Abstract
Background
Treatment‐related toxicities and decreased levels of patient performance during cancer therapy might contribute to body composition changes and thereby impact outcomes. However, the effect of longitudinal body composition changes on outcomes in patients with advanced endometrial cancer is unknown. This study investigated the association between body composition changes during staging surgery and adjuvant chemoradiotherapy and outcomes in patients with stage III endometrial cancer.
Methods
Pretreatment and post‐treatment computed tomography (CT) images of 131 patients with stage III endometrial cancer who were treated between 2008 and 2016 were analysed. All CT images were contrast enhanced and acquired according to the standardized protocol. The skeletal muscle index (SMI), skeletal muscle radiodensity (SMD), and total adipose tissue index were measured from two sets of CT images obtained at the level of the third lumbar vertebra. The skeletal muscle gauge was calculated by multiplying SMI by SMD (SMI × SMD). Predictors of overall survival and progression‐free survival were identified using Cox regression models.
Results
The median follow‐up was 50.6 (range 12.1–117.0) months. Overall, body mass index (BMI) changes during treatment were 0.4% per 210 days (95% confidence interval: −0.6 to 1.4; P = 0.41), and patients experienced an average SMD loss of 2.1% per 210 days (95% confidence interval: −4.0 to −0.2; P = 0.03). Weight loss and SMD loss ≥5% were observed in 23 (17.6%) and 54 (41.2%) patients, respectively. The changes in SMD did not correlate with those in BMI (Spearman's ρ for SMD, −0.13; P = 0.13). SMD change (per 1 Hounsfield unit/210 days decrease) was independently associated with poorer overall survival (hazard ratio: 1.32, 95% confidence interval: 1.14–1.52; P < 0.001) and progression‐free survival (hazard ratio: 1.28, 95% confidence interval: 1.12–1.43; P < 0.001). Our results did not show association between survival and pretreatment myosteatosis and sarcopenia or changes in SMI and total adipose tissue index during treatment. The pretreatment skeletal muscle gauge was associated with treatment modifications such as delays, dose reductions, and discontinuation of chemotherapy.
Conclusions
Skeletal muscle radiodensity decreased significantly during treatment and was independently associated with poorer survival in patients with stage III endometrial cancer who underwent staging surgery and adjuvant chemoradiotherapy. SMD loss was occult and occurred independently of BMI change.
Keywords: Endometrial cancer, Sarcopenia, Myosteatosis, Chemoradiotherapy
Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy, and its incidence is on the rise, with an estimated 63 230 new cases and 11 350 deaths occurring in 2018. Per current estimates, approximately 20% of women with EC will be diagnosed with locally advanced disease.1
Endometrial cancer is staged surgically, as recommended by the International Federation of Gynecology and Obstetrics (FIGO), since 1988.2 Patients with stage III EC are at increased risk of distant metastases and cancer‐related death3; however, the optimal adjuvant treatment is yet to be determined for these patients.4 In a randomized trial comparing adjuvant chemotherapy with radiotherapy, the radiotherapy was shown to achieve better pelvic control, while chemotherapy was shown to be more effective in controlling distant metastases. Neither approach significantly affected survival.5 Combining radiotherapy and chemotherapy (CRT) might maximize treatment efficiency and be the preferred treatment for stage III EC.6, 7, 8, 9, 10, 11, 12, 13 Recently, PORTEC‐3 trial showed that CRT improved failure‐free survival over radiotherapy alone for stage III EC, although the improvement in overall survival (OS) was marginal.14 The GOG 258 trial revealed that CRT reduced locoregional recurrence compared with chemotherapy alone.15 However, both PORTEC‐3 and GOG 258 trials reported that CRT significantly increased the incidences of toxicities and decreased the patients' functioning levels during treatment.16, 17
Changes in body composition during cancer therapy have been associated with treatment‐related toxicity, physical inactivity, malnutrition, cancer invasiveness, and cancer therapy, which in turn could influence patient outcomes.18, 19, 20, 21, 22, 23, 24, 25, 26 Therefore, the prognostic value of body composition measurements in stage III EC needs to be evaluated. It has been shown that the cross‐sectional areas of skeletal muscle and adipose tissue on a single computed tomography (CT) slice at the level of the third lumbar vertebra are strongly correlated with the total body skeletal muscle and adipose tissues.27, 28, 29 CT images could provide objective quantitative [skeletal muscle index (SMI)] and qualitative [skeletal muscle radiodensity (SMD)] measures of skeletal muscle. Skeletal muscle with low SMI is indicative of skeletal muscle mass depletion, also known as sarcopenia.21 SMD is a radiological characteristic, and skeletal muscle with low SMD is suggestive of fatty infiltration of the skeletal muscle (myosteatosis) leading to poor ‘quality’ skeletal muscle.30 SMI and SMD are defined independent of the other, and both are demonstrated prognostic indicators for cancer outcomes; integrating the SMI and SMD into a novel measure of skeletal muscle gauge (SMG) could lead to a more unified reporting for body composition and disease outcomes.31 Recently, SMG was reported as a better predictor of chemotherapy toxicities than either SMI or SMD.32, 33, 34 Adipose tissue content, including subcutaneous and visceral adipose tissues, has also been associated with cancer outcomes and can be assessed on CT imaging.35, 36, 37
Sarcopenia and myosteatosis at the time of cancer diagnosis were previously shown to be associated with the outcome in EC.38, 39 A longitudinal study of changes in body composition during cancer therapy may provide a more comprehensive understanding of the influences of body composition on cancer outcomes40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51; however, the changes in body composition during cancer therapy and its impact on outcomes in stage III EC patients were unclear. Moreover, current doses of chemotherapy are calculated based on body surface area (BSA), which takes only height and weight into account and not other potentially important body composition parameters.32, 33 It has been shown that pharmacokinetics and chemotherapy toxicities are more related to lean body mass.52, 53, 54 Chemotherapy toxicities have been reported as common causes for treatment modifications in CRT, including delays, dose reductions, or discontinuation of chemotherapy, in advanced EC.11, 12, 13, 14 However, the association between body composition and treatment modifications due to CRT toxicities was also unclear in stage III EC patients undergoing staging surgery and adjuvant CRT.
The aim of this study was to longitudinally assess the changes in body composition parameters using routine CT images acquired during staging and follow‐up of EC patients and to determine whether body composition measures were associated with (i) survival outcomes and (ii) treatment modifications in patients with stage III EC undergoing staging surgery and adjuvant CRT.
Materials and methods
Patients
This retrospective study was conducted in accordance with the Declaration of Helsinki. Our Institutional Review Board approved the study and waived the requirement for patients' informed consent owing to the retrospective and observational nature of this study. Patients at either of the two tertiary institutions with FIGO stage III EC, who had undergone surgical staging following hysterectomy, bilateral salpingo‐oophorectomy, and lymphadenectomy and had also undergone adjuvant CRT between 2008 and 2016, were included. Patients were eligible for inclusion when the following criteria were met: (i) a routine abdominal CT scan was performed before staging surgery and a second abdominal CT scan after the completion of the adjuvant CRT course, (ii) both CT scans were of sufficient quality to perform accurate measurements of tissue area, and (iii) sufficient relevant clinical data could be retrieved from the patient's medical chart. Patients who received adjuvant chemotherapy alone (n = 4) or radiotherapy alone (n = 2) were excluded. Demographic, disease, and treatment characteristics were obtained from the patients' medical records.
Treatment
The standard of care for patients with stage III EC at our institutions at the time of the study was adjuvant CRT. The timeline of diagnosis of EC, CT scans, and treatments for stage III EC is shown in Figure 1. Chemotherapy consisted of 3 cycles of intravenous paclitaxel 175 mg/m2 and carboplatin AUC5 every 3 weeks prior to radiotherapy. Intensity‐modulated radiotherapy was initiated within 4 weeks of completing the third cycle of chemotherapy. The standard radiation field was the pelvis with a prescribed dose of 45.0 to 50.4 Gy. Extended‐field radiotherapy was considered for patients with positive para‐aortic lymph nodes or at the physicians' discretion. The prescribed dose for vaginal‐cuff brachytherapy was 5 Gy at 0.5 cm below the vaginal surface for 4–6 fractions. Post‐radiation chemotherapy was initiated within 4 weeks of completing radiotherapy and included 3 identical cycles of paclitaxel and carboplatin.
Figure 1.
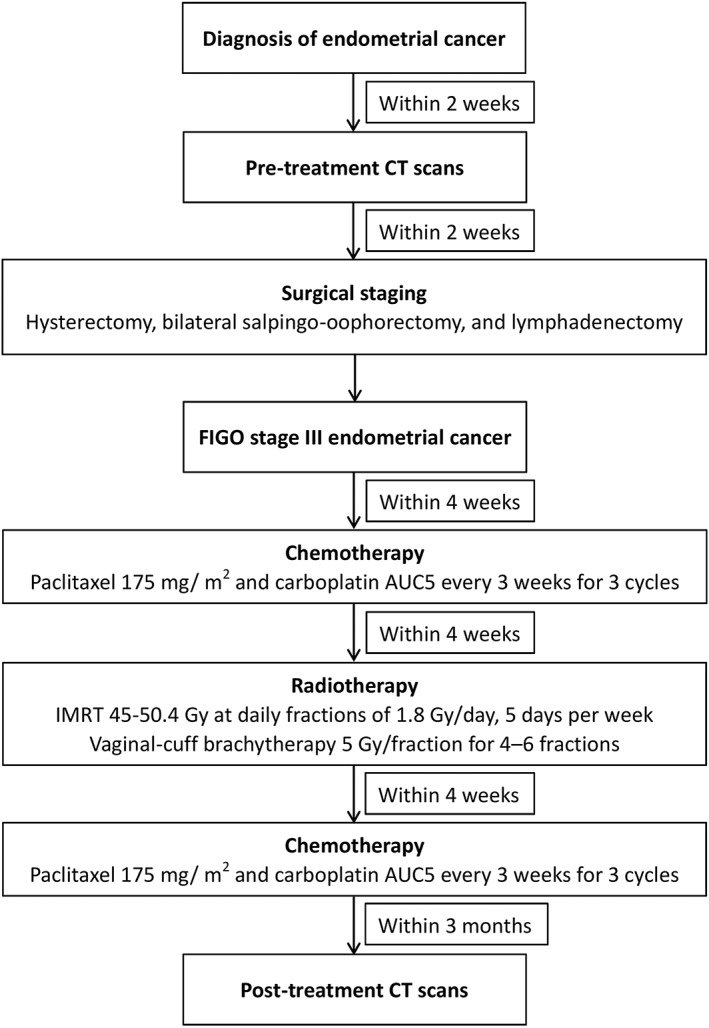
Timeline of endometrial cancer diagnosis, CT scans, and treatments for stage III endometrial cancer. CT, computed tomography; FIGO, International Federation of Gynecology and Obstetrics; IMRT, intensity‐modulated radiotherapy.
Computed tomography‐based body composition analysis
Pretreatment and post‐treatment CT images were retrieved for analysis; a routine pretreatment CT scan was performed before staging surgery, and a post‐treatment CT scan was performed within 3 months after completing the course of adjuvant CRT (Figure 1). Body weight and height were also obtained from medical records within 2 weeks of the date of the initial and follow‐up CT scans. All CT examinations were performed according to a standardized protocol. In our institutions, routine CT of the female abdomen and pelvis scans was obtained after intravenous administration of iohexol 300 (Omnipaque 300, GE Healthcare) or iopromide 300 (Ultravist 300, Bayer HealthCare) in a single uniphasic bolus dose of 80–100 mL via a power injector at 2 mL/s. The portal‐venous phase was obtained with a fixed delay of 70 s after administration of the contrast material and a pitch between 1.0 and 1.5 before the contrast medium was excreted into the bladder. CT image parameters included the following information: contrast‐enhanced, 5 mm slice thickness, 120 kVp, and approximately 290 mA. Two consecutive axial CT images extending from the third lumbar to the iliac crest were analysed using the Varian Eclipse software (Varian Medical Systems Inc., Palo Alto, CA, USA).55 The skeletal muscle area (including psoas, paraspinal, transversus abdominis, rectus abdominis, and internal and external oblique muscles) was calculated by using Hounsfield unit (HU) thresholds of −29 and +150.27, 28, 56 The mean skeletal muscle radiation attenuation of the entire cross‐sectional muscle area was reported as SMD. Subcutaneous and intermuscular adipose tissues were calculated with a radiodensity between −190 and −30 HU, whereas visceral adipose tissue was calculated with a radiodensity between −150 and −50 HU.28 Total adipose tissue (TAT) was calculated by summing the subcutaneous, intermuscular, and visceral adipose tissue. Tissue cross‐sectional areas (cm2) were calculated by summing the given tissue pixels and multiplying by the pixel surface area. Mean tissue areas for two consecutive images were calculated. One researcher, blinded to the patients' information, measured the body composition parameters. The intraobserver coefficients of variation were 0.8%, 1.0%, and 0.9% for the skeletal muscle area, SMD, and TAT area, respectively, in a sample of 50 patients randomly selected from this cohort. The cross‐sectional areas of skeletal muscle and TAT were normalized for the patients' heights to calculate the indexes (cm2/m2) for skeletal muscle (SMI) and TAT (TATI). SMG was calculated by multiplying SMI by SMD (SMI × SMD).31 The actual units for SMG are (cm2 tissue × average HU)/(m2 height); for simplicity, we present them as arbitrary units.32, 33
Given that the body composition varies greatly with the type and stage of the cancer, geographical regions, and ethnicities,57, 58 we defined our own cut‐off values for this cohort based on previous studies with similar population sizes.42, 59 Cut‐off values were set at the lowest tertile for SMI, SMD, and SMG and at the highest tertile for TATI.
Body composition change was assessed based on the differences between the pretreatment and post‐treatment CT images. Overall, a complete course of treatment in an EC patient, including staging surgery and adjuvant CRT, would take 6 months or more.11, 12, 13, 14, 15 In this study, the median duration to complete these treatments was 198 days (interquartile range: 179–209 days); the median duration between pretreatment and post‐treatment CT scans was 223 days (interquartile range: 205–236 days). To account for variations in the durations of scan intervals, changes in body composition were calculated as the change per 210 days to provide a standardized method with which to compare data between patients. The consensus definition of cachexia in patients with cancer is weight loss greater than 5% or weight loss greater than 2% in already symptomatic patients who have a body mass index (BMI) less than 20 kg/m2.60 To simulate the definition of cachexia, patients with a reduction or increase in SMI, SMD, SMG, or TATI of ≥5.0% were classified as having ‘loss’ or ‘gain’, respectively.45
Study endpoint
The primary endpoint was OS, defined as the time from the date of diagnosis to that of death from any cause, and progression‐free survival (PFS), defined as the time from the date of diagnosis to that of disease recurrence, progression, or death from any cause. The secondary endpoint was treatment modifications because of reported toxicities. Treatment modifications taken into account were delays, dose reductions, or discontinuation of chemotherapy because of toxicities during the course of CRT, whereas delays due to patient preference or vacation were not taken into consideration. Information on treatment modifications were obtained from medical records.
Statistical analysis
Continuous data are presented as the mean ± standard deviation or median and range, as applicable, while categorical data are presented as numbers and percentages. The distributions of patient and clinical characteristics were compared using the chi‐square test for categorical variables and analysis of variance for continuous variables. Tukey's method was performed when the analysis of variance revealed a difference to identify where the difference occurred. Paired t‐tests and the Wilcoxon signed‐rank test were used to assess changes in body composition. Spearman's correlation coefficient was used to assess relationships between BMI and body composition. Logistic regression analyses were used to test associations between treatment modification and body composition measurement; BSA was also assessed, because in previous studies, lean body mass was reportedly better correlated with drug clearance pharmacokinetics than BSA.52, 53, 54
Survival curves were constructed using the Kaplan–Meier method with log‐rank tests. Cox proportional hazards models were used to estimate the hazard ratios (HRs) and 95% confidence interval (CIs) of body composition and risk of outcomes. All variables with a P < 0.05 on univariable analysis or with clinical relevance were subjected to multivariable analysis. The data were analysed using IBM SPSS software (version 21.0; IBM Corp., Armonk, NY, USA). A P < 0.05 was considered statistically significant.
Results
We identified 148 patients treated for stage III EC between 2008 and 2016 (Figure 2). After exclusion of 10 patients (patients with adjuvant chemotherapy alone, adjuvant radiotherapy alone, and/or patients without sufficient clinical data), CT measurements were evaluated in 138 patients. Another seven patients were excluded either because of missing post‐treatment CT scans or because of insufficient quality of the scans. Final analysis was conducted on 131 patients with 262 CT scans. Baseline characteristics for the included patients are presented in Table 1. The median duration between diagnosis and pretreatment CT scans was 8 days (interquartile range: 4–11 days). The median duration between pretreatment CT scans and staging surgery was 7 days (interquartile range: 3–11 days).
Figure 2.
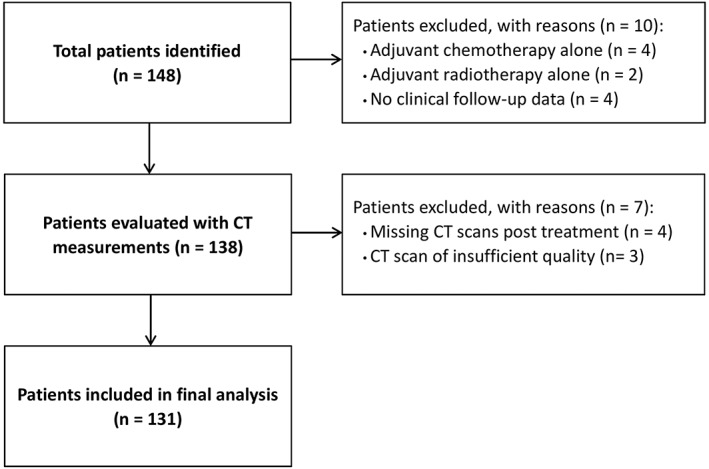
Flow chart for patient inclusion. CT, computed tomography.
Table 1.
Patient and tumor characteristics, values expressed as mean ± standard deviation, unless stated otherwise
| Characteristics | Overall (n = 131) | SMD loss (n = 54) | Stable SMD (n = 42) | SMD gain (n = 35) | P‐value |
|---|---|---|---|---|---|
| Age (years) | 54.3 ± 9.6 | 55.8 ± 9.0 | 52.3 ± 9.1 | 54.4 ± 10.9 | 0.21 |
| ECOG performance status, n (%) | 0.36 | ||||
| 0 | 124 (94.7) | 52 (96.3) | 38 (90.5) | 34 (97.1) | |
| 1 | 7 (5.3) | 2 (3.7) | 4 (9.5) | 1 (2.9) | |
| Body surface area (m2) | 1.57 ± 0.1 | 1.57 ± 0.1 | 1.57 ± 0.1 | 1.57 ± 0.1 | 0.95 |
| Pretreatment BMI (kg/m2) | 24.5 ± 3.7 | 24.6 ± 3.9 | 23.8 ± 3.2 | 25.0 ± 3.8 | 0.34 |
| BMI change (% per 210 days) | 0.4 ± 5.8 | 1.3 ± 6.7 | 0.5 ± 5.7 | −1.2 ± 4.3 | 0.17 |
| Weight change, categorical | 0.95 | ||||
| Weight gain or loss <5.0% | 108 (82.4) | 45 (83.3) | 34 (81.0) | 29 (82.9) | |
| Weight loss ≥5.0% | 23 (17.6) | 9 (16.7) | 8 (19.0) | 6 (17.1) | |
| Pretreatment SMI (cm2/m2) | 42.4 ± 6.5 | 41.6 ± 5.2 | 42.8 ± 7.1 | 42.9 ± 7.7 | 0.56 |
| Pretreatment sarcopenia,a n (%) | 44 (33.6) | 18 (33.3) | 12 (28.6) | 14 (40.0) | 0.57 |
| SMI change (% per 210 days) | −0.2 ± 7.2 | −2.3 ± 8.9 | 1.2 ± 4.2 | 1.2 ± 6.6 | <0.001 |
| SMI loss | 29 (22.1) | 21 (38.9) | 2 (4.8) | 6 (17.1) | 0.001 |
| Stable SMI | 78 (59.5) | 27 (50.0) | 32 (76.2) | 19 (54.3) | |
| SMI gain | 24 (18.3) | 6 (11.1) | 8 (19.0) | 10 (28.6) | |
| Pretreatment SMD (HU) | 38.0 ± 6.2 | 38.5 ± 5.6 | 40.4 ± 5.4 | 34.3 ± 6.5 | <0.001 |
| Pretreatment myosteatosis,a n (%) | 44 (33.6) | 16 (29.6) | 9 (21.4) | 19 (54.3) | 0.01 |
| Post‐treatment SMD (HU) | 37.1 ± 6.6 | 34.1 ± 6.0 | 40.1 ± 5.7 | 38.0 ± 6.7 | <0.001 |
| Post‐treatment myosteatosis,a n (%) | 51 (38.9) | 33 (61.1) | 8 (19.0) | 10 (28.6) | <0.001 |
| Pretreatment TATI (cm2/m2) | 120.8 ± 56.9 | 121.4 ± 55.1 | 114.0 ± 51.9 | 128.3 ± 65.6 | 0.55 |
| Pretreatment low TATI,a n (%) | 87 (66.4) | 39 (72.2) | 28 (66.7) | 20 (57.1) | 0.34 |
| TATI change (% per 210 days) | 3.2 ± 20.0 | 7.7 ± 22.2 | 1.7 ± 20.0 | −1.8 ± 14.9 | 0.08 |
| TATI loss | 47 (35.9) | 15 (27.8) | 15 (35.7) | 17 (48.6) | 0.14 |
| Stable TATI | 49 (37.4) | 19 (35.2) | 18 (42.9) | 12 (34.3) | |
| TATI gain | 35 (26.7) | 20 (37.0) | 9 (21.4) | 6 (17.1) | |
| FIGO stage, n (%) | 0.54 | ||||
| IIIA | 14 (10.7) | 4 (7.4) | 5 (11.9) | 5 (14.3) | |
| IIIB | 5 (3.8) | 1 (1.9) | 3 (7.1) | 1 (2.9) | |
| IIIC | 112 (85.5) | 49 (90.7) | 34 (81.0) | 29 (82.9) | |
| Histological grade and type, n (%) | 0.17 | ||||
| Endometrioid grade 1–2 | 70 (53.4) | 22 (40.7) | 25 (59.5) | 23 (65.7) | |
| Endometrioid grade 3 | 26 (19.8) | 13 (24.1) | 8 (19.0) | 5 (14.3) | |
| Non‐endometrioidb | 35 (26.7) | 19 (35.2) | 9 (24.1) | 7 (20.0) | |
| Tumor size (cm) | 5.6 ± 3.1 | 5.5 ± 2.8 | 5.6 ± 2.9 | 5.7 ± 3.7 | 0.96 |
| Myometrial invasion, n (%) | 0.91 | ||||
| <50% | 27 (20.6) | 11 (20.4) | 8 (19.0) | 8 (22.9) | |
| ≥ 50% | 104 (79.4) | 43 (79.6) | 34 (81.0) | 27 (77.1) | |
| Lymphovascular space invasion, n (%) | 0.92 | ||||
| Yes | 102 (77.9) | 43 (79.6) | 32 (76.2) | 27 (77.1) | |
| No | 29 (22.1) | 11 (20.4) | 10 (23.8) | 8 (22.9) | |
| Cervical stromal involvement, n (%) | 0.60 | ||||
| Yes | 54 (41.2) | 23 (42.6) | 19 (45.2) | 12 (34.3) | |
| No | 77 (58.8) | 31 (57.4) | 23 (54.8) | 23 (65.7) | |
| Radiation field, n (%) | 0.90 | ||||
| Extended‐field radiotherapy | 75 (57.3) | 31 (57.4) | 25 (59.5) | 19 (54.3) | |
| Pelvic radiotherapy | 56 (42.7) | 23 (42.6) | 17 (40.5) | 16 (45.7) | |
| Brachytherapy, n (%) | 0.79 | ||||
| Yes | 94 (71.8) | 37 (68.5) | 31 (73.8) | 26 (74.3) | |
| No | 37 (28.2) | 17 (31.5) | 11 (26.2) | 9 (25.7) | |
| Median (interquartile range) duration between CT scans (days) | 223 (205–236) | 219 (198–233) | 224 (215–232) | 228 (204–241) | 0.45 |
BMI, body mass index; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; HU, Hounsfield unit; IMATI, intra‐muscular adipose tissue index; SATI, subcutaneous adipose tissue index; SD, standard deviation; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index; TATI, total adipose tissue index; VATI, visceral adipose tissue index.
SMD < 35.1 HU, SMI < 39.3 cm2/m2, and TATI < 134.0 cm2/m2 were defined as myosteatosis, sarcopenia, and low TATI, respectively.
Non‐endometrioid includes clear cell, mucinous, undifferentiated, and papillary serous carcinoma.
Body composition at baseline and change during treatment
Table 2 summarizes the body composition at baseline and its changes during treatment. Overall, BMI changes during treatment were 0.4% per 210 days (95% CI: −0.6 to 1.4; P = 0.41), and 23 (17.6%) patients experienced weight loss of ≥5%. Patients lost an average of 2.1% of SMD per 210 days (95% CI: −4.0 to −0.2; P = 0.03), 0.2% of SMI per 210 days (95% CI: −1.5 to 1.0; P = 0.70), and 2.2% of SMG per 210 days (95% CI: −4.4 to 0.1; P = 0.06). Patients gained an average of 3.2% of TATI per 210 days (95% CI: −0.2 to 6.7; P = 0.07). Changes in SMD and SMG did not correlate with change in BMI (Spearman's ρ for SMD, −0.13; P = 0.13; ρ for SMG, −0.01; P = 0.93). Changes in SMI and TATI weakly correlated with changes in BMI (Spearman's ρ for SMI, 0.20; P = 0.02; ρ for TATI, 0.40; P = 0.001). Changes in SMD weakly correlated with changes in SMI and TATI (Spearman's ρ for SMI, 0.31; P = 0.001; ρ for TATI, −0.22; P = 0.01).
Table 2.
Body composition parameters changes during treatment (n = 131)
| First CT scan | Second CT scan | Absolute change per 210 days | Relative change per 210 days (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean ± SD | Mean ± SD | Mean | 95% CI | P‐value | Mean | 95% CI | P‐value |
| BMI (kg/m2) | ||||||||
| Overall | 24.5 ± 3.7 | 24.5 ± 3.8 | 0.1 | −0.2 to 0.3 | 0.56 | 0.4 | −0.6 to 1.4 | 0.41 |
| Non‐myosteatosis | 24.2 ± 3.8 | 24.3 ± 4.0 | 0.1 | −0.2 to 0.4 | 0.74 | 0.6 | −0.8 to 2.0 | 0.66 |
| Myosteatosis | 25.0 ± 3.3 | 25.0 ± 3.5 | 0.02 | −0.3 to 0.3 | 0.81 | 0.1 | −1.1 to 1.3 | 0.93 |
| SMI (cm2/m2) | ||||||||
| Overall | 42.4 ± 6.5 | 42.3 ± 7.6 | −0.1 | −0.6 to 0.5 | 0.82 | −0.2 | −1.5 to 1.0 | 0.70 |
| Non‐myosteatosis | 41.8 ± 6.0 | 41.9 ± 6.4 | 0.05 | −0.6 to 0.7 | 0.83 | 0.2 | −1.2 to 1.7 | 0.88 |
| Myosteatosis | 43.4 ± 7.4 | 43.2 ± 9.5 | −0.3 | −1.3 to 0.7 | 0.55 | −1.2 | −3.6 to 1.1 | 0.41 |
| SMD (HU) | ||||||||
| Overall | 38.0 ± 6.2 | 37.1 ± 6.6 | −1.0 | −1.6 to −0.3 | 0.004 | −2.1 | −4.0 to −0.2 | 0.03 |
| Non‐myosteatosis | 41.5 ± 4.2 | 40.0 ± 5.4 | −1.4 | −2.2 to −0.7 | <0.001 | −3.5 | −5.2 to −1.8 | <0.001 |
| Myosteatosis | 31.1 ± 3.2 | 31.3 ± 4.8 | 0.01 | −1.3 to 1.3 | 0.96 | 0.6 | −4.0 to 5.1 | 0.86 |
| SMG (AU) | ||||||||
| Overall | 1605.9 ± 337.0 | 1571.4 ± 378.8 | −37.2 | −71.3 to −3.0 | 0.03 | −2.2 | −4.4 to 0.1 | 0.06 |
| Non‐myosteatosis | 1732.7 ± 294.5 | 1673.5 ± 323.5 | −59.5 | −100.4 to −18.5 | 0.02 | −3.1 | −5.5 to −0.8 | 0.02 |
| Myosteatosis | 1355.1 ± 270.0 | 1369.3 ± 402.0 | 7.0 | −54.8 to 68.7 | 0.63 | −0.2 | −5.2 to 4.7 | 0.66 |
| TATI (cm2/m2) | ||||||||
| Overall | 120.9 ± 56.9 | 122.7 ± 58.1 | 1.8 | −1.6 to 5.1 | 0.30 | 3.2 | −0.2 to 6.7 | 0.07 |
| Non‐myosteatosis | 107.7 ± 51.8 | 110.9 ± 54.3 | 2.9 | −1.1 to 6.9 | 0.15 | 4.4 | −0.1 to 8.8 | 0.051 |
| Myosteatosis | 146.8 ± 58.4 | 146.2 ± 58.7 | −0.5 | −6.7 to 5.7 | 0.88 | 0.9 | −4.8 to 6.6 | 0.74 |
AU, arbitrary unit; BMI, body mass index; CI, confidence interval; CT, computed tomography; HU, Hounsfield unit; SD, standard deviation; SMD, skeletal muscle radiodensity; SMG, skeletal muscle gauge; SMI, skeletal muscle index; TATI, total adipose tissue index.
The cut‐off values for myosteatosis, sarcopenia, low SMG, and low TATI were SMD < 35.1 HU, SMI < 39.3 cm2/m2, SMG < 1408.1 arbitrary unit, and TATI < 134.0 cm2/m2, respectively. Patient and tumor characteristics according to pretreatment myosteatosis are summarized in Table S1. Age, BMI, and SMI were not significantly higher in the pretreatment myosteatosis group compared with the pretreatment non‐myosteatosis group while the TATI were. In terms of SMD changes during treatment, 54 (41.2%), 42 (32.1%), and 35 (26.7%) patients were diagnosed with SMD loss, stable SMD, and SMD gain, respectively. The patient characteristics according to SMD change are presented in Table 1. Patients in the SMD loss group lost significantly more SMI during treatment (P < 0.001). Pretreatment SMD was significantly lower in the SMD gain group, and pretreatment myosteatosis was present in 19 (54.3%) patients in the SMD gain group compared with 9 (21.4%) and 16 (29.6%) patients in the stable SMD and the SMD loss groups, respectively (P = 0.01). Post‐treatment myosteatosis was present in 33 (61.1%) patients in the SMD loss group compared with 8 (19.0%) and 10 (28.6%) patients in the stable SMD and SMD gain groups, respectively (P < 0.001). Patients in the SMD loss group gain marginally more TATI during treatment than the stable SMD and SMD gain groups (P = 0.08). Demographic characteristics such as FIGO stage, histological grade and type, myometrial invasion, lymphovascular space invasion, cervical stromal involvement, and treatment were comparable among the SMD change groups. When integrating SMI and SMD into SMG, 51 (38.9%), 42 (32.1%), and 38 (29.0%) of the patients were diagnosed with SMG loss, stable SMG, and SMG gain, respectively.
Treatment modifications
One hundred and twenty‐seven patients (96.9%) received 6 cycles of chemotherapy; all completed their planned radiotherapy. Treatment modifications resulting from toxicity occurred in 32 patients (24.4%), including delay in treatment in 6 (4.6%), chemotherapy dose reduction in 27 (20.6%), and discontinuation of chemotherapy in 4 (3.1%). When adjusting for age, BSA, and Eastern Cooperative Oncology Group performance status, the pretreatment SMG was associated with treatment modification (odds ratio: 0.80, 95% CI: 0.69–0.93; P = 0.004). The SMI and SMD at baseline were marginally associated with treatment modification (Table 3).
Table 3.
Multiple logistic regression analysis for body composition parameters associated with treatment modificationa
| Parameter | Odds ratio (95% CI) | P‐value |
|---|---|---|
| Pretreatment | ||
| SMI (5 cm2/m2 increase) | 0.70 (0.48–1.03) | 0.07 |
| SMD (5 HU increase) | 0.70 (0.49–1.01) | 0.06 |
| TATI (5 cm2/m2 increase) | 0.98 (0.94–1.02) | 0.36 |
| SMG (100 AU increase) | 0.80 (0.69–0.93) | 0.004 |
AU, arbitrary unit; CI, confidence interval; SMD, skeletal muscle radiodensity; SMG, skeletal muscle gauge; SMI, skeletal muscle index; TATI, total adipose tissue index.
Adjusted for age, body surface area, and Eastern Cooperative Oncology Group performance status.
Body composition and outcomes
The median follow‐up period was 50.6 (range 12.1–117.0) months. The 5 year OS and PFS rates for all patients combined were 79.7% and 76.1%, respectively. All of the recurrences were distant metastases, as none of the patients were diagnosed with vaginal, pelvic, or para‐aortic recurrences within the radiation field. The 5 year OS rates were 76.7% among patients in the pretreatment myosteatosis group and 81.3% among those in the pretreatment non‐myosteatosis group (P = 0.47; Figure 3A), and the corresponding 5 year PFS rates were 70.5% and 80.7%, respectively (P = 0.24; Figure 3B). In terms of SMD changes during treatment, the 5 year OS rates for patients with SMD loss, stable SMD, and SMD gain were 59.7%, 94.0%, and 90.5%, respectively (P < 0.001; Figure 3C), and the corresponding PFS rates were 55.9%, 92.8%, and 85.7%, respectively (P < 0.001; Figure 3D). There were no significant differences in OS and PFS between the groups based on SMI change (Figure 3E and 3F). When integrating SMI and SMD into SMG, patients with SMG loss had significantly poorer OS and PFS compared with those in the stable SMG and SMG gain groups (Figure 3G and 3H). There were no differences in OS and PFS between the groups based on pretreatment sarcopenia, low SMG, low TATI, and TATI changes (Figure S1). On further subgroup analysis, patients with SMD loss had significantly poorer OS and non‐statistically significant lower PFS in the pretreatment myosteatosis groups (Figure S2A). In the pretreatment non‐myosteatosis group, patients with SMD loss had significantly poorer OS and PFS (Figure S2B).
Figure 3.
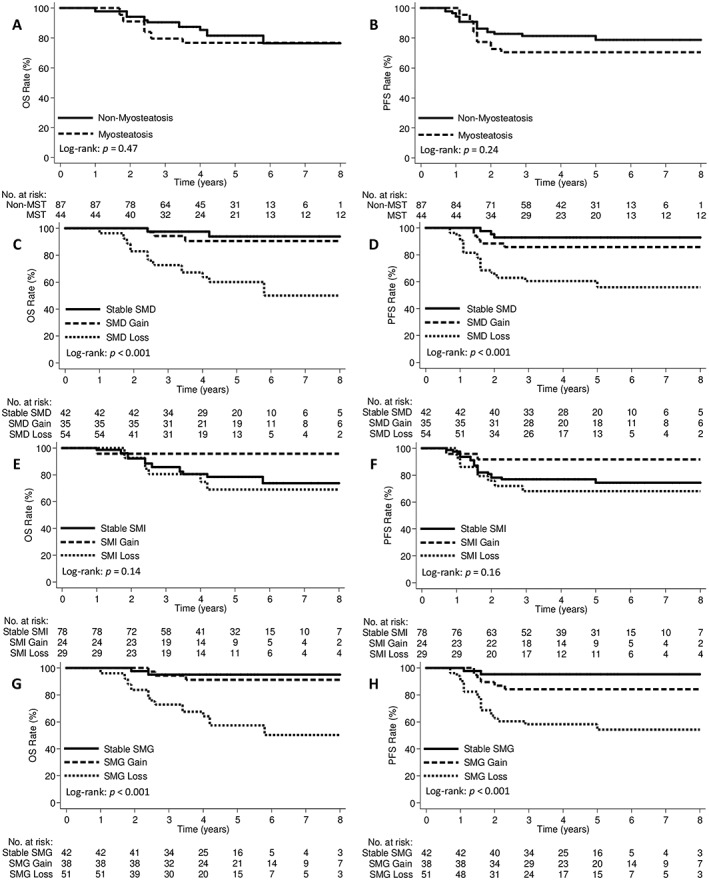
Kaplan–Meier curves demonstrating overall survival and progression‐free survival according to (A, B) pretreatment myosteatosis, (C, D) SMD change, (E, F) SMI change, and (G, H) SMG change groups. MST, myosteatosis; OS, overall survival; PFS, progression‐free survival; SMD, skeletal muscle radiodensity; SMG, skeletal muscle gauge; SMI, skeletal muscle index.
On univariable analysis, changes in SMI, SMD, and SMG; histological grade and type; and cervical stromal involvement were predictors of OS and PFS ( Table S2). Age was a predictor of PFS, but not of OS. On multivariable analysis (Table 4), SMD change (per 1 HU/210 days decrease) was independently associated with significantly poorer OS (HR: 1.32, 95% CI: 1.14–1.52; P < 0.001) and PFS (HR: 1.28, 95% CI: 1.12–1.43; P < 0.001). SMD loss was an independent prognostic factor for OS (HR: 11.08, 95% CI: 2.43–50.58; P = 0.002) and PFS (HR: 8.24, 95% CI: 2.32–29.23; P = 0.001). When integrating the SMI and SMD into SMG, the SMG loss was independently associated with significantly worse OS (HR: 10.63, 95% CI: 2.45–46.21; P = 0.002) and PFS (HR: 11.36, 95% CI: 2.67–48.35; P = 0.001). Pretreatment sarcopenia; myosteatosis; low SMG; and changes in weight, SMI, and TATI during treatment were not associated with OS or PFS.
Table 4.
Multivariable Cox proportional hazards model for overall survival and progression‐free survival (n = 131)
| Variable | OSa | PFSb | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Model A | ||||
| Pretreatment SMI, categorical | ||||
| Non‐sarcopenia | Reference | Reference | ||
| Sarcopenia | 0.63 (0.23–1.72) | 0.37 | 0.61 (0.25–1.48) | 0.28 |
| SMI change (% per 210 days) | 1.00 (0.94–1.06) | 0.94 | 1.00 (0.95–1.05) | 0.85 |
| Pretreatment SMD, categorical | ||||
| Non‐myosteatosis | Reference | Reference | ||
| Myosteatosis | 1.18 (0.48–2.86) | 0.72 | 1.19 (0.54–2.64) | 0.67 |
| SMD change (per 1 HU/210 days decrease) | 1.32 (1.14–1.52) | <0.001 | 1.28 (1.12–1.43) | <0.001 |
| TATI change (% per 210 days) | 0.99 (0.97–1.01) | 0.15 | 0.99 (0.97–1.01) | 0.13 |
| Model B | ||||
| Pretreatment SMI, categorical | ||||
| Non‐sarcopenia | Reference | Reference | ||
| Sarcopenia | 0.67 (0.27–1.70) | 0.40 | 0.67 (0.29–1.52) | 0.33 |
| SMI change (% per 210 days) | 0.99 (0.93–1.04) | 0.60 | 0.99 (0.94–1.04) | 0.67 |
| Pretreatment SMD, categorical | ||||
| Non‐myosteatosis | Reference | Reference | ||
| Myosteatosis | 1.33 (0.54–3.28) | 0.54 | 1.19 (0.54–2.65) | 0.66 |
| SMD change, categorical | ||||
| Stable SMD (<±5.0%) | Reference | Reference | ||
| SMD loss (≥−5.0%) | 11.08 (2.43–50.58) | 0.002 | 8.24 (2.32–29.23) | 0.001 |
| SMD gain (≥+5.0%) | 1.68 (0.28–10.27) | 0.57 | 2.26 (0.53–9.62) | 0.27 |
| TATI change (% per 210 days) | 0.99 (0.96–1.01) | 0.19 | 0.99 (0.97–1.01) | 0.21 |
| Model C | ||||
| Pretreatment SMGc, categorical | ||||
| High SMG | Reference | Reference | ||
| Low SMG | 0.73 (0.29–1.79) | 0.49 | 0.63 (0.28–1.42) | 0.27 |
| SMG change, categorical | ||||
| Stable SMG (<±5.0%) | Reference | Reference | ||
| SMG loss (≥−5.0%) | 10.63 (2.45–46.21) | 0.002 | 11.36 (2.67–48.35) | 0.001 |
| SMG gain (≥+5.0%) | 1.92 (0.31–11.74) | 0.48 | 4.93 (0.97–25.09) | 0.06 |
| TATI change (% per 210 days) | 0.99 (0.97–1.02) | 0.48 | 0.99 (0.97–1.01) | 0.39 |
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival; SMD, skeletal muscle radiodensity; SMG, skeletal muscle gauge; SMI, skeletal muscle index; TATI, total adipose tissue index.
Hazard ratio for death estimated by Cox model, adjusted for histological grade and type, and cervical stromal involvement.
Hazard ratio for death estimated by Cox model, adjusted for age, histological grade and type, and cervical stromal involvement.
SMD < 35.1 HU, SMI < 39.3 cm2/m2, and SMG < 1408.1 arbitrary unit were defined as myosteatosis, sarcopenia, and low SMG, respectively.
Discussion
This is the first study to examine the longitudinal changes in body composition and their associations with prognoses in patients with stage III EC who underwent staging surgery and adjuvant CRT. We found that patients experienced SMD loss during treatment and that this was associated with poorer outcomes independent of pretreatment sarcopenia and myosteatosis. The changes in SMD were not correlated with altered BMI, suggesting that SMD loss could be occult and occurring independently of BMI change. Moreover, pretreatment SMG was associated with treatment modification.
The optimal adjuvant treatment for stage III EC has been unclear. The GOG 258 and PORTEC‐3 trials found that adjuvant CRT did not improve OS, although radiotherapy significantly reduced vaginal and pelvic recurrences.14, 15 In this study, the 5 year OS and PFS were similar to the final results of the PORTEC‐3 trial. Moreover, none of the patients in our study had vaginal, pelvic, or para‐aortic recurrences within the radiation field, highlighting the benefits of locoregional control using radiotherapy in patients with stage III EC.7, 8 Additional trials aimed at identifying regimens that can maximize locoregional control using radiotherapy and also produce improvements in distant control (as seen with chemotherapy for patients with stage III EC) are needed.61
The body composition measurement using clinically available CT images could be imaging biomarkers of survival outcomes in advanced stage EC.38, 39, 47 CT images can provide objective body composition measures and are widely used in staging, radiotherapy planning, and follow‐up in patients with advanced EC. Rodrigues and Chaves reported that pretreatment sarcopenia and myosteatosis were predictors of poor short‐term survival.38 We measured pretreatment and post‐treatment body compositions. The deterioration in SMD during treatment was found to be an indicator of poor outcomes; with the increase in fat infiltration to the muscle (e.g. decreased in SMD), both PFS and OS are significantly reduced. However, pretreatment sarcopenia or myosteatosis was not a predictor of outcomes in this study. In addition, we found pretreatment non‐myosteatosis patients significantly lost SMD during treatment, while the SMD change was not significant in pretreatment myosteatosis patients (Table 2). Evaluating muscle at a single specific time point may not help in predicting survival and could not evaluate muscle loss.49, 62 These findings suggested that a longitudinal study of changes in body composition may provide a more comprehensive understanding of the impacts of body composition on outcomes.40, 41, 42, 43, 44, 45, 46, 47, 48, 49
Body mass index is an imprecise measure of body composition, and monitoring BMI changes may be insufficient to promptly identify occult body composition changes.18, 19 We found that changes in SMI and TATI were weakly correlated with the change in BMI, and changes in SMD and SMG showed no correlation with the BMI. In addition, BSA has traditionally been used to determine the dosages for chemotherapy; however, determining the dose based only on weight and height does not take into account body composition and its relation to weight. It has been shown that lean body mass correlates better with pharmacokinetics and drug toxicities.52, 53, 54 The measurements of body composition other than BMI using readily available CT images might help guide individualized treatment and supportive care.18, 58
Skeletal muscle gauge is a recently devised metric that incorporates both SMD and SMI and has already been shown to be very useful in predicting treatment‐related toxicities.31, 32, 33, 34 Shachar et al. have shown that SMG was a better predictor of grade ≥3 chemotherapy toxicity compared with SMI or SMD in women with early‐stage breast cancer undergoing anthracycline and taxane‐based chemotherapy.33 In colorectal cancer patients, SMG was better correlated with 5‐fluorouracil toxicity than either SMI or SMD alone.34 In the present study, pretreatment SMG was significantly associated with treatment modifications due to toxicities, although the association of SMD and SMI with such changes in the treatment plan was of borderline significance. However, it should be noted that SMG was derived by simply multiplying the skeletal measures (SMI × SMD); we hypothesized SMI and SMD to be equally weighted. Whether such hypothesis can affect outcomes is not known. The prognostic values of SMG need to be validated in future studies with larger patient populations.
The most commonly used cut‐off values for identifying sarcopenia and myosteatosis were published by Prado et al. and Martin et al.20, 21 These cut‐offs were established from Canadian population with respiratory or gastrointestinal tract cancer. However, the body composition may be affected by the type and stage of the cancer and by demographic factors such as age, sex, and ethnicity.37, 58, 63 The BMI of the Asian population is relatively low compared with that of the Canadian population.64 Hence, the cut‐off values of Martin et al. may not be applicable to the Asian cohort, as they might skew the results. Fujiwara et al. have reported different cut‐offs in a large Japanese cohort study compared with the study of Martin et al.21, 37 They also reported that SMD was negatively correlated with BMI and adipose tissue indexes, which will result in the myosteatosis group having higher BMI and adipose tissue indexes. In this study, SMD at baseline was negatively correlated with BMI and TATI and weakly correlated with SMI (Figure S3). We defined our own cut‐off values for our cohort based on previous studies with similar population sizes.42, 59 Using the present cut‐off value of SMD, the myosteatosis group had significantly higher adipose tissue indexes and non‐significantly higher BMI. These findings suggested that the currently used cut‐off values defining sarcopenia and myosteatosis need to be refined depending on ethnicity and the type and stage of the cancer.
Contrast‐enhanced CT images may confound assessment of SMD. van Vugt et al. revealed that contrast enhancement strongly influenced SMD values. In their study, mean SMD was significantly lower for the unenhanced phase (30.9 ± 8.0 HU) compared with the arterial (38.0 ± 9.9 HU) and portal‐venous (38.7 ± 9.2 HU) phases (both P < 0.001). No significant difference was found between SMD in the portal‐venous and arterial phases (P = 0.161). van Vugt et al. also recommended using the portal‐venous phase of contrast‐enhanced CT for studies that describe the association between SMD and outcome measures to improve comparability between studies.65 Evaluating the association between SMD and outcomes, using CT images acquired with standardized protocol, would provide more comparable results in body composition studies.66 In this study, all CT images of patients were contrast enhanced and acquired with standardized protocol. We observed that SMD change was independently associated with poor survival, based on our choice of 5% change, which might not be a clinically useful cut‐off value. The clinically optimal and practical cut‐off values need to be determined in a larger cohort.67 In addition, it should also be noted that SMD change during treatment may not be comparable between unenhanced and contrast‐enhanced CT images.65, 66 For example, contrast‐enhanced pretreatment CT images are not comparable with unenhanced post‐treatment CT images, as significant SMD decrease might be reported, which might be mainly due to the non‐enhanced image and not muscle loss. Therefore, documentation of the phase of CT analysed and incorporation of standardized CT protocol may provide more comparable results in future studies.
The optimal intervention that best preserves skeletal muscles has yet to be determined. Pharmacotherapy, physical activity, structured nutritional supplements, and proper guidance may all help preserve skeletal muscle. Wright et al. reported that, in patients with advanced cancer undergoing early‐phase standard‐of‐care therapy, 7 weeks of adjunct testosterone treatment improved lean body mass as well as the quality of life and physical activity.68 Dieli‐Conwright et al. recently reported that combined resistance and aerobic exercises significantly attenuated sarcopenic obesity in patients with breast cancer.69 A phase III clinical trial (NCT02330926) is currently evaluating whether multimodal interventions can improve the outcomes of cancer patients. Future studies are needed to investigate the role of any such interventions in patients with advanced EC.
Our study had several limitations. First, it was a retrospective investigation involving a small number of patients, which likely limited our statistical power to detect significant differences in some of the body composition measurements and outcomes. We were not able to identify any biomarkers that could predict which patients will have muscle loss during treatment at baseline. Second, measurements of food intake and physical activity were not available, even though they may also influence body composition. Muscle deterioration might also be due to reverse causality, wherein it may be considered as a marker for more invasive tumors which are in turn associated with higher mortality and more rapid progression. However, we were unable to identify a causal relationship between SMD loss and poor survival and could only reveal an association between them. It is unknown whether muscle deterioration can be reversed in cancer patients and if this reversal can affect prognosis. Third, our study included only Asian patients. Further studies of body composition changes during CRT in Western patients treated for advanced EC are needed to validate the findings of this study more broadly. Despite these limitations, the strength of our study is that patients received very similar treatments and consistently underwent pretreatment and post‐treatment CT. The treatment outcomes were comparable with previous studies,11, 12, 13, 14, 15 and the follow‐up period was also adequate. Taken together, our findings contribute strongly to the increasing body of research showing that prognoses are clearly associated with skeletal muscle changes during treatment.
In conclusion, SMD loss occurred in patients with stage III EC during staging surgery and adjuvant CRT; SMD loss was independently associated with poorer outcomes. The adipose tissue at baseline or its change during treatment did not influence outcomes. Changes in SMD did not correlate with changes in BMI, suggesting that body composition measurements using CT images obtained for staging and follow‐up should be incorporated into the clinical setting as patient‐specific indicators that can predict outcomes and potentially guide interventions aimed at preserving skeletal muscle. Future studies are required to devise optimal cancer therapy based on individual body composition phenotypes characteristics; this would serve to improve survival outcomes in patients with advanced stage EC.
Author contributions
J.L. and Y.‐J.C. contributed to the conception and design of the study. Y.‐T.J. contributed to the imaging of the data analysis. All authors contributed to the acquisition, analysis, or interpretation of the data and critically revised the manuscript for important intellectual content. J.L. drafted the manuscript. J.L. and F.‐J.S. contributed to the statistical analysis.
Conflict of interest
None declared.
Funding
None.
Supporting information
Table S1 Patient and tumor characteristics according to pre‐treatment myosteatosis groups, values expressed as mean ± standard deviation, unless stated otherwise
Table S2 Univariable Cox proportional hazards model for overall survival and progression‐free survival.
Figure S1. Kaplan‐Meier curve demonstrating overall survival and progression‐free survival for overall patients according to pretreatment (A) sarcopenia, (B) SMG, and (C) TATI and (D) TATI change groups, OS, overall survival; PFS, progression‐free survival; SCP, sarcopenia; SMG, skeletal muscle gauge; TATI, total adipose tissue index.
Figure S2. Kaplan‐Meier curve demonstrating overall survival and progression‐free survival according to SMD change groups for the (A) pre‐treatment mysoteatosis and (B) pre‐treatment non‐myosteatosis patients. OS, overall survival; PFS, progression‐free survival; SMD, skeletal muscle radiodensity.
Figure S3. Scatter plots of BMI, SMI, SMD, and TATI. (A) SMD vs. BMI, (B) SMD vs. SMI, and (C) SMD vs. TATI. Correlations were assessed using Pearson correlation coefficients (rho). rho and slopes (m) for the correlations are indicated on individual graphs. BMI, body mass index; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index; TATI, total adipose tissue index.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.70
Lee J., Lin J.‐B., Wu M.‐H., Jan Y.‐T., Chang C.‐L., Huang C.‐Y., Sun F.‐J., and Chen Y.‐J. (2019) Muscle radiodensity loss during cancer therapy is predictive for poor survival in advanced endometrial cancer, Journal of Cachexia, Sarcopenia and Muscle, 10, 814–826, doi: 10.1002/jcsm.12440.
Contributor Information
Jie Lee, Email: sinus.5706@mmh.org.tw, Email: sinus125125@gmail.com.
Yu‐Jen Chen, Email: chenmdphd@gmai.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Shepherd JH. Revised FIGO staging for gynaecological cancer. Br J Obstet Gynaecol. 1989;96:889–892. [DOI] [PubMed] [Google Scholar]
- 3. Greven KM, Lanciano RM, Corn B, Case D, Randall ME. Pathologic stage III endometrial carcinoma. Prognostic factors and patterns of recurrence. Cancer. 1993;71:3697–3702. [DOI] [PubMed] [Google Scholar]
- 4. Morice P, Leary A, Creutzberg C, Abu‐Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. [DOI] [PubMed] [Google Scholar]
- 5. Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, et al. Adjuvant chemotherapy vs radiotherapy in high‐risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer—results from two randomised studies. Eur J Cancer. 2010;46:2422–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Binder PS, Kuroki LM, Zhao P, Cusworth S, Divine LM, Hagemann AR, et al. Benefit of combination chemotherapy and radiation stratified by grade of stage IIIC endometrial cancer. Gynecol Oncol. 2017;147:309–314. [DOI] [PubMed] [Google Scholar]
- 8. Lester‐Coll NH, Park HS, Rutter CE, Corso CD, Young MR, Ratner ES, et al. Who benefits from chemoradiation in stage III‐IVA endometrial cancer? An analysis of the National Cancer Data Base. Gynecol Oncol. 2016;142:54–61. [DOI] [PubMed] [Google Scholar]
- 9. Secord AA, Geller MA, Broadwater G, Holloway R, Shuler K, Dao NY, et al. A multicenter evaluation of adjuvant therapy in women with optimally resected stage IIIC endometrial cancer. Gynecol Oncol. 2013;128:65–70. [DOI] [PubMed] [Google Scholar]
- 10. Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high‐risk endometrial cancer. Gynecol Oncol. 2006;103:155–159. [DOI] [PubMed] [Google Scholar]
- 11. Geller MA, Ivy JJ, Ghebre R, Downs LS Jr, Judson PL, Carson LF, et al. A phase II trial of carboplatin and docetaxel followed by radiotherapy given in a “Sandwich” method for stage III, IV, and recurrent endometrial cancer. Gynecol Oncol. 2011;121:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scribner DR Jr, Puls LE, Gold MA. A phase II evaluation of docetaxel and carboplatin followed by tumor volume directed pelvic plus or minus paraaortic irradiation for stage III endometrial cancer. Gynecol Oncol. 2012;125:388–393. [DOI] [PubMed] [Google Scholar]
- 13. Lupe K, Kwon J, D'Souza D, Gawlik C, Stitt L, Whiston F, et al. Adjuvant paclitaxel and carboplatin chemotherapy with involved field radiation in advanced endometrial cancer: a sequential approach. Int J Radiat Oncol Biol Phys. 2007;67:110–116. [DOI] [PubMed] [Google Scholar]
- 14. de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie‐Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high‐risk endometrial cancer (PORTEC‐3): final results of an international, open‐label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matei D, Filiaci VL, Randall M, Steinhoff M, DiSilvestro P, Moxley KM, et al. A randomized phase III trial of cisplatin and tumor volume directed irradiation followed by carboplatin and paclitaxel vs. carboplatin and paclitaxel for optimally debulked, advanced endometrial carcinoma. J Clin Oncol 2017;35:5505. [Google Scholar]
- 16. de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie‐Meder C, et al. Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high‐risk endometrial cancer (PORTEC‐3): an open‐label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17:1114–1126. [DOI] [PubMed] [Google Scholar]
- 17. Matulonis UA, Filiaci VL, Huang HQ, Randall M, Kim B, DiSilvestro P, et al. Analysis of patient‐reported outcomes (PROs) for GOG‐258, a randomized phase III trial of cisplatin and tumor volume directed irradiation followed by carboplatin and paclitaxel (Cis‐RT+CP) vs. carboplatin and paclitaxel (CP) for optimally debulked, locally advanced endometrial carcinoma: a Gynecologic Oncology Group/NRG study. J Clin Oncol 2018;36:5589. [Google Scholar]
- 18. Caan BJ, Cespedes Feliciano EM, Kroenke CH. The importance of body composition in explaining the overweight paradox in cancer‐counterpoint. Cancer Res. 2018;78:1906–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strulov Shachar S, Williams GR. The obesity paradox in cancer‐moving beyond BMI. Cancer Epidemiol Biomarkers Prev. 2017;26:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol. 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 21. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 22. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta‐analysis and systematic review. Eur J Cancer. 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 23. Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74. [DOI] [PubMed] [Google Scholar]
- 24. Pin F, Barreto R, Couch ME, Bonetto A, O'Connell TM. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle. 2019; 10.1002/jcsm.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown JL, Lee DE, Rosa‐Caldwell ME, Brown LA, Perry RA, Haynie WS, et al. Protein imbalance in the development of skeletal muscle wasting in tumour‐bearing mice. J Cachexia Sarcopenia Muscle. 2018;9:987–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pin F, Barreto R, Kitase Y, Mitra S, Erne CE, Novinger LJ, et al. Growth of ovarian cancer xenografts causes loss of muscle and bone mass: a new model for the study of cancer cachexia. J Cachexia Sarcopenia Muscle. 2018;9:685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 28. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–122. [DOI] [PubMed] [Google Scholar]
- 29. Shen W, Punyanitya M, Wang Z, Gallagher D, St‐Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol (1985) 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 30. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014;210:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weinberg MS, Shachar SS, Muss HB, Deal AM, Popuri K, Yu H, et al. Beyond sarcopenia: characterization and integration of skeletal muscle quantity and radiodensity in a curable breast cancer population. Breast J. 2018;24:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shachar SS, Deal AM, Weinberg M, Nyrop KA, Williams GR, Nishijima TF, et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane‐based chemotherapy. Clin Cancer Res. 2017;23:658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shachar SS, Deal AM, Weinberg M, Williams GR, Nyrop KA, Popuri K, et al. Body composition as a predictor of toxicity in patients receiving anthracycline and taxane‐based chemotherapy for early‐stage breast cancer. Clin Cancer Res. 2017;23:3537–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Williams GR, Deal AM, Shachar SS, Walko CM, Patel JN, O'Neil BH, et al. The impact of sarcopenia on toxicity and pharmacokinetics of 5‐fluorouracil (5FU) in colorectal cancer. J Clin Oncol. 2017;35:633. [Google Scholar]
- 35. Ebadi M, Martin L, Ghosh S, Field CJ, Lehner R, Baracos VE, et al. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br J Cancer. 2017;117:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131–140. [DOI] [PubMed] [Google Scholar]
- 38. Rodrigues CS, Chaves GV. Skeletal muscle quality beyond average muscle attenuation: a proposal of skeletal muscle phenotypes to predict short‐term survival in patients with endometrial cancer. J Natl Compr Canc Netw. 2018;16:153–160. [DOI] [PubMed] [Google Scholar]
- 39. Silva de Paula N, de Aguiar BK, Azevedo Aredes M, Villaca Chaves G. Sarcopenia and skeletal muscle quality as predictors of postoperative complication and early mortality in gynecologic cancer. Int J Gynecol Cancer. 2018;28:412–420. [DOI] [PubMed] [Google Scholar]
- 40. Daly LE, Ni Bhuachalla EB, Power DG, Cushen SJ, James K, Ryan AM. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle. 2018;9:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blauwhoff‐Buskermolen S, Versteeg KS, de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol. 2016;34:1339–1344. [DOI] [PubMed] [Google Scholar]
- 42. Choi MH, Yoon SB, Lee K, Song M, Lee IS, Lee MA, et al. Preoperative sarcopenia and post‐operative accelerated muscle loss negatively impact survival after resection of pancreatic cancer. J Cachexia Sarcopenia Muscle. 2018;9:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kiyotoki T, Nakamura K, Haraga J, Omichi C, Ida N, Saijo M, et al. Sarcopenia is an important prognostic factor in patients with cervical cancer undergoing concurrent chemoradiotherapy. Int J Gynecol Cancer. 2018;28:168–175. [DOI] [PubMed] [Google Scholar]
- 44. Lee J, Chang CL, Lin JB, Wu MH, Sun FJ, Jan YT, et al. Skeletal muscle loss is an imaging biomarker of outcome after definitive chemoradiotherapy for locally advanced cervical cancer. Clin Cancer Res. 2018;24:5028–5036. [DOI] [PubMed] [Google Scholar]
- 45. Kays JK, Shahda S, Stanley M, Bell TM, O'Neill BH, Kohli MD, et al. Three cachexia phenotypes and the impact of fat‐only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J Cachexia Sarcopenia Muscle. 2018;9:673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brown JC, Caan BJ, Meyerhardt JA, Weltzien E, Xiao J, Cespedes Feliciano EM, et al. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I‐III colorectal cancer: a population‐based cohort study (C‐SCANS). J Cachexia Sarcopenia Muscle. 2018;9:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology‐epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle. 2019;9:1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scherbakov N, Doehner W. Cachexia as a common characteristic in multiple chronic disease. J Cachexia Sarcopenia Muscle. 2019;9:1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rutten IJ, van Dijk DP, Kruitwagen RF, Beets‐Tan RG, Olde Damink SW, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle. 2016;7:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee J, Chang CL, Lin JB, Wu MH, Sun FJ, Wu CJ, et al. The effect of body mass index and weight change on late gastrointestinal toxicity in locally advanced cervical cancer treated with intensity‐modulated radiotherapy. Int J Gynecol Cancer. 2018;28:1377–1386. [DOI] [PubMed] [Google Scholar]
- 51. Mytelka DS, Li L, Benoit K. Post‐diagnosis weight loss as a prognostic factor in non‐small cell lung cancer. J Cachexia Sarcopenia Muscle. 2018;9:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5‐fluorouracil‐based chemotherapy toxicity. Clin Cancer Res. 2007;13:3264–3268. [DOI] [PubMed] [Google Scholar]
- 53. Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920–2926. [DOI] [PubMed] [Google Scholar]
- 54. Prado CM, Lima IS, Baracos VE, Bies RR, McCargar LJ, Reiman T, et al. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol. 2011;67:93–101. [DOI] [PubMed] [Google Scholar]
- 55. McDonald AM, Swain TA, Mayhew DL, Cardan RA, Baker CB, Harris DM, et al. CT measures of bone mineral density and muscle mass can be used to predict noncancer death in men with prostate cancer. Radiology. 2017;282:475–483. [DOI] [PubMed] [Google Scholar]
- 56. Rutten IJG, Ubachs J, Kruitwagen R, Beets‐Tan RGH, Olde Damink SWM, Van Gorp T. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle. 2017;8:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Martin L. Diagnostic criteria for cancer cachexia: data versus dogma. Curr Opin Clin Nutr Metab Care. 2016;19:188–198. [DOI] [PubMed] [Google Scholar]
- 58. Hilmi M, Jouinot A, Burns R, Pigneur F, Mounier R, Gondin J, et al. Body composition and sarcopenia: the next‐generation of personalized oncology and pharmacology? Pharmacol Ther. 2018; 10.1016/j.pharmthera.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 59. van Dijk DP, Bakens MJ, Coolsen MM, Rensen SS, van Dam RM, Bours MJ, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 61. Dowdy SC, Glaser GE. Adjuvant therapy for women with high‐risk endometrial carcinoma. Lancet Oncol. 2018;19:268–269. [DOI] [PubMed] [Google Scholar]
- 62. Rutten IJ, Ubachs J, Kruitwagen RF, van Dijk DP, Beets‐Tan RG, Massuger LF, et al. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur J Surg Oncol. 2017;43:717–724. [DOI] [PubMed] [Google Scholar]
- 63. Zengin A, Jarjou LM, Prentice A, Cooper C, Ebeling PR, Ward KA. The prevalence of sarcopenia and relationships between muscle and bone in ageing West‐African Gambian men and women. J Cachexia Sarcopenia Muscle. 2018;9:920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19.2 million participants. Lancet 2016;387:1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van Vugt JLA, van den Coebergh Braak RRJ, Schippers HJW, Veen KM, Levolger S, de Bruin RWF, et al. Contrast‐enhancement influences skeletal muscle density, but not skeletal muscle mass, measurements on computed tomography. Clin Nutr. 2018;37:1707–1714. [DOI] [PubMed] [Google Scholar]
- 66. Rollins KE, Javanmard‐Emamghissi H, Awwad A, Macdonald IA, Fearon KCH, Lobo DN. Body composition measurement using computed tomography: does the phase of the scan matter? Nutrition. 2017;41:37–44. [DOI] [PubMed] [Google Scholar]
- 67. Williams BA, Mandrekar JN, Mandrekar SJ, Cha SS, Furth AF. Finding optimal cutpoints for continuous covariates with binary and time‐to‐event outcomes. Rochester, MN, Mayo Foundation, Technical Report Series 79. 2006.
- 68. Wright TJ, Dillon EL, Durham WJ, Chamberlain A, Randolph KM, Danesi C, et al. A randomized trial of adjunct testosterone for cancer‐related muscle loss in men and women. J Cachexia Sarcopenia Muscle. 2018;9:482–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dieli‐Conwright CM, Courneya KS, Demark‐Wahnefried W, Sami N, Lee K, Buchanan TA, et al. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: a randomized controlled trial. J Clin Oncol. 2018;36:875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle. 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Patient and tumor characteristics according to pre‐treatment myosteatosis groups, values expressed as mean ± standard deviation, unless stated otherwise
Table S2 Univariable Cox proportional hazards model for overall survival and progression‐free survival.
Figure S1. Kaplan‐Meier curve demonstrating overall survival and progression‐free survival for overall patients according to pretreatment (A) sarcopenia, (B) SMG, and (C) TATI and (D) TATI change groups, OS, overall survival; PFS, progression‐free survival; SCP, sarcopenia; SMG, skeletal muscle gauge; TATI, total adipose tissue index.
Figure S2. Kaplan‐Meier curve demonstrating overall survival and progression‐free survival according to SMD change groups for the (A) pre‐treatment mysoteatosis and (B) pre‐treatment non‐myosteatosis patients. OS, overall survival; PFS, progression‐free survival; SMD, skeletal muscle radiodensity.
Figure S3. Scatter plots of BMI, SMI, SMD, and TATI. (A) SMD vs. BMI, (B) SMD vs. SMI, and (C) SMD vs. TATI. Correlations were assessed using Pearson correlation coefficients (rho). rho and slopes (m) for the correlations are indicated on individual graphs. BMI, body mass index; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index; TATI, total adipose tissue index.


