Abstract
Objective
Previous research conducted in Russia showed that the number of patients with non-alcoholic fatty liver disease (NAFLD) and associated metabolic comorbidities is large. We conducted an observational study to describe the management of NAFLD in patients with metabolic syndrome in Russia.
Design
A total of 2843 adult patients from 174 medical sites across 6 federal districts of Russia with newly diagnosed NAFLD, who had at least one of four comorbidities, namely overweight/obesity, hypertension, type 2 diabetes mellitus, and hypercholesterolaemia, and who received phosphatidylcholine (PPC) as an adjunctive treatment to standard care, were enrolled during 2015–2016.
Results
Overall, 2263 patients (79.6%) had at least two metabolic comorbidities associated with NAFLD; overweight/obesity was the most common comorbidity reported in 2298 patients (80.8%). Simple steatosis was the most frequently identified clinical form of NAFLD, diagnosed in 2128 patients (74.9%). Among hypertensive patients, ACE inhibitors, statins, and sartans were most commonly prescribed. Biguanides were administered in more than half of diabetic patients. In patients with overweight/obesity and hypercholesterolaemia, statins were the most frequently prescribed medications. Almost all patients (2837/2843; 99.8%) were treated with 1.8 g of PPC three times per day. PPC therapy was associated with a 90.5% 6-month compliance rate, high treatment satisfaction, and a favourable safety profile. However, almost 15% of diabetic patients and 40% of overweight/obese patients received no further treatment.
Conclusions
In Russia, patients with newly diagnosed NAFLD represent a population heavily burdened by comorbidities, mainly overweight/obesity and hypercholesterolaemia. A significant part of these patients did not receive a comprehensive pharmacotherapy, highlighting the existing unmet need in the current management of NAFLD patients with metabolic syndrome in Russia.
Keywords: non-alcoholic fatty liver disease, comorbidities, overweight, obesity, Russia, phosphatidylcholine, essential phospholipids
Summary box.
What is already known about this subject?
The prevalence of non-alcoholic fatty liver disease (NAFLD) being usually considered as a component of metabolic syndrome is alarmingly growing worldwide with 37% prevalence in Russianadult population. Since no medicine can be considered today as the standard of care for the treatment of NAFLD, pharmacotherapy that targets fat accumulation in the liver and ameliorates hepatic histology would be beneficial for the management of NAFLD.
Due to their membranous, antioxidative, and antifibrotic effects, administration of essential phospholipids (EPLs), which are hepatoprotective natural products, has been pathogenically justified in hepatic steatosis.
What are the new findings?
In Russia, patients with newly diagnosed NAFLD represent a population heavily burdened by comorbidities, mainly overweight/obesity and hypercholesterolaemia and more than one-third of them experienced a lack of appropriate treatment related to their comorbidities
EPL administration was associated with a favourable safety profile, high treatment adherence, and patient satisfaction.
How might it impact on clinical practice in the foreseeable future?
Liver protection with EPLs might have a place in the management plan of NAFLD complementing lifestyle modification and other medications that the patient may be taking.
Screening strategies and disease management practices in NAFLD patients with associated metabolic comorbidities in Russia should be improved.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disorder worldwide.1 It is an acquired metabolic stress-induced liver disease, caused by multifactorial pathogenic mechanisms.2 NAFLD encompasses a broad spectrum of liver clinical pathologies, including simple steatosis (fatty infiltration in more than 5% of hepatocytes) and non-alcoholic steatohepatitis (NASH), which could advance to fibrosis and cirrhosis, and may lead to liver failure, or even hepatocellular carcinoma.1 3
The prevalence of NAFLD is alarmingly growing worldwide, reaching 16.5% in lean individuals and up to 75% in obese individuals in both the adult and paediatric populations.4–6 The rising prevalence is mainly attributed to changes in dietary habits and sedentary lifestyle.7
NAFLD is increasingly recognised as the hepatic manifestation of the metabolic syndrome that represents a cluster of metabolic abnormalities, such as hyperlipidaemia, glucose intolerance, obesity, and systemic hypertension.2 3 It has been demonstrated that the risk and severity of NAFLD increase with the number of components of metabolic syndrome present.2 8 Obesity is considered as the biggest risk factor for NAFLD, with up to 74% of the obese population having diagnosed NAFLD.9–11
Moreover, the growing evidence suggests that NAFLD may also be considered as an independent risk factor for the metabolic syndrome.11 It has been shown that NAFLD increases the risk of type 2 diabetes mellitus (T2DM) and atherogenic dyslipidaemia,12 13 exacerbates cardiovascular disorders, and increases the risk of cardiovascular mortality.13–15
Current therapy of NAFLD is mainly directed at treating the components of the metabolic syndrome and includes correction of obesity with a hypocaloric diet and physical exercise, and control of hyperglycaemia with diet, insulin, or oral hypoglycaemic agents.16 Lifestyle modifications, such as weight reduction and physical activity, may reverse steatosis, decrease liver enzyme levels, and reduce fibrosis to some extent.17–19 However, only a small proportion of patients adhere to lifestyle modifications, such as regular exercise and a long-term healthy diet.20 Thus, pharmacotherapy that targets fat accumulation in the liver and ameliorates hepatic histology could be beneficial for the management of NAFLD.21 Essential phospholipids (EPLs) are naturally occurring hepatoprotectors that represent a well-defined, highly purified extract of the semen of soybeans with the standardised content of 72%–96% of 3-sn-phosphatidylcholine, the active molecule of EPLs. EPLs have been consistently associated with clinical improvement of steatosis based on ultrasound, CT, and liver biopsy.2 22–25 Due to the membranous, antioxidative, and antifibrotic effects, administration of EPLs in hepatic steatosis and NASH has been pathogenically justified,16 and EPLs are recommended as an antioxidant therapy for NAFLD in the Russian guidelines for the diagnosis and management of NAFLD.26
In Russia, NAFLD is present in up to 37% of the adult population27 and is frequently associated with the metabolic syndrome.28 Due to its high prevalence and associated risk factors, it seemed valuable to conduct an observational study aimed at describing the current clinical practices in the management of NAFLD in high-risk patients in Russia. We defined ‘high-risk’ patients as a group diagnosed with NAFLD and one or more associated metabolic comorbidities (arterial hypertension and/or overweight/obesity and/or high cholesterol and/or T2DM). The primary study objective was to describe the management patterns of NAFLD in subgroups of patients with concomitant diseases who were already receiving EPLs as an adjunctive treatment. The secondary objectives aimed at evaluating the safety of EPLs, compliance to EPL therapy, and treatment satisfaction; the effectiveness of EPLs was also evaluated but will be reported in detail elsewhere.
Methods
Study design and setting
This was an observational, multicenter study, which enrolled patients at 174 medical sites across 6 major federal districts of the Russian Federation between September 2015 and September 2016. Patients who met the study eligibility criteria were enrolled and followed-up by general practitioners and gastroenterologists.
The study was conducted under real-life conditions of daily clinical practice and in accordance with the Declaration of Helsinki, the Good Clinical Practice guidelines, and all applicable Russian laws and regulations. All patients were informed about the nature of the study.
Patients
Male and female outpatients, aged between 18 and 60 years, with newly diagnosed NAFLD (within 30 days before study inclusion) were enrolled. In addition, eligible patients had at least one of the following concomitant diseases: high blood pressure diagnosed by a cardiologist, T2DM diagnosed by an endocrinologist, high serum cholesterol (defined as a total cholesterol level of ≥5.0 mmol/L), and/or overweight/obesity (body mass index≥27 kg/m2). Patients had also been receiving EPL therapy (Essentiale Forte N: 300 mg of EPLs) prescribed by a physician as an adjunctive treatment to standard care in routine clinical practice. Since this was an observational study, no therapeutic or diagnostic intervention was performed in the frame of the study. Major exclusion criteria were the presence of other severe acute or chronic conditions (including other liver diseases and neoplasms), and treatment with other hepatoprotectors, intravenous phosphatidylcholine (PPC), or other concomitant EPLs within 30 days before study entry.
Study endpoints
The primary objective of this study was to describe the management patterns of NAFLD in subgroups of patients with concomitant diseases (arterial hypertension and/or overweight/obesity and/or high cholesterol and/or T2DM) who were receiving PPC as an adjunctive treatment to standard care. The differentiation between ‘pure’ steatosis, steatohepatitis, and fibrosis was made based on available ultrasonographic data, liver function tests, and transient elastography/liver biopsy (for liver fibrosis staging). Furthermore, the study aimed to estimate the average daily dose, and evaluate the 6-month medication adherence and safety of PPC administered as an adjunctive treatment of NAFLD in daily clinical practice. The effectiveness of PPC adjunctive therapy in improving ultrasonographic findings and liver function tests was also evaluated. However, these outcomes are beyond the scope of this publication, and will be presented elsewhere.
Data collection
Individual patient data were collected using electronic case report forms at three time points: at baseline, 12 weeks, and 24 weeks. At baseline, information on NAFLD/liver disease symptoms, the nature and number of comorbidities associated with NAFLD, patients’ previous pharmacological treatments, and the prescribed dosage and duration of PPC therapy was collected. Moreover, at each study visit, results of the most recent laboratory tests (which include liver function tests, and blood glucose and lipid profile), ultrasonography findings (if available), and current pharmacological treatments were assessed. In addition, at 12 and 24 weeks of the study, reasons for study withdrawal, patients’ compliance to PPC therapy (which was evaluated through the use of a patient diary, or by calculating the ratio of the number of PPC capsules taken to the number of PPC capsules prescribed over the study period in case of patient diary loss), and adverse events (AEs)/serious AEs (SAEs) were evaluated. Furthermore, at 24 weeks of the study, both patients and physicians were asked to assess their satisfaction with PPC therapy using a 4-point Likert scale (where 1 is somewhat dissatisfied or low level of satisfaction, 2 somewhat satisfied or moderate satisfaction level, 3 very satisfied or high satisfaction level, and 4 extremely satisfied or very high satisfaction level).
Statistical analyses
Since this study aimed to describe the real-world treatment patterns in four subgroups of NAFLD patients with comorbidities, most findings were reported as proportions. To detect a proportion of 50.0%±3.7%, at a two-sided significance level of 5%, with 90% power, in any of the four comorbidity subgroups of patients with one, two, three, or four concomitant diseases, a sample size of 701 subjects per subgroup was calculated as appropriate. Assuming a dropout rate of less than 3%, the number of patients per subgroup was 720. Consequently, a total sample size of 2880 patients was required for this study.
The population set used for statistical analysis comprised all eligible enrolled patients who provided adequate evaluable data at each study visit. The Wilcoxon-Mann-Whitney, the Student’s t, or the analysis of variance tests were applied for continuous variables to assess the statistical significance of subgroup differences. The association between categorical variables was examined by Pearson’s χ2 or Fisher’s exact two-tailed test, when appropriate. In general, findings were reported for categorical variables using percentage distributions, while mean, SD, median, and IQR were used to describe continuous variables. All statistical tests were two-sided and were performed at a 0.05 significance level. Statistical analyses were performed using SAS V.9.3.
Results
Patient characteristics
A total of 2843 patients with newly diagnosed NAFLD were recruited by 174 qualified general practitioners and gastroenterologists, from 18 cities located in 6 different regions of Russia (Northwestern Federal District, Volga Federal District, Southern Federal District, Ural Federal District, Siberian Federal District, and Central Federal District). A total of 16 subjects (0.56%) dropped out of the study due to logistic issues.
The majority of the study population were female (62.2%) who had a significantly higher mean±SD age than their male counterparts (49.7±8.2 vs 47.2±9.0 years; p<0.001). The patients’ demographic and baseline characteristics are presented in table 1. The vast majority (2434/2843; 85.6%) of the study participants were non-smokers, and only 201 patients (7.1%) consumed alcohol at least once a week.
Table 1.
Demographic and baseline characteristics of the study population (N=2843)
| Baseline characteristic | Study population (N=2843) |
| Mean±SD age (median; IQR), years |
48.7±8.6 (50.7; 43.6–55.6) |
| Male/female, n (%) | 1076 (37.8)/1767 (62.2) |
| Mean±SD weight (median; IQR), kg |
91.0±14.1 (90.0; 82.0–99.5) |
| Mean±SD BMI (median; IQR), kg/m2 |
32.0±4.6 (31.8; 29.2–34.6) |
| Mean±SD waist circumference (median; IQR), cm |
98.4±12.4 (98.0; 90.0–105.0) |
| Comorbid condition | |
| According to the nature of the comorbidity, n (%)* | |
| Hypertension | 1642 (57.8) |
| Overweight/obesity | 2298 (80.8) |
| Elevated cholesterol | 2122 (74.6) |
| T2DM† | 477 (16.8) |
| According to the number of comorbidities, n (%) | |
| 1 | 580 (20.4) |
| 2 | 1112 (39.1) |
| 3 | 869 (30.6) |
| 4 | 282 (9.9) |
Percentages are calculated as n/N.
*Patients may have more than one comorbid condition.
†In this study, mean±SD haemoglobin A1c level at baseline was 6.1%±1.4% (this parameter was available in 843 subjects).
BMI, body mass index;IQR, interquartile range;; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Diagnosis and assessment of NAFLD
Based on physical examination at baseline, several signs of chronic liver disease were detected in half of the study population (1429/2843; 50.3%) by simple observation of the patients.
In total, 1813 out of 2843 patients (63.8%) had an enlarged liver based on clinical examination (percussion and palpation of the liver), and further confirmed by ultrasound examination performed for all study participants, transient elastography performed in 125 patients (4.4%), and liver biopsy performed in 2 patients (0.07%). Based on the combined findings of these three diagnostic methods, hepatomegaly was detected in 1774 out of 2843 patients (62.4%). In addition, hepatic steatosis was observed in almost all study participants (2747/2843; 96.6%).
Staging of NAFLD showed that simple steatosis was the most frequently seen clinical form of NAFLD, diagnosed in 2128 patients (74.9%). NASH was detected in 712 patients (25.0%), and only 3 patients (0.1%) suffered from fibrosis. Cirrhosis was not detected in any of the patients.
Prevalence and treatment patterns of comorbid conditions
Overweight/obesity was the most common comorbid condition in the overall study population, with a prevalence rate of more than 80%. High serum cholesterol was also a major comorbid condition, reported in nearly 75% of the study participants. High blood pressure was diagnosed in 57.8% of patients, and T2DM in 16.8%. Overall, 2263 patients (79.6%) had at least two metabolic comorbidities present (table 1).
Table 2 presents the prescription patterns of comorbidity-related medications at baseline and after 24 weeks in the overall study population. At baseline, the proportion of patients who did not receive any comorbidity-related medication was the lowest in patients with T2DM (14.9%), and the highest in patients with overweight/obesity (39.1%). After 24 weeks, we observed a decrease in the use of concomitant medication in patients with NAFLD receiving EPLs as an adjunctive treatment for all four comorbidities (table 2).
Table 2.
Prescription patterns of comorbidity-related medication at baseline and after 24 weeks in the study population
| Pharmacological class, n (%) | HT (N=1642) | HT (N=1635) | Obesity* (N=2298) | Obesity* (N=2285) |
T2DM (N=477) | T2DM (N=475) | High cholesterol (N=2122) | High cholesterol (N=2119) |
| Baseline | At 24 weeks | Baseline | At 24 weeks | Baseline | At 24 weeks | Baseline | At 24 weeks | |
| Beta-blockers | 234 (14.3) | 228 (13.9) | 186 (8.1) | 179 (7.8) | 51 (10.7) | 50 (10.5) | 195 (9.2) | 192 (9.1) |
| Diuretics | 181 (11.0) | 185 (11.3) | 152 (6.6) | 160 (7.0) | 26 (5.5) | 31 (6.5) | 141 (6.6) | 146 (6.9) |
| ACE inhibitors | 588 (35.8) | 585 (35.8) | 467 (20.3) | 465 (20.4) | 105 (22.0) | 105 (22.1) | 445 (21.0) | 452 (21.3) |
| Sartans | 368 (22.4) | 364 (22.3) | 307 (13.4) | 304 (13.3) | 81 (17.0) | 77 (16.2) | 291 (13.7) | 294 (13.9) |
| Other hypotensive medications | 221 (13.5) | 217 (13.3) | 188 (8.2) | 183 (8.0) | 56 (11.7) | 56 (11.8) | 182 (8.6) | 178 (8.4) |
| Statins | 466 (28.4) | 468 (28.6) | 601 (26.2) | 583 (25.5) | 111 (23.3) | 115 (24.2) | 698 (32.9) | 677 (32.0) |
| Biguanides | 162 (9.9) | 173 (10.6) | 239 (10.4) | 246 (10.8) | 241 (50.5) | 247 (52.0) | 208 (9.8) | 218 (10.3) |
| Other hypoglycaemic medications† | 72 (4.4) | 68 (4.2) | 93 (4.0) | 85 (3.7) | 107 (22.4) | 104 (21.9) | 83 (3.9) | 77 (3.6) |
| Others | 268 (16.3) | 172 (10.5) | 332 (14.5) | 208 (9.1) | 73 (15.3) | 44 (9.3) | 334 (15.7) | 211 (10.0) |
| No treatment | 343 (20.9) | 371 (22.7) | 898 (39.1) | 954 (41.8) | 71 (14.9) | 77 (16.2) | 756 (35.6) | 810 (38.2) |
Percentages are calculated as n/N. Some patients (%) with multiple comorbidities were included in several columns.
*Or overweight.
†Including sulfonylureas (ie, gliclazide, gliquidone, glimepiride, and glibenclamide), insulin, inhibitors of dipeptidyl peptidase-4 (ie, vildagliptin, sitagliptin, saxagliptin, and linagliptin), repaglinide, and acarbose.
HT, hypertension; T2DM, type 2 diabetes mellitus.
In patients with all four comorbidities, biguanides were the most frequently prescribed concomitant medications (in 126/282 patients (44.7%) at baseline vs 137/281 patients (48.8%) at 24 weeks), followed by ACE inhibitors (in 95/282 (33.7%) at baseline vs 96/281 (34.2%) at 24 weeks), and statins (85/282 (30.1%) at baseline vs 88/281 (31.3%) at 24 weeks). Statins were practically the only class of prescribed lipid-lowering agents; besides statins, only two patients with hypercholesterolaemia were treated with fenofibrate.
On average, 62% of patients with one comorbidity did not receive any concomitant medication (357/580 (61.6%) patients at baseline vs 363/570 (63.7%) patients at 24 weeks). The number of patients with all four comorbidities who were not treated with any concomitant medication was 37/282 (13.1%) and 33/281 (11.7%) at baseline and 24 weeks of the study, respectively.
Overall, 86.2% of all patients were not compliant with strict diets or starvation recommended by their physicians, even for a few times a month.
Dosing regimens, safety, and adherence to PPC therapy
Almost all study participants (2837/2843; 99.8%) were prescribed 1.8 g of PPC administered three times per day. The 6-month compliance rate to PPC therapy was estimated at 90.5%. The majority (81.7%) of attending physicians were either extremely satisfied (616/2827; 21.8%) or very satisfied (1693/2827; 59.9%) with the patients’ PPC therapy. Similarly, patient satisfaction with PPC therapy was very high (82%) (figure 1). PPC showed a good safety profile, and no AEs/SAEs were reported during the study period.
Figure 1.
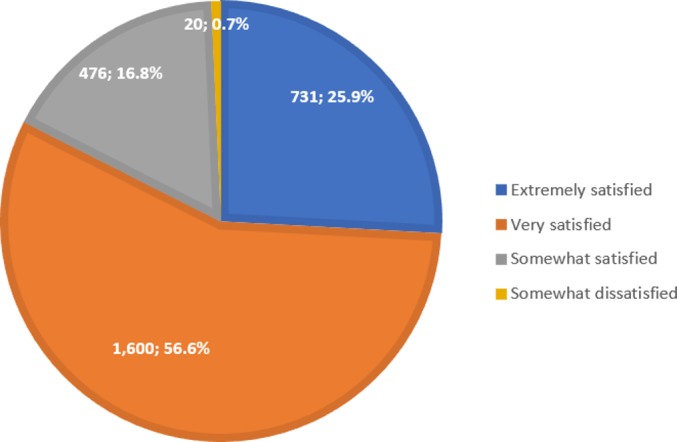
Patient satisfaction with phosphatidylcholine therapy (N=2827). Percentages are calculated as n/N.
Discussion
Our study expands existing knowledge on the prevalence of comorbid conditions in patients with newly diagnosed NAFLD and on the management of these conditions in a real-life setting in Russia. The nationwide screening study, DIREG 1 (Non-alcoholic Fatty Liver Disease morbidity rate and its correlations with risk factors assessment among the GP-s patients flow, DIsease REGistry, DIREG_L_01903), on the prevalence of metabolic comorbidities in 8315 NAFLD patients in Russia, found that hypercholesterolaemia, arterial hypertension, and obesity were all frequently observed and prevalent in more than 50% of the NAFLD patients; while T2DM was detected in 23.1% of patients.28 Our study confirms that overweight/obesity, hypertension, and hypercholesterolaemia are the most prevalent NAFLD comorbidities with a prevalence rate of more than 57%. Overweight/obesity was the most frequent metabolic comorbidity of NAFLD reported in 80.8% of patients. This is in line with the recently published data from the American Association for the Study of Liver Diseases which found that obesity (from overweight to obese and severely obese states) is the most common and well-documented risk factor of NAFLD.29 Our study showed that almost 80% of high-risk patients with recently diagnosed NAFLD had at least two metabolic comorbidities. This is consistent with the previously published findings where approximately 90% of NAFLD patients had more than one component of the metabolic syndrome.10 29 30 A complex relationship between NAFLD and the metabolic syndrome is frequently discussed in the literature.29 It has been established that accumulation of hepatic fat, which is closely linked to insulin resistance, increases lipolysis of peripheral adipose tissue and increases fat influx into the liver in the form of free fatty acids. Insulin resistance was shown to promote de novo triglyceride synthesis within the liver and to inhibit fatty acid oxidation, thereby promoting triglyceride accumulation.21 31 Therefore, improving insulin sensitivity could be a key strategy in the treatment of NAFLD.21
The majority of patients diagnosed with NAFLD are asymptomatic.32 Clinical symptoms and physical findings, when present, are usually non-specific and unreliable for diagnosing and assessing NAFLD severity.33 34 In our study, simple steatosis was the most frequently seen clinical form of NAFLD, diagnosed in approximately 75% of patients. Frequently, hepatomegaly is the only physical finding in NAFLD patients.32 In our study, hepatomegaly was observed in 62.4% of patients, based on the combined findings of liver ultrasonography, transient elastography, and liver biopsy. This was additionally supported by percussion and palpation of the liver, which detected hepatomegaly in 63.8% of patients, stressing the diagnostic value of physical examination and non-invasive tests, such as palpation and percussion.
More than 25% of NAFLD patients with overweight/obesity, high serum cholesterol, and hypertension were treated with statins. Statins are usually the first-line treatment for NAFLD patients with dyslipidaemia and elevated serum cholesterol. Moreover, they are the most commonly prescribed lipid-lowering agents in Russia, which was highlighted in our study. Yet, there has long been a reluctance to treat patients with liver disease with lipid-lowering therapy, and specifically with statins, because of hepatotoxicity concerns.35 However, the incidence of serious hepatotoxicity with statins in patients with NAFLD is exceedingly low, and statins are safely used in patients with liver disease.36 Moreover, statins have a well-established role in the primary and secondary prevention of cardiovascular diseases.35
Our results also show that hypertension was most commonly treated with ACE inhibitors and sartans, which is in accordance with the current treatment recommendations for hypertension management. Both sartans and ACE inhibitors have been shown to reduce fibrosis progression in chronic liver disease.35
Biguanides (specifically metformin) were prescribed to 50.5% of NAFLD patients with T2DM in this study. All currently approved diabetes medications are deemed safe in patients with compensated liver disease, and NAFLD should not limit the patient’s therapeutic options.35 Although metformin has been shown to be beneficial in improving peripheral tissue insulin sensitivity, it should be noted that two published meta-analyses of randomised controlled trials have reported that metformin therapy does not improve liver histology in patients with NAFLD and NASH.37 38 Thus, metformin is not recommended for treating NASH in adult patients.29
Alarmingly, almost 15% of diabetic patients did not receive any further treatment except for the prescribed 1.8 g of PPC three times per day. This could be related to inadequate evaluation of the disease and its comorbidities; low adherence to anti-diabetic medications which might be due, among other reasons, to patient out-of-pocket costs.39 40 In addition, according to the latest Russian guidelines which were designed to standardise and facilitate T2DM care in all regions of the Russian Federation, drug treatment for T2DM is recommended in patients with haemoglobin A1c above 6.5%,41 while in our study, mean±SD haemoglobin A1c level at baseline was 6.1%±1.4%.
Moreover, we found that approximately 62% of NAFLD patients with one metabolic component did not receive any concomitant medication. This is a particularly high figure which highlights the need to improve knowledge among physicians on metabolic comorbidity treatment in NAFLD patients.
Almost 40% of overweight/obese patients did not receive any further treatment for their obesity. This was expected, given that the adoption of healthy lifestyle habits is the foundation of managing obesity. Yet, 86.2% of all patients were not compliant with the recommended strict diets. Although the use of pharmacotherapy in the management of obesity remains controversial, pharmacological agents may be beneficial in the treatment of obese patients with NAFLD, since visceral fat is strongly associated with hepatic steatosis.42 43 Thus, improving hepatic steatosis in obese patients should be the focus for future intervention trials.
At 24 weeks of the study, we observed a decrease in the use of concomitant medications in all four comorbidity subgroups (hypertension, overweight/obesity, T2DM, and high cholesterol). This finding is rather worrying since the current treatment of chronic metabolic disorders is a life-long commitment requiring administration of daily doses of medication. Thus, it is important to increase patient adherence to life-long medication by improving patient–physician communication and simplification of drug regimens.44 45
Management of NAFLD should aim at preventing progressive liver injury in high-risk patients. EPLs belong to a class of hepatoprotectors with diverse pharmacological properties and are recommended in the Russian guidelines for the management of NAFLD.26 In in-vitro and animal investigations, EPLs showed anti-inflammatory, antioxidant, antifibrogenic, antiapoptotic, membrane-protective, and lipid-regulating effects.46 The review of 25 clinical studies evaluating EPL effectiveness in fatty liver disease showed that EPLs accelerated the improvement or normalisation of subjective symptoms and pathological findings, such as pain in the right hypochondrium, dyspeptic symptoms, and hepatomegaly.16 In our study, EPL administration (99.8% of patients received 1.8 g of PPC three times per day) was associated with high levels of treatment adherence and satisfaction, and a very good safety profile (no reported AEs/SAEs during the study period).
This study had some limitations inherent to its observational nature, mainly patient selection bias associated with the enrolment of patients with specific comorbidities. Therefore, the prevalence and treatment patterns of the comorbidities might not accurately reflect the actual situation in Russia. However, in order to reduce patient selection bias, each physician was requested to enrol a consistent set of patients (16 consecutive patients per physician) that met the study eligibility criteria. Additionally, the possible influence of confounding factors on the outcomes of this study has been accounted for in the statistical analyses by use of multivariate analyses.
Among other limitations, it can be mentioned that liver biopsy, which is still considered the gold standard for the diagnosis of NAFLD, and elastography were rarely performed, and differentiation between NAFLD stages was achieved using available data (mainly ultrasonography and liver enzyme activity). It is impractical to perform liver biopsy since it has many drawbacks, such as sampling error, cost, and risk of complications.2 Furthermore, because of the complexity of the analyses, we were unable to assess patient adherence to PPC therapy and to comorbidity-related medications, according to the number and nature of associated metabolic comorbidities. In addition, histological evaluation of liver tissue was not performed.
There are also several key strengths to this study. The large sample size and the large number of study centres (n=174) located in 18 different cities throughout the country make it possible to extrapolate the results to the general population of high-risk patients with newly diagnosed NAFLD in Russia.
In conclusion, the present study indicates that, in Russia, patients with newly diagnosed NAFLD represent a population heavily burdened by comorbidities, mainly obesity and hypercholesterolaemia. Importantly, more than 40% of patients included in the study may experience a lack of appropriate treatment related to their conditions. EPL administration was associated with high levels of treatment compliance and satisfaction, and a very good safety profile among NAFLD patients with comorbidities. However, there remains an urgent need to improve the screening strategies and disease management practices in the high-risk population.
Acknowledgments
The authors would like to thank Thomas Rohban, MD (Partner 4 Health, France) for providing medical writing support (sponsored by Sanofi) in accordance with the Good Publication Practice (GPP3) guidelines.
Footnotes
Contributors: IVM was the study’s national coordinator, who contributed to the design and supervision of the study. All the authors participated in the acquisition, analysis, and interpretation of the data. CSP, ES, LKP, AAS, and KMS contributed to the drafting of the initial manuscript and its finalisation. All the authors have read and approved the final version of the manuscript.
Funding: This study was funded by Sanofi-Aventis, France.
Competing interests: KMS is a Sanofi employee.
Patient consent for publication: Obtained.
Ethics approval: The study was approved by the Independent Interdisciplinary Ethics Committee on Ethical Review for Clinical Studies, which is the operating ethics committee in the Russian Federation (Protocol #13 dated 28 August 2015).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request.
References
- 1.Mavrogiannaki AN, Migdalis IN. Nonalcoholic fatty liver disease, diabetes mellitus and cardiovascular disease: newer data. Int J Endocrinol 2013;2013:450639 10.1155/2013/450639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dajani AIM, Abu Hammour AM, Zakaria MA, et al. Essential phospholipids as a supportive adjunct in the management of patients with NAFLD. Arab J Gastroenterol 2015;16:99–104. 10.1016/j.ajg.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 3.Munteanu MA, Nagy GA, Mircea PA. Current management of NAFLD. Med Pharm Rep 2016;89:19–23. 10.15386/cjmed-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldridge AD, Perez-Atayde AR, Graeme-Cook F, et al. Idiopathic steatohepatitis in childhood: a multicenter retrospective study. J Pediatr 1995;127:700–4. 10.1016/S0022-3476(95)70156-7 [DOI] [PubMed] [Google Scholar]
- 5.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388–93. 10.1542/peds.2006-1212 [DOI] [PubMed] [Google Scholar]
- 6.Leoni S, Tovoli F, Napoli L, et al. Current guidelines for the management of non-alcoholic fatty liver disease: a systematic review with comparative analysis. WJG 2018;24:3361–73. 10.3748/wjg.v24.i30.3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dajani A, AbuHammour A. Treatment of nonalcoholic fatty liver disease: where do we stand? an overview. Saudi J Gastroenterol 2016;22:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill HK, Wu GY. Non-alcoholic fatty liver disease and the metabolic syndrome: effects of weight loss and a review of popular diets. are low carbohydrate diets the answer? World J Gastroenterol 2006;12:345–53. 10.3748/wjg.v12.i3.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2002;17:S186–90. 10.1046/j.1440-1746.17.s1.10.x [DOI] [PubMed] [Google Scholar]
- 10.El-Kader SMA, El-Den Ashmawy EM. Non-alcoholic fatty liver disease: the diagnosis and management. World J Hepatol 2015;7:846–58. 10.4254/wjh.v7.i6.846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang KC, Hung H-F, Lu C-W, et al. Association of non-alcoholic fatty liver disease with metabolic syndrome independently of central obesity and insulin resistance. Sci Rep 2016;6:27034 10.1038/srep27034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibata M, Kihara Y, Taguchi M, et al. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care 2007;30:2940–4. 10.2337/dc07-0792 [DOI] [PubMed] [Google Scholar]
- 13.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015;62:S47–64. 10.1016/j.jhep.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 14.Zhou YJ, LI YY, Nie YQ, et al. Natural course of nonalcoholic fatty liver disease in southern China: a prospective cohort study. J Dig Dis 2012;13:153–60. 10.1111/j.1751-2980.2011.00571.x [DOI] [PubMed] [Google Scholar]
- 15.Pisto P, Santaniemi M, Bloigu R, et al. Fatty liver predicts the risk for cardiovascular events in middle-aged population: a population-based cohort study. BMJ Open 2014;4:e004973 10.1136/bmjopen-2014-004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gundermann K-J, Gundermann S, Drozdzik M, et al. Essential phospholipids in fatty liver: a scientific update. Clin Exp Gastroenterol 2016;9:105–17. 10.2147/CEG.S96362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho J-Y, Chung T-H, Lim K-M, et al. The impact of weight changes on nonalcoholic fatty liver disease in adult men with normal weight. Korean J Fam Med 2014;35:243–50. 10.4082/kjfm.2014.35.5.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H-J, He J, Pan L-L, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: a randomized clinical trial. JAMA Intern Med 2016;176:1074–82. 10.1001/jamainternmed.2016.3202 [DOI] [PubMed] [Google Scholar]
- 19.Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 2017;67:829–46. 10.1016/j.jhep.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 20.Baratta F, Pastori D, Polimeni L, et al. Adherence to Mediterranean diet and non-alcoholic fatty liver disease: effect on insulin resistance. Am J Gastroenterol 2017;112:1832–9. 10.1038/ajg.2017.371 [DOI] [PubMed] [Google Scholar]
- 21.Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J 2006;82:315–22. 10.1136/pgmj.2005.042200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonciarz Z, Besser P, Lelek E, et al. Randomised placebo-controlled double blind trial on “essential” phospholipids in the treatment of fatty liver associated with diabetes. Méd Chir Dig 1988;17:61–5. [Google Scholar]
- 23.JH L, Chen XY, Zhong CF, et al. [A randomized controlled study of essential phospholipids (Essentiale capsules) in the treatment of fatty liver]. Infect Dis Info 2000;13:180–1. [Google Scholar]
- 24.Arvind N, Savaikar P, Rajkumar JS. Therapy for NAFLD – a comparative study of essential phospholipids vs. ursodeoxycholic acid. Ind J Clin Pract 2006;16:21–4. [Google Scholar]
- 25.Sas E, Grinevich V, Efimov O, et al. 1366 beneficial influence of polyunsaturated phosphatidylcholine enhances functional liver condition and liver structure in patients with nonalcoholic steatohepatitis. Results of prolonged randomized blinded prospective clinical study. J Hepatol 2013;58:S549 10.1016/S0168-8278(13)61365-3 [DOI] [Google Scholar]
- 26.Lazebnik LB, Radchenko VG, Golovanova EV, et al. [Nonalcoholic fatty liver disease: diagnostic, symptoms, treatment. Guidelines were approved by the XV gastroenterological scientific society of Russia in 2015]. Eksp Klin Gastroenterol 2015;(7):85–96. [PubMed] [Google Scholar]
- 27.Ivashkin VT, Drapkina OM, Mayev IV, et al. [The prevalence of non-alcoholic fatty liver disease in patients of outpatient practice in the Russian Federation: the results of the study DIREG 2]. Russian Journal of Gastroenterology, Hepatology, Coloproctology 2015;25:31–8. [Google Scholar]
- 28.Drapkina O, Evsyutina Y, Ivashkin V. Prevalence of non-alcoholic fatty liver disease in the Russian Federation: the open, multicenter, prospective study, DIREG 1. AJCMR 2015;3:31–6. 10.12691/ajcmr-3-2-3 [DOI] [Google Scholar]
- 29.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology 2018;67:328–57. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 30.Bang KB, Cho YK. Comorbidities and metabolic derangement of NAFLD. J Lifestyle Med 2015;5:7–13. 10.15280/jlm.2015.5.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 2004;114:147–52. 10.1172/JCI200422422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221–31. 10.1056/NEJMra011775 [DOI] [PubMed] [Google Scholar]
- 33.Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp Diabetes Res 2012;2012:145754 10.1155/2012/145754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoonsari M, Mohammad Hosseini Azar M, Ghavam R, et al. Clinical manifestations and diagnosis of nonalcoholic fatty liver disease. Iran J Pathol 2017;12:99–105. [PMC free article] [PubMed] [Google Scholar]
- 35.Corey KE, Vuppalanchi R. Assessment and management of comorbidities (including cardiovascular disease) in patients with nonalcoholic fatty liver disease. Clin Liver Dis 2012;1:114–6. 10.1002/cld.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chalasani N, Aljadhey H, Kesterson J, et al. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology 2004;126:1287–92. 10.1053/j.gastro.2004.02.015 [DOI] [PubMed] [Google Scholar]
- 37.Musso G, Gambino R, Cassader M, et al. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology 2010;52:79–104. 10.1002/hep.23623 [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Liu L, Wang B, et al. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep 2013;1:57–64. 10.3892/br.2012.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirkman MS, Rowan-Martin MT, Levin R, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care 2015;38:dc142098 10.2337/dc14-2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Machado-Alba JE, Torres-Rodríguez S, Farm Q, et al. Effectiveness the pharmaceutical care in diabetic patients. Colomb Med 2011;42:72–80. [Google Scholar]
- 41.Dedov II, Shestakova MV, Mayorov AY, et al. Standards of specialized diabetes care. Edited by Dedov II, Shestakova mV, Mayorov AY. 8th edition. Diabetes Mellitus 2017;20:1–112. 10.14341/DM20171S8 [DOI] [Google Scholar]
- 42.Yousef MH, Juboori AA, Albarrak AA, et al. Fatty liver without a large “belly”: Magnified review of non-alcoholic fatty liver disease in non-obese patients. World J Gastrointest Pathophysiol 2017;8:100–7. 10.4291/wjgp.v8.i3.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol 2018;6:237–48. 10.1016/S2213-8587(17)30236-X [DOI] [PubMed] [Google Scholar]
- 44.Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long-term statin therapy? Curr Atheroscler Rep 2013;15:291 10.1007/s11883-012-0291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Usherwood T. Encouraging adherence to long-term medication. Aust Prescr 2017;40:147–50. 10.18773/austprescr.2017.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gundermann K-J, Kuenker A, Kuntz E, et al. Activity of essential phospholipids (EPL) from soybean in liver diseases. Pharmacol Rep 2011;63:643–59. 10.1016/S1734-1140(11)70576-X [DOI] [PubMed] [Google Scholar]


