Abstract
Objective
This study aimed to demonstrate that large-scale visual field (VF) data can be extracted from electronic medical records (EMRs) and to assess the feasibility of calculating metrics from these data that could be used to audit aspects of service delivery of glaucoma care.
Method and analysis
Humphrey visual field analyser (HFA) data were extracted from Medisoft EMRs from five regionally different clinics in England in November 2015, resulting in 602 439 records from 73 994 people. Target patients were defined as people in glaucoma clinics with measurable and sustained VF loss in at least one eye (HFA mean deviation (MD) outside normal limits ≥2 VFs). Metrics for VF reliability, stage of VF loss at presentation, speed of MD loss, predicted loss of sight years (bilateral VF impairment) and frequency of VFs were calculated.
Results
One-third of people (34.8%) in the EMRs had measurable and repeatable VF loss and were subject to analyses (n=25 760 patients). Median (IQR) age and presenting MD in these patients were 71 (61, 78) years and −6 (–10, –4) dB, respectively. In 19 264 patients with >4 years follow-up, median (IQR) MD loss was −0.2 (−0.8, 0.3) dB/year and median (IQR) intervals between VF examinations was 11 (8, 16) months. Metrics predicting loss of sight years and reliability of examinations varied between centres (p<0.001).
Conclusion
This study illustrates the feasibility of assessing aspects of health service delivery in glaucoma clinics through analysis of VF databases. Proposed metrics could be useful for blindness prevention from glaucoma in secondary care centres.
Keywords: audit, glaucoma, perimetry, visual fields, electronic medical records
Key messages.
What is already known about the subject?
Electronic medical record (EMR) systems are routinely used for patient management in eye clinics. Data from these EMRs have been used effectively in clinical services (such as cardiovascular healthcare) to audit/manage both patients and clinics, but less so in ocular healthcare.
What are the new findings?
The potential use of visual field data for audit in glaucoma was demonstrated by successful download and aggregated analysis of an anonymised data extract. We propose several metrics that can be used to monitor various aspects of glaucoma clinics. We compare these metrics in five glaucoma clinics in England.
How might these results change the focus of research or clinical practice?
This type of electronic monitoring of glaucoma service delivery could be used to assess, in real time, how a glaucoma clinic is performing and highlight potential areas for improvement. Identification of patients at high risk of blindness could facilitate targeting of care towards those most at need.
Introduction
Electronic medical records (EMRs) have potential to form a repository of data on patient encounters that can be directly used in research and clinical audit.1 The latter can be used as a first step towards improving health service delivery and improving patient care. Indeed, it is only possible to gauge improvements in a process after it has been measured in the first place.
Glaucoma clinics in Hospital Eye Services (HES) in England handle more than 1 million patient visits per year.2 3 Most of these visits are for monitoring people with established glaucoma. Once diagnosed, all patients with glaucoma require lifelong monitoring so that any worsening of disease can be detected and treatment intensified accordingly. Patient management focuses on monitoring the visual field (VF), assessment of the optic nerve and measurement of the intraocular pressure (IOP). The latter is critical because it is the only modifiable risk factor for the condition worsening (progression).4 Research evidence from clinics in England suggest measures of IOP are the main determinant of how often a patient is monitored over a period of time.5 6 In contrast to IOP, a measurement of the VF is the closest surrogate to what matters to the patient in the glaucoma clinic, that is, preservation of their vision.7 VF monitoring is therefore recognised to be critically important for the clinical management of the patient but it is also perceived to be difficult to do unless it is well implemented.8–10 The computerised technology used to measure VFs (standard automated perimetry (SAP)) has been in clinics for 20–30 years and has remained largely unchanged. Data from SAP are stored electronically, often in an EMR. These records, which are likely to be historically rich, should be amenable to easy electronic auditing. Therefore, at different clinical centres it might be possible, for example, to audit measures of disease severity (VF loss) of patients at diagnosis. Moreover, it might be possible to audit speed at which patients in different clinics might be losing vision and, for example, whether frequency of VF monitoring is consistent across clinics. It is these ideas that we explore in this report.
The National Ophthalmology Database (NOD) was established under the auspices of the Royal College of Ophthalmologists (RCOphth) in 2010. The NOD aimed to collate pseudonymised data collected as a by-product of routine clinical care using EMR systems for the purposes of national audit, research and establishing meaningful measures for revalidation of ophthalmologists.11 In 2014, the Healthcare Quality Improvement Partnership (HQIP) commissioned NOD to do a feasibility study to investigate the use VF data to audit activity in glaucoma clinics. The results of the study are reported here with the specific aim of examining the viability of extracting meaningful metrics of health service delivery that might in the future allow comparison between glaucoma clinics.
Methods
VF data were extracted from the Medisoft EMR system (Medisoft, Leeds, UK) from five regionally different National Health Service (NHS) Hospital Trust glaucoma clinics in England. The extraction was done in November 2015 and data transferred to the RCOphth NOD. All patient data were anonymised and subsequently transferred to a single secure database held at City university. For the purpose of this report, the five centres are anonymised. No other clinical data were used in this study apart from patient’s age, gender and the dates of the VF examinations. Subsequent analyses of the data were approved by a research ethics committee of City, University of London; the study adhered to the Declaration of Helsinki and the General Data Protection Regulation of the European Union. The database material contained 602 439 separate VF records from 73 994 people (table 1) recorded between April 2000 and March 2015.
Table 1.
Total number of visual fields per centre. Each centre is simply labelled 1–5 and represented by a specific colour. This colour coding is used throughout this report (every centre had data recorded between April 2000 and March 2015 apart from centre 5 where data were first recorded in May 2000).
| Centre 1 | Centre 2 | Centre 3 | Centre 4 | Centre 5 | |
| People, n | 3423 | 8459 | 27 921 | 18 636 | 15 555 |
| VF records, n | 16 162 | 65 355 | 285 552 | 113 847 | 121 523 |
VF, visual field.
Patient involvement
This research was done without patient involvement. Patients were not invited to contribute to the editing or writing of this document. Patients were not asked to comment on the study design, consulted to develop patient relevant outcomes or to interpret the results.
Inclusion and exclusion criteria
SAP in these clinics, and most others in England, is routinely performed on a Humphrey visual field analyser (HFA, Carl Zeiss Meditec, Dublin, California, USA). Only VFs recorded on the HFA using a Goldmann size III stimulus with a 24-2 test pattern acquired with the Swedish Interactive Testing Algorithm (SITA Standard or SITA Fast) were included, reducing the aggregated database to 576 615 VFs from 71 361 people. SITA fast is commonly used in clinics in England. (Although SITA Standard is a more precise testing algorithm than SITA Fast at lower VF sensitivities, it is unlikely to make a sizeable difference to improving the time to detect VF progression.12) In our study population, 83% of the recorded VFs were SITA Fast and the rest were SITA Standard.
For the purpose of this report, our study population was defined as people in glaucoma clinics with measurable and sustained VF loss in at least one eye. This definition aims to exclude people suspected of having possible glaucoma (glaucoma suspects) and people with normal VFs and raised eye pressure (ocular hypertension (OHT)). Therefore, patients were only included if they had a VF with an HFA mean deviation (MD) flagged as outside the 95% normative limits in the HFA VF analysis software in at least one eye. (MD is a standard measure of the overall severity of VF loss, relative to healthy peers, with more negative values indicating greater VF loss.) Moreover, this proxy criterion for measurable VF loss had to be satisfied for both of the first two VFs recorded in the clinic; this was done in order to improve the precision of the estimate of an individual likely to have real VF loss at their presentation to secondary care. The number of patients satisfying these criteria expressed as a percentage of the total number of people with a VF record was calculated. This can be thought of as a simple count of people in clinics with actual VF loss at presentation to secondary care (diagnosis) as opposed to, for example, being a glaucoma suspect, a false-positive referral or having OHT.
Metrics for assessing service delivery
Six different metrics were calculated to characterise and estimate aspects of patient monitoring and outcomes in the clinics.
Age at presentation was estimated by the age of the patient (years) at the time of their first VF record.
Reliability of VFs was estimated by using the HFA false-positive (FP) measure. It is accepted that HFA FP is a useful measure of a reliable examination.13 The HFA flags VFs as unreliable if there are more than 15% FP errors. Percentage of all VFs considered as unreliable due to FP errors was therefore determined for each centre.
Stage of VF loss at presentation was estimated by MD in the worse eye (the one with the more negative MD) at the second VF examination. The second VF was used to ameliorate the bias of the perimetry learning effect.14 15 We chose the worse eye as a surrogate of the most ‘detectable’ level of VF loss at the stage of case finding in primary care. Patients with MD worse than −12 dB in this eye were defined as having advanced VF loss. Patients with MD better than −6 dB in this most affected eye were defined as having early VF loss, with all other patients classified as having moderate VF loss. These VF criteria have been used in health economic investigations of service delivery of glaucoma.16 17 The proportion of patients within each of these three categories (early, moderate, advanced) can be summarised in a traffic-light waffle plot (green, yellow, red) for each centre.
Next, a subset of the study patients with sufficient series of VF examinations were defined as those with at least five VFs recorded over a period of 4 or more years. This subset of data were used for three more metrics summarising patient follow-up activity at each clinic.
Speed (rate) of VF loss in clinics was determined by using simple linear regression of MD against time of follow-up (dB/year) and was only calculated in patients with series of data. The first VF examination in each series was removed to account for perimetric learning effects.
Risk of VF loss blindness in clinics was estimated by a Loss of Sight Years (LSY) metric as described elsewhere.18 In short, LSY estimates the number of years that a patient will have bilateral VF loss worse than MD of −22 dB (binocular VF impairment) in their predicted remaining lifetime.19 The metric considers rate of VF loss in both eyes and the patient’s residual life expectancy based on age and sex as reported in UK Office of National Statistics.20 Residual life expectancy takes into account that a person aged, for example, 80 years is more likely to live to age 81 years than someone aged 70 years and is a useful measure of relative life expectancy. For patients with two eligible eyes with series of VF data, we determined whether LSY would be predicted to be longer than 3 years. We then calculated the percentage of patients in each centre with this attribute.
Frequency of VF examination in clinics was simply estimated as the average interval (months) between recorded VF examinations during the follow-up period in those patients with series of VF data.
Summary measures and distributions of these metrics were evaluated and compared for the five glaucoma clinics. We used medians and IQRs along with conservative non-parametric tests to make simple, illustrative comparisons between clinics. All analyses were done using R (R Development Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, URL: http://www.R-project.org, 2008).21
Results
Application of the inclusion and exclusion criteria resulted in 223 379 VFs from 25 760 patients. Therefore 65.2% (n=48 234) of people were excluded because they only had one VF examination or normal VFs in both eyes in their first or second VF examination. These people were excluded from further analysis. Series of VFs (more than 4 years of follow-up) were available for 19 264 patients. Summary measures of the metrics for assessing glaucoma service delivery for the five centres (and the aggregate data) are given in table 2.
Table 2.
This table shows the summary measures of a number of metrics across the five centres (and a total column). The six main metrics highlighted in the Methods section are given in bold font. Median (and IQR) values or percentages are reported as summary measures.
| Centre 1 | Centre 2 | Centre 3 | Centre 4 | Centre 5 | TOTAL | |
| Patients with VF loss, n | 1373 | 2404 | 11 589 | 5713 | 4681 | 25 760 |
| VFs, n | 7813 | 19 654 | 121 939 | 36 011 | 35 737 | 223 379 |
| Sex (% men) | 45.6 | 45.2 | 47.1 | 47.7 | 44.8 | 46.5 |
| Median (IQR) Age (years) at presentation | 75 (67, 80) | 71 (61, 78) | 71 (61, 78) | 71 (62, 79) | 69 (58, 77) | 71 (61, 78) |
| % of unreliable VFs | 3.0 | 4.4 | 5.1 | 4.3 | 4.1 | 4.6 |
| Median (IQR) MD at presentation (dB) | −6.5 (−11.7, -3.9) | −5.8 (−10.6, −3.6) | −5.7 (−10.1, −3.6) | −5.4 (−9.9, −3.5) | −5.4 (−9.9, −3.5) | −5.6 (−10.2, −3.6) |
| % patients with advanced VF loss at presentation | 29.6 | 25.0 | 24.2 | 23.0 | 22.8 | 24.0 |
| Patients* with VF series >4 years, n | 843 | 1786 | 9208 | 3917 | 3480 | 19 264 |
| Median (IQR) MD loss per year (dB/year)* | − 0.37 ( − 1.2, 0.32) | − 0.26 ( − 0.90, 0.20) | − 0.23 ( − 0.83, 0.21) | − 0.19 ( − 0.81, 0.27) | − 0.10 ( − 0.71, 0.37) | − 0.21 ( − 0.83, 0.26) |
| % Patients LSY >3 years | 12.5 | 12.9 | 11.8 | 10.1 | 10.0 | 11.2 |
| Median (IQR) interval between VFs (months) | 12.6 (8.6, 18.1) | 10.3 (7.6, 13.6) | 9.5 (7.2, 13.0) | 15.4 (11.5, 21.1) | 12.3 (9.6, 16.5) | 11.2 (8.1, 15.8) |
LSY, Loss of Sight Years; MD, mean deviation; VF, visual field.
There was a statistically significant difference in the median age at presentation between the centres (p<0.001; Kruskal-Wallis test). Centre 1 had slightly older patients with little difference between the other centres. There was also a statistically significant difference between centres for percent of unreliable VFs (p<0.001; χ2 test). Centre 1 (despite having older test takers) returned the lowest proportion of unreliable VFs. Differences between centres were generally small. Yet, for example, the rate of unreliable VFs recorded at centre 3 was 1.7 times higher than that of centre 1 (95% CI for the relative risk of 1.4 to 1.9). It is noteworthy that around 1 in 20 VF examinations were unreliable according to this proxy measure across all centres.
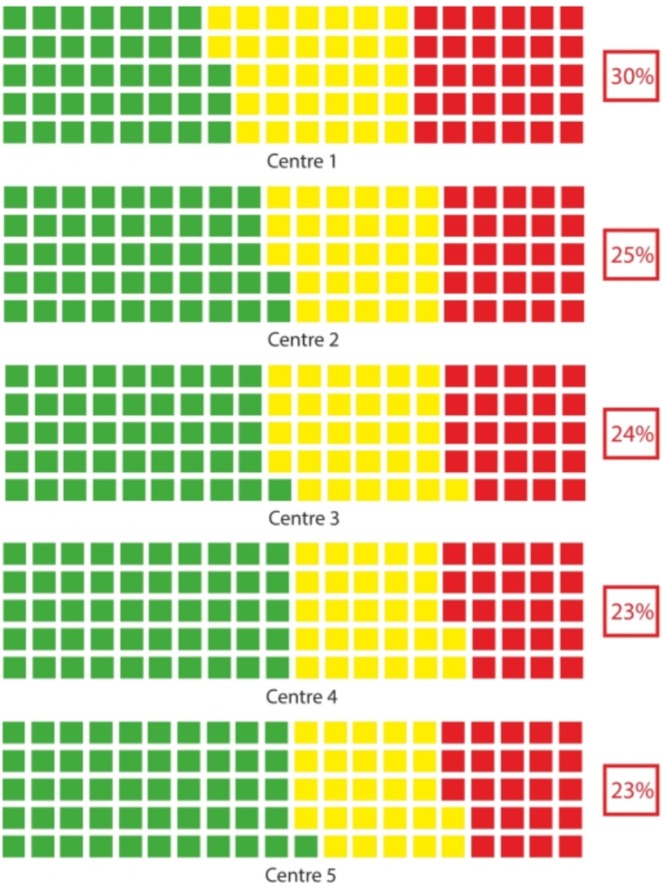
Stage of VF loss at presentation is also summarised as a series waffle (traffic light) plots for each centre in figure 1. Centre 1 had notably more patients presenting with advanced VF loss when compared with the other centres. (Note this value summarises percent with advanced loss for those who present with some sort of VF loss. The value would be lower if the denominator was all people in the clinics.22)
Figure 1.
A traffic-light (waffle) plot showing the classification of visual field (VF) loss of newly presenting patients in each of the five centres. Each square represents 1% of the patients being classified as early (green), moderate (yellow) or advanced (red) VF loss on presentation.
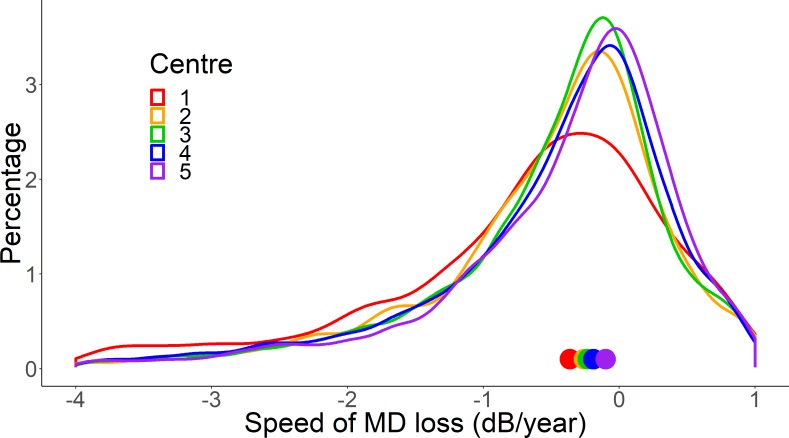
Speed (rate) of VF loss in the five clinics are summarised as distributions in figure 2. The distributions were generally similar with the exception of centre 1 (and to a lesser extent centre 2); these distributions exhibited much heavier tails which means there were more eyes experiencing faster speed of loss VF loss at these centres compared with the others.
Figure 2.
A plot showing the distribution of the speed of mean deviation (MD) loss (dB/year) in each of the five centres. Each line represents one of the centres. The distribution curves are obtained by using kernel density estimation to fit a smoothed curve to a histogram. Centre 1 (red) has a heavier tail compared with the other centres, indicating a higher proportion of patients with higher speeds of loss. Centre 1 also has a lower kurtosis (tail-to-peak ratio) and more negative skewness than the others. The coloured symbols on the x-axis indicate the median value for each centre (see table 2).
Risk of VF loss blindness as estimated by percentage of patients predicted to have LSY >3 years was slightly greater in centre 2 (12.9%) compared with the other centres and this was statistically significant (p<0.001; Kruskal-Wallis test).
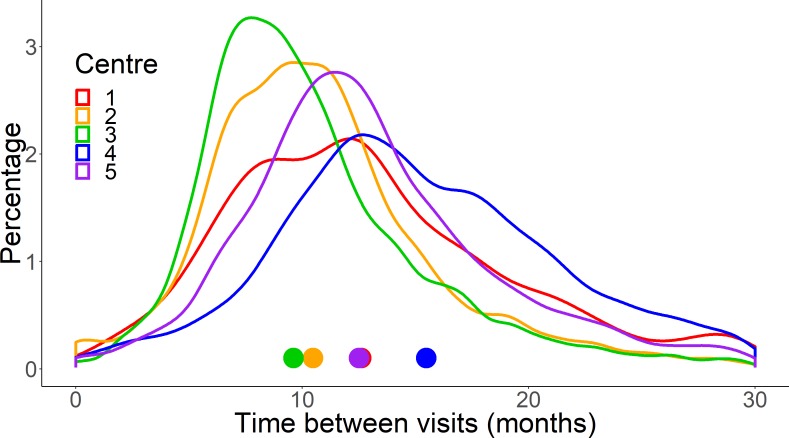
Frequency of VF examination at the five clinics is summarised by distributions of the time interval in months between VF tests in figure 3. Centre 4 had a noteworthy longer median time interval between VF tests (15.4 months) compared with the other centres and this was statistically significant (p<0.001; Kruskal-Wallis test).
Figure 3.
A plot showing the distributions of the time interval between patient visits (in months) in each of the five centres. Each line represents one of the centres. Centre 4 is more positively skewed, indicating a higher proportion of patients with longer intervals between visits. The coloured symbols indicate the median time interval between visits for each centre (see table 2).
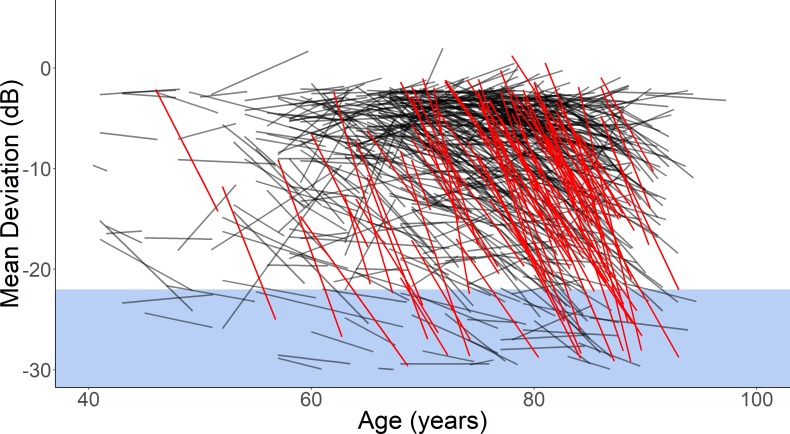
One way of visualising speed of VF loss in clinics is to use a Hedgehog plot.18 One of these is shown for centre 1 in figure 4.
Figure 4.
A hedgehog plot showing the mean deviation loss over time of each eye in centre 1. Eyes highlighted in red indicate a speed of loss worse than −1.5 dB/year. The region marked in blue is a threshold for a severely impaired visual field. More detail on these plots can be found in Bryan et al.18
Discussion
This work confirms that routinely collected VF data from glaucoma clinics can be downloaded, aggregated and anonymised from an EMR and be made available for analysis. This has previously been done for research purposes yet, here we illustrate how it could be done to potentially monitor and compare health service delivery at different glaucoma clinics.12 19 22–24 For this exercise, assessments were done on anonymised centres with a key point of this work showing that this approach is feasible for future implementation. We did not assess the logistics of the VF data extraction, which in this instance was carried out as part of the NOD work commissioned by HQIP. We have shown that assessment of VF records in glaucoma clinics could provide a first step towards quality improvement of services; this is a novel idea. For example, we have demonstrated how VF metrics of late presentation of glaucoma, or speed of loss of VF in people in glaucoma clinics during follow-up could be easily summarised for a clinical centre. We also have shown how it might be possible to compare the reliability and use of VF measurement between centres—for instance, it is feasible to identify centres that are doing more or fewer VF examinations compared with others. The latter is important because it has been shown previously, via health economic modelling of retrospective data, that optimising use of VF resources could improve clinical management of patients and save money at the same time.3 Since VF data can be held in EMRs this makes them amenable to automated and live analysis. Our feasibility study indicates that this approach could be used to monitor what happens to people in different glaucoma clinics in real time. Assessment of quality improvement of glaucoma services with an implementation of this idea could be the subject of future work.
The results from this report illustrate the feasibility of calculating metrics for assessing service delivery between centres using VF records alone. Nevertheless, some discussion about the differences in these metrics between the five anonymous centres is worthwhile. For example, centre 1 had more patients presenting with advanced VF loss and had more patients losing VF at a faster speed than other centres. These variables are highly associated in people with glaucoma and are, in turn, positively associated with older age.3 24 25 It is noteworthy that patients in centre 1 were generally older than those in the other centres and this might, at least in part, explain these observations. Still, our chosen metric for risk of VF loss blindness as estimated by percentage of patients predicted to have LSY >3 years was 12.5% in centre 1. This observation underlines the importance of preventing late detection of VF loss for prevention of avoidable blindness.19
Our report reveals some other interesting subsidiary findings. Our population for this report is people with measurable VF loss in glaucoma clinics (at least two VFs with actual VF loss as measured by MD). These inclusion criteria reduced our sample by 65.2%. In other words, around two-thirds of people with HFA 24-2 VF records in these data sets had single ‘one-off’ VFs or had normal VFs at presentation to the clinics. This figure illustrates the huge volume of likely false-positive glaucoma referrals, glaucoma suspects and ocular hypertensive that glaucoma clinics deal with on a daily basis. Moreover, we found that around 1 in 20 VFs are recorded with reliability indices outside normal limits. In addition, the median interval between VF tests in people being followed over time was 11.2 months and as high as 15.4 months in one centre. This supports previous findings that annual VF testing is the norm for most patients in glaucoma clinics in England.6 Heath economic modelling has highlighted the benefits of stratifying patients to more or less VF monitoring based on age and stage of disease at diagnosis; a prospective study is needed to prove these findings.3
Using EMRs for research or audit is not a new idea, having been implemented in many fields of medicine to study diseases such as diabetes, heart failure, cancer and asthma.26 In eye clinics EMRs have been used for audit of cataract surgical outcomes but importantly also have potential for making healthcare delivery more efficient by facilitating more streamlined clinical work flow, better patient management and improved data tracking.11 27–32 However, ideas and implementation are different entities and meaningful use of EMRs in ophthalmology is still a work in progress.33–35 At present, certainly in clinics in England, there are challenges about how clinical data are recorded, archived and stored. Moreover, issues such as non-collated data sets, duplicate IDs and differing databases add to the challenge of moving towards comprehensive use of EMRs.
Our approach to the concept of assessing service delivery in glaucoma clinics has several strengths. In theory, VF records should be easily stored on EMRs, making them amenable to easy extraction, analysis and audit. Whether this happens in practice is dependent on the motivation of implementing electronic archiving of VF examinations and use of EMRs in HES. Another rational for using VF metrics is their relevance to measuring vision. IOP is the only modifiable risk factor for glaucoma progression and is crucial to patient management but VF metrics will best estimate the status of people’s vision loss in the clinic. Our approach does not consider everyone in glaucoma clinics but centres on those with a proxy measure of sustained VF loss. These patients are at higher risk of further significant vision loss in their lifetime compared, for example, with people with OHT.19 33–35
There are limitations to the ideas that we report in this study. The main problem is reliance on the VF data alone. A more complete assessment of what is happening in a glaucoma clinic would be achieved by considering exact diagnosis, treatment regimens, IOP, optic nerve head characteristics, individual patient history or other risk factors. For example, much can be learnt about what is happening in glaucoma clinics by reviewing repositories of data on prescribed medications.36 However, EMRs need an established and standardised minimum data set for glaucoma care and this is the subject of future work. Our idea will also be limited to how well VF records can be archived at a centre. The five sites chosen for this study were all EMR enabled and known to run large glaucoma services with aggregated electronic VF databases. Alternative options could include separate data extractions from individual machines with subsequent aggregation into a single database, but this would be time consuming and carry significant cost, in particular if these services were delivered in different settings such as outreach clinics.
There are also limitations to some of the metrics we have proposed for assessing service delivery. First, while MD is a useful summary measure of how much sensitivity loss there is in a VF and particularly convenient to monitor changes over time, it is not a perfect measure for glaucomatous VF loss. MD can be affected by non-glaucomatous changes such as a general reduction in VF sensitivity caused by, for example, cataract. Second, as noted previously, we only used the FP reliability index as a measure of patient test taking performance.13 Of course, this measure, or any other similar measure, would not capture patients failing to complete an examination or those excluded because of a previous failure to reliably conduct a VF examination. Third, our measure of “risk of blindness” (LSY) makes a number of assumptions around residual life expectancy and progression of VF loss being constantly linear. Finally, when comparing metrics it will also be important to consider some centres simply differ in terms of population factors (eg, racial and socioeconomic profile) and audits using the methods proposed in our report would have to take this into account.
In conclusion, this study illustrates the feasibility of assessing some aspects of quality of care in glaucoma clinics through analysis of VF databases from EMR enabled centres. This approach, which is outcome focused, is a potentially useful method for assessing blindness prevention from glaucoma in secondary care centres. VF testing technology is standardised in the UK NHS, and although in many centres the electronic VF tests will be distributed across several VF testing machines, it is feasible to aggregate these fields into a central database located in each centre for central analysis. Ideally, such a central field database would reside within a specialty specific EMR implementation serving both clinical and quality assurance needs. Secondary benefits from such an approach would include the ability to more easily detect patients whose VF loss is progressing rapidly in order to intensify their treatment as well as detection of those patients whose VFs are stable who may require less intensive monitoring once VF stability has been documented. By shifting focus towards those in most need, health services resources can be more effectively used. In the current NHS digital environment, a variety of challenges would need to be overcome in order to extend this audit approach into a national audit of vision preservation in people with glaucoma.
Acknowledgments
The authors would like to thank Beth Barnes (head of professional support), Paul Donachie (medical statistician) and the rest of the audit team at the national ophthalmology database https://www.nodaudit.org.uk/about/team.
Footnotes
Contributors: SRK: design, analysis and interpretation of data, drafting the article, revising the article. SRB: design, analysis and interpretation of data, drafting the article. JS: conception, design, revising the article. DPC: conception, design, interpretation of data, drafting the article, revising the article.
Funding: Data were electronically extracted from contributing hospitals by the Royal College of Ophthalmologists audit team as part of the Healthcare Quality Improvement Partnership commissioned National Ophthalmology Database Audit (which forms part of the National Clinical Audit and Patient Outcomes Programme). SRK received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie Grant Agreement No. 675033. The listed funding organisations had no role in the design or conduct of this research.
Competing interests: DPC reports speaker fees from Allergan, Bayer, Santen; unrestricted funding from Allergan, Roche, Santen; consultancy with Centervue—all outside the remit of the submitted work.
Patient consent for publication: Not required.
Ethics approval: All patient data were anonymised and transferred to a single secure database. No other clinical data were made available apart from each patient's age. Subsequent analyses of the data were approved by a research ethics committee of City, University of London and this study adhered to the tenets of the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Bodagh N, Archbold RA, Weerackody R, et al. Feasibility of real-time capture of routine clinical data in the electronic health record: a hospital-based, observational service-evaluation study. BMJ Open 2018;8:e019790 10.1136/bmjopen-2017-019790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Institute for Health and Clinical Excellence Glaucoma diagnosis and management of chronic open angle glaucoma and ocular hypertension, 2012. Available: http://www.nice.org.uk/guidance/cg85
- 3. Boodhna T, Crabb DP. More frequent, more costly? health economic modelling aspects of monitoring glaucoma patients in England. BMC Health Serv Res 2016;16:1–13. 10.1186/s12913-016-1849-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet 2015;385:1295–304. 10.1016/S0140-6736(14)62111-5 [DOI] [PubMed] [Google Scholar]
- 5. Crabb DP, Russell RA, Malik R, et al. Frequency of visual field testing when monitoring patients newly diagnosed with glaucoma: mixed methods and modelling. Heal Serv Deliv Res 2014;2:1–102. 10.3310/hsdr02270 [DOI] [PubMed] [Google Scholar]
- 6. Fung SSM, Lemer C, Russell RA, et al. Are practical recommendations practiced? A national multi-centre cross-sectional study on frequency of visual field testing in glaucoma. Br J Ophthalmol 2013;97:843–7. 10.1136/bjophthalmol-2012-302903 [DOI] [PubMed] [Google Scholar]
- 7. Crabb DP. A view on glaucoma--are we seeing it clearly? Eye 2016;30:304–13. 10.1038/eye.2015.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malik R, Baker H, Russell RA, et al. A survey of attitudes of glaucoma subspecialists in England and Wales to visual field test intervals in relation to NICE guidelines. BMJ Open 2013;3:e002067–5. 10.1136/bmjopen-2012-002067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glen FC, Baker H, Crabb DP. A qualitative investigation into patients' views on visual field testing for glaucoma monitoring. BMJ Open 2014;4:e003996 10.1136/bmjopen-2013-003996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chauhan BC, Garway-Heath DF, Goñi FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol 2008;92:569–73. 10.1136/bjo.2007.135012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Day AC, Donachie PHJ, Sparrow JM, et al. The Royal College of ophthalmologists' national ophthalmology database study of cataract surgery: report 1, visual outcomes and complications. Eye 2015;29:552–60. 10.1038/eye.2015.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saunders LJ, Russell RA, Crabb DP. Measurement precision in a series of visual fields acquired by the standard and fast versions of the Swedish interactive thresholding algorithm: analysis of large-scale data from clinics. JAMA Ophthalmol 2015;133:74–80. 10.1001/jamaophthalmol.2014.4237 [DOI] [PubMed] [Google Scholar]
- 13. Junoy Montolio FG, Wesselink C, Gordijn M, et al. Factors that influence standard automated perimetry test results in glaucoma: test reliability, technician experience, time of day, and season. Invest Ophthalmol Vis Sci 2012;53:7010–7. 10.1167/iovs.12-10268 [DOI] [PubMed] [Google Scholar]
- 14. Gardiner SK, Demirel S, Johnson CA. Is there evidence for continued learning over multiple years in perimetry? Optom Vis Sci 2008;85:1043–8. 10.1097/OPX.0b013e31818b9b40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wild JM, Searle AET, Dengler-Harles M, et al. Long-Term follow-up of baseline learning and fatigue effects in the automated perimetry of glaucoma and ocular hypertensive patients. Acta Ophthalmol 1991;69:210–6. 10.1111/j.1755-3768.1991.tb02713.x [DOI] [PubMed] [Google Scholar]
- 16. Mills RP, Budenz DL, Lee PP, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol 2006;141:24–30. 10.1016/j.ajo.2005.07.044 [DOI] [PubMed] [Google Scholar]
- 17. Burr JM, Mowatt G, Hernández R, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess 2007;11:iii-iv, ix-x, 1-190 10.3310/hta11410 [DOI] [PubMed] [Google Scholar]
- 18. Bryan SR, Crabb DP. A new graphical tool for assessing visual field progression in clinical populations. Transl Vis Sci Technol 2018;7:22 10.1167/tvst.7.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saunders LJ, Russell RA, Kirwan JF, et al. Examining visual field loss in patients in glaucoma clinics during their predicted remaining lifetime. Invest Ophthalmol Vis Sci 2014;55:102–9. 10.1167/iovs.13-13006 [DOI] [PubMed] [Google Scholar]
- 20. Office for National Statistics National life tables, UK, 2018. Available: https://www.ons.gov.uk/peoplepopulationandcommunitypeoplepopulationandcommunity/birthsdeathsandmarriagesbirthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdomnationallifetablesunitedkingdom/2015to2017
- 21. R Core Team R: a language and environment for statistical computing, 2018. Available: http://www.r-project.org/
- 22. Boodhna T, Crabb DP. Disease severity in newly diagnosed glaucoma patients with visual field loss: trends from more than a decade of data. Ophthalmic Physiol Opt 2015;35:225–30. 10.1111/opo.12187 [DOI] [PubMed] [Google Scholar]
- 23. Crabb DP, Saunders LJ, Edwards LA. Cases of advanced visual field loss at referral to glaucoma clinics - more men than women? Ophthalmic Physiol Opt 2017;37:82–7. 10.1111/opo.12328 [DOI] [PubMed] [Google Scholar]
- 24. Boodhna T, Saunders LJ, Crabb DP. Are rates of vision loss in patients in English glaucoma clinics slowing down over time? Trends from a decade of data. Eye 2015;29:1613–9. 10.1038/eye.2015.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quigley HA, West SK, Rodriguez J, et al. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol 2001;119:1819–26. 10.1001/archopht.119.12.1819 [DOI] [PubMed] [Google Scholar]
- 26. Dean BB, Lam J, Natoli JL, et al. Review: use of electronic medical records for health outcomes research. Med Care Res Rev 2009;66:611–38. 10.1177/1077558709332440 [DOI] [PubMed] [Google Scholar]
- 27. Hanna KE, Anderson SM, Maddox SD. Think research: using electronic medical records to bridge patient care and research; 2005.
- 28. Chiang MF, Boland MV, Brewer A, et al. Special requirements for electronic health record systems in ophthalmology. Ophthalmology 2011;118:1681–7. 10.1016/j.ophtha.2011.04.015 [DOI] [PubMed] [Google Scholar]
- 29. Nghiem AZ, Canning C, Eason J, et al. Going paperless: improved cataract surgery outcome data quality in a new fully electronic unit. Eye 2019;33:948–52. 10.1038/s41433-019-0350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sparrow JM, Taylor H, Qureshi K, et al. The cataract national dataset electronic multi-centre audit of 55,567 operations: risk indicators for monocular visual acuity outcomes. Eye 2012;26:821–6. 10.1038/eye.2012.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnston RL, Taylor H, Smith R, et al. The cataract national dataset electronic multi-centre audit of 55,567 operations: variation in posterior capsule rupture rates between surgeons. Eye 2010;24:888–93. 10.1038/eye.2009.195 [DOI] [PubMed] [Google Scholar]
- 32. Jaycock P, Johnston RL, Taylor H, et al. The cataract national dataset electronic multi-centre audit of 55,567 operations: updating benchmark standards of care in the United Kingdom and internationally. Eye 2009;23:38-49 10.1038/sj.eye.6703015 [DOI] [PubMed] [Google Scholar]
- 33. Boland MV. Electronic health records and ophthalmology: a work in progress. JAMA Ophthalmol 2015;133:633-4 10.1001/jamaophthalmol.2015.0913 [DOI] [PubMed] [Google Scholar]
- 34. Boland MV, Chiang MF, Lim MC, et al. Adoption of electronic health records and preparations for demonstrating meaningful use: an American Academy of ophthalmology survey. Ophthalmology 2013;120:1702–10. 10.1016/j.ophtha.2013.04.029 [DOI] [PubMed] [Google Scholar]
- 35. Sanders DS, Read-Brown S, Tu DC, et al. Impact of an electronic health record operating room management system in ophthalmology on documentation time, surgical volume, and staffing. JAMA Ophthalmol 2014;132:586–92. 10.1001/jamaophthalmol.2013.8196 [DOI] [PubMed] [Google Scholar]
- 36. Rotchford AP, Hughes J, Agarwal PK, et al. Prevalence of treatment with glaucoma medication in Scotland, 2010-2017. Br J Ophthalmol 2019:bjophthalmol-2019-314206 10.1136/bjophthalmol-2019-314206 [DOI] [PubMed] [Google Scholar]