Abstract
Background
Increasing evidence suggests that severe skeletal muscle index (SMI) loss (sarcopenia) is associated with poor overall survival in metastatic colorectal cancer patients, but its mechanisms are unknown. We recently found, using data of the randomized phase 3 CAIRO3 study, that SMI loss was related with shorter time to disease progression and overall survival during first‐line maintenance treatment with capecitabine + bevacizumab (CAP‐B) or observation and during more intensive capecitabine + oxaliplatin + bevacizumab (CAPOX‐B) reintroduction treatment. As a potential risk factor for reduced survival, we explored whether sarcopenia and SMI loss were associated with dose‐limiting toxicities (DLTs) during CAP‐B and CAPOX‐B.
Methods
Sarcopenia status and SMI loss were assessed by using consecutive computed tomography scans. DLTs were defined as any dose delay/reduction/discontinuation of systemic treatment because of reported CTCAE (version 3.0) toxicities at the start or during treatment. Poisson regression models were used to study whether sarcopenia and body mass index (BMI) at the start of treatment and SMI and BMI loss during treatment were associated with DLTs.
Results
One hundred eighty‐two patients (mean age 63.0 ± 8.8 years, 37% female) received CAP‐B, and 232 patients (mean age 63.0 ± 9.0 years, 34% female) received CAPOX‐B. At the start of CAP‐B and CAPOX‐B, 54% and 46% of patients were sarcopenic, respectively. Mean BMI was lower in sarcopenic patients, although patients were on average still overweight (sarcopenic vs. non‐sarcopenic at the start of CAP‐B 25.0 ± 3.9 vs. 26.7 ± 4.1 and CAPOX‐B 25.8 ± 3.8 vs. 27.1 ± 3.8 kg/m2). Sarcopenia at the start of CAP‐B was not associated with DLTs [relative risk 0.87 (95% confidence interval 0.64–1.19)], whereas patients with >2% SMI loss had a significantly higher risk of DLTs [1.29 (1.01–1.66)]. At the start of subsequent CAPOX‐B, 25% of patients received a dose reduction, and the risk of dose reduction was significantly higher for patients with preceding SMI loss [1.78 (1.06–3.01)] or sarcopenia [1.75 (1.08–2.86)]. After the received dose reductions, sarcopenia or SMI loss was not significantly associated with a higher risk of DLTs during CAPOX‐B [sarcopenia vs. non‐sarcopenic: 0.86 (0.69–1.08) and SMI loss vs. stable/gain: 0.83 (0.65–1.07)]. In contrast, BMI (loss) at the start or during either treatment was not associated with an increased risk of DLTs.
Conclusions
In this large longitudinal study in metastatic colorectal cancer patients during palliative systemic treatment, sarcopenia and/or muscle loss was associated with an increased risk of DLTs. BMI was not associated with DLTs and could not detect sarcopenia or SMI loss. Prospective (randomized) studies should reveal whether normalizing chemotherapeutic doses to muscle mass or muscle mass preservation (by exercise and nutritional interventions) increases chemotherapeutic tolerance and improves survival.
Keywords: Body composition, Metastatic colorectal cancer, Dose‐limiting toxicity, Chemotherapy, Skeletal muscle mass, Sarcopenia
Introduction
Muscle mass loss in cancer patients, including metastatic colorectal cancer (mCRC), is common and not exclusive to underweight patients.1, 2 Muscle mass loss and low muscle mass (sarcopenia) are associated with poor treatment outcomes. A recent meta‐analysis found that 19–71% of mCRC patients suffer from sarcopenia and that sarcopenia was associated with poor overall survival and increased treatment‐related toxicities.3 In several studies, the loss of muscle mass was also associated with reduced survival.4, 5, 6
The mechanisms by which muscle depletion links to poor survival are unknown. It is hypothesized that the increased treatment‐related toxicities risk in these patients may be a major contributor.3 Excess toxicities could lead to more frequent dose reductions, dose delays, and treatment discontinuations [i.e. dose‐limiting toxicities (DLTs)]. DLTs may lead to a reduced dose intensity (i.e. dose of chemotherapy delivered per unit of time) and dose density (i.e. time of intervals between dose administration of chemotherapy), which may be important determinants for the effects of systemic treatment on survival.7, 8 Low muscle mass may be related to excess toxicities due to an increased fragility or a pharmacokinetic effect.9, 10 Currently, systemic treatment is dosed on the patients' weight and height [body surface area (BSA)] and does not incorporate body composition. In sarcopenic patients, altered distribution parameters, due to lower muscularity for hydrophilic drugs such as capecitabine, could lead to higher drug concentrations (in a shorter amount of time) and subsequent more frequent toxicities.9, 10
Several studies in (m)CRC patients found an association between low muscle mass and an increased toxicity risk during systemic treatment.11, 12, 13, 14 However, most of these studies were limited in sample size or did not investigate the occurrence of subsequent dose reductions, delays, and discontinuations, which may have more impact on survival than toxicity in general. Although muscle mass is influenced by type of cancer and/or treatment and the occurrence of toxicities depends on the type of drugs and their intensity,3 most studies used only one time point to assess muscle mass during the course of the disease and were heterogeneous in terms of drugs administered.
Here, as a potential risk factor for reduced survival in mCRC patients, we investigate whether sarcopenia at the start of treatment and longitudinal muscle mass loss during treatment are associated with increased risk of DLTs and other treatment‐related toxicities during capecitabine + bevacizumab (CAP‐B) and subsequent capecitabine + oxaliplatin + bevacizumab (CAPOX‐B). We used data of the randomized phase 3 mCRC CAIRO3 study,15 in which we previously found that muscle loss during these subsequent treatment phases was related to early disease progression and reduced overall survival.16
Materials and methods
Study population
In CAIRO3, mCRC patients with stable disease or better after first‐line systemic treatment with 6 cycles CAPOX‐B were randomized to CAP‐B maintenance treatment or observation. Eligible patients were aged ≥18 years, had histological proof of CRC, had World Health Organization performance status ≤1, and received no previous systemic treatment for mCRC. After first progression on CAP‐B or observation (PD1), patients received CAPOX‐B reintroduction treatment or, if not feasible, any other treatment until second progression (PD2). Patients were assessed for disease status by computed tomography (CT) scans every 9 weeks according to RECIST criteria17 or at any time when disease progression was suspected based on clinical symptoms. Data on a patient's height, body weight, and the occurrence of DLTs were available for the two consecutive treatment phases within CAIRO3 (i.e. CAP‐B and CAPOX‐B reintroduction). Here, we selected patients with an available abdominal CT scan at the start and end of these regimens. Ethical approval for CAIRO3 was acquired by the Medical Ethics Committee of Nijmegen, The Netherlands. The CAIRO3 trial protocol was registered at ClinicalTrials.gov (NCT00442637).
Skeletal muscle mass analysis
Computed tomography scans were analysed for muscle mass by a trained analyst and a software tool (Slice‐o‐Matic, version 5.0; Tomovision). Detailed information on muscle analysis is reported elsewhere.2 In short, we used cross‐sectional evaluation of single slices with the third vertebral level (L3) as a landmark, which highly correlates with total body muscle mass.18 Before selecting the L3 slice, a rigid fusion method was used to rotate and fuse repeated CT scans to reduce measurement errors due to variation in the positioning of patients during consecutive CT scans over time. We used pre‐established thresholds for Hounsfield units (−29 to 150 HU) to quantify the muscle compartments (i.e. psoas, paraspinal, and abdominal wall muscles). Cross‐sectional areas (cm2) were computed for each image and used to calculate the skeletal muscle index (SMI):
Absolute SMI changes were calculated for any two consecutive CT scans. We used a cut‐off point of >2% SMI loss to discriminate between patients who lost SMI vs. patients who remained stable or increased in SMI during the two treatment periods. This cut‐off was arbitrarily chosen, based on the distribution of patients with SMI loss during the different regimens.
Data on body weight and height were used to calculate body mass index (BMI) = [weight (kg)/height (m)2]. Presence of sarcopenia was determined by applying published sex‐specific cut‐off points for SMI and BMI.1
Treatment
Maintenance CAP‐B treatment consisted of a low‐dose, continuous schedule of capecitabine orally of 625 mg/m2 twice daily continuously plus bevacizumab 7.5 mg/kg intravenously once every 3 weeks.
Reintroduction CAPOX‐B treatment consisted of oxaliplatin 130 mg/m2 and bevacizumab 7.5 mg/kg, both given intravenously every 3 weeks given on the first day of each cycle plus capecitabine orally 1000 mg/m2, Days 1–14.
If dose reductions were applied during the pre‐randomization (initial CAPOX‐B) or maintenance (CAP‐B) period, these were continued during CAPOX‐B reintroduction treatment. BSA was calculated using the Mosteller formula: BSA (m2) = [height (cm) × weight (kg)]/3600.19
Assessment of toxicities
Dose‐limiting toxicities were defined as any dose delay (>3 days), reduction, or discontinuation of systemic treatment because of reported toxicities. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria (CTCAE), version 3.0.
Recommendations on dose adjustments were described in the CAIRO3 study protocol (Table S1). A dose delay was recommended in case of grade ≥2 non‐haematologic toxicity, and treatment administration was not resumed before improvement to grade 1. A dose reduction was recommended when grade ≥3 toxicity occurred or in case of grade ≥2 neuropathy. Specific dose adaptations depended on both the type of agent and the specific toxicity. Dose reductions for bevacizumab were not applied. Doses reduced for toxicity were not re‐escalated. Treatment was discontinued after the occurrence of progression of disease, unacceptable toxicities that prevented the further safe administration of treatment, if the patient refused to receive further treatment or when another treatment was indicated (e.g. metastasectomy, another primary tumour, and start radiation therapy).
Statistical analysis
Poisson regression models were used to estimate the relative risks (RRs) for the association between sarcopenia, SMI loss, and the presence of DLTs or other toxicities during treatment.20 Each treatment period was analysed separately for the association of any DLT (0 vs. ≥1) or any treatment‐related toxicity (0 vs. ≥1) with sarcopenia (yes/no) SMI loss [>2% loss vs. ≤2% SMI loss (i.e. loss vs. stable/gain)]. All models were adjusted for the following, empirically selected, potential confounders: age (continuously), sex (male/female), primary tumour resection (yes/no), and dose reduction during initial (pre‐randomization) CAPOX‐B treatment (yes/no). During CAPOX‐B reintroduction treatment, we additionally adjusted for treatment received after CAIRO3 randomization (maintenance CAP‐B vs. observation). To correct for possible overdispersion (more variation in the outcome than is expected by the model, which can result in an underestimation of the standard errors of model parameters), we used robust standard errors.20 As a secondary analysis, we investigated the association between BMI (normal weight/overweight/obese) at the start of treatment and BMI loss during treatment with DLTs or other toxicities. BMI loss was continuously included in the analysis due to the small number of patients who lost >2% BMI (i.e. n = 10 during CAP‐B and n = 28 during CAPOX‐B). All P‐values were two‐sided, and the level of significance was considered at P < 0.05. For all statistical analyses, we used SPSS version 21.
Results
Based on the availability of CT scans or BMI measures, 182 patients who received CAP‐B and 232 patients who received CAPOX‐B were included. Figure 1 displays reasons for missing or non‐evaluable CT scans. During both regimens, baseline characteristics of patients with available CT scans were comparable with patients without available CT scans (P > 0.05).
Figure 1.
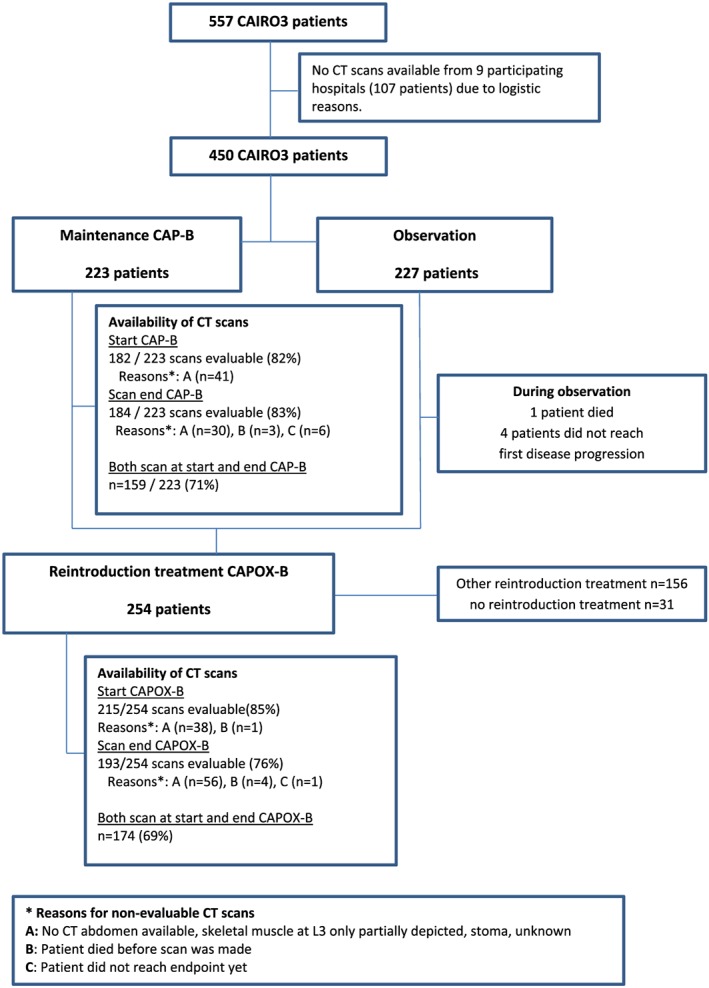
Flowchart. CAP‐B, capecitabine + bevacizumab; CAPOX‐B, capecitabine + oxaliplatin + bevacizumab; CT, computed tomography.
Sarcopenia was observed in 54% and 46% of patients at the start of CAP‐B and CAPOX‐B, respectively (Table 1). Sarcopenic patients were less frequently obese compared with non‐sarcopenic patients (at the start of CAP‐B 11% vs. 22% and CAPOX‐B 9% vs. 21%, respectively). Mean BMI was lower in sarcopenic patients, although patients were on average still overweight (sarcopenic vs. non‐sarcopenic patients at the start of CAP‐B 25.0 ± 3.9 vs. 26.7 ± 4.1 and at the start of CAPOX‐B 25.8 ± 3.8 vs. 27.1 ± 3.8 kg/m2).
Table 1.
Patient characteristics at the start of two treatment regimens
| Maintenance CAP‐B treatment | Reintroduction CAPOX‐B treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Sarcopenic at the start of Tx (n = 99) | Non‐sarcopenic at the start of Tx (n = 83) | SMI loss (>2%) (n = 45) | SMI stable/increase (n = 114) | Sarcopenic at the start of Tx (n = 99) | Non‐sarcopenic at the start of Tx (n = 116) | SMI loss (>2%) (n = 114) | SMI stable/increase (n = 60) | |
| Age, mean in years (±SD) | 64.2 (±8.9) | 63.1 (±8.4) | 64.7 (±7.4) | 63.1 (±8.6) | 64.2 (±8.6) | 62.4 (±9.3) | 62.9 (±8.8) | 64.7 (±9.2) |
| ≤70 | 68 (69%) | 68 (82%) | 31 (69%) | 91 (80%) | 72 (73%) | 91 (78%) | 89 (78%) | 40 (67%) |
| >70 | 31 (31%) | 15 (18%) | 14 (31%) | 23 (20%) | 27 (27%) | 25 (22%) | 25 (22%) | 20 (33%) |
| Sex | ||||||||
| Female | 36 (36%) | 31 (37%) | 15 (33%) | 44 (39%) | 38 (38%) | 36 (31%) | 32 (28%) | 30 (50%) |
| Male | 63 (63%) | 52 (63%) | 30 (67%) | 70 (61%) | 61 (62%) | 80 (69%) | 82 (72%) | 30 (50%) |
| Primary site | ||||||||
| Colon only | 47 (48%) | 44 (53%) | 17 (38%) | 56 (49%) | 50 (51%) | 59 (51%) | 53 (47%) | 37 (62%) |
| Rectum only | 32 (32%) | 20 (24%) | 16 (36%) | 31 (27%) | 27 (27%) | 33 (28%) | 34 (30%) | 13 (22%) |
| Rectosigmoid | 20 (20%) | 19 (23%) | 12 (27%) | 27 (24%) | 22 (22%) | 24 (21%) | 27 (24%) | 10 (17%) |
| Resection primary tumour | ||||||||
| Yes | 52 (53%) | 59 (71%) | 29 (64%) | 65 (57%) | 59 (60%) | 79 (68%) | 71 (62%) | 33 (55%) |
| No | 47 (48%) | 24 (29%) | 16 (36%) | 49 (43%) | 40 (40%) | 37 (32%) | 43 (38%) | 27 (45%) |
| WHO performance score | ||||||||
| 0 | 60 (61%) | 48 (58%) | 29(64%) | 66 (58%) | 46 (55%) | 53 (62%) | 41 (60%) | 34 (61%) |
| 1 | 39 (39%) | 35 (42%) | 16 (36%) | 48 (42%) | 33 (40%) | 31 (37%) | 26 (38%) | 19 (34%) |
| 2 | 0 (0%) | (0%) | 0 (0%) | 0 (0%) | 4 (5%) | 0 (0%) | 2 (3%) | 3 (5%) |
| 3 | 0 (0%) | (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) |
| Missing | 0 | 0 | 0 | 0 | 16 | 31 | 45 | 3 |
| LDH (IU/L)a | ||||||||
| Elevated | 51 (52%) | 48 (58%) | 27 (60%) | 58 (51%) | NAa | NAa | NAa | NAa |
| Normal | 48 (49%) | 35 (42%) | 18 (40%) | 56 (49%) | ||||
| BMI, mean ± SD | 25.0 (±3.9) | 26.7 (±4.1) | 25.9 (±4.2) | 25.8 (±4.0) | 25.8 (±3.8) | 27.1 (±3.8) | 26.7 (±3.9) | 25.5 (±3.6) |
| Underweight (<18.5) | 5 (5%) | 0 (0%) | 0 (0%) | 3 (3%) | 1 (1%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Normal (18.5–25) | 45 (46%) | 37 (45%) | 18 (41%) | 53 (47%) | 34 (34%) | 29 (26%) | 33 (30%) | 23 (39%) |
| Overweight (25–30) | 38 (38%) | 28 (34%) | 18 (41%) | 40 (35%) | 55 (56%) | 60 (54%) | 60 (54%) | 30 (51%) |
| Obese (30+) | 11 (11%) | 18 (22%) | 8 (18%) | 18 (16%) | 9 (9%) | 23 (21%) | 18 (16%) | 5 (9%) |
| Unknown | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 1 |
| Sarcopenia | ||||||||
| Yes | NA | NA | 21 (48%) | 63 (55%) | NA | NA | 50 (44%) | 32 (47%) |
| No | 23 (52%) | 51 (45%) | 63 (56%) | 28 (47%) | ||||
| Unknown | 1 | 0 | 1 | 0 | ||||
| Body surface area, mean in m2 | 1.9 (±0.23) | 2.0 (±0.22) | 1.9 (±0.23) | 1.9 (±0.22) | 2.0 (±0.22) | 2.0 (±0.20) | 2.0 (±0.22) | 1.9 (±0.20) |
| CAIRO3 treatment arm | ||||||||
| CAP‐B maintenance | NA | NA | NA | NA | 55 (56%) | 61 (53%) | 57 (50%) | 40 (67%) |
| Observation | 44 (44%) | 55 (47%) | 57 (50%) | 20 (33%) | ||||
| Reintroduction Tx | ||||||||
| CAPOX‐B | 50 (51%) | 45 (54%) | 20 (44%) | 67 (59%) | NA | NA | NA | NA |
| Other | 49 (50%) | 38 (46%) | 25 (56%) | 47 (41%) | ||||
Sarcopenia was defined as skeletal muscle index (SMI) of <43 cm2/m2 for men with body mass index (BMI) <25 kg/m2, <53 cm2/m2 for men with BMI ≥25 kg/m2, and <41 cm2/m2 for women of any BMI. CAP‐B, capecitabine + bevacizumab; CAPOX‐B, capecitabine + oxaliplatin + bevacizumab; LDH, lactate dehydrogenasis; NA, not applicable; SD, standard deviation; Tx, treatment; WHO, World Health Organization.
Not available at the time of the start of reintroduction treatment.
Detailed information on muscle mass and body weight changes during CAIRO3 treatments were reported elsewhere.2, 16 In short, during CAP‐B, 28% of patients lost >2% SMI, and 66% of patients lost >2% SMI during CAPOX‐B.
Dose‐limiting toxicities during treatment
During both regimens, DLTs were frequently observed. Overall, more than half of patients experienced ≥1 DLTs (Table 2, Figure 2).
Table 2.
Associations of skeletal muscle index and body mass index loss and (dose‐limiting) toxicities
| n | Number of cycles received (median, range) | Dose reduction at the start of Tx | RRa dose reduction at the start of Tx | ≥1 DLT during Tx | RR ≥1 DLTa | At least 1 non‐haematologic toxicity grade ≥2 during Tx | RR grade ≥2a toxicity | At least 1 non‐haematologic toxicity grade ≥3 during Tx | RR grade ≥3a toxicity | |
|---|---|---|---|---|---|---|---|---|---|---|
| Maintenance CAP‐B treatment | ||||||||||
| BMI at the start of Txb | ||||||||||
| Underweight (<18.5) | 5 | 4 (3–33) | NA | NA | 3 (60%) | NAc | 3 (60%) | NAc | 1 (20%) | NAc |
| Normal (18.5–25) | 81 | 9 (1–55) | NA | NA | 50 (62%) | 1 [reference] | 50 (62%) | 1 [reference] | 41 (51%) | 1 [reference] |
| Overweight (25–30) | 65 | 12 (1–38) | NA | NA | 42 (65%) | 1.09 (0.85–1.40) | 42 (65%) | 0.95 (0.87–1.04) | 33 (51%) | 1.27 (0.88–1.84) |
| Obese (30+) | 29 | 9 (1–54) | NA | NA | 16 (55%) | 0.90 (0.62–1.31) | 16 (55%) | 0.87 (0.78–0.98) | 12 (41%) | 1.40 (0.89–2.21) |
| Sarcopenia status at the start of Tx | ||||||||||
| Sarcopenic | 99 | 9 (1–54) | NA | NA | 58 (59%) | 0.87 (0.64–1.19) | 87 (88%) | 0.93 (0.84–1.02) | 49 (50%) | 1.01 (0.69–1.47) |
| Non‐sarcopenic | 83 | 12 (1–55) | NA | NA | 53 (64%) | 1 [reference] | 77 (93%) | 1 [reference] | 38 (46%) | 1 [reference] |
| SMI change during Tx | ||||||||||
| SMI >2% loss | 45 | 12.0 (1–54) | NA | NA | 32 (71%) | 1.29 (1.01–1.66) | 43 (96%) | 1.07 (0.97–1.17) | 26 (58%) | 1.83 (1.22–2.75) |
| SMI stable gain | 114 | 9.0 (1–44) | NA | NA | 61 (54%) | 1 [reference] | 100 (88%) | 1 [reference] | 47 (41%) | 1 [reference] |
| BMI loss during Tx per unit continuouslyd | 113 | 12.0 (3–54) | NA | NA | 74 (66%) | 1.01 (0.95–1.06) | 103 (91%) | 1.01 (0.99–1.03) | 49 (43%) | 1.01 (0.91–1.10) |
| Reintroduction CAPOX‐B treatment | ||||||||||
| BMI at the start of Tx | ||||||||||
| Underweight (<18.5) | 1 | 3 (3–3) | 0 (0%) | NAc | 1 (100%) | NAc | 1 (100%) | NAc | 0 (0%) | NAc |
| Normal (18.5–25) | 63 | 6 (1–56) | 13 (21%) | 1 [reference] | 38 (60%) | 1 [reference] | 53 (84%) | 1 [reference] | 31 (49%) | 1 [reference] |
| Overweight (25–30) | 115 | 6 (1–36) | 30 (26%) | 1.39 (0.79–2.44) | 67 (58%) | 0.95 (0.73–1.22) | 91 (79%) | 0.95 (0.83–1.10) | 51 (44%) | 1.07.98 (0.81–1.41) |
| Obese (30+) | 32 | 6 (1–23) | 8 (25%) | 1.29 (0.58–2.89) | 21 (66%) | 1.07 (0.77–1.49) | 25 (78%) | 0.97 (0.79–1.19) | 16 (50%) | 1.30 (0.89–1.90) |
| Sarcopenia status at the start of Tx | ||||||||||
| Sarcopenic | 99 | 6 (1–56) | 31 (31%) | 1.75 (1.08–2.86) | 56 (56%) | 0.86 [0.69–1.08] | 79 (80%) | 1.00 (0.87–1.15) | 47 (48%) | 0.99 (0.78–1.26) |
| Non‐sarcopenic | 116 | 6 (2–33) | 20 (17%) | 1 [reference] | 74 (64%) | 1 [reference] | 91 (78%) | 1 [reference] | 52 (45%) | 1 [reference] |
| SMI change during Tx | ||||||||||
| SMI >2% loss | 114 | 6 (1–27) | 41(36%)d | 1.78 (1.06–3.01) e | 63 (55%) | 0.83 (0.65–1.07) | 86 (75%) | 0.93 (0.79–1.10) | 56 (49%) | 1.22 (0.86–1.73) |
| SMI stable gain | 60 | 6 (3–56) | 13(21%)d | 1 [reference]e | 42 (69%) | 1 [reference] | 50 (83%) | 1 [reference] | 26 (43%) | 1 [reference] |
| BMI loss during Tx per unit continuouslyd , f | 232 | 6 (1–56) | 54 (23%) | 1.02 (0.90–1.16)e | 142 (61%) | 0.99 (0.93–1.06) | 217 (94%) | 1.01 (0.99–1.02) | 125 (54%) | 1.10 (1.05–1.16) |
Statistical significant relative risks are in bold. BMI, body mass index; CAP‐B, capecitabine + bevacizumab; CAPOX‐B, capecitabine + oxaliplatin + bevacizumab; DLT, dose‐limiting toxicity; NA, not applicable; RR, relative risk; SMI, skeletal muscle index; Tx, treatment.
Relative risks determined by Poisson regression analysis, all models were adjusted for age, sex, resection primary tumour, and dose reduction during initial CAPOX‐B treatment. During CAPOX‐B reintroduction treatment, models were additionally adjusted for treatment received after CAIRO3 randomization (maintenance CAP‐B vs. observation).
In two women, BMI was missing.
Because of small numbers, patients were not included in Poisson regression analysis.
Because of the low percentage of patients with >2% BMI loss, BMI change was continuously included in the analysis.
SMI or BMI loss during CAP‐B treatment.
BMI loss during previous CAP‐B treatment.
Figure 2.
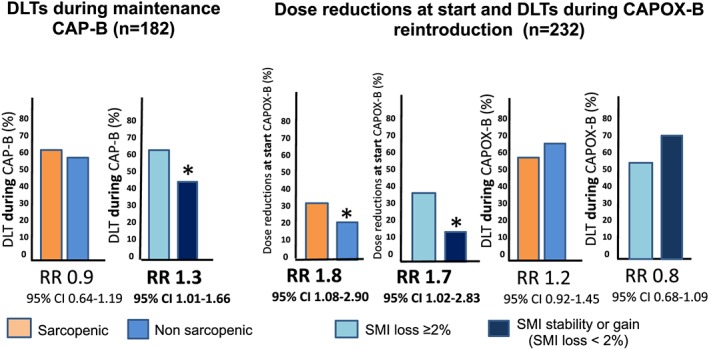
Associations of sarcopenia, skeletal muscle index (SMI) loss, and dose‐limiting toxicities (DLTs). This figure displays the association of sarcopenia and skeletal muscle index loss with dose‐limiting toxicities during treatment with capecitabine + bevacizumab (CAP‐B) and capecitabine + oxaliplatin + bevacizumab (CAPOX‐B). Relative risks (RRs) are determined by Poisson regression models. All models were adjusted for age, sex, resection primary tumour, and dose reduction during initial capecitabine + oxaliplatin + bevacizumab treatment. *Statistically significant results.
Sarcopenia at the start of CAP‐B was not associated with an increased risk of DLTs [RR 0.87 (95% confidence interval 0.64–1.19)]. Patients with >2% SMI loss during CAP‐B had a significantly higher risk of DLTs [SMI loss vs. SMI stable/gain; RR 1.29 (1.01–1.66)].
Sarcopenic patients and patients who lost >2% SMI during previous CAP‐B treatment or observation had significantly more risk of receiving a dose reduction at the start of CAPOX‐B (RR 1.75 [1.08–2.86] and RR 1.78 [1.06–3.01], respectively). Among patients who received a dose reduction at the start of CAPOX‐B reintroduction, 70% of patients had received a dose reduction during previous treatments (i.e. during initial CAPOX‐B or CAP‐B maintenance treatment), whereas 30% had not. After the administered dose reductions, we found no significantly higher risk of DLTs for sarcopenic patients during CAPOX‐B [RR 0.86 (0.69–1.098)] or for patients with >2% SMI loss [RR 0.83 (0.65–1.07)].
Toxicities during treatment
During both regimens, grade ≥2 and ≥3 toxicities occurred frequently (Table 2). During CAP‐B, neither sarcopenia nor SMI loss was associated with an increased risk of grade ≥2 toxicities (RR 0.93 [0.84–1.02] and RR 1.07 [0.97–1.17], respectively). We also did not observe an association between sarcopenia at the start of CAP‐B and grade ≥3 toxicities [RR 1.01 (0.69–1.47)], in contrast to >2% SMI loss during CAP‐B, which carried a significantly higher risk of grade ≥3 toxicities [RR 1.83 (1.22–2.75)]. After initial dose reductions at the start of CAPOX‐B, we observed no significant differences in the occurrence of toxicities grade ≥2 or ≥3 during CAPOX‐B for patients with sarcopenia or SMI loss (Table 2).
Body mass index and toxicities
Body mass index at the start of either regimen was not associated with an increased risk of DLTs or toxicities, except for obese patients and a lower risk of grade ≥2 toxicities during CAP‐B [RR 0.87 (0.78–0.98)]. Also, BMI loss during either regimen was not associated with an increased risk of DLTs or toxicities, except for BMI loss during CAPOX‐B and an increased risk of grade ≥3 toxicities [RR 1.08 (1.10–1.16)] (Table 2).
Discussion
In this large longitudinal study, we evaluated the association between sarcopenia and SMI loss and the occurrence of DLTs during two consecutive palliative treatment regimens in mCRC patients. We found that, in patients with good tolerance of 6 cycles initial CAPOX‐B treatment, >2% SMI loss during subsequent less intensive CAP‐B treatment was significantly associated with an increased risk of DLTs. Furthermore, patients with sarcopenia at the start of CAPOX‐B reintroduction treatment or with >2% SMI loss during previous CAP‐B treatment or observation more frequently received a dose reduction at the start of CAPOX‐B reintroduction. Finally, during CAP‐B, patients with >2% SMI loss had a significantly increased risk of grade ≥3 toxicities.
Our study is the first on this topic that uses both cross‐sectional SMI and longitudinal SMI change during treatment to investigate the relation between SMI loss and DLTs during two consecutive regimens. Another important strength of this study is that we used data of a large randomized phase 3 trial, implying high‐quality data inherent to data collection in trials, with standardized toxicity assessments and protocol recommendations on dose adjustments. Our findings that sarcopenia at the start of treatment was associated with an increased toxicity risk are in line with previous, mostly small, and cross‐sectional studies.11, 12, 13, 14 The increased risk in these studies ranged from a factor 1.5 to 13.5, mainly depending on type of treatment and the definition of sarcopenia used [e.g. some studies determined cut‐off points for the dose of systemic treatment/kg lean body mass (LBM) and found that patients who received a dosage above the cut‐off points have an increased toxicity risk toxicities]. Our data additionally show that SMI loss over time is associated with an increased toxicity risk. Hence, our data support the observation that the loss of muscle mass is associated with increased DLTs and may contribute to the observed reduced survival in these patients.
In contrast to SMI loss, BMI (loss) was not associated with an increased risk of DLTs, although obese compared with normal weight patients had a lower risk of grade ≥2 toxicities during CAP‐B, which might be explained by their on average higher SMI,21 and although patients who lost BMI (i.e. both fat and muscle mass loss) during CAPOX‐B had an increased risk of grade ≥3 toxicities. We conclude that BMI is not an appropriate tool to identify patients at risk of poor outcome and may wrongly reassure clinicians about patients' nutritional status. Indeed, at the start of both regimens, 54% of patients were sarcopenic, despite the fact that these patients were on average overweight. In mCRC patients, CT scans are routinely performed for diagnosis and treatment evaluation and therefore readily available for body composition analysis. Therefore, muscle mass analysis using routine CT scans is a potential tool to identify patients at risk for poor outcome, without additional costs or patient burden.
We did not find an association between sarcopenia and toxicities during CAP‐B. A possible explanation is that in CAIRO3, patients with intolerance for initial CAPOX‐B (i.e. severe toxicity that prevented its further safe administration) were excluded from participation.
Sarcopenia at the start of CAPOX‐B and SMI loss during previous CAP‐B were associated with dose reductions at the start of CAPOX‐B. Of patients who received a dose reduction, 70% had received a dose reduction during previous treatments (i.e. initial CAPOX‐B or CAP‐B), whereas 30% had not. Of these 30%, reasons for dose reductions were not specified. Sarcopenia and SMI loss are associated with poor physical functioning, increased fatigue, and reduced quality of life.22 We hypothesize that patients with SMI loss present in less fit condition, which may cause physicians to initiate dose reductions at the start of treatment in order to prevent toxicity.
A potential method to reduce DLTs in sarcopenic patients is to adjust chemotherapy dosing to body composition, as most anticancer agents distribute and metabolize within the fat‐free mass or LBM.10, 23 Sarcopenic patients may have altered pharmacokinetic parameters, including area under the time concentration curve and clearance.23 Currently, doses of systemic treatment are determined by BSA, which is too simplified to describe body composition. Indeed, several studies in patients with cancer showed that LBM has a low correlation with BSA,13, 23 especially in obese patients.9 One study in CRC patients who received 5‐fluorouracil intravenously found that low LBM was associated with a decrease in distribution volume and clearance.24 Two studies investigated the relation between BSA dosed capecitabine and oxaliplatin normalized to LBM and found that treatment concentrations above the cut‐off points were associated with severe toxicity, including grade ≥3 toxicities for capecitabine and dose‐limiting neuropathy for oxaliplatin.12, 13 Even more, in both studies, negligible toxicity was observed in patients who received doses below the cut‐off points, which may imply that they may benefit from higher doses. Normalizing chemotherapy to LBM may be a potent tool to individualize chemotherapy treatment and to prevent toxicity. This concept awaits verification, and the first prospective feasibility study is initiated in patients with metastatic lung cancer.25
Another potential tool to increase chemotherapeutic tolerance is muscle mass preservation. In advanced cancer patients, muscle loss is a multifactorial syndrome in which a reduced nutritional intake, metabolic changes (due to the tumour and treatment), often combined with low physical activity levels, lead to alterations in body composition and eventually to cancer cachexia.26 Muscle mass preservation during later stages of advanced cancer, after (extensive) weight loss has occurred, is difficult as the progressive metabolic derangements that occur during cancer (cachexia) may no longer be reversible, at least not by the conventional interventions such as nutritional support.26, 27 However, there might be a window of opportunity in the initial phase of metastatic disease, as during this phase, patients may still have exploitable anabolic potential.2, 6 Indeed, in CAIRO3, we observed that muscle loss that occurred during initial 6 cycles CAPOX‐B treatment was reversible during subsequent less intensive CAP‐B treatment or observation.2 Possible therapeutic approaches to increase muscle mass include physical exercise (aerobic and resistance training), nutritional supplements (high energy/high protein), and orexigenic agents.3 However, due to the lack of well‐powered clinical trials, solid evidence is lacking on the effects of muscle mass preservation on oncologic outcomes, including DLTs.3 Several previous trials however did investigate the effects of high‐protein intake and/or aerobic or resistance training mainly in patients with early prostatic of breast cancer (and one study in mCRC), and showed that both muscle mass and strength increased, but did not evaluate the effect on oncologic outcomes such as DLTs.28, 29 Also, pharmacologic treatments that utilize or target ghrelin,29 androgene receptors,30 interleukin‐1α,31 β‐receptor blockade,32 testosterone,33 and myostatin34 were able to increase muscle mass but failed to show improvement in functional or oncologic outcomes, likely because they were initiated during the (very) late stage of metastatic cancer.35 Finally, fish oil (i.e. N‐3 fatty acids) supplementation may have protective effects for chemotherapy‐induced toxicities.36 Two randomized trials in breast (n = 20)37 and lung cancer patients (n = 90)38 receiving chemotherapy with paclitaxel and/or platinum found a significant reduction in neuropathy incidence after fish oil supplementation, although in the lung cancer trial, this was not the primary endpoint. These results have now led to several ongoing randomized trials to study the effects of muscle mass preservation on oncologic outcomes,35, 39 including a randomized trial that examines the efficacy of resistance exercise and protein supplementation on lean mass to reduce DLTs in patients with (non‐metastatic) colon cancer (ClinicalTrials.gov number: NCT03291951).
Furthermore, it was previously shown that high‐aged patients have an increased risk of toxicities and of being sarcopenic. However, whether older patients have a different risk of experiencing toxicities due to sarcopenia/SMI change compared with younger patients is unknown. Because of the low number of patients aged 70 years or older in this study (n = 45 during CAP‐B and n = 52 during CAPOX‐B), we could not analyse the effects of sarcopenia or SMI loss on DLT and toxicity risk in high‐aged patients in this study. However, due to the increasing number of elderly patients with cancer, future research on this topic seems important, including studies that investigate the effects of interventions that aim to increase muscle mass in elderly cancer populations.
We are aware of some limitations of the current research. Firstly, patients were excluded if CT scans were unavailable, for example, because of treatment discontinuation or disease progression. This may have resulted in a selection (bias) and an underestimation of the observed effect (i.e. when excluded patients with poor prognosis had an increased risk of DLTs). However, baseline characteristics of patients with and without evaluable CT scans were comparable, and we adjusted for potential confounders in our analysis. Secondly, data on lifestyle measures and nutritional intake were not available in CAIRO3, and both may have impact on SMI loss and toxicity risk. Thirdly, because this is an observational analysis, we cannot draw conclusions on the causal relationship between SMI loss and toxicity, and we cannot exclude residual confounding.
Conclusions
In mCRC patients during palliative systemic treatment, sarcopenia and SMI loss are associated with an increased risk of DLTs, which may contribute to the observed worse survival in this group of patients. BMI was not associated with DLTs and could not accurately detect sarcopenia or SMI loss. Muscle mass analysis by using routine CT scans has the potential to identify patients at risk of poor outcomes. Prospective studies should reveal whether normalizing chemotherapeutic doses to muscle mass or preservation of muscle mass (by exercise and nutritional interventions) increases chemotherapeutic tolerance and improves survival.
Conflict of interest
B. Dorresteijn and M. Jourdan work at Nutricia Research. S.A. Kurk, P.H.M. Peeters, R.K. Stellato, P.A. de Jong, G.‐J.M. Creemers, F.L.G. Erdkamp, F.E. de Jongh, P.A.M. Kint, L.H.J. Simkens, B.C. Tanis, M.L.R. Tjin‐A‐Ton, A. Van Der Velden, C.J.A. Punt, M. Koopman, and A.M. May declare that they have no conflict of interest.40
Supporting information
Table S1a. Dose adjustments according to the CAIRO3 protocol for oxaliplatin and capecitabine if toxicity occurred during a previous cycle.
Table S1b. Dose adjustments for capecitabine according to the CAIRO3 study protocol for non‐hematological toxicity.
Acknowledgement
We thank all patients and staff at each of the study centres. The authors certify that they complywith the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017.40 This study was funded by the Dutch Colorectal Cancer Group (DCCG) and the province of Utrecht, The Netherlands.
Kurk S., Peeters P., Stellato R., Dorresteijn B., de Jong P., Jourdan M., Creemers G.‐J., Erdkamp F., de Jongh F., Kint P., Simkens L., Tanis B., Tjin‐A‐Ton M., Van Der Velden A., Punt C., Koopman M., and May A. (2019) Skeletal muscle mass loss and dose‐limiting toxicities in metastatic colorectal cancer patients, Journal of Cachexia, Sarcopenia and Muscle, 10, 803–813, doi: 10.1002/jcsm.12436.
List of where and when the study has been presented in part elsewhere, if applicable: (i) poster discussion presentation, ESMO annual conference, September 2017, Madrid, Spain, and (ii) oral abstract presentation during the Young Investigator Award Session, 10th International Conference on Cachexia, Sarcopenia and Muscle Wasting, December 2017, Rome, Italy.
Trial registration: The original CAIRO3 trial protocol was registered at ClinicalTrials.gov, number NCT00442637.
References
- 1. Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 2. Kurk S, Peeters P, Dorresteijn B, de Jong P, Jourdan M, Kuijf H, et al. Impact of different palliative systemic treatments on skeletal muscle mass in metastatic colorectal cancer patients. J Cachexia Sarcopenia Muscle 2018;9:909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bozzetti F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol 2017;28:2107–2118. [DOI] [PubMed] [Google Scholar]
- 4. Blauwhoff‐Buskermolen S, Versteeg KS, de van der Schueren MAE, den Braver NR, Berkhof J, Langius JAE, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 2016;34:1339–1344. [DOI] [PubMed] [Google Scholar]
- 5. Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, et al. Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS ONE 2015;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prado CM, Sawyer MB, Ghosh S, Lieffers JR, Esfandiari N, Antoun S, et al. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 2013;98:1012–1019. [DOI] [PubMed] [Google Scholar]
- 7. Havrilesky LJ, Reiner M, Morrow PK, Watson H, Crawford J. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol 2015;93:203–210. [DOI] [PubMed] [Google Scholar]
- 8. Bonilla L, Ben‐Aharon I, Vidal L, Gafter‐Gvili A, Leibovici L, Stemmer SM. Dose‐Dense chemotherapy in nonmetastatic breast cancer: a systematic review and meta‐analysis of randomized controlled trials. J Natl Cancer Inst 2010;102:1845–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 10. Antoun S, Borget I, Lanoy E. Impact of sarcopenia on the prognosis and treatment toxicities in patients diagnosed with cancer. Curr Opin Support Palliat Care 2013;7:383–389. [DOI] [PubMed] [Google Scholar]
- 11. Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 2014;66:583–589. [DOI] [PubMed] [Google Scholar]
- 12. Prado CMM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5‐fluorouracil‐based chemotherapy toxicity. Clin Cancer Res 2007;13:3264–3268. [DOI] [PubMed] [Google Scholar]
- 13. Ali R, Baracos VE, Sawyer MB, Bianchi L, Roberts S, Assenat E, et al. Lean body mass as an independent determinant of dose‐limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med 2016;5:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cespedes EM, Scd F, Lee VS, Prado CM. Muscle mass at the time of diagnosis of nonmetastatic colon cancer and early discontinuation of chemotherapy, delays, and dose reductions on adjuvant FOLFOX: the C‐SCANS study. Cancer 2017. Dec 15;123(24):4868–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simkens LHJ, van Tinteren H, May A, ten Tije AJ, Creemers G‐JM, Loosveld OJL, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet 2015;6736:1–10. [DOI] [PubMed] [Google Scholar]
- 16. Kurk S, Peeters P, Dorresteijn B, de Jong P, Creemers MJ, Erdkamp F, et al. Impact of skeletal muscle index (SMI) loss during palliative systemic treatment (Tx) on time to progression and overall survival (OS) in metastatic colorectal cancer (mCRC) patients. J Clin Oncol 2017;35:10087–10087 (manuscript submitted). [Google Scholar]
- 17. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2008;45:228–247. [DOI] [PubMed] [Google Scholar]
- 18. Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 19. Mosteller R. Simplified calculation of body‐surface area. N Engl J Med 1987;317:1098. [DOI] [PubMed] [Google Scholar]
- 20. Knol MJ, Le Cessie S, Algra A, Vandenbroucke JP, Groenwold RHH. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. CMAJ 2012;184:895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, et al. Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C‐SCANS study). Cancer Epidemiol Biomarkers Prev 2017;26:1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malietzis G, Aziz O, Bagnall NM, Johns N, Fearon KC, Jenkins JT. The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: a systematic review. Eur J Surg Oncol 2015;41:186–196. [DOI] [PubMed] [Google Scholar]
- 23. Prado C, Maia Y, Ormsbee M, Sawyer M, Baracos V. Assessment of nutritional status in cancer—the relationship between body composition and pharmacokinetics. Anticancer Agents Med Chem 2013;13:1197–1203. [DOI] [PubMed] [Google Scholar]
- 24. Gusella M, Toso S, Ferrazzi E, Ferrari M, Padrini R. Relationships between body composition parameters and fluorouracil pharmacokinetics. Br J Clin Pharmacol 2002;54:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crosby V, D'Souza C, Bristow C, Proffitt A, Hussain A, Potter V, et al. Can body composition be used to optimize the dose of platinum chemotherapy in lung cancer? A feasibility study. Support Care Cancer 2017;25:1257–1261. [DOI] [PubMed] [Google Scholar]
- 26. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 27. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2012;10:80–89. [DOI] [PubMed] [Google Scholar]
- 28. Focht B, Clinton S, Devor S. Resistance exercise interventions during and following cancer treatment: a systematic review. J Support Oncol 2013;11:45–60. [PubMed] [Google Scholar]
- 29. Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non‐small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double‐blind, phase 3 trials. Lancet Oncol 2016;17:519–531. [DOI] [PubMed] [Google Scholar]
- 30. Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, Hancock ML, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double‐blind, randomised controlled phase 2 trial. Lancet Oncol 2013;14:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hickish T, Andre T, Wyrwicz L, Saunders M, Sarosiek T, Kocsis J, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Oncol 2017;18:192–201. [DOI] [PubMed] [Google Scholar]
- 32. Stewart Coats AJ, Ho GF, Prabhash K, von Haehling S, Tilson J, Brown R, et al. Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non‐small cell lung cancer or colorectal cancer: a randomized, double‐blind, placebo‐controlled, international multicentre phase II study (the ACT‐ONE trial). J Cachexia Sarcopenia Muscle 2016;7:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wright TJ, Dillon EL, Durham WJ, Chamberlain A, Randolph KM, Danesi C, et al. A randomized trial of adjunct testosterone for cancer‐related muscle loss in men and women. J Cachexia Sarcopenia Muscle 2018;9:482–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Golan T, Geva R, Richards D, Madhusudan S, Lin BK, Wang HT, et al. LY2495655, an antimyostatin antibody, in pancreatic cancer: a randomized, phase 2 trial. J Cachexia Sarcopenia Muscle 2018;9:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology‐epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle 2019;9:1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11–48. [DOI] [PubMed] [Google Scholar]
- 37. Ghoreishi Z, Esfahani A, Djazayeri A, Djalali M, Golestan B, Ayromlou H, et al. Omega‐3 fatty acids are protective against paclitaxel‐induced peripheral neuropathy: a randomized double‐blind placebo controlled trial. BMC Cancer 2012;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sánchez‐lara K, Turcott JG, Juárez‐hernández E, Nuñez‐valencia C, Villanueva G, Guevara P, et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non‐small cell lung cancer: randomised trial. Clin Nutr 2014;33:1017–1023. [DOI] [PubMed] [Google Scholar]
- 39. van der Werf A, Blauwhoff‐Buskermolen S, Langius JAE, Berkhof J, Verheul HMW, de van der Schueren MAE. The effect of individualized NUTritional counseling on muscle mass and treatment outcome in patients with metastatic COLOrectal cancer undergoing chemotherapy: a randomized controlled trial protocol. BMC Cancer 2015;15:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1a. Dose adjustments according to the CAIRO3 protocol for oxaliplatin and capecitabine if toxicity occurred during a previous cycle.
Table S1b. Dose adjustments for capecitabine according to the CAIRO3 study protocol for non‐hematological toxicity.


