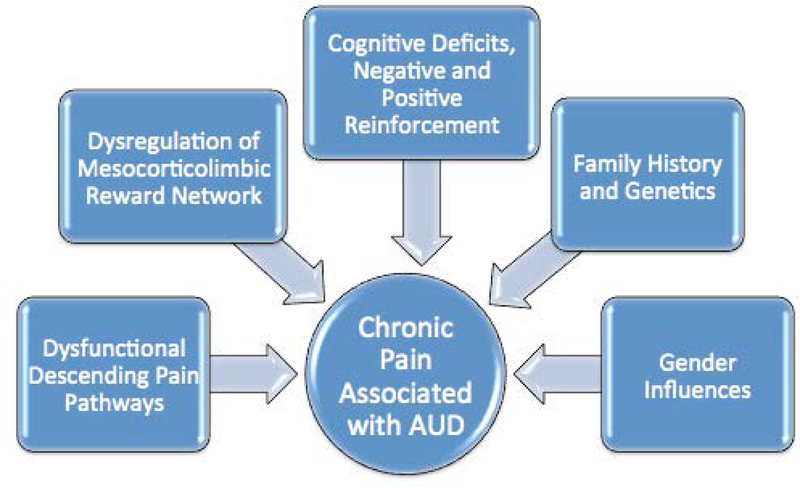
Chronic pain is characterized by pathophysiological, structural, and functional changes within the peripheral and central nervous systems. While alcohol can exert direct or indirect neurotoxic effects on nerve cells, neither the specific locations nor the processes controlling the damage are fully understood in relation to increased risk of chronic pain in individuals with AUD. If AUD-related neuropathy results in direct damage to nociceptors, patients can develop neuropathic pain (Chopra & Tiwari, 2012). However, AUD-related damage involves central, as well as peripheral nervous system circuitry, and it is the brain damage itself that is thought to be especially involved in the chronification of pain. In fact, as is true for AUD, pain is a multifactorial experience, involving complex interactions of multiple biological, neurological, psychological, cultural, social, and other environmental factors. Accordingly, each of these factors provides a substrate for modulatory effects of alcohol on pain experiences and vice versa. Below, we describe a number of major mediating influences of AUD on pain processing that may eventually lead to the development of chronic pain (summarized in Figure 1). It is important to note, however, that because of the etiological complexity of AUD — and pain — additional influences also are likely to modulate the interaction of the two conditions, among which are: age; temperament; education, cultural background and other sociodemographic characteristics; parameters of alcohol and drug use (amount, duration, abstinence); medical and psychiatric comorbidities; and general health status (Oscar-Berman et al., 2014; Zale et al., 2015).
Figure 1.
Major influences underlying the development of chronic pain in Alcohol Use Disorder. Alcohol Use Disorder and pain are complex conditions having multiple additional etiological impacts reviewed elsewhere (Oscar-Berman et al., 2014; Zale et al., 2015).
Dysfunctional Descending Pain Pathways.
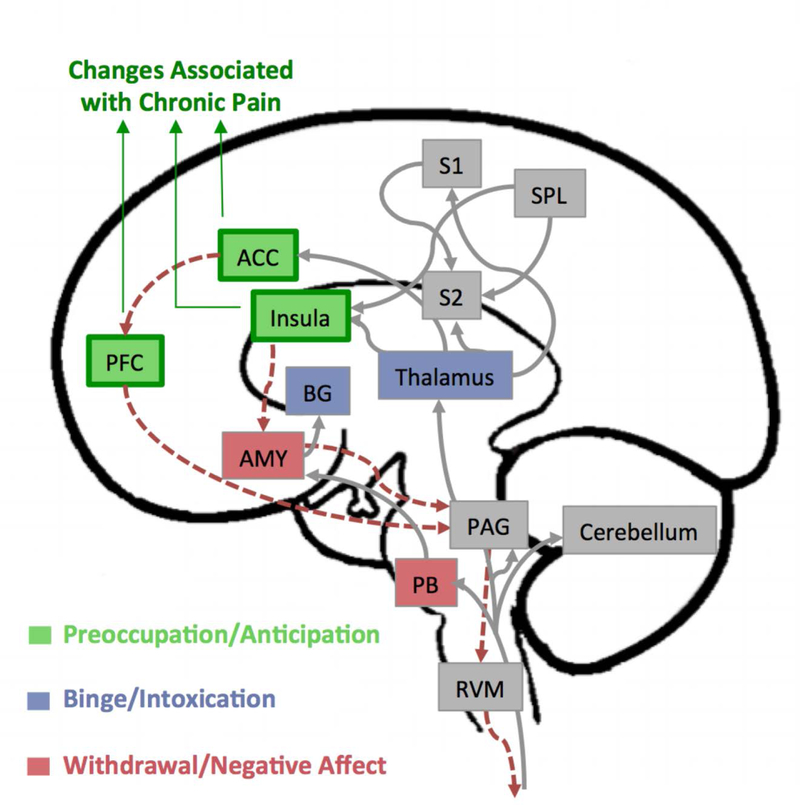
Dysfunction in descending pain modulatory circuits is thought to play an important role in the chronification of pain (Ossipov et al., 2014). This circuit, which controls top-down modulation of pain, receives inputs arising from multiple regions in the brain, including the hypothalamus, amygdala, and the rostral anterior cingulate cortex (Figure 2). These regions feed into the rostral ventromedial medulla, which includes the midline nucleus raphe and periaqueductal gray matter that have neural pathways to the spinal dorsal horn. Together, they form the descending pain modulatory system from the brain to the spinal cord and can modulate nociceptive processing by providing a substrate for cortical and subcortical structures to exert their influence.
Figure 2.
Overlap between pain and alcoholism neurocircuitry. Brain regions and pathways involved in the experience of pain are distributed throughout the brain. Gray arrows show afferent pain pathways that carry the nociceptive information from the spinal cord to the brain, and the red dotted arrows show the descending modulatory pathways connecting the regions involved in such processes (adapted from Bushnell et al., (Bushnell, Ceko, & Low, 2013)). Regions highlighted in green, purple, and red, represent overlaps with regions implicated in the three stages of development and maintenance of Alcohol Use Disorder (AUD) that include a binge intoxication stage, a withdrawal-negative-affect stage, and a stage of preoccupation-anticipation (based on a model by Koob and Volkow, (Koob & Volkow, 2010)). Particularly, changes in prefrontal cortex (PFC), anterior cingulate cortex (ACC), and insula are consistently reported in the brains of patients with chronic pain. These regions control descending pain pathways, which may undergo neuroplasticity changes associated with AUD. In chronic pain patients, changes in these regions include structural abnormalities in the gray matter and in white matter pathways connecting to these regions, as well as chemical and receptor level changes. It is likely that similar changes mediated through alcohol abuse could increase the susceptibility to chronic pain in AUD patients. Abbreviations: PFC – prefrontal cortex; AMY – amygdala; ACC – anterior cingulate cortex; S1 – primary somatosensory cortex; S2 – secondary somatosensory cortex; SPL – superior parietal lobe; BG – basal ganglia; PAG – periaqueductal grey; PB – parabrachial nucleus; RVM – rostroventral medulla.
Direct or indirect damage caused by alcohol to higher centers in the brain can affect descending pain pathways to the brainstem and spinal cord, which in turn, can modulate the activity of ascending signals and thus the pain experience (Bushnell, Ceko et al. 2013, Ossipov, Morimura et al. 2014). Both AUD and chronic pain are thought to involve dysregulation of the brain’s extended reward and oversight system and stress systems (S. Edwards & Koob, 2010; Koob & Le Moal, 2001; Sawyer et al., 2017), especially involving forebrain areas such as the prefrontal cortex (Gilpin & Koob, 2008). As noted earlier, disorders that alter affect such as depression and anxiety are associated prominently with both AUD and chronic pain. In part, this may be due to allostatic changes in the mesolimbic dopaminergic pathway as a result of dysregulation of the reward system and up-regulation in the brain stress system in both conditions (Egli et al., 2012). Such alterations may modulate or exacerbate the experience of pain and also motivate alcohol-seeking behaviors (Egli et al., 2012).
Alcohol interacts with various neurotransmitters such as gamma-aminobutyric acid (GABA), glutamate, dopamine, acetylcholine, and serotonin, or their receptors in the central nervous system, particularly within the descending pain modulatory system interfering with the balance between excitatory and inhibitory neurotransmitters (Valenzuela, 1997). For instance, while alcohol consumption initially potentiates GABA, a major inhibitory neurotransmitter, the number of GABA receptors declines with excessive drinking over a long period of time (Davies, 2003; Oscar-Berman & Marinkovic, 2003; Valenzuela, 1997). As another example, descending inhibition of nociceptive inputs by the midbrain periaqueductal gray is mediated by opioids (Ossipov, Dussor, & Porreca, 2010; Yeung, Yaksh, & Rudy, 1977), and there is evidence that alcohol may alter release of opioid peptides, as well as the binding of endogenous ligands to opioid receptors (Mendez & Morales-Mulia, 2008). This also may interfere with efficiency in descending pain inhibition at the midbrain level and precipitate development of chronic pain conditions in which deficiency in descending pain modulatory system is thought to be a central cause (Ossipov et al., 2014).
Dysregulation of the Mesocorticolimbic Reward Network.
The mesocorticolimbic reward network plays a major role in the reinforcing effects of alcohol, and in the modulation of pain, nociception, and analgesia. Structural and functional connectivity alterations within the mesocorticolimbic reward network, especially in the hippocampus, nucleus accumbens, ventral tegmental area, and prefrontal cortex, are thought to contribute significantly to the transition from acute to chronic pain (Apkarian et al., 2011; Baliki et al., 2012; Navratilova & Porreca, 2014), as well as in the manifestation of chronic pain syndromes (Mitsi & Zachariou, 2016). According to the allostatic model of addiction (Koob, 2013; Koob & Le Moal, 2001), progression from occasional social and/or recreational alcohol use to AUD is associated with distinctive neuroadaptations thought to emerge initially in order to neutralize the effects of the chronic influx of extracellular dopamine induced by persistent alcohol administration (George & Koob, 2017). In particular, the reward system (particularly dopaminergic neurons and receptors) undergoes down-regulation, whereas the stress systems (hypothalamic-pituitary-adrenal axis) undergo up-regulation (Brodie, Pesold, & Appel, 1999; Deehan, Hauser, Wilden, Truitt, & Rodd, 2013; George & Koob, 2017). Evidence supporting this view has come from positron emission tomography (PET) neuroimaging studies showing a decrease in striatal dopamine release among individuals with AUD (Volkow et al., 2007). Indeed, dopamine and its receptors are central components of the mesolimbic reward system, and activation of mesolimbic dopaminergic pathways is implicated in positive reinforcement. With respect to pain processing, PET neuroimaging also has revealed that patients with burning mouth syndrome, a chronic pain condition, exhibited significantly decreased striatal dopaminergic activity, indicative of decreased dopaminergic inhibition or decreased sensorimotor gating of nociceptive information (Jaaskelainen et al., 2001).
Both the down-regulation of reward systems and up-regulation of stress systems are associated with negative affective experiences that can modulate pain perception, as well as anxiety and depression – common comorbid disorders among patients suffering from chronic pain. Moreover, the extent of both types of neuroadaptations may depend upon the emotional profiles of individuals (George & Koob, 2017), as well as their sex, social, cultural, or religious beliefs (R. R. Edwards, Dworkin, Sullivan, Turk, & Wasan, 2016), in addition to their age and genetic makeup. Whether AUD-related down-regulation of the mesocorticolimbic reward network facilitates the development of chronic pain through dysregulation of dopaminergic inhibition is yet to be determined, but the overlap between the two may suggest an important link.
Cognitive Processes.
One important aspect of pain is the cognitive-evaluative dimension, which is a higher-order process related to expectation and anticipation. Numerous studies using postmortem pathological analyses of brain tissue (Harper, 2009), and in vivo magnetic resonance imaging have demonstrated abnormalities in the structure and activation patterns of prefrontal cortex in relation to cognitive deficits in AUD (Dupuy & Chanraud, 2016; Oscar-Berman & Marinkovic, 2007; Oscar-Berman et al., 2014). In particular, damage to the dorsolateral prefrontal cortex is reported to be associated with cognitive deficits in AUD patients, and structural and functional abnormalities of dorsolateral prefrontal cortex have been reported in chronic pain conditions (Seminowicz & Moayedi, 2017).
Pain perception is a subjective, complex, and distributed process that involves multiple structures involved in sensory, emotional, and cognitive processing that interact together concurrently to form the perceived pain experience (Chapman, 2005). Therefore, effective measurement of pain perception can be challenging (Chapman, 2005; Rosier, Iadarola, & Coghill, 2002; Younger, McCue, & Mackey, 2009). Despite this challenge, there are a number of validated for assessments of pain intensity and for evaluating multiple dimensions of the pain experience, as well as overall functioning, that rely on subjective perceptions of pain apart from physiologic or neurologic measurements (Younger et al., 2009).
Impaired cognition can modulate the cognitive-evaluative dimension of pain experiences, both as a reinforcing factor for alcohol-seeking behavior (as alcohol is known to alleviate pain) and also in how pain is perceived. Additionally, physiological cues accompanying alcohol consumption can influence drinkers through modulating their expectancy. This may be the main enabling factor in developing chronic pain through reinforcement in susceptible individuals, and a behavioral model of chronic pain (the operant model; (Fordyce, 1976; Sharp, 2001)), suggests that positive and negative reinforcement of acute pain behaviors may lead to the development of chronic pain. It should be noted that this model does not rule out or ignore the role of biological factors in the development of chronic pain, but instead emphasizes the significance of reinforcement and learning in the development and maintenance of chronic pain (Gatzounis, Schrooten, Crombez, & Vlaeyen, 2012). For instance, it is likely that dopamine release in the mesocorticolimbic dopamine system (precipitated by consuming alcohol) is responsible for relief from acute pain. In turn, relief from acute pain can be a positive reinforcing factor for maintenance of the pain state as it will lead to reward (alcohol intake and resulting dopamine release), with the alcohol itself acting then as a negative reinforcing factor.
Studies also have shown chronic pain itself is associated with significant cognitive deficits, including attention, learning and memory, psychomotor speed, information processing, and general executive functioning (Moriarty, McGuire, & Finn, 2011). More specifically, deficits have been shown in attention switching among patients with chronic pain, which has been attributed to pain overriding some of one’s limited attentional resources, thereby interfering with other attempts to direct attention (Eccleston & Crombez, 1999; Grisart & Van der Linden, 2001). Chronic pain patients also have shown deficits on explicit memory, particularly working memory, decreased reaction time, lower perceptual-motor coordination, and impaired executive functioning processes such as goal-directed behavior, planning, decision making, and evaluation of the consequences of one’s actions (Berryman et al., 2013; Moriarty et al., 2011).
The onset of chronic pain may precede memory problems, and chronic pain has been shown to increase the risk of dementia in older adults (Whitlock et al., 2017). Unfortunately, the assessment of pain in patients who already have been diagnosed with varying types or combinations of types of dementia and amnesia, is especially challenging, and therefore, research and clinical treatment with these populations has been limited and inadequate (Buffum, Hutt, Chang, Craine, & Snow, 2007). Compared to healthy controls, individuals suffering from chronic back pain or complex regional pain syndrome have a smaller hippocampus, a brain structure that is involved in memory formation and consolidation (Mutso et al., 2012). In a mouse model of chronic pain, it was shown that production of new neurons in the hippocampus failed. This finding was surprising given that the hippocampus is a brain region in which new neurons can grow both in adult humans and in adult mice (Mutso et al., 2012). Recent comprehensive reviews of studies evaluating working memory and long-term memory in chronic pain patients reported that the patients commonly complained about poor memory, and that there was a moderate decline in both working memory and long-term memory in chronic pain patients (Berryman et al., 2013; Mazza, Frot, & Rey, 2018). While based on these studies, it seems reasonable to conclude that chronic pain causes memory problems, it is also likely that chronic pain and memory problems may occur in parallel due to damage in brain structures (such as hippocampus) shared between the two, and caused by something else, without either necessarily causing the other. For instance, in a longitudinal study on sub-acute back pain patients, of whom some recovered and the others transitioned to chronic back pain, it was found that intra-corticolimbic connectivity within the dorsomedial prefrontal cortex-amygdala-accumbens circuit, as well as smaller amygdala volume, predetermined risk for chronic pain, accounting for 60% of the variance (Vachon-Presseau et al., 2016). The prefrontal cortex, amygdala, and nucleus accumbens are all essential components of the alcoholism/addiction circuitry (Volkow & McLellan, 2016).
Impairments in cognitive processes are particularly relevant to chronic pain patients with AUD, as alcohol also interferes with these processes during intoxication and, perhaps more importantly, can be associated with lasting impairments among individuals with AUD (Oscar-Berman & Marinkovic, 2007; Peterson, Rothfleisch, Zelazo, & Pihl, 1990). In addition, the cognitive functions noted above are important for decision-making involved in alcohol consumption, self-control of alcohol use, and goal-directed behavior such as treatment engagement and maintenance of abstinence or recovery (Bates, Bowden, & Barry, 2002; Field, Wiers, Christiansen, Fillmore, & Verster, 2010). Some research has suggested targeting such cognitive deficits in AUD treatment may have a positive impact on treatment outcomes (Bates, Buckman, & Nguyen, 2013). Therefore, the additive impact of pain on cognition may be another mechanism by which pain interferes with recovery from AUD.
Family History and Genetics.
It is estimated that 50% to 60% of the total variance in risk for AUD is accounted for by variation in genetic factors (Rietschel & Treutlein, 2013). Twin studies and studies of the offspring of individuals with AUD have shown that family history of AUD mediates the risk of AUD. Children of patients with AUD are at as much as four times higher risk of developing AUD. But controversy exists regarding whether family history is a risk factor through genetic mechanisms, or through environmental mechanisms (e.g., growing up in a household with parents with AUD), or through the interaction of genes and environment. Irrespective of the mechanism involved, family history of AUD is a profound risk for AUD.
Family history of AUD also influences pain experience in offspring. In one study, family history of AUD was associated with higher sensitivity to noxious stimulation with electric shocks, even in individuals who do not have a drinking problem themselves (Stewart et al., 1995). Another study reported that family history of AUD, together with the personality trait of neuroticism, mediated the analgesic effect of alcohol in response to noxious thermal stimulation (Ralevski et al., 2010). Individuals with both a positive family history of AUD and high neuroticism scores showed a strong analgesic effect even at low doses of alcohol. Similar to AUD, neuroticism is regulated by genetic and environmental factors including developmental, and social, but in contrast to AUD, it can be a more dynamic and time-varying trait that changes throughout life.
Family history of AUD also could be a mediating risk factor for comorbid affective disorders in pain patients. In a study on the relationship between fibromyalgia and familial history of depression and AUD in first-degree relatives (Katz & Kravitz, 1996), patients who had both fibromyalgia and depression also had higher odds of AUD in their first-degree relatives. Another family history study on prepubertal children suggested that the risk of prepubertal onset of major depressive disorder in families with a high aggregation of affective disorders is higher when there also is a high prevalence of AUD in the families (Puig-Antich et al., 1989).
Gender Influences.
Differences in clinical manifestations of AUD between men and women have been recognized for decades (Cloninger, Sigvardsson, & Bohman, 1996; Johnson, Gruenewald, Treno, & Taff, 1998; Ruiz & Oscar-Berman, 2013; Ruiz & Oscar-Berman, 2015), but neuroscience research on abnormalities associated with AUD in conjunction with pain syndromes have not adequately considered gender differences. Nevertheless, a number of studies have suggested that women may be more prone than men to drink to self-medicate for mood problems (Brady & Randall, 1999), or to drink to decrease aversive affect in general (perhaps pain-related), while men might drink to enhance favorable emotional states (Buckner, Eggleston, & Schmidt, 2006; Crutzen, Kuntsche, & Schelleman-Offermans, 2013; Mosher Ruiz, Oscar-Berman, Kemppainen, Valmas, & Sawyer, 2017; Ruiz & Oscar-Berman, 2015).
When considering chronic bodily pain in community residents over age 65, Brennan and colleagues (2005), asked 227 current problem drinkers (161 men) and 174 nonproblem drinkers (86 men) to rate the amount of bodily pain they had experienced in the past month, and the extent to which bodily pain interfered with their normal activities, on 5- and 6-point scales, respectively. The investigators found that, of the problem drinkers, approximately 43% of men and 44% of women reported experiencing moderate to severe pain, but in nonproblem drinkers, only 28% of men and 33% of women reported that level of pain. Likewise, pain interfered with daily activities ‘moderately’ to ‘extremely’ among 34% of men and 29% of women with drinking problems, compared to 16% and 19% of the men and women without drinking problems. Importantly, almost 38% of current problem drinkers reported using alcohol to manage pain, whereas in contrast, only 15% of nonproblem-drinking men and 13% of nonproblem-drinking women did so. Among the problem drinkers who experienced moderate to severe pain, almost 57% of men and 59% of women reported using alcohol for pain management, compared to 21% of nonproblem-drinking men and women with the same level of pain.
In a more recent study characterizing the prevalence and severity of recurrent pain in at least 11 chronic pain conditions endorsed by 451 (41% women) treatment-seeking individuals with AUD (Boissoneault et al., 2018), chronic pain was found to be highly prevalent (54%), especially among women (63% women vs. 47% men). On average, those with pain endorsed having more than a single chronic pain condition, and current pain severity was described as moderate. And in addition to women being more likely than men to report recurrent pain episodes and greater pain severity, women also reported having more concurrent pain conditions than men (mean number of 3.8 vs. 2.4). Although both men and women with pain indicated that pain had affected their substance use, pain tended to be more severe among women than men. Of note, later age and delays to first treatment were associated with pain presence and intensity, but more so in men than in women. The investigators suggested that biopsychosocial influences, such as hormones and beliefs about gender-roles regarding pain may underlie the higher prevalence and severity in women. According to the results of a study of the clinical profiles of 302 (216 men), treatment-seeking individuals with cannabis use disorder (Sherman et al., 2017), women reported higher pain scores, greater withdrawal intensity, and negative consequences of withdrawal. Women also reported poor physical health and drug-related medical problems. While that study is not directly relevant to AUD, it clearly supports the value of applying gender-specific treatment strategies to substance abuse in general.
Impulsivity.
One of the important risk factors for relapse to drinking and for the development of AUD and other substance use disorders, is impulsivity. Impulsivity is multidimensional construct referring to a predisposition for individuals to react quickly in response to an internal or external stimulus, without consideration of the possible negative consequences (Lejuez et al., 2010). While not a prominent trait in chronic pain patients, impulsivity may be especially relevant to individuals with AUD who suffer from chronic pain. These individuals would be in a situation that is analogous to what has been described for opioid analgesic misuse risk in chronic, low-back pain patients who had been prescribed opioid analgesics (Marino et al., 2013). The experience of physical pain also has been reported to be elevated in alcohol dependent patients having high levels of impulsivity, with physical pain being an independent correlate of both subjectively reported and objectively measured levels of impulsivity (Jakubczyk, Brower, et al., 2016). In particular, there seems to be a role for an attention dimension of impulsivity that represents heightened distractibility and compromised cognitive control, both in AUD (Jakubczyk, Brower, et al., 2016) and in opioid analgesic misuse in chronic pain patients (Marino et al., 2013). In other words, a high level of distractibility, together with poor cognitive control, would indicate that a person has a more difficult time cognitively regulating pain perception, as well as lower control over increasing the likelihood that s/he would engage in substance use rather than attempting to engage in self-control behavior.
Alcohol and Pain Management: A Bidirectional Relationship
Given the analgesic effects of alcohol on pain, pervasiveness of alcohol use as a pain management strategy has proven to be substantial among individuals exhibiting pain. For example, in a study of older adult (ages 55–65) problem drinkers and healthy controls, the drinkers were more likely to report more severe pain, greater pain interference, and more frequent use of alcohol to manage pain (Brennan et al., 2005). In a recent large study (Alford et al., 2016), the investigators identified 589 adult primary care patients who screened positive for illegal drug use and misuse of prescription medications. They found that 87% of those who screened positive suffered from chronic pain as well. Of those, the majority (79%) of the individuals identified self-medication for pain as the reason for heavy alcohol use.
In another study conducted to characterize the prevalence and severity of significant recurrent pain and various chronic pain conditions in individuals with AUD, later age of first treatment and longer transition time from alcohol dependence to treatment were associated with greater pain severity (Boissoneault et al., 2018). In addition, the frequency of using alcohol to manage pain was predictive of drinking problems up to three years later among women, and of health problems and injury among men (Brennan et al., 2005). Studies of individuals with diagnosed AUD have shown that pain also plays a significant role in alcohol treatment outcomes. Reduction in physical pain during treatment for AUD has been associated with lower risk for relapse (Jakubczyk, Ashrafioun, et al., 2016), as well as frequency and quantity of drinking 12 months following treatment, despite no change in pain scores (Witkiewitz et al., 2015). Additionally, reduction in physical pain can improve sleep quality (Finan, Goodin, & Smith, 2013). Indeed, sleep complaints are highly reported in chronic pain disorders (Finan et al., 2013; M. T. Smith & Haythornthwaite, 2004). As sleep related problems may increase the risk of relapse among abstinent alcoholic Individuals (Brower, 2001), improving sleep quality through reduction in physical pain can in turn, reduce the risk of AUD.
Because pain has a negative impact on alcohol overconsumption among individuals in treatment for AUD, researchers have investigated whether addressing pain within the context of treatment for alcohol or substance use disorders may be beneficial for drinking outcomes. Among patients receiving pain management cognitive behavioral therapy (CBT), lower pain ratings (Morley et al., 1999) and greater self-efficacy in managing pain, were seen among individuals in treatment for substance use disorders (Ilgen et al., 2011). This type of pain management treatment typically involves skills that foster acceptance of pain, physical skills to cope with pain, and cognitive skills such as addressing thought processes that may prolong and exacerbate the experience of pain (Cucciare, Sorrell, & Trafton, 2009; Morley et al., 1999; Vowles & McCracken, 2008). Together, research findings support the importance of including both pain and drinking behavior jointly in the context of treatment for AUD. Consideration of conjoint treatment of AUD and pain is essential, especially given the bidirectional relationship between the two, including the dampening effect of alcohol on pain perception, which may lead to drinking as a coping mechanism, and thus, poor AUD treatment outcomes. This point may be particularly relevant for individuals exhibiting pain within the context of a more severe health problem, such as HIV or sickle cell disease (Levenson et al., 2007; Merlin et al., 2015; Merlin et al., 2014).
Pain and Recovery from AUD
It is not surprising that research on the interrelation between pain and recovery from AUD has been limited, because of a definitional ambiguity related to the construct of recovery (The Betty Ford Institute Consensus Panel, 2007; White, 2007). In behavioral terms, recovery can be characterized by a reduction or elimination of the behaviors associated with addictive involvement, which can be represented by a continuum of changes, ranging from use of pharmacological adjuncts, to moderation in substance use, and to total abstinence. Abstinence, therefore, represents only one aspect of the recovery experience and is not necessarily synonymous with or equivalent to Recovery as a broad concept. In fact, the construct of recovery constitutes improvements among several qualitative dimensions that cannot be reduced to mere abstinence (Kelly, Greene, & Bergman, 2018). Abstinence does, however, represent a convenient metric of recovery, demonstrating in quantifiable terms the remission of an addictive behavior. In this context, acute withdrawal and early abstinence refer to the initial detoxification period, and are defined as 48–72 hours and 3–6 weeks, respectively. Protracted abstinence typically is defined as three months or more (Heilig, Egli, Crabbe, & Becker, 2010). Long-term recovery is defined as more than five years of continued remission.
Both early and protracted abstinence are characterized by symptoms associated with withdrawal syndrome, which includes intense negative affective states with awful emotional and somatic pain (Heilig et al., 2010). A particular type of craving emerges during early and protracted abstinence, termed withdrawal relief craving, as a result of the negative affective experiences (Verheul, van den Brink, & Geerlings, 1999), making relapse more likely during this early stage of recovery. This craving is hypothesized to be mediated by a decreased sensitivity to reward, through down-regulation of dopaminergic neurotransmission, and a greater sensitivity to stress or pain, through the up-regulation of the hypothalamus-pituitary axis (Koob, 2013).
Not only does early and protracted abstinence induce a type of pain characteristic of early recovery, but it also has the tendency to exacerbate dysregulated nociception (Egli et al., 2012). In cases where pain among AUD individuals results from a comorbid condition (e.g., cancer, neuralgia, fibromyalgia), abstinence of any duration can reveal the presence and intensity of pain that was previously being masked by the analgesic effects of alcohol. This dynamic can present unique challenges for recovering individuals suffering from acute and/or chronic pain, as well as for the physicians responsible for treating both conditions.
A common pattern of recovery is for the affected individual to become abstinent from his or her primary drug of addiction (e.g., alcohol), while use or misuse of a secondary or tertiary drug (e.g., nicotine, cannabis, opiates) may be initiated, continued, or escalated (White & Kurtz, 2006). This dynamic may be due in part to the fact that use of alternative substances may help the individual cope with the negative experiences associated with withdrawal from his or her primary substance of misuse. When affected individuals also suffer from acute or chronic pain, use of an alternative substance may provide additional analgesic benefit and therefore, may serve some therapeutic purpose. This scenario however, represents an increased risk for the individual to become re-addicted to an alternative substance.
Physicians responsible for treating pain in patients with co-occurring AUD may be reluctant to prescribe opiate medications, known to be associated with the potential for addiction (Markowitz, Francis, & Gonzales-Nolas, 2010). Alternatively, such patients may also be reluctant to take medication with a potential for addiction, even when prescribed, for fear of becoming re-involved in the addictive cycle, or for fear of jeopardizing the work they have put into sustaining their own recovery (Weaver & Schnoll, 2002). For this reason, patients in recovery from AUD, requiring pain management, should first be tried on non-pharmacological therapies (e.g., heat, ice, physical therapy, chiropractic, and massage therapy) (Markowitz et al., 2010).
Finally, management of chronic pain in AUD patients cannot be optimized without considering the reciprocal risks and benefits of the treatment choices on exacerbating drinking patterns or increasing the risk of relapse. Opioids in particular may not be appropriate for managing pain in individuals with AUD, as they probably engage the same brain reward pathways as in AUD. Indeed, there is evidence for the involvement of the endogenous cannabinoid system in the pharmacological and behavioral effects of alcohol (Perra et al., 2005). However, gabapentin, a GABA analogue anticonvulsant medication that also is used to treat pain, has been shown to have the benefit of reducing cravings and to significantly delay relapse in individuals with AUD (Brower et al., 2008). By contrast, in a review of the risks and benefits of different approaches to the pharmacological management of chronic pain in patients with AUD (Murphy et al., 2015), the authors recommended that nonopioid medications be given priority, because they may offer a more favorable risk profile, as well as additional benefits, such as improvement in anxiety, depression, or insomnia.
Conclusions
Chronic pain syndromes have the propensity to initiate alcohol abuse, or to trigger relapse in individuals who have attained abstinence. Similarly, AUD-related brain changes can precipitate chronic pain. Therefore, for early detection and treatment of patients at risk for developing chronic pain conditions, and for preemptive interventional approaches to reduce the risk of consequent AUD, it is essential to characterize the interrelatedness of pain related functional and structural abnormalities associated with AUD. This can be achieved by understanding neural links between AUD and chronic pain, and ultimately identifying markers that predict the risk of development of both conditions. Since central neural mechanisms are thought to play a key role in the chronification of pain, identifying structural and functional abnormalities in the brain in relation to AUD is vital for recognizing links between AUD and chronic pain. AUD mediated changes in descending pain pathways, the mesocorticolimbic reward network, and particularly prefrontal cortex, may be involved in the initiation and maintenance of chronic pain states in AUD. Characterization of brain deficits in AUD precipitating chronic pain allows for early detection of AUD patients at risk for developing chronic pain conditions, and for preemptive interventional approaches to reduce the risk of consequent alcohol abuse.
Public Significance Statement.
Chronic pain syndromes have the propensity to trigger the risk of initiating alcohol abuse, or triggering relapse in individuals who had attained abstinence. Similarly, alcoholism-related brain changes can precipitate chronic pain. Characterization of the interrelatedness of alcoholism and pain allows for early detection and treatment of patients at risk for developing chronic pain conditions, and for preemptive interventional approaches to reduce the risk of consequent alcohol abuse.
Acknowledgements
The writing of this paper was supported in part by grants from: National Institute on Alcohol Abuse and Alcoholism (NIAAA) R01AA07112 and K05AA00219, and by the US Department of Veterans Affairs Clinical Science Research and Development grant I01CX000326 to MOB; NIAAA grant F31 AA025522-01A1 to KT; and Burrows Wellcome Fund Training Program Awards to KT and BLT. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Department of Veterans Affairs, or the Burrows Wellcome Fund.
References
- Abrams K, & Kushner MG (2004). The moderating effects of tension-reduction alcohol outcome expectancies on placebo responding in individuals with social phobia. Addict Behav, 29(6), 1221–1224. doi: 10.1016/j.addbeh.2004.03.020 [DOI] [PubMed] [Google Scholar]
- Alford DP, German JS, Samet JH, Cheng DM, Lloyd-Travaglini CA, & Saitz R (2016). Primary Care Patients with Drug Use Report Chronic Pain and Self-Medicate with Alcohol and Other Drugs. J Gen Intern Med, 31(5), 486–491. doi: 10.1007/s11606-016-3586-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV (2011). The brain in chronic pain: clinical implications. Pain Manag, 1(6), 577–586. doi: 10.2217/pmt.11.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, & Geha PY (2009). Towards a theory of chronic pain. Prog Neurobiol, 87(2), 81–97. doi: 10.1016/j.pneurobio.2008.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, & Zubieta JK (2005). Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain, 9(4), 463–484. doi: 10.1016/j.ejpain.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Hashmi JA, & Baliki MN (2011). Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain, 152(3 Suppl), S49–64. doi: 10.1016/j.pain.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Neugebauer V, Koob G, Edwards S, Levine JD, Ferrari L, … Regunathan S (2013). Neural mechanisms of pain and alcohol dependence. Pharmacol Biochem Behav, 112, 34–41. doi: 10.1016/j.pbb.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvray M, Myin E, & Spence C (2010). The sensory-discriminative and affective-motivational aspects of pain. Neurosci Biobehav Rev, 34(2), 214–223. doi: 10.1016/j.neubiorev.2008.07.008 [DOI] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, … Apkarian AV (2012). Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci, 15(8), 1117–1119. doi: 10.1038/nn.3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, & Barry D (2002). Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Exp Clin Psychopharmacol, 10(3), 193–212. [DOI] [PubMed] [Google Scholar]
- Bates ME, Buckman JF, & Nguyen TT (2013). A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychol Rev, 23(1), 27–47. doi: 10.1007/s11065-013-9228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeson SE, Blanton H, Martinez JM, Curtis DC, Sherfey C, Seegmiller B, … Guindon J (2016). Binge ethanol consumption increases inflammatory pain responses and mechanical and cold sensitivity: Tigecycline treatment efficacy shows sex differences. Alcohol Clin Exp Res, 40(12), 2506–2515. doi: 10.1111/acer.13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman C, Stanton TR, Jane Bowering K, Tabor A, McFarlane A, & Lorimer Moseley G (2013). Evidence for working memory deficits in chronic pain: a systematic review and meta-analysis. Pain, 154(8), 1181–1196. doi: 10.1016/j.pain.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Boissoneault J, Lewis B, & Nixon SJ (2018). Characterizing chronic pain and alcohol use trajectory among treatment-seeking alcoholics. Alcohol, 75, 47–54. doi: 10.1016/j.alcohol.2018.05.009 [DOI] [PubMed] [Google Scholar]
- Brady KT, & Randall CL (1999). Gender differences in substance use disorders. Psychiatr Clin North Am, 22(2), 241–252. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, & Moos RH (2005). Pain and use of alcohol to manage pain: prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction, 100(6), 777–786. doi: 10.1111/j.1360-0443.2005.01074.x [DOI] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, & Appel SB (1999). Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res, 23(11), 1848–1852. [PubMed] [Google Scholar]
- Brower KJ (2001). Alcohol’s effects on sleep in alcoholics. Alcohol Res Health, 25(2), 110–125. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, & Zucker RA (2008). A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res, 32(8), 1429–1438. doi: 10.1111/j.1530-0277.2008.00706.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, & Cutter HSG (1977). Alcohol, customary drinking behavior, and pain. J Abnorm Psychol, 86(2), 179–188. doi: 10.1037/0021-843X.86.2.179 [DOI] [PubMed] [Google Scholar]
- Buckner JD, Eggleston AM, & Schmidt NB (2006). Social anxiety and problematic alcohol consumption: the mediating role of drinking motives and situations. Behav Ther, 37(4), 381–391. doi: 10.1016/j.beth.2006.02.007 [DOI] [PubMed] [Google Scholar]
- Buffum MD, Hutt E, Chang VT, Craine MH, & Snow AL (2007). Cognitive impairment and pain management: review of issues and challenges. J Rehabil Res Dev, 44(2), 315–330. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Ceko M, & Low LA (2013). Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci, 14(7), 502–511. doi: 10.1038/nrn3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, & Carrier B (1999). Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A, 96(14), 7705–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan MH, & Randall CL (2003). Self-medication in social phobia: a review of the alcohol literature. Addict Behav, 28(2), 269–284. [DOI] [PubMed] [Google Scholar]
- Chapman CR (2005). Pain perception and assessment. Minerva Anestesiol, 71(7–8), 413–417. [PubMed] [Google Scholar]
- Chavarria J, Allan NP, Boffa JW, Albanese BJ, Schmidt NB, & Zvolensky MJ (2015). Decomposing the Relationship Between Anxiety Sensitivity and Alcohol Use. J Stud Alcohol Drugs, 76(6), 957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra K, & Tiwari V (2012). Alcoholic neuropathy: possible mechanisms and future treatment possibilities. Br J Clin Pharmacol, 73(3), 348–362. doi: 10.1111/j.1365-2125.2011.04111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, & Bohman M (1996). Type I and type II alcoholism: An update. Alcohol Health & Research World, 20(1), 18–24. [PMC free article] [PubMed] [Google Scholar]
- Crutzen R, Kuntsche E, & Schelleman-Offermans K (2013). Drinking motives and drinking behavior over time: a full cross-lagged panel study among adults. Psychol Addict Behav, 27(1), 197–201. doi: 10.1037/a0029824 [DOI] [PubMed] [Google Scholar]
- Cucciare MA, Sorrell JT, & Trafton JA (2009). Predicting response to cognitive-behavioral therapy in a sample of HIV-positive patients with chronic pain. J Behav Med, 32(4), 340–348. doi: 10.1007/s10865-009-9208-5 [DOI] [PubMed] [Google Scholar]
- Cutter HS, Maloof B, Kurtz NR, & Jones WC (1976). “Feeling no pain” differential responses to pain by alcoholics and nonalcoholics before and after drinking. J Stud Alcohol, 37(3), 273–277. [DOI] [PubMed] [Google Scholar]
- Davies M (2003). The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci, 28(4), 263–274. [PMC free article] [PubMed] [Google Scholar]
- Davis KD, & Moayedi M (2013). Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol, 8(3), 518–534. doi: 10.1007/s11481-012-9386-8 [DOI] [PubMed] [Google Scholar]
- Deehan GA Jr., Hauser SR, Wilden JA, Truitt WA, & Rodd ZA (2013). Elucidating the biological basis for the reinforcing actions of alcohol in the mesolimbic dopamine system: the role of active metabolites of alcohol. Front Behav Neurosci, 7, 104. doi: 10.3389/fnbeh.2013.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartini KS, & Carey KB (2011). The role of anxiety sensitivity and drinking motives in predicting alcohol use: a critical review. Clin Psychol Rev, 31(1), 169–177. doi: 10.1016/j.cpr.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Duarte R, McNeill A, Drummond G, & Tiplady B (2008). Comparison of the sedative, cognitive, and analgesic effects of nitrous oxide, sevoflurane, and ethanol. Br J Anaesth, 100(2), 203–210. doi: 10.1093/bja/aem369 [DOI] [PubMed] [Google Scholar]
- Duerden EG, & Albanese MC (2013). Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum Brain Mapp, 34(1), 109–149. doi: 10.1002/hbm.21416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy M, & Chanraud S (2016). Imaging the addicted brain: Alcohol. Int Rev Neurobiol, 129, 1–31. doi: 10.1016/bs.irn.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Eccleston C, & Crombez G (1999). Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull, 125(3), 356–366. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Dworkin RH, Sullivan MD, Turk DC, & Wasan AD (2016). The role of psychosocial processes in the development and maintenance of chronic pain. J Pain, 17(9 Suppl), T70–92. doi: 10.1016/j.jpain.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, & Koob GF (2010). Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol, 5(3), 393–401. doi: 10.2217/fnl.10.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Koob GF, & Edwards S (2012). Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev, 36(10), 2179–2192. doi: 10.1016/j.neubiorev.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Wiers RW, Christiansen P, Fillmore MT, & Verster JC (2010). Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcohol Clin Exp Res, 34(8), 1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Goodin BR, & Smith MT (2013). The association of sleep and pain: an update and a path forward. J Pain, 14(12), 1539–1552. doi: 10.1016/j.jpain.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordyce Wilbert E. (1976). Behavioral methods for chronic pain and illness Saint Louis: Mosby. [Google Scholar]
- Gatch MB, & Lal H (1999). Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin Exp Res, 23(2), 328–333. [PubMed] [Google Scholar]
- Gatzounis R, Schrooten MG, Crombez G, & Vlaeyen JW (2012). Operant learning theory in pain and chronic pain rehabilitation. Curr Pain Headache Rep, 16(2), 117–126. doi: 10.1007/s11916-012-0247-1 [DOI] [PubMed] [Google Scholar]
- George O, & Koob GF (2017). Individual differences in the neuropsychopathology of addiction. Dialogues Clin Neurosci, 19(3), 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, & Koob GF (2008). Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res Health, 31(3), 185–195. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, … Kaplan K (2004). Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry, 61(8), 807–816. doi: 10.1001/archpsyc.61.8.807 [DOI] [PubMed] [Google Scholar]
- Grisart JM, & Van der Linden M (2001). Conscious and automatic uses of memory in chronic pain patients. Pain, 94(3), 305–313. [DOI] [PubMed] [Google Scholar]
- Gu X, Gao Z, Wang X, Liu X, Knight RT, Hof PR, & Fan J (2012). Anterior insular cortex is necessary for empathetic pain perception. Brain, 135(Pt 9), 2726–2735. doi: 10.1093/brain/aws199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson R, & Kallmen H (1988). Alcohol and unpleasant stimulation: subjective shock calibration and pain and discomfort perception. Percept Mot Skills, 66(3), 739–742. doi: 10.2466/pms.1988.66.3.739 [DOI] [PubMed] [Google Scholar]
- Harper C (2009). The neuropathology of alcohol-related brain damage. Alcohol Alcohol, 44(2), 136–140. doi: 10.1093/alcalc/agn102 [DOI] [PubMed] [Google Scholar]
- Hasin DS, & Grant BF (2015). The national epidemiologic survey on alcohol and related conditions (NESARC) waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol, 50(11), 1609–1640. doi: 10.1007/s00127-015-1088-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, & Becker HC (2010). Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol, 15(2), 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer RK, Rainville P, Duncan GH, & Bushnell MC (2001). Cortical representation of the sensory dimension of pain. J Neurophysiol, 86(1), 402–411. doi: 10.1152/jn.2001.86.1.402 [DOI] [PubMed] [Google Scholar]
- Hoffmann NG, Olofsson O, Salen B, & Wickstrom L (1995). Prevalence of abuse and dependency in chronic pain patients. Int J Addict, 30(8), 919–927. [DOI] [PubMed] [Google Scholar]
- Horn-Hofmann C, Buscher P, Lautenbacher S, & Wolstein J (2015). The effect of nonrecurring alcohol administration on pain perception in humans: a systematic review. J Pain Res, 8, 175–187. doi: 10.2147/JPR.S79618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IASP Terminology. from http://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698-Pain.
- Ilgen MA, Haas E, Czyz E, Webster L, Sorrell JT, & Chermack S . (2011). Treating chronic pain in veterans presenting to an addictions treatment program. Cognitive and Behavioral Practice, 18(1), 149–160. [Google Scholar]
- Jaaskelainen SK, Rinne JO, Forssell H, Tenovuo O, Kaasinen V, Sonninen P, & Bergman J (2001). Role of the dopaminergic system in chronic pain -- a fluorodopa-PET study. Pain, 90(3), 257–260. [DOI] [PubMed] [Google Scholar]
- Jakubczyk A, Ashrafioun L, Ilgen M, Kopera M, Klimkiewicz A, Krasowska A, … Wojnar M (2016). Physical pain and history of suicidal behaviors in alcohol-dependent patients entering treatment in Poland. Subst Use Misuse, 51(10), 1307–1317. doi: 10.3109/10826084.2016.1168444 [DOI] [PubMed] [Google Scholar]
- Jakubczyk A, Brower KJ, Kopera M, Krasowska A, Michalska A, Łoczewska A, … Wojnar M (2016). Physical pain and impulsivity in alcohol-dependent patients. Addict Res & Theory, 24(6), 458–465. doi: 10.3109/16066359.2016.1164844 [DOI] [Google Scholar]
- Jakubczyk A, Ilgen MA, Kopera M, Krasowska A, Klimkiewicz A, Bohnert A, … Wojnar M (2016). Reductions in physical pain predict lower risk of relapse following alcohol treatment. Drug Alcohol Depend, 158, 167–171. doi: 10.1016/j.drugalcdep.2015.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochum T, Boettger MK, Burkhardt C, Juckel G, & Bar KJ (2010). Increased pain sensitivity in alcohol withdrawal syndrome. Eur J Pain, 14(7), 713–718. doi: 10.1016/j.ejpain.2009.11.008 [DOI] [PubMed] [Google Scholar]
- Johnson FW, Gruenewald PJ, Treno AJ, & Taff GA (1998). Drinking over the life course within gender and ethnic groups: a hyperparametric analysis. J Stud Alcohol, 59(5), 568–580. [DOI] [PubMed] [Google Scholar]
- Katz RS, & Kravitz HM (1996). Fibromyalgia, depression, and alcoholism: a family history study. J Rheumatol, 23(1), 149–154. [PubMed] [Google Scholar]
- Kelly J, Greene MC, & Bergman BG (2018). Beyond abstinence: changes in indices of quality of life with time in recovery in a nationally representative sample of U.S. adults. Alcohol Clin Exp Res, 42(4), 770–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyne DM, Moseley GL, Sterling M, Barbe MF, & Hodges PW (2018). Individual variation in pain sensitivity and conditioned pain modulation in acute low back pain: Effect of stimulus type, sleep, and psychological and lifestyle factors. J Pain, 19(8), 942 e941–942 e918. doi: 10.1016/j.jpain.2018.02.017 [DOI] [PubMed] [Google Scholar]
- Koob GF (2013). Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci, 13, 3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 24(2), 97–129. doi: 10.1016/S0893-133X(00)00195-0 [DOI] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217–238. doi: 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, & Borchardt C (2000). The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev, 20(2), 149–171. [DOI] [PubMed] [Google Scholar]
- Kyle BN, & McNeil DW (2014). Autonomic arousal and experimentally induced pain: a critical review of the literature. Pain Res Manag, 19(3), 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer ME, Palmer RS, & Marlatt GA (1999). Relapse prevention. An overview of Marlatt’s cognitive-behavioral model. Alcohol Res Health, 23(2), 151–160. [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, & de Wit H (2010). Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcohol Clin Exp Res, 34(8), 1334–1345. doi: 10.1111/j.1530-0277.2010.01217.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JL, McClish DK, Dahman BA, Penberthy LT, Bovbjerg VE, Aisiku IP, … Smith WR (2007). Alcohol abuse in sickle cell disease: the Pisces Project. Am J Addict, 16(5), 383–388. doi: 10.1080/10550490701525434 [DOI] [PubMed] [Google Scholar]
- Loeser John D., & Bonica John J. (2001). Bonica’s management of pain (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Marino EN, Rosen KD, Gutierrez A, Eckmann M, Ramamurthy S, & Potter JS (2013). Impulsivity but not sensation seeking is associated with opioid analgesic misuse risk in patients with chronic pain. Addict Behav, 38(5), 2154–2157. doi: 10.1016/j.addbeh.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JD, Francis EM, & Gonzales-Nolas C (2010). Managing acute and chronic pain in a substance abuse treatment program for the addicted individual early in recovery: a current controversy. J Psychoactive Drugs, 42(2), 193–198. doi: 10.1080/02791072.2010.10400691 [DOI] [PubMed] [Google Scholar]
- Mazza S, Frot M, & Rey AE (2018). A comprehensive literature review of chronic pain and memory. Prog Neuropsychopharmacol Biol Psychiatry, 87(Pt B), 183–192. doi: 10.1016/j.pnpbp.2017.08.006 [DOI] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, & Boyd CJ (2006). The relationship between past-year drinking behaviors and nonmedical use of prescription drugs: prevalence of co-occurrence in a national sample. Drug Alcohol Depend, 84(3), 281–288. doi: 10.1016/j.drugalcdep.2006.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M, & Morales-Mulia M (2008). Role of mu and delta opioid receptors in alcohol drinking behaviour. Curr Drug Abuse Rev, 1(2), 239–252. [DOI] [PubMed] [Google Scholar]
- Merlin JS, Walcott M, Kerns R, Bair MJ, Burgio KL, & Turan JM (2015). Pain self-management in HIV-infected individuals with chronic pain: a qualitative study. Pain Med, 16(4), 706–714. doi: 10.1111/pme.12701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin JS, Walcott M, Ritchie C, Herbey I, Kertesz SG, Chamot E, … Turan JM (2014). ‘Two pains together’: patient perspectives on psychological aspects of chronic pain while living with HIV. PLoS One, 9(11), e111765. doi: 10.1371/journal.pone.0111765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merskey Harold, Bogduk Nikolai, & International Association for the Study of Pain. Task Force on Taxonomy. (1994). Classification of chronic pain : descriptions of chronic pain syndromes and definitions of pain terms (2nd ed.). Seattle: IASP Press. [Google Scholar]
- Mitsi V, & Zachariou V (2016). Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience, 338, 81–92. doi: 10.1016/j.neuroscience.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty O, McGuire BE, & Finn DP (2011). The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol, 93(3), 385–404. doi: 10.1016/j.pneurobio.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Morley S, Eccleston C, & Williams A (1999). Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain, 80(1–2), 1–13. [DOI] [PubMed] [Google Scholar]
- Mosher Ruiz S, Oscar-Berman M, Kemppainen MI, Valmas MM, & Sawyer KS (2017). Associations between personality and drinking motives among abstinent adult alcoholic men and women. Alcohol Alcohol, 52(4), 496–505. doi: 10.1093/alcalc/agx016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy L, Ng KW, Su VCh, Woodworth-Giroux S, Levy TS, Sproule BA, & Furlan AD (2015). Approach to the pharmacological management of chronic pain in patients with an alcohol use disorder. J Pain Res, 8, 851–857. doi: 10.2147/JPR.S88900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, … Apkarian AV (2012). Abnormalities in hippocampal functioning with persistent pain. J Neurosci, 32(17), 5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National survey on drug use and health - SAMHSA. (2015): Dept. of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies
- Navratilova E, & Porreca F (2014). Reward and motivation in pain and pain relief. Nat Neurosci, 17(10), 1304–1312. doi: 10.1038/nn.3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak SP, Herman-Stahl M, Flannery B, & Zimmerman M (2009). Physical pain, common psychiatric and substance use disorders, and the non-medical use of prescription analgesics in the United States. Drug Alcohol Depend, 100(1–2), 63–70. doi: 10.1016/j.drugalcdep.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, & Marinkovic K (2003). Alcoholism and the brain: an overview. Alcohol Res Health, 27(2), 125–133. [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, & Marinkovic K (2007). Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev, 17(3), 239–257. doi: 10.1007/s11065-007-9038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer KS, Ruiz SM, Luhar RB, & Gravitz ZR (2014). Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. Handb Clin Neurol, 125, 183–210. doi: 10.1016/B978-0-444-62619-6.00012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, & Porreca F (2010). Central modulation of pain. J Clin Invest, 120(11), 3779–3787. doi: 10.1172/JCI43766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Morimura K, & Porreca F (2014). Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care, 8(2), 143–151. doi: 10.1097/SPC.0000000000000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL, & Pistis M (2005). Involvement of the endogenous cannabinoid system in the effects of alcohol in the mesolimbic reward circuit: electrophysiological evidence in vivo. Psychopharmacology (Berl), 183(3), 368–377. doi: 10.1007/s00213-005-0195-0 [DOI] [PubMed] [Google Scholar]
- Peterson JB, Rothfleisch J, Zelazo PD, & Pihl RO (1990). Acute alcohol intoxication and cognitive functioning. J Stud Alcohol, 51(2), 114–122. [DOI] [PubMed] [Google Scholar]
- Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, & Nichelli P (2002). Does anticipation of pain affect cortical nociceptive systems? J Neurosci, 22(8), 3206–3214. doi: 20026310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter JS, Prather K, & Weiss RD (2008). Physical pain and associated clinical characteristics in treatment-seeking patients in four substance use disorder treatment modalities. Am J Addict, 17(2), 121–125. doi: 10.1080/10550490701862902 [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Goetz D, Davies M, Kaplan T, Davies S, Ostrow L, … Ryan ND (1989). A controlled family history study of prepubertal major depressive disorder. Arch Gen Psychiatry, 46(5), 406–418. [DOI] [PubMed] [Google Scholar]
- Ralevski E, Perrino A, Acampora G, Koretski J, Limoncelli D, & Petrakis I (2010). Analgesic effects of ethanol are influenced by family history of alcoholism and neuroticism. Alcohol Clin Exp Res, 34(8), 1433–1441. doi: 10.1111/j.1530-0277.2010.01228.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhudy JL, Dubbert PM, Parker JD, Burke RS, & Williams AE (2006). Affective modulation of pain in substance-dependent veterans. Pain Med, 7(6), 483–500. doi: 10.1111/j.1526-4637.2006.00237.x [DOI] [PubMed] [Google Scholar]
- Rietschel M, & Treutlein J (2013). The genetics of alcohol dependence. Ann N Y Acad Sci, 1282, 39–70. doi: 10.1111/j.1749-6632.2012.06794.x [DOI] [PubMed] [Google Scholar]
- Rosier EM, Iadarola MJ, & Coghill RC (2002). Reproducibility of pain measurement and pain perception. Pain, 98(1–2), 205–216. [DOI] [PubMed] [Google Scholar]
- Ruiz SM, & Oscar-Berman M (2013). Closing the gender gap: The case for gender-specific alcoholism research. J Alcohol Drug Depend, 1(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz SM, & Oscar-Berman M (2015). Gender and alcohol abuse. . In Martin S (Ed.), The SAGE Encyclopedia of Alcohol: Social, Cultural, and Historical Perspectives . 2455 Teller Road, Thousand Oaks California 91320 United States: SAGE Publications, Inc. [Google Scholar]
- Sawyer KS, Oscar-Berman M, Barthelemy OJ, Papadimitriou GM, Harris GJ, & Makris N (2017). Gender dimorphism of brain reward system volumes in alcoholism. Psychiatry Res Neuroimaging, 263, 15–25. doi: 10.1016/j.pscychresns.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, & Moayedi M (2017). The dorsolateral prefrontal cortex in acute and chronic pain. J Pain, 18(9), 1027–1035. doi: 10.1016/j.jpain.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, … Naylor MR (2013). Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain, 14(12), 1573–1584. doi: 10.1016/j.jpain.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp TJ (2001). Chronic pain: a reformulation of the cognitive-behavioural model. Behav Res Ther, 39(7), 787–800. [DOI] [PubMed] [Google Scholar]
- Sherman BJ, McRae-Clark AL, Baker NL, Sonne SC, Killeen TK, Cloud K, & Gray KM (2017). Gender differences among treatment-seeking adults with cannabis use disorder: Clinical profiles of women and men enrolled in the achieving cannabis cessation-evaluating N-acetylcysteine treatment (ACCENT) study. Am J Addict, 26(2), 136–144. doi: 10.1111/ajad.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu R, Lussier D, Rosenblum A, Fong C, Portenoy J, Joseph H, & Portenoy RK (2008). Prevalence and characteristics of chronic pain in patients admitted to an outpatient drug and alcohol treatment program. Pain Med, 9(7), 911–917. doi: 10.1111/j.1526-4637.2008.00420.x [DOI] [PubMed] [Google Scholar]
- Shumilla JA, Liron T, Mochly-Rosen D, Kendig JJ, & Sweitzer SM (2005). Ethanol withdrawal-associated allodynia and hyperalgesia: age-dependent regulation by protein kinase C epsilon and gamma isoenzymes. J Pain, 6(8), 535–549. doi: 10.1016/j.jpain.2005.03.005 [DOI] [PubMed] [Google Scholar]
- Smith JP, & Randall CL (2012). Anxiety and alcohol use disorders: comorbidity and treatment considerations. Alcohol Res, 34(4), 414–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, & Haythornthwaite JA (2004). How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev, 8(2), 119–132. doi: 10.1016/S1087-0792(03)00044-3 [DOI] [PubMed] [Google Scholar]
- Stewart SH, Finn PR, & Pihl RO (1995). A dose-response study of the effects of alcohol on the perceptions of pain and discomfort due to electric shock in men at high familial-genetic risk for alcoholism. Psychopharmacology (Berl), 119(3), 261–267. [DOI] [PubMed] [Google Scholar]
- The Betty Ford Institute Consensus Panel. (2007). What is recovery? A working definition from the Betty Ford Institute. Journal of Substance Abuse Treatment, 33. [DOI] [PubMed] [Google Scholar]
- Thompson T, Oram C, Correll CU, Tsermentseli S, & Stubbs B (2017). Analgesic effects of alcohol: A systematic review and meta-analysis of controlled experimental studies in healthy participants. J Pain, 18(5), 499–510. doi: 10.1016/j.jpain.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Tracey I, & Bushnell MC (2009). How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J Pain, 10(11), 1113–1120. doi: 10.1016/j.jpain.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Trafton JA, Oliva EM, Horst DA, Minkel JD, & Humphreys K (2004). Treatment needs associated with pain in substance use disorder patients: implications for concurrent treatment. Drug Alcohol Depend, 73(1), 23–31. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E, Tetreault P, Petre B, Huang L, Berger SE, Torbey S, … Apkarian AV (2016). Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain, 139(Pt 7), 1958–1970. doi: 10.1093/brain/aww100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela CF (1997). Alcohol and neurotransmitter interactions. Alcohol Health Res World, 21(2), 144–148. [PMC free article] [PubMed] [Google Scholar]
- Verheul R, van den Brink W, & Geerlings P (1999). A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol, 34(2), 197–222. [DOI] [PubMed] [Google Scholar]
- Volkow ND, & McLellan AT (2016). Opioid Abuse in Chronic Pain--Misconceptions and Mitigation Strategies. N Engl J Med, 374(13), 1253–1263. doi: 10.1056/NEJMra1507771 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, … Wong C (2007). Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci, 27(46), 12700–12706. doi: 10.1523/jneurosci.3371-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowles KE, & McCracken LM (2008). Acceptance and values-based action in chronic pain: a study of treatment effectiveness and process. J Consult Clin Psychol, 76(3), 397–407. doi: 10.1037/0022-006X.76.3.397 [DOI] [PubMed] [Google Scholar]
- Wagner G, Koschke M, Leuf T, Schlosser R, & Bar KJ (2009). Reduced heat pain thresholds after sad-mood induction are associated with changes in thalamic activity. Neuropsychologia, 47(4), 980–987. doi: 10.1016/j.neuropsychologia.2008.10.021 [DOI] [PubMed] [Google Scholar]
- Weaver MF, & Schnoll SH (2002). Opioid treatment of chronic pain in patients with addiction. J Pain Palliat Care Pharmacother, 16(3), 5–26. [DOI] [PubMed] [Google Scholar]
- White WL (2007). Addiction recovery: Its definition and conceptual boundaries. Journal of Substance Abuse Treatment, 33, 229–241. [DOI] [PubMed] [Google Scholar]
- White WL, & Kurtz E (2006). The varieties of recovery experience. International Journal of Self-Help & Self-Care, 3(1–2), 21–61. [Google Scholar]
- Whitlock EL, Diaz-Ramirez LG, Glymour MM, Boscardin WJ, Covinsky KE, & Smith AK (2017). Association Between Persistent Pain and Memory Decline and Dementia in a Longitudinal Cohort of Elders. JAMA Intern Med, 177(8), 1146–1153. doi: 10.1001/jamainternmed.2017.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Ploner M, & Tracey I (2008). Neurocognitive aspects of pain perception. Trends Cogn Sci, 12(8), 306–313. doi: 10.1016/j.tics.2008.05.005 [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, McCallion E, Vowles KE, Kirouac M, Frohe T, Maisto SA, … Heather N (2015). Association between physical pain and alcohol treatment outcomes: The mediating role of negative affect. J Consult Clin Psychol, 83(6), 1044–1057. doi: 10.1037/ccp0000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, & Vowles KE (2018). Alcohol and Opioid Use, Co-Use, and Chronic Pain in the Context of the Opioid Epidemic: A Critical Review. Alcohol Clin Exp Res, 42(3), 478–488. doi: 10.1111/acer.13594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ (2010). What is this thing called pain? J Clin Invest, 120(11), 3742–3744. doi: 10.1172/JCI45178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ (2011). Central sensitization: implications for the diagnosis and treatment of pain. Pain, 152(3 Suppl), S2–15. doi: 10.1016/j.pain.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung JC, Yaksh TL, & Rudy TA (1977). Concurrent mapping of brain sites for sensitivity to the direct application of morphine and focal electrical stimulation in the production of antinociception in the rat. Pain, 4(1), 23–40. [DOI] [PubMed] [Google Scholar]
- Younger J, McCue R, & Mackey S (2009). Pain outcomes: a brief review of instruments and techniques. Curr Pain Headache Rep, 13(1), 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Camarillo VM, Sadeghi P, & Black M (1998). Effects of ethanol and nitrous oxide, alone and in combination, on mood, psychomotor performance and pain reports in healthy volunteers. Drug Alcohol Depend, 52(2), 115–123. [DOI] [PubMed] [Google Scholar]
- Zale EL, Maisto SA, & Ditre JW (2015). Interrelations between pain and alcohol: An integrative review. Clin Psychol Rev, 37, 57–71. doi: 10.1016/j.cpr.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]