Abstract
This study evaluated effects of differing gel volumes on pharmacokinetics (PK). IQB4012, a gel containing the non-nucleoside reverse transcriptase inhibitor IQP-0528 and tenofovir (TFV), was applied to the pigtailed macaque vagina and rectum. Vaginal gel volumes (1% loading of both drugs) were 0.5 or 1.5 ml; following wash-out, 1 or 4 ml of gel were then applied rectally. Blood, vaginal, and rectal fluids were collected at 0, 2, 4, and 24 h. Vaginal and rectal tissue biopsies were collected at 4 and 24 h. There were no statistically significant differences in concentrations for either drug between gel volumes within compartments at matched time points. After vaginal gel application, median IQP-0528 concentrations were ~ 104–105 ng/g, 105–106 ng/ml, and 103–105 ng/ml in vaginal tissues, vaginal fluids, and rectal fluids, respectively (over 24 h). Median vaginal TFV concentrations were 1–2 logs lower than IQP-0528 levels at matched time points. After rectal gel application, median IQP-0528 and TFV concentrations in rectal fluids were ~ 103–105 ng/ml and ~ 102–103 ng/ml, respectively. Concentrations of both drugs sampled in rectal tissues were low (~ 101–103 ng/g). For 1 ml gel, half of sampled rectal tissues had undetectable concentrations of either drug, and over half of sampled rectal fluids had undetectable TFV concentrations. These results indicate differences in drug delivery between the vaginal and rectal compartments, and that smaller vaginal gel volumes may not significantly compromise microbicide PK and prophylactic potential. However, effects of rectal gel volume on PK for both drugs were less definitive.
Keywords: HIV prevention, Vaginal gel, Rectal gel, Macaque, Pharmacokinetics, IQP0528, PrEP
Introduction
Among the HIV preexposure prophylaxis (PrEP) modalities in development today, microbicide gels remain a significant arm of the antiretroviral (ARV)-based prevention pipeline. Although oral PrEP in the form of once-daily Truvada® (emtricitabine and tenofovir disoproxil fumarate) is now FDA approved in the USA [1] and has been shown to be effective at preventing HIV acquisition by 44–86% among various demographics [2–8] particularly when adherence is high, other biomedical prevention tools are also being developed to expand affordable and reliable options for at-risk populations. Alternative PrEP methods such as 30–90-day intravaginal rings, long-acting injectables, and on-demand products containing one or more ARVs are under research and development, to broaden users’ choices and to help circumvent issues related to product adherence, potential long-term toxicity, accessibility, and/or cost concerns that can be associated with oral PrEP [4, 9–13].
Microbicide gels are intended to be an on-demand option for men and women who engage in vaginal and/or receptive anal intercourse (RAI). By coating the vaginal and rectal mucosa, they deliver drug directly down into the tissue, through which it migrates to infectible host cells along the length of the vaginal and rectal canals that may be exposed to semen [14]. They may also serve as physical barriers to HIV migration into the tissue [15, 16]. However, despite extensive studies of the vaginal gels, research on rectal gels has proceeded at a slower rate, and safe and efficacious rectal microbicide gels are still in the early stages of development [17, 18]. Extension and application of our knowledge of vaginal gels to rectal microbicide gels must address biological and behavioral challenges. Hyperosmolar vaginal gel formulations or personal lubricants are deemed unsuitable for rectal use due to epithelial damage and potentially increased risk of infection [19–21]. Vaginal gels as a PrEP modality in women have had low efficacy rates to date, for multiple reasons [4, 22–27]. Recent findings point to vaginal microflora as a contributing factor, with potential effects on genital inflammation, and ARV uptake and/or stability in vaginal tissues after gel application, but further studies of this phenomenon are needed [28–31]. A pattern that does clearly emerge from these clinical trials is behavior-based: low adherence is cited as a common factor across trials [4, 22, 25, 26, 32]. In light of these findings, emphasis is increasingly placed on participants’ personal experiences and preferences in an effort to understand and overcome barriers associated with poor adherence. Studies have revealed product-related issues such as gel leakage and messiness, as well as undesirable effects on intercourse [32–38]. Recommended microbicide gel volumes for vaginal application have been typically 4–5 ml [4, 7, 22, 25, 26], and acceptability studies involving rectal gels have evaluated volumes ranging from 1.5 to 35 ml [39–44]. While smaller gel volumes might address some user concerns regarding gel leakage, it is unclear whether these will achieve adequate gel distribution within the vaginal canal, and efficacious drug levels in vivo to protect against HIV infection. However, mathematical models evaluating the effects of gel volume on vaginal spreading and consequent mucosal drug delivery have predicted that smaller volumes may provide nearly equivalent drug delivery while reducing gel leakage [14].
While modeling and imaging studies are valuable for guiding microbicide gel design and use, the experimental in vivo pharmacokinetic (PK) implications of variable gel volumes have not previously been evaluated. Using reduced gel volumes is also motivated by the prior behavioral studies. We have previously described PK profiles of a dual chamber gel, IQB3002, which contains 1% of the NNRTI IQP-0528, a small molecule pyrimidinedione that inhibits a number of HIV strains at low EC50 concentrations [45, 46]. The gels’ properties, particularly its low osmolality, are specifically formulated for safe application in both the vaginal and rectal compartments [47, 48]. Preliminary study of vaginal application in female rhesus macaques showed that IQP-0528 concentrations achieved in vaginal tissues and fluids were several orders of magnitude above the in vitro EC50 value [45]. Ex vivo virus infection assays further demonstrated that human peripheral blood mononuclear cells (PBMC) were protected from HIV-1 challenge when co-cultured with vaginal tissue biopsies from the gel-treated macaques [45]. Importantly, this level of protection was achieved with a reduced vaginal dose of 1.5 ml. That volume was allometrically scaled down versus a typical 4-ml clinically applied volume in humans via the ratio of approximate surface area of the rhesus macaque vagina to that in women. Collectively, our findings prompted further investigation of effects of gel volume in microbicide PK evaluations, for both rectal and vaginal gels.
The current study extends our previous work and evaluates PK following application of four different volumes of a related IQP-0528 gel, IQB4012, in female pigtailed macaques (PT). Two volumes (0.5 or 1.5 ml) were tested in the vaginal compartment and two volumes (1 or 4 ml) were applied in the rectum. Gel IQB4012 is similar to IQB3002 evaluated in our previous study in that it is formulated for dual application in the vagina and rectum (Table 1). In addition to 1% IQP-0528, IQB4012 also contains 1% of the NRTI tenofovir (TFV).
Table 1.
DuoGel formulations
| Ingredient (w/w, %) | 3002 | 4012 |
|---|---|---|
| Tenofovir | / | 1.00 |
| IQP-0528 | 1.00 | 1.00 |
| Phosphate buffer | 93.90 | 93.15 |
| Glycerin | 2.50 | 2.50 |
| Hydroxyethyl cellulose | 2.10 | 1.00 |
| Carbopol | 0.25 | 1.00 |
| Methylparaben | 0.20 | 0.20 |
| Propylparaben | 0.05 | 0.05 |
| EDTA | / | 0.05 |
| Lactic acid | / | 0.05 |
Materials and methods
Drug and product formulation
IQB4012 was manufactured by ImQuest BioSciences (Frederick, MD). The gel (Table 1) contains 1% (w/w) IQP-0528 [49–51], 1% (w/w) TFV, 10 mM sodium phosphate solution in sterile molecular grade water (93.15% w/w), glycerol (2.5% w/w), methylparaben (0.2% w/w), propyl paraben (0.05% w/w), hydroxyethyl cellulose (1% w/w), carbopol (1% w/w), EDTA (0.05% w/w), DL-lactic acid (0.05% w/w) [48]. It has a pH of 6.00, osmolality of 360 mOsm/kg and viscosity @ 1 s−1 = 128.7 Pa s. The release properties of IQP-0528 and TFV from the gel into a liquid sink (Franz Cell) are 53.93 and 82.51 μg/cm 3h, respectively, with tissue permeability of 118.36 and 206.86 μg/cm3, respectively. The product was stored at room temperature (25 °C) in a dark, moisture-free environment until use.
Pigtailed macaques and study design
All procedures in this study were approved by the Centers for Disease Control and Prevention IACUC (Institutional Animal Care and Use Committee). Animals were housed under approved biosafety level 2 containment conditions at the Centers for Disease Control and Prevention (CDC), and their diet, care, and maintenance conformed to “Guide for the Care and Use of Laboratory Animals” guidelines [52]. Six sexually mature, naive female pigtailed macaques (PT) with an average body mass of 8.7 kg were utilized in this study. All PT were anesthetized prior to procedures using 10 mg/kg ketamine mixture intramuscularly, or other approved anesthetic such as Telazol, 2–6 mg/kg intramuscularly, as determined by CDC Animal Resources Branch (ARB) standard operating protocol and ARB staff including the attending veterinarian. Ketamine and Telazol were supplied by the staff pharmacist in the Animal Resources Branch in accordance with CDC guidelines. For vaginal PK experiments, each PT received 0.5 ml or 1.5 ml of IQB4012 gel following anesthesia. Rationale for dosing (gel volume) was based on relative scaling of the size of the macaque female reproductive tract relative to humans. The upper volume is therefore equivalent by volume to a human dose of 4 mL. Gel was delivered into the posterior vagina, near the cervix, using a sterile 10-ml syringe attached to a sterile gastric feeding tube (size, 5 or 9 French; 7 to 8 cm in length). Syringes were weighed pre and post gel application to verify weight and volume of gel delivered. Animals were maintained recumbent under anesthesia with the pelvis slightly elevated for 30 min to minimize gel leakage. Animals were placed in two groups of three each. One group received 0.5 mL of IQB4012, while the other group received 1.5 mL IQB4012 once weekly for 4 consecutive weeks. After a 2-week rest period, the doses of the groups were then switched for another 4 weeks so that all six macaques received each dose four times. The data presented are therefore a summary of 24 applications of 0.5 and 1.5 mL of IQB4012.
For rectal PK experiments, a similar syringe apparatus was used to deliver 1 or 4 ml of IQB4012 into the rectum 2 in. past the sphincter of each PT after performing a rectal wash with 10 ml physiological saline. As with the vaginal PK study, the same crossover design was used with rectal gel application performed once weekly for 4 weeks with a 2-week rest period before the crossover and an additional 4 weeks of application. The rectal PK experiments were initiated several weeks after the vaginal PK study, to allow for a suitable wash-out period.
Specimen collections
For the vaginal gel application experiments, specimens collected for PK assessments included blood plasma, vaginal and rectal 3-mm punch biopsy specimens (n = 3 punch biopsies per site), vaginal secretions (multiswab device fitted with Weck-Cel® sponges, collected proximally and distally relative to the cervix), and rectal secretions approximately 5 cm into the rectum (Weck-Cel® spears). Spears and sponges were weighed before and after specimen collection. Vaginal pH was determined by collecting a swab of vaginal fluid and rolling it on a pH colorimetric indicator strip (EMD Millipore). Blood and swabs were collected immediately prior to (0 h) and at specific time intervals (2 h, 4 h, and 24 h) after IQB4012 application (Table 2). Tissue biopsies were collected at 4 h and 24 h only. Identical time points and specimens were used for collection of blood plasma, rectal fluids, and rectal biopsies following rectal application of IQB4012 (Table 2). Blood was obtained via the saphenous or femoral veins using a 21–22 gauge vacutainer needle set-up into cell preparatory tubes (CPTs) and processed to obtain plasma and peripheral blood mononuclear cells. All specimens were collected, processed, and stored at − 70 °C until drug analysis as detailed previously [45].
Table 2.
Outline of study design and specimen collections. For the vaginal PK study, female pigtailed macaques (N = 6) were treated with 0.5 or 1.5 ml IQB4012 that was applied in the posterior vagina, proximal to the cervix. For the rectal PK study, 1 or 4 ml IQB4012 was applied in the rectum of N = 6 female pigtailed macaques. Specimens indicated were collected immediately before (0 h) and at 2, 4, and 24 h after each weekly gel application
| Sample | 0 h* | 2 h | 4 h | 24 h |
|---|---|---|---|---|
| Blooda (vaginal and rectal PK) | X | X | X | X |
| Vaginal multiswabb (vaginal PK) | X | X | X | X |
| Rectal spearsc (vaginal and rectal PK) | X | X | X | X |
| Vaginal pinch biopsiesd (vaginal PK) | - | - | X | X |
| Rectal pinch biopsiese (vaginal and rectal PK) | - | - | X | X |
| Vaginal pHf (vaginal PK) | X | X | X | X |
2-ml CPT tubes
Vaginal multiswab device loaded with eight Weck-Cel sponges; four proximal and four distal
Two rectal wicks/animal/time point
VAGINAL: three pinch biopsies/animal/time point (one prox, one med, one dist)
RECTAL: two pinch biopsies/animal/time point
Vaginal pH: pH indicator strips
Measurement of IQP-0528 and TFV concentrations
Vaginal fluids in individual multiswab Weck-Cel® sponges (two proximal and two distal per animal per time point each week), and rectal fluids in individual Weck-Cel spears (two spears per animal per time point each week) were analyzed for IQP-0528 and TFV levels. Each week, biopsy samples that included three punches from vaginal tissues (one proximal, one medial, one distal per time point per animal), and two punches from rectal tissues collected approximately 5 cm into the rectum were analyzed per animal per time point. Unlike the active form of TFV, IQP-0528 is rapidly transported bi-directionally across the cell membrane and is not restricted within the cell, and so tissues were not washed prior to analysis to avoid drug loss [46]. For plasma, 100 μl aliquots per animal per time point from each week were analyzed. The concentrations of IQP-0528 and TFV in the biological samples were determined based on a previously described liquid chromatography-tandem mass spectrometry (LC-MS) method [45, 53, 54]. The minimum level at which quantitative results could be obtained, the lower limit of quantitation (LLOQ), is ten times the standard deviation of injections at the lowest concentration which were statistically different from blank injections using a 99% confidence interval. The LLOQ of IQP-0528 for tissues, vaginal or rectal fluids, and plasma were determined to be 10 ng/sample for biopsies and 10 ng/ml for fluids. Vaginal fluid and tissue densities of 1.0 g/ml were used to convert weight to volume concentrations of IQP-0528 and TFV (ng/g of fluid or tissue). Reported concentrations in vaginal secretions were corrected to account for sponge net weight and dilution factors. Data for rectal fluids are reported as ng/ml of spear eluate.
Statistical analyses
Data shown are medians with minimum and maximum values unless indicated otherwise. To analyze differences in IQP-0528 and TFV levels across multiple time points and animals, one-way analyses of variance (ANOVA) were applied, with Dunns post-test analysis. Significant paired data sets (p < 0.05) were further analyzed ad-hoc using the Wilcoxon matched-pairs signed-rank t test. Analyses were performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA).
Results
Vaginal application of IQB4012
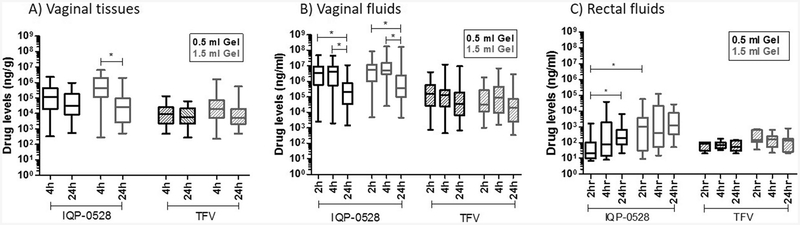
Specimens collected over a 24-h period after 0.5 ml gel application showed that median IQP-0528 concentrations in vaginal tissues and vaginal fluids at 4 h (open black boxes, Fig. 1a, b; Table 3) were of the order of 105 ng/g (95,000–290,086 ng/g) and 106 ng/ml (2,038,462−6,758,116 ng/ml), respectively. These were followed by a statistically significant (p < 0.0001) one log decrease in vaginal fluids at 24 h (257,071–328,205 ng/ml). IQP-0528 levels in vaginal fluids at 2 h were similar to those observed at 4 h. The graphs (Fig. 1a, b) illustrate pooled data from proximal, medial, and distal sites relative to the cervix, with Table 3 listing median concentrations at each of those sites. Data were grouped for analysis (Fig. 1a, b) as there was no significant difference in IQP-0528 concentrations across those sites at matched time points. Interestingly, application of 1.5 ml gel (open gray boxes, Fig. 1a, b; Table 3) yielded similar results in vaginal tissues and fluids, with IQP-0528 concentrations being in the same range as that following 0.5 ml gel treatment (open black boxes, Fig. 1a, b; Table 3). Slight amounts of gel were observed to be present at the 2-h collection, but gel was not present in the vast majority of the 4-h samples. Low concentrations of IQP-0528 were detected in rectal fluids (Fig. 1c and Table 3) following vaginal application of either volume of IQB4012, with median levels being in the range of 101–103 ng/ml over 24 h (20–1258 ng/ml). A significant difference between the two gel volumes was noted for IQP-0528 concentrations in rectal fluids at 2 h (open black and open gray boxes, Fig. 1c), but not at later time points. Analysis of rectal tissue biopsies revealed IQP-0528 levels below LLOQ (data not shown).
Fig. 1.
Analysis of IQP-0528 (open) and TFV (striped) levels in a vaginal tissues, b vaginal fluids, and c rectal fluids from N = 6 macaques treated vaginally with 0.5 (black) or 1.5 ml (gray) IQB-4012 gel. Results shown for vaginal samples include pooled data from proximal, medial, and distal sites. Concentrations at each of these sites are listed in Table 2. One-way ANOVA test with Dunns post-test analysis was performed. Significant paired data sets (p < 0.05) were further analyzed ad-hoc using Wilcoxon matched-pairs signed-rank t test, and yielded p values < 0.0001 where indicated (*)
Table 3.
Drug concentrations in vaginal tissues, vaginal fluids, and rectal fluids from N = 6 female pigtailed macaques treated vaginally with 0.5 or 1.5 ml IQB4012 gel containing 1% wt/wt each of IQP-0528 and TFV
| IQP-0528 median drug concentration (min—max) | |||||||
|---|---|---|---|---|---|---|---|
| Vaginal tissues (ng/g) | Vaginal fluids (ng/ml) | Rectal fluids (ng/ml) | |||||
| Proximalb | Medial | Distal | Proximal | Distal | - | ||
| 0.5 ml Gel | 2 h | N/A | N/A | N/A | 6,758,116 (2474–46,372,550) | 2,319,091 (BLLOQ-17,500,000) | 20 (BLLOQ-1583) |
| 4 h | 128,443 (323–2,344,444) | 290,086 (887–1,503,333) | 95,000 (BLLOQ-1,839,7441 | 6,014,248 (1946–43,815,790) | 2,038,462 (BLLOQ-17,891,890) | 77 (BLLOQ-37,600) | |
| 24 h | 43,878 (1622–962,500) | 22,527 (714–458,763) | 20,915 (545–224,753) | 257,071 (BLLOQ-5,200,000) | 328,205 (BLLOQ-11,034,8801 | 199 (BLLOQ-6288) | |
| Ratio (measured/EC50a) | N/A | N/A | N/A | 2 h-6,758,116 | 2 h-2,319,091 | 2 h-20 | |
| 4 h-6,014,248 | 4 h-2,038,462 | 4 h-77 | |||||
| 24 h-257,071 | 24 h-328,205 | 24 h-199 | |||||
| 1.5 ml Gel | 2 h | N/A | N/A | N/A | 5,654,526 (4714–57,865,860) | 6,022,388 (BLLOQ-83,227,270) | 1004 (BLLOQ-56,000) |
| 4 h | 611,547 (449–4,760,736) | 739,886 (278–6,377,273) | 213,000 (BLLOQ-6,404,545) | 4,925,373 (BLLOQ-60,750,000) | 4,636,643 (BLLOQ-173,000,000) | 411 (BLLOQ-120,000) | |
| 24 h | 23,873 (1489–1,054,000) | 46,315 (1087–2,031,746) | 24,167 (BLLOQ-517,742) | 475,000 (BLLOQ-159,000,000) | 375,385 (BLLOQ-32,714,2901 | 1258 (BLLOQ-26,475) | |
| Ratio (measured/EC50) | N/A | N/A | N/A | 2 h-5,654,526 | 2 h-6,022,388 | 2 h-1004 | |
| 4 h-4,925,373 | 4 h-4,636,643 | 4 h-411 | |||||
| 24 h-475,000 | 24 h-375,385 | 24 h-1258 | |||||
| TFV median drug concentration (min—maxi | |||||||
| Vaginal tissues (ng/g) | Vaginal fluids (ng/ml) | Rectal fluids (ng/ml) | |||||
| Proximal | Medial | Distal | Proximal | Distal | - | ||
| 0.5 ml Gel | 2 h | N/A | N/A | N/A | 158,146 (BLLOQ2-3,025,157) | 232,128 (BLLOQ-3,968,750) | 80 (BLLOQ-104) |
| 4 h | 4486 (484–60,000) | 8857 (714–130,909) | 13,367 (BLLOQ-72,051) | 124,304 (BLLOQ-12,113,400) | 132,458 (BLLOQ-1,971,250) | 69 (BLLOQ-176) | |
| 24 h | 16,932 (270–61,786) | 3553 (BLLOQ-30,000) | 5906 (BLLOQ-37,030) | 48,942 (BLLOQ-9,600,000) | 18,709 (BLLOQ-267,794) | 53 (BLLOQ-139) | |
| Ratio (measured/EC50) | N/A | N/A | N/A | 2 h-230 | 2 h-1009 | 2 h-0.12 | |
| 4 h-180 | 4 h-192 | 4 h-0 | |||||
| 24 h-71 | 24 h-27 | 24 h-0.08 | |||||
| 1.5 ml Gel | 2 h | N/A | N/A | N/A | 30,394 (BLLOQ-1,162,921) | 37,183 (BLLOQ-1,776,786) | 138 (BLLOQ-726) |
| 4 h | 35,081 (225–1,613,636) | 10,181 (417–835,784) | 19,250 (BLLOQ-1,200,000) | 54,269 (BLLOQ-2,645,455) | 152,548 (BLLOQ-6,993,750) | 156 (BLLOQ-624) | |
| 24 h | 2390 (BLLOQ202.466) | 12,200 (BLLOQ-546,825) | 5377 (BLLOQ-23,226) | 17,976 (BLLOQ-2,883,333) | 56,977 (BLLOQ-830,952) | 127 (BLLOQ-791) | |
| Ratio (measured/EC50) | N/A | N/A | N/A | 2 h-44.1 | 2 h-54 | 2 h-0.20 | |
| 4 h-78.8 | 4 h-221 | 4 h-0.23 | |||||
| 24 h-26.1 | 24 h-82.7 | 24 h-0.18 | |||||
N/A not applicable, BLLOQ below lower limit of quantitation
EC50 values of 1.0 ng/ml for IQP-0528 and 689 ng/ml for TFV were used to calculate measured drug/EC50 ratios
P,M, and D refer to location of sample collection in relation to cervix. P proximal, M medial, D distal
Similar to the trends observed for IQP-0528 PK, there were no significant differences in TFV concentrations across all time points when comparing applications of 0.5 and 1.5 ml gel in the vaginal canal. Median levels of TFV were 1–2 logs lower than IQP-0528 concentrations at matched time points over the 24-h period. They were 103–104 ng/g in vaginal tissues (4486–13,367 ng/g for 0.5 ml gel; 10,181–35,081 ng/g for 1.5 ml gel) and 104–105 ng/ml in vaginal secretions (124,304–132,458 ng/ml for 0.5 ml gel; 54,269–152,548 ng/ml for 1.5 ml gel) at 4 h, followed by a negligible decrease in concentrations at 24 h (striped black and striped gray boxes, Fig. 1a, b; Table 3). Similar to IQP-0528 levels in the rectum, median concentrations of TFV in rectal secretions were low and not always detectable and did not exceed 200 ng/ml over 24 h (striped black and striped gray boxes, Fig. 1c; Table 3). Concentrations in rectal biopsy tissues were below the LLOQ (1 ng/sample, data not shown). In summary, these data collectively show that a twofold difference in gel volume (0.5 versus 1.5 ml) did not have a significant impact on the PK of either IQP-0528 or TFV in the vaginal compartment. Plasma concentrations of both compounds were below the LLOQ (10 ng/ml, data not shown).
Rectal application of IQB4012
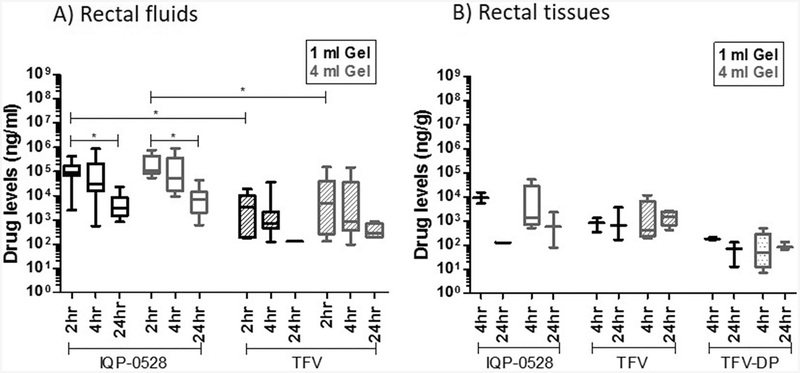
After a wash-out period following the vaginal PK study, rectal PK experiments were conducted in the same macaques. Either 1 or 4 ml of IQB4012 gel was applied rectally. Rectal spears were collected at 2, 4, and 24 h, and rectal pinch biopsies were collected at 4 and 24 h, which are time points identical to those considered in the vaginal PK study. Application of 1 ml IQB4012 gel yielded median IQP-0528 concentrations in the two-log range, 103–105 ng/ml in rectal fluids. There was a statistically significant (p < 0.0001) decrease from 93,375 ng/ml at 2 h to 2975 ng/ml at 24 h (open black bars, Fig. 2a; Table 4). Following application of 4 ml of gel, similar IQP-0528 concentrations were observed in rectal fluids over the 24-h period (open gray bars, Fig. 2a; Table 4). Interestingly, despite the relatively high IQP-0528 concentrations present in rectal fluids, approximately half of the rectal tissue samples analyzed were found to have IQP-0528 concentrations below the LLOQ. This was primarily found in the low volume group, where nearly two-thirds of rectal biopsies from animals treated with 1 ml had undetectable levels of IQP-0528. Tissue samples with detectable concentrations were in the range of 102–103 ng/g for both gel volumes tested (black open bars and gray open bars, Fig. 2b; Table 4), and there was no significant difference in IQP-0528 concentrations between the two gel volumes at matched time points.
Fig. 2.
Analysis of IQP-0528 (open), TFV (striped), and TFV-DP (dotted) levels in a rectal fluids and b rectal tissues from N = 6 macaques following rectal application of 1 (black) or 4 ml (gray) IQB-4012 gel. Concentrations at each of these sites are detailed in Table 3. One-way ANOVA test with Dunns post-test analysis was performed. Significant paired data sets (p < 0.05) were further analyzed ad-hoc using Wilcoxon matched-pairs signed-rank t test, and yielded EC50 < 0.0001 where indicated (*)
Table 4.
Drug concentrations in rectal tissues and rectal fluids from N = 6 female pigtailedmacaques following rectal application of 1 or 4 ml IQB4012 gel containing 1% wt/wt each of IQP-0528 and TFV+
| IQP-0528 median drug concentration (min-max) | ||||
|---|---|---|---|---|
| Rectal tissues (ng/g) | Rectal fluids (ng/ml) | |||
| 1 ml Gel | 2h | N/A | 93,375 (2480–420,500) | |
| 4h | 9087 (BLLOQ2-15,150) | 31,000 (545–873,500) | ||
| 24 h | 127* | 2975 (815–22,900) | ||
| Ratio (measured/EC50a) | N/A | 2 h-93,375 | ||
| 4 h-31,000 | ||||
| 24 h-2975 | ||||
| 4 ml Gel | 2h | N/A | 106,500 (54,600–769,000) | |
| 4h | 1277 (BLLOQ-51,304) | 53,775 (9570–930,500) | ||
| 24 h | 566 (BLLOQ-2228) | 7065 (608–43,750) | ||
| Ratio (measured/EC50) | N/A | 2 h-106,500 | ||
| 4 h-53,775 | ||||
| 24 h-7065 | ||||
| TFV/TFV-DP median drug concentration (min-max) | ||||
| Rectal tissues (ng/g) | Rectal fluids (ng/ml) | |||
| TFV | TFV-DP | |||
| 1 ml Gel | 2h | N/A | N/A | 3230 (BLLOQ-18,600) |
| 4h | 811 (BLLOQ-1283) | 176 (BLLOQ-207) | 705 (BLLOQ-34,650) | |
| 24 h | 659 (BLLOQ-3586) | 70 (BLLOQ-128) | 132* | |
| Ratio (Measured /EC50) | N/A | N/A | 2 h-4.69 | |
| 4 h-1.02 | ||||
| 24 h-0.19 | ||||
| 4 ml Gel | 2h | N/A | N/A | 5065 (BLLOQ-151,500) |
| 4h | 393 (BLLOQ-11,464) | 47 (BLLOQ-480) | 864 (BLLOQ-147,000) | |
| 24 h | 1411 (BLLOQ-2648) | 81 (BLLOQ-131) | 277 (BLLOQ-816) | |
| Ratio (measured/EC50) | NA | NA | 2 h-7.35 | |
| 4 h-0.32 | ||||
| 24 h-0.12 | ||||
N/A not applicable, BLLOQ below lower limit of quantitation
EC50 values of 1.0 ng/ml for IQP-0528 and 689 ng/ml for TFV were used to calculate measured drug/EC50 ratios
Drug detected in only one sample
Analysis of TFV levels in rectal fluids showed similar patterns to those for IQP-0528. Volumes of 1 ml (striped black bars, Fig. 2a; Table 4) and 4 ml (striped gray bars, Fig. 2a; Table 4) yielded comparable drug concentrations at matched time points. Specifically, median fluid TFV concentrations were on the order of 103 ng/ml (3230 ng/ml for 1 ml gel; 5065 ng/ml for 4 ml gel) at 2 h, and 102 ng/ml at 4 h (705 ng/ml for 1 ml gel; 864 ng/ml for 4 ml gel) and 24 h (132 ng/ml for 1 ml gel; 277 ng/ml for 4 ml gel). It should be noted that approximately two-thirds of rectal fluid samples from the low volume group yielded TFV levels below the LLOQ. The levels of TFV and TFV-diphosphate (TFV-DP), the active intracellular form of TFV, in approximately half of the rectal tissue samples from animals treated with 1 ml gel were also below the LLOQ, with detectable concentrations (Table 4) being at or near the magnitude of 102 ng/g at 4 h (811 ng/g TFV; 176 ng/g TFV-DP) and 24 h (659 ng/g TFV; 70 ng/g TFV-DP). Median concentrations of TFVand TFV-DP in rectal tissues from the 4-ml gel volume group (striped gray bars and dotted gray bars, Fig. 2b; Table 4) were not significantly different from the low volume group at matched time points. Median TFV and TFV-DP concentrations were near 102–103 ng/g or less at 4 h (393 ng/g TFV; 47 ng/g TFV-DP) and 24 h (1,411 ng/g TFV; 81 ng/g TFV-DP). To summarize, the PK results showed that IQP-0528 and TFV/TFV-DP concentrations in the rectum were each comparable between the 1 and 4 ml gel groups over a 24-h period, with a notable number of the rectal specimens yielding values below LLOQ (10 ng/sample), particularly for TFVand the low volume (1 ml) group. Systemic drug absorption was not apparent after rectal application of IQB4012, as levels of both drugs were below the LLOQ (10 ng/ml) in blood plasma (data not shown).
Discussion
Microbicide gel volume is one of many factors that influence user acceptability, but its impact on in vivo PK has not been fully elucidated. The impact of gel volume may differ among microbicide drugs with different physicochemical properties and may be different for vaginal versus rectal gel application. The present study addressed three factors; gel volume, drug and target compartment. The test gel, IQB4012, has been developed specifically to address the need for a safe and effective rectal microbicide product, particularly given the prevalence of receptive anal intercourse in heterosexual populations and its impact on HIV transmission [55–64]. Because IQB4012 is formulated for safe application both in the vagina and rectum [47, 48], convenience to user(s) is promoted, bypassing the need for separate products for vaginal and rectal use and potentially improving product acceptability and adherence. This formulation was engineered as an improvement on its predecessor, IQB3002, with rheological properties designed not simply to promote good initial spreading along the vaginal and rectal canals, but improved retention after initial application (Table 1).
MRI-based in vivo imaging studies of two volumes (2.5 versus 3.5 ml) of up to seven different microbicide gels and personal lubricants in women showed that there were no statistically significant differences between the volumes tested [65]. Recently, an optical imaging study comparing vaginal applications of 2 versus 4 ml of the HEC gel used as a placebo in microbicide trials, found differences in coating thickness but not extent of coating along the canal; computational modeling inferences from those data suggested small differences in mucosal drug delivery between the two volumes [66]. However, it is important to note that besides volume and gel composition, host factors such as ambulation, presence of semen, and local dilution of gel (estimated to range 10–30%) due to ambient fluid in the vaginal compartment, also impact a gel’s rheological properties and consequent deployment and drug delivery [67–71].
Co-formulation of IQB4012 with both IQP-0528 and TFV provides two drugs with contrasting physicochemical properties: IQB4012 is hydrophobic and lipophilic, while TFV is relatively hydrophilic. Those distinctions impact the relative transport rates of the drugs out from the gel and into and through the mucosal tissue. These two drugs have exhibited additive to synergistic activity that is concentration dependent when tested with primary HIV-1 isolates in vitro [72]. IQP-0528 diffuses bi-directionally across cell membranes, whereas TFV is phosphorylated within cells and retained with a long half-life [46, 73, 74]. This allows for one of the active drugs to be readily available to trafficking cells. These differing characteristics may well be advantageous for better protection when the two drugs are delivered together. The susceptible cells in the mucosa are loaded with TFV-DP, and this has been a good correlate of protection in macaque efficacy studies with gels and intravaginal rings [73, 75]. This formulation advantage may be similar to what has been observed for the combination of TFVand FTC in macaque efficacy studies where the PK of the two compounds lead to a more broad window of protective efficacy than a single drug alone [76].
Results from this study show that the concentrations of IQP-0528 measured in fluids from the vaginal compartment after vaginal application were 5–6 logs higher than the reported EC50/IC50 value for IQP-0528 [46, 47, 49–51, 73, 77] up to 24 h post gel application, and were 1–3 logs higher than the EC50 in rectal fluids (Table 3, ratio of measured/EC50). In contrast, the measured drug/EC50 ratio for TFV when applied vaginally was substantially lower than (range 26–1009) reflecting the difference in potency of the two drugs (Table 3). A very similar trend was evident when the gel was applied rectally (Table 3), with IQP-0528 ratios being 3–5 logs higher than the EC50 and the TFV ratios being substantially lower in rectal fluids (range 0.12–7.35).
Results showed no significant differences between applied gel volumes in fluid and tissue levels for both IQP-0528 and for TFV in the vagina and in the rectum. Further, rectal tissue TFV-DP levels did not differ between volumes when applied rectally. Differences were not detected in tissue concentrations of both drugs sampled at proximal versus medial versus distal locations along the vaginal canal, i.e., their longitudinal drug delivery was uniform. Furthermore, bi-directional dosing was observed as shown by presence of drug, albeit at low concentrations, in rectal fluids following vaginal application of both 0.5 and 1.5 ml IQB4012. Similar effects were observed when comparing PK data for the two volumes (1 versus 4 ml) tested in the rectal PK study, although the majority of specimens collected from the low volume (1 ml) group had undetectable levels of IQP-0528 and TFV. These results were somewhat surprising given that the total drug in the lower volumes was one fourth of that in the higher volumes. However, the range of measured values was such that a fourfold difference would be difficult to detect.
We emphasize, however, that IQP-0528 (and other microbicides, more generally) delivery and potency within the rectal compartment may be different from those for the vagina. Many factors could contribute to this, including: compartment size and openness; composition of luminal fluids and luminal drug clearance processes; mucosal tissue structure and cell types, including target cells and their distributions; mucosal tissue permeability; and pH and the microbiome. In addition to differences in length and mucosal surface area, the mucosal structures of the two organs are different. The vagina has a multilayer, non-secretory, stratified squamous epithelium with thickness of hundreds of micrometers. Infectible host cells populate the lamina propria below it, which is primarily connective tissue. The rectal mucosa is characterized by a single layer of mucus-secreting columnar epithelial cells arranged in rugae that extend millimeters down into the mucosa; the lamina propria wraps around the rugae, and extends downward, containing pockets of lipids within its connective tissue. These qualitatively different environments present different permeabilities to migrating molecules, and virions. Vaginalrectal contrasts in TFV delivery have been predicted by a deterministic computational PK model [78].
The threshold for minimum volumes may be higher for the rectum than the vaginal canal because of physiological and anatomical differences. If potentially efficacious drug levels can be achieved with lower gel volumes as suggested by these results, a “less is more” approach presents a feasible option that could help improve user perceptibility and experience of this gel, thereby encouraging product adherence. However, user sensory perceptions are multifaceted and what might be acceptable to some may not be to others. The data reported herein are only intended to provide information about the viability of additional choices. Further investigation is needed, particularly to determine the optimal volume for use in the rectal compartment. There is currently no consensus for rectal microbicide gel volumes, unlike the relatively narrow range used for vaginal gels (3.5–4 ml in clinical trials); a wide range (1.5–35 ml) of volumes has been tested for rectal use thus far. Other gel formulation properties that govern spreading and absorption may have to be adjusted accordingly to account for volume, especially when user-dependent characteristics come into play. Indeed, a caveat of the current study is that it is not designed to factor in ambulation due to necessary sedation of macaques during experiments. Thus, its effect on gel spreading and subsequent PK relative to gel volume remains to be elucidated. In addition, we also cleansed the area with a 10-ml saline wash prior to insertion. Cleansing prior to RAI is common, but not always the case. The IQB4012 gel formulation has demonstrated good safety profiles in human ectocervical and colorectal tissue explants, while also effectively inhibiting HIV-1 replication in these tissue models at concentrations as low as 10 μM (A. Ham et al., unpublished data). The findings from the in vivo study described herein build upon these promising characteristics of IQB4012. They complement computational PK models and imaging studies and provide further insight on ARV PK in the context of gel volume. This, in turn, can inform microbicide gel applications in clinical trial settings.
In summary, our results collectively show that the dual compartment IQB4012 gel is capable of delivering both IQP-0528 and TFV in vaginal and rectal tissues to levels well above concentrations that are inhibitory to HIV-1 in vitro. Lowering gel volumes by a factor of 3–4, and a corresponding reduction of total drug, did not appear to significantly impact PK of IQP-0528 and TFVin the vaginal and rectal compartments. Additional studies using a reduction in total drug of tenfold or more might result in a significant difference in drug levels, but a reduction of tenfold in volume was beyond the scope of this study. Importantly, our findings are consistent with our previous report evaluating vaginal application of 1.5 ml IQB3002 gel formulation, and also align with optical imaging and computational modeling studies that showed pharmacologically small effects when varying gel volume [79]. These findings provide relevant information for clinical trials of microbicide gels, and lay a foundation for future studies that include investigating the impact of lower gel volumes on efficacy.
Acknowledgments
Research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number 5U19AI101961. We acknowledge the following members of the CDC DHAP Laboratory Branch/Preclinical Evaluation Team for their contributions to our nonhuman primate research: David Garber, James Mitchell, Frank Deyounks, Shanon Ellis, and Kristen Kelley.
All experiments comply with the current laws of the country in which they were performed. All institutional and national guidelines for the care and use of laboratory animals were followed.
Footnotes
Conflict of interest Lara E. Pereira, Tyana Singletary, Amy Martin, Chuong T. Dinh, Angela Holder, and Janet McNicholl declare no conflicts of interest. Karen W. Buckheit, Robert W. Buckheit, Jr., and Anthony S. Ham are employed by ImQuest Biosciences and received funding from NIH grant # 5U19AI101961 and declare no conflicts of interest. David F. Katz and James M. Smith received funding from NIH grant # 5U19AI101961 and declare no conflicts of interest.
Publisher's Disclaimer: Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.FDA approves first drug for reducing the risk of sexually acquired HIV infection [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm2012.
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6): 509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373(23):2237–46. [DOI] [PubMed] [Google Scholar]
- 6.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. [DOI] [PubMed] [Google Scholar]
- 7.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013): 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51(5):496–505. [DOI] [PubMed] [Google Scholar]
- 10.Horberg M, Raymond B. Financial policy issues for HIV pre-exposure prophylaxis: cost and access to insurance. Am J Prev Med. 2013;44(1 Suppl 2):S125–8. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson DL, Spiegelman D, Knox TK, Wilson IB. Evolution and predictors of change in total bone mineral density over time in HIV-infected men and women in the nutrition for healthy living study. J Acquir Immune Defic Syndr. 2008;49(3):298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahon JM, Myers JE, Kurth AE, Cohen SE, Mannheimer SB, Simmons J, et al. Oral pre-exposure prophylaxis (PrEP) for prevention of HIV in serodiscordant heterosexual couples in the United States: opportunities and challenges. AIDS Patient Care STDs. 2014;28(9):462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mack N, Odhiambo J, Wong CM, Agot K. Barriers and facilitators to pre-exposure prophylaxis (PrEP) eligibility screening and ongoing HIV testing among target populations in Bondo and Rarieda, Kenya: results of a consultation with community stakeholders. BMC Health Serv Res. 2014;14:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz DF, Yuan A, Gao Y. Vaginal drug distribution modeling. Adv Drug Deliv Rev. 2015;92:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai BE, Henderson MH, Peters JJ, Walmer DK, Katz DF. Transport theory for HIV diffusion through in vivo distributions of topical microbicide gels. Biophys J. 2009;97(9):2379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai BE, Geonnotti AR, Desoto MG, Montefiori DC, Katz DF. Semi-solid gels function as physical barriers to human immunodeficiency virus transport in vitro. Antivir Res. 2010;88(2):143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiruy H, Fuchs EJ, Marzinke MA, Bakshi RP, Breakey JC, Aung WS, et al. A phase 1 randomized, blinded comparison of the pharmacokinetics and colonic distribution of three candidate rectal microbicide formulations of tenofovir 1% gel with simulated unprotected sex (CHARM-02). AIDS Res Hum Retrovir. 2015;31(11): 1098–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGowan I, Cranston RD, Duffill K, Siegel A, Engstrom JC, Nikiforov A, et al. A phase 1 randomized, open label, rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of three formulations of tenofovir 1% gel (the CHARM-01 study). PLoS One. 2015;10(5):e0125363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs EJ, Lee LA, Torbenson MS, Parsons TL, Bakshi RP, Guidos AM, et al. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: potential implication for HIV transmission. J Infect Dis. 2007;195(5):703–10. [DOI] [PubMed] [Google Scholar]
- 20.Rebe KB, De Swardt G, Berman PA, Struthers H, McIntyre JA. Sexual lubricants in South Africa may potentially disrupt mucosal surfaces and increase HIV transmission risk among men who have sex with men. S Afr Med J. 2013;104(1):49–51. [DOI] [PubMed] [Google Scholar]
- 21.Vishwanathan SA, Morris MR, Wolitski RJ, Luo W, Rose CE, Blau DM, et al. Rectal application of a highly osmolar personal lubricant in a macaque model induces acute cytotoxicity but does not increase risk of SHIV infection. PLoS One. 2015;10(4):e0120021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashuba AD, Gengiah TN, Werner L, Yang KH, White NR, Karim QA, et al. Genital tenofovir concentrations correlate with protection against HIV infection in the CAPRISA 004 trial: importance of adherence for microbicide effectiveness. J Acquir Immune Defic Syndr. 2015;69(3):264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Straten A, Brown ER, Marrazzo JM, Chirenje MZ, Liu K, Gomez K, et al. Divergent adherence estimates with pharmacokinetic and behavioural measures in the MTN-003 (VOICE) study. J Int AIDS Soc. 2016;19(1):20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rees H, Delany-Moretlwe S, Baron D, Lombard C, Gray G, Myer L, et al. FACTS 001 Phase III trial of pericoital tenofovir 1% gel for HIV prevention in women [abstract 26LB]. Program and abstracts of the 2015 Conference on Retroviruses and Opportunistic Infections (CROI) Seattle: CROI 2015. [Google Scholar]
- 26.CONRAD. FACTS 001 results presented at CROI 2015. Available at www.conradorg/news-pressreleases-107html. 2015. [Google Scholar]
- 27.McGowan I, Taylor DJ. Heterosexual anal intercourse has the potential to cause a significant loss of power in vaginal microbicide effectiveness studies. Sex Transm Dis. 2010;37(6):361–4. [PubMed] [Google Scholar]
- 28.Abdool Karim S HIV infection in young women in Africa: an overview. . 21st International AIDS Conference, Durban 2016;Presentation TUSS0602. [Google Scholar]
- 29.Abdool Karim S Understanding the high rates of HIV in young women in Africa: implications of new epidemiological, phylogenetic, genomic and proteomic evidence. 21st Internaional AIDS Conference, Durban 2016;Presentation TUSS0606. [Google Scholar]
- 30.Burgener A, Klatt N. Uncovering the role of the vaginal microbiome in undermining PrEP efficacy in women. 21st Internaional AIDS Conference, Durban 2016;Presentation TUSS0605. [Google Scholar]
- 31.Passmore J-A, Williams B. Role of vaginal microbiota in genital inflammation and enhancing HIV acquisition in women. 21st Internaional AIDS Conference, Durban 2016;Presentation TUSS0604. [Google Scholar]
- 32.van der Straten A, Stadler J, Montgomery E, Hartmann M, Magazi B, Mathebula F, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PLoS One. 2014;9(2):e89118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carballo-Dieguez A, Giguere R, Dolezal C, Chen BA, Kahn J, Zimet G, et al. “Tell Juliana”: acceptability of the candidate microbicide VivaGel(R) and two placebo gels among ethnically diverse, sexually active young women participating in a phase 1 microbicide study. AIDS Behav. 2012;16(7):1761–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahan ED, Zaveri T, Ziegler GR, Hayes JE. Relationships between perceptual attributes and rheology in over-the-counter vaginal products: a potential tool for microbicide development. PLoS One. 2014;9(9):e105614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrow KM, Fava JL, Rosen RK, Vargas S, Shaw JG, Kojic EM, et al. Designing preclinical perceptibility measures to evaluate topical vaginal gel formulations: relating user sensory perceptions and experiences to formulation properties. AIDS Res Hum Retrovir. 2014;30(1):78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrow KM, Ruiz MS. Assessing microbicide acceptability: a comprehensive and integrated approach. AIDS Behav. 2008;12(2):272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Straten A, Stadler J, Luecke E, Laborde N, Hartmann M, Montgomery ET, et al. Perspectives on use of oral and vaginal antiretrovirals for HIV prevention: the VOICE-C qualitative study in Johannesburg, South Africa. J Int AIDS Soc. 2014;17(3 Suppl 2): 19146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrow KM, Rosen RK, Vargas S, Katz DF, Fava JL, Kojic EM, et al. More…? Less…? Just right…? The role of perceived volume in gel and film perceptibility during intercourse, and its impact on product preference. AIDS Res Hum Retrovir. 2014;30(S1):A145. [Google Scholar]
- 39.Carballo-Dieguez A, Dolezal C, Bauermeister JA, O’Brien W, Ventuneac A, Mayer K. Preference for gel over suppository as delivery vehicle for a rectal microbicide: results of a randomised, crossover acceptability trial among men who have sex with men. Sex Transm Infect. 2008;84(6):483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carballo-Dieguez A, Exner T, Dolezal C, Pickard R, Lin P, Mayer KH. Rectal microbicide acceptability: results of a volume escalation trial. Sex Transm Dis. 2007;34(4):224–9. [DOI] [PubMed] [Google Scholar]
- 41.Gross M, Celum CL, Tabet SR, Kelly CW, Coletti AS, Chesney MA. Acceptability of a bioadhesive nonoxynol-9 gel delivered by an applicator as a rectal microbicide. Sex Transm Dis. 1999;26(10): 572–8. [DOI] [PubMed] [Google Scholar]
- 42.Tabet SR, Surawicz C, Horton S, Paradise M, Coletti AS, Gross M, et al. Safety and toxicity of nonoxynol-9 gel as a rectal microbicide. Sex Transm Dis. 1999;26(10):564–71. [DOI] [PubMed] [Google Scholar]
- 43.McGowan I, Hoesley C, Cranston RD, Andrew P, Janocko L, Dai JY, et al. A phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel (MTN-007). PLoS One. 2013;8(4):e60147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carballo-Dieguez A, Giguere R, Dolezal C, Bauermeister J, Leu CS, Valladares J, et al. Rectal-specific microbicide applicator: evaluation and comparison with a vaginal applicator used rectally. AIDS Behav. 2014;18(9):1734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pereira LE, Mesquita PM, Ham A, Singletary T, Deyounks F, Martin A, et al. Pharmacokinetic and pharmacodynamic evaluation following vaginal application of IQB3002, a dual-chamber microbicide gel containing the nonnucleoside reverse transcriptase inhibitor IQP-0528 in rhesus macaques. Antimicrob Agents Chemother. 2015;60(3):1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mesquita PM, Srinivasan P, Johnson TJ, Rastogi R, Evans-Strickfaden T, Kay MS, et al. Novel preclinical models of topical PrEP pharmacodynamics provide rationale for combination of drugs with complementary properties. Retrovirology. 2013;10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dezzutti CS, Shetler C, Mahalingam A, Ugaonkar SR, Gwozdz G, Buckheit KW, et al. Safety and efficacy of tenofovir/IQP-0528 combination gels—a dual compartment microbicide for HIV-1 prevention. Antivir Res. 2012;96:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ham AS, Nugent ST, Peters JJ, Katz DF, Shelter CM, Dezzutti CS, et al. The rational design and development of a dual chamber vaginal/rectal microbicide gel formulation for HIV prevention. Antivir Res. 2015;120:153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckheit RJ, Tracy LH, Karen MW, Chung SG, Cho EH. Comparative evaluation of the inhibitory activities of a series of pyrimidinedione congeners that inhibit human immunodeficiency virus types 1 and 2. Antimicrob Agents Chemother. 2008;52:225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buckheit RWJ, Hartman TL, Watson KM, Kwon HS, Lee SH, Lee JW, et al. The structure-activity relationships of 2,4(1H,3H)-pyrimidinedione derivatives as potent HIV type 1 and type 2 inhibitors. Antivir Chem Chemother. 2007;18:259–75. [DOI] [PubMed] [Google Scholar]
- 51.Buckheit RWJ, Watson K, Fliakas-Boltz V, Russell J, Loftus TL, Osterling MC, et al. SJ-3366, a unique and highly potent nonnucleoside reverse transcriptase inhibitor of human immunodeficiency virus type 1 (HIV-1) that also inhibits HIV-2. Antimicrob Agents Chemother. 2001;45:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. Eighth Edition. Eighth ed. Washington, D.C.: National Academies Press; 2011. [PubMed] [Google Scholar]
- 53.Kuklenyik Z, Martin A, Pau CP, Garcia-Lerma JG, Heneine W, Pirkle JL, et al. Effect of mobile phase pH and organic content on LC-MS analysis of nucleoside and nucleotide HIV reverse transcriptase inhibitors. J Chromatogr Sci. 2009;47:365–72. [DOI] [PubMed] [Google Scholar]
- 54.Johnson TJ, Srinivasan P, Albright TH, Watson-Buckheit K, Rabe L, Martin A, et al. Safe and sustained vaginal delivery of pyrimidinedione HIV-1 inhibitors from polyurethane intravaginal rings. Antimicrob Agents Chemother. 2012;56(3):1291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baggaley RF, Dimitrov D, Owen BN, Pickles M, Butler AR, Masse B, et al. Heterosexual anal intercourse: a neglected risk factor for HIV? Am J Reprod Immunol. 2013;69:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brody S, Weiss P. Heterosexual anal intercourse: increasing prevalence, and association with sexual dysfunction, bisexual behavior, and venereal disease history. J Sex Marital Ther. 2011;37:298–306. [DOI] [PubMed] [Google Scholar]
- 57.Duby Z, Colvin C. Conceptualizations of heterosexual anal sex and HIV risk in five East African communities. J Sex Res. 2014;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 58.Gorbach PM, Manhart LE, Hess KL, Stoner BP, Martin DH, Holmes KK. Anal intercourse among young heterosexuals in three sexually transmitted disease clinics in the United States. Sex Transm Dis. 2009;36:193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jenness SM, Begier EM, Neaigus A, Murrill CS, Wendel T, Hagan H. Unprotected anal intercourse and sexually transmitted diseases in high-risk heterosexual women. Am J Public Health. 2011;101: 745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalichman SC, Pinkerton SD, Carey MP, Cain D, Mehlomakulu V, Carey KB, et al. Heterosexual anal intercourse and HIV infection risks in the context of alcohol serving venues, cape town, South Africa. BMC Public Health. 2011;11:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalichman SC, Simbayi LC, Cain D, Jooste S. Heterosexual anal intercourse among community and clinical settings in cape town, South Africa. Sex Transm Infect. 2009;85:411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLellan-Lemal E, O’Daniels CM, Marks G, Villar-Loubet O, Doherty IA, Simpson C, et al. Sexual risk behaviors among African-American and Hispanic women in five counties in the southeastern United States: 2008–2009. Womens Health Issues. 2012;22:e9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28:1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reynolds GL, Fisher DG, Napper LE, Fremming BW, Jansen MA. Heterosexual anal sex reported by women receiving HIV prevention services in Los Angeles County. Womens Health Issues. 2010;20:414–9. [DOI] [PubMed] [Google Scholar]
- 65.Barnhart KT, Pretorius ES, Shaunik A, Timbers K, Nasution M, Mauck C. Vaginal distribution of two volumes of the novel microbicide gel cellulose sulfate (2.5 and 3.5 mL). Contraception. 2005;72(1):65–70. [DOI] [PubMed] [Google Scholar]
- 66.Katz DF, Ham A, Smith JM, Guthrie K, Simons M, Gao Y, et al. , editors. P07.09 Do Microbicide Gel Volume and Properties Matter? Effects on Deployment, PK and User Sensory Perceptions and Experiences HIV Research for Prevention 2016; 2016; Sheraton Grand Chicago, Chicago. [Google Scholar]
- 67.Barnhart KT, Pretorius ES, Shera DM, Shabbout M, Shaunik A. The optimal analysis of MRI data to quantify the distribution of a microbicide. Contraception. 2006;73(1):82–7. [DOI] [PubMed] [Google Scholar]
- 68.Kieweg SL, Katz DF. Squeezing flows of vaginal gel formulations relevant to microbicide drug delivery. J Biomech Eng. 2006;128(4): 540–53. [DOI] [PubMed] [Google Scholar]
- 69.Lai BE, Xie YQ, Lavine ML, Szeri AJ, Owen DH, Katz DF. Dilution of microbicide gels with vaginal fluid and semen simulants: effect on rheological properties and coating flow. J Pharm Sci. 2008;97(2):1030–8. [DOI] [PubMed] [Google Scholar]
- 70.Mauck CK, Katz D, Sandefer EP, Nasution MD, Henderson M, Digenis GA, et al. Vaginal distribution of Replens and K-Y jelly using three imaging techniques. Contraception. 2008;77(3):195–204. [DOI] [PubMed] [Google Scholar]
- 71.Mitchell C, Paul K, Agnew K, Gaussman R, Coombs RW, Hitti J. Estimating volume of cervicovaginal secretions in cervicovaginal lavage fluid collected for measurement of genital HIV-1 RNA levels in women. J Clin Microbiol. 2011;49(2):735–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hartman TL, Yang L, Buckheit RW Jr. Antiviral interactions of combinations of highly potent 2,4(1H,3H)-pyrimidinedione congeners and other anti-HIV agents. Antivir Res. 2011;92(3):505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dobard C, Sharma S, Martin A, Pau CP, Holder A, Kuklenyik Z, et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J Virol. 2012;86(2):718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burns RN, Hendrix CW, Chaturvedula A. Population pharmacokinetics of tenofovir and tenofovir-diphosphate in healthy women. J Clin Pharmacol. 2015;55(6):629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith JM, Rastogi R, Teller RS, Srinivasan P, Mesquita PM, Nagaraja U, et al. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci U S A. 2013;110(40): 16145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia-Lerma JG, Otten RA, Qari SH, Jackson E, Cong ME, Masciotra S, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5(2):e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dobard C, Sharma S, Parikh UM, West R, Taylor A, Martin A, et al. Postexposure protection of macaques from vaginal SHIV infection by topical integrase inhibitors. Sci Transl Med. 2014;6:227ra35. [DOI] [PubMed] [Google Scholar]
- 78.Gao Y, Katz DF. Multicompartmental pharmacokinetic model of tenofovir delivery to the rectal mucosa by an enema. PLoS One. 2017;12(1):e0167696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katz DF, Gao Y, Kang M. Using modeling to help understand vaginal microbicide functionality and create better products. Drug Deliv Transl Res. 2011;1(3):256–76. [DOI] [PMC free article] [PubMed] [Google Scholar]