Abstract
Heart rate variability (HRV) is a measure of autonomic nervous system activity, which reflects an individual’s ability to adapt to physiological and environmental changes. Low resting HRV has been linked to several mental health conditions, including depression, anxiety, and alcohol dependence (Kemp et al. in Biological Psychiatry 67(11):1067–1074, 2010. doi:10.1016/j.biopsych.2009.12.012; Kemp et al. in PloS One, 7(2):e30777, 2012; Quintana et al. in Drug and Alcohol Dependence, 132(1–2):395–398, 2013. doi:10.1016/j.drugalcdep.2013.02.025). HRV has also been used as a method for indexing the relative balance of sympathetic nervous system (SNS) activity to parasympathetic nervous system activity. This balance—in particular, moderately dominant SNS activity—has been shown to play a significant role in women’s genital sexual arousal in the laboratory; however, the role of SNS activity in clinically relevant sexual arousal function is unknown. The present study assessed the feasibility of using HRV as an index of women’s self-reported sexual arousal function outside the laboratory. Sexual arousal function, overall sexual function, and resting HRV were assessed in 72 women, aged 18–39. Women with below average HRV were significantly more likely to report sexual arousal dysfunction (p<.001) and overall sexual dysfunction (p<.001) than both women with average HRV and women with above average HRV. In conclusion, low HRV may be a risk factor for female sexual arousal dysfunction and overall sexual dysfunction.
Keywords: Heart rate variability, Sympathetic nervous system, Female sexual dysfunction, Female sexual arousal
Introduction
Heart rate variability (HRV) has emerged as a valuable non-invasive test to assess autonomic nervous system (ANS) activity (Xhyheri et al. 2012). Several studies have linked low resting HRV to mental health conditions including depression, anxiety, and alcohol dependence, indicating these disorders may be related to an imbalance in autonomic activity (Kemp et al. 2010; Kemp et al. 2012; Quintana et al. 2013). As HRV is an index of the balance of sympathetic nervous system (SNS) and parasympathetic nervous system activity (PNS), it has proven a useful tool for examining the relative role of SNS activity in female sexual arousal (Lorenz et al. 2012). Moderate SNS dominance (relative to PNS activity) has been shown to predict women’s genital arousal in the laboratory (Lorenz et al. 2012; Meston and Gorzalka 1996a, b; Meston and Heiman 1998; Meston and Gorzallka 1995a, b). Given these findings, it is reasonable to expect ANS activity (indexed by HRV) may be related to women’s self-reported, real-life sexual arousal function outside of the laboratory. The present study is the first to examine HRV as a potential marker of clinically relevant sexual arousal function and overall sexual function in women.
Heart rate variability refers to the variation over time between consecutive heartbeats and is one of the most sensitive and objective measures of the interplay between the SNS and the PNS, the two major branches of the ANS. Autonomic balance of these two branches creates a dynamic equilibrium of vital functions. When the body is physiologically and psychologically stable, PNS input into heart rate is greater than SNS input, resulting in a lower heart rate and relatively greater influence of breathing-related fluctuations (the respiratory sinus arrhythmia) on heart rate (i.e., higher HRV). When physiological or psychological stress is high, increased SNS activity helps co-ordinate increased rates of heartbeats and breathing, leading to relatively less influence of breathing-related fluctuations on heart rate (i.e., lower HRV).
Increasing evidence indicates autonomic balance, specifically SNS dominance, plays a significant role in female sexual function. In a series of studies by Meston and colleagues (Lorenz et al. 2012; Meston and Gorzalka 1996a, b; Meston and Heiman 1998; Meston et al. 1997; Meston and Gorzallka 1995a, b), moderate activation of the SNS using either exercise (Meston and Gorzalka 1996a; Meston and Gorzalka 1995a, b) or ephedrine (Meston and Heiman 1998) facilitated genital sexual arousal, and suppression of the SNS using clonidine inhibited genital arousal (Meston et al. 1997). In order to further examine the role of autonomic balance and to test the hypothesis that there may be an optimal level of SNS activation for facilitating genital sexual arousal, Meston and Gorzalka (1996a) induced SNS activation via exercise and measured the effect of low, moderate, and high levels of SNS activation on genital arousal by assessing arousal at 5, 15, and 30 min post-exercise. They found that high SNS activation (5 min post-exercise) inhibited genital arousal; the opposite effect of what was seen in earlier literature. Moderate SNS activation (15 min post-exercise) and low SNS activation (30 min post-exercise) still facilitated significant increases in physiological arousal (Meston and Gorzalka 1996b). Moreover, a recent study demonstrated an optimal level of SNS dominance for facilitating women’s physiological sexual arousal (Lorenz et al. 2012).
Heart rate variability, specifically, plays both a direct and an indirect role in female sexual arousal function. Directly, HRV may index cardiovascular health, which is critical for genital sexual arousal. Sexual arousal is largely a matter of selective manipulation of blood pressure in the genitals. As HRV is a marker of heart health (for review, see Rajendra Acharya et al. 2006), it is also a marker of the body’s ability to modulate blood pressure appropriately to context. Indirectly, HRV’s role in sexual arousal function relates to the processing of emotional cues. A proven indicator of emotional responding (Appelhans and Luecken 2006), HRV reflects an individual’s capacity to respond to emotional cues, which is particularly relevant to sexual arousal function (Heiman 1980). In this context, low resting HRV may be more reflective of poor emotional health than above average resting HRV is indicative of good emotional health.
The laboratory studies by Meston and colleagues examined the relationship between women’s genital sexual arousal and different levels of experimentally induced SNS activation. However, there is currently no research on the effect of variations in resting autonomic balance on sexual arousal function and sexual function in general. Clinical literature has shown that low resting HRV, which is generally indicative of relative SNS dominance, is a marker of various negative physical and mental health outcomes (Kemp and Quintana 2013). The present study is the first to determine the effect of resting state autonomic balance, indexed by HRV, on validated measures of sexual arousal function and sexual function at large. To date, there are no validated physiological markers of sexual dysfunction in women. If HRV proves to be a risk factor of sexual dysfunction, it could have widespread implications for developing a cost effective, objective, and empirically validated means for monitoring changes in female sexual arousal function.
In sum, based both on evidence that moderate and low levels of SNS activation facilitated genital arousal (Meston and Gorzalka 1995a, b; Meston and Gorzalka 1996a) yet high levels of SNS activation inhibited genital arousal (Meston and Gorzalka 1996b) and on a growing clinical literature indicating that low HRV (generally indicative of high SNS), is associated with negative health outcomes (Kemp and Quintana 2013), we predicted a positive linear relationship between HRV and sexual arousal function. That is, we predicted that women with autonomic balance indicating moderate or low resting SNS activity (relative to PNS activity) would be less likely than women with autonomic balance indicating high resting SNS to report clinically relevant sexual arousal dysfunction on an empirically validated scale of sexual function. We also predicted that this relationship would hold for overall sexual function (Table 1).
Table 1.
Participant Characteristics (N = 72)
| M | SD | |
|---|---|---|
| Age (years) | 22.7 | 4.3 |
| FSFI (total score) | 28.0 | 5.2 |
| HRV neutral | 67.5 | 19.8 |
| HRV resting norm, female, age 10–29a | 66.0 | 18.0 |
| HRV resting norm, female, age 30–49a | 58.0 | 13.0 |
| n | (%) | |
| Race | ||
| Caucasian | 45 | 62.5 |
| African American | 2 | 2.8 |
| Asian American | 8 | 11.1 |
| Other/missing | 17 | 23.6 |
| Relationship status | ||
| Single/dating | 40 | 55.6 |
| Married or in a committed relationship | 30 | 41.7 |
| Divorced | 1 | 1.4 |
| Other | 1 | 1.4 |
| Sexual identity | ||
| Exclusively or predominately heterosexual | 63 | 87.5 |
| Equally heterosexual and lesbian | 7 | 9.7 |
| Exclusively or predominately lesbian | 2 | 2.8 |
Normative resting HRV values established by Umetani et al. (1998)
Method
Participants
Participants were selected from three experiments, one previously published elsewhere (Lorenz et al. 2012), contributing n = 39) and two unpublished studies (contributing n = 33). No participant’s data was used more than once. In each study, participants were recruited from The University of Texas at Austin psychology subject pool and from the Austin area community using flyers, online advertisements, and print advertisements that highlighted the sexual nature of the experiments. Potential participants were screened over the phone to ensure that they met the inclusion and exclusion criteria. Please see Table 2 for general inclusion and exclusion criteria. One of the unpublished studies recruited both women with and without histories of childhood sexual abuse; however, because differences in sexual distress and sexual satisfaction have been observed between women with and without abuse histories (Rellini and Meston 2007), only data from women without abuse histories were used in the present sample.
Table 2.
Inclusion and exclusion criteria
| Inclusion criteria | |
|---|---|
| At least 18 years of age Currently sexually active |
|
| Exclusion criteria | N |
| Lorenz et al. (2012) | 39 |
| Current self-reported sexual complaints within domains of sexual desire, sexual arousal, and/or sexual pain, and/or a history of treatment for sexual dysfunction | |
| History of sexual trauma | |
| Use of medication known to affect sexual or vascular functioning, with the exception of hormonal contraceptives | |
| Untreated Axis I disorders | |
| Medical conditions likely to affect sexual arousal | |
| Unpublished study 1 | 21 |
| History of sexually transmitted diseases | |
| Past pelvic surgery | |
| Current pelvic, vaginal, or urinary tract infection | |
| Neurological impairment | |
| Unpublished study 2 | 12 |
| Current self-reported sexual complaints within domains of sexual desire, sexual arousal, and/or sexual pain, and/or a history of treatment for sexual dysfunction | |
| History of sexually transmitted diseases | |
| Past pelvic surgery | |
| Current pelvic, vaginal or urinary tract infection | |
| Neurological impairment |
Procedure
Although the studies included in this paper had different objectives, they were all conducted within the same laboratory and followed the same general procedure. Testing sessions took place in a private room with an intercom that participants used to communicate with the researcher. Participants were instructed in how to attach the wires for an electrocardiogram (ECG) before the session began. In all three studies, vaginal photoplethysmography was used to assess physiological sexual arousal; these data are not considered in the present manuscript but some of the main findings can be found in Lorenz et al. (2012). After participants attached the wires and inserted the vaginal probe, they underwent a 5–10 min habituation period where no measurements were taken. Participants then watched a 3-min neutral (nonsexual) film followed by one of a set of erotic films validated to produce sexual arousal. While all participants viewed a neutral film followed by an erotic film, only HRV data collected from the entire 3-min neutral film segments were used in analyses as our index of resting HRV. Following the neutral film, participants in all three studies viewed an 8–10 min erotic film clip, during which HRV was also measured. The two film segments (neutral, then erotic) were always presented in the same order. All participants completed measures on demographics and sexual function (see below). Participants gave informed consent and were compensated between $10 and $50, depending on the number of sessions and on the study completed.
Measures
Heart Rate Variability
In the Lorenz et al. (2012) study, heart rate was measured during the neutral film segment at a rate of 80 samples/s. In the other two studies, heart rate was measured at a rate of 200 samples/s. These sampling rates are adequate to produce a minimally biased estimate of time domain measures of HRV, such as those presented here (Hejjel and Roth 2004; Ziemssen et al. 2008). The three leads of the ECG were placed under the participant’s right collarbone, below the lowermost left rib, and on the right ankle. The signal from the leads was collected with AcqKnowledge software, and movement artifacts were removed manually. The AcqKnowledge peak finder function was used to isolate the beat-to-beat (NN) intervals.
Heart rate variability was calculated using the standard deviation of the NN intervals (SDNN), one of the most widely used techniques to analyze HRV (Xhyheri et al. 2012). Research has shown that SDNN, a time domain index, is an effective and accurate marker of HRV and can reflect the relative contribution of the SNS to the regulation of heart rate. Specifically, a high SDNN is thought to reflect low SNS (and/or high PNS) activity, while a low SDNN is thought to reflect high SNS activity (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). After collecting all of the NN intervals for each participant, SDNN for the neutral film segment was computed using Kubios HRV Analysis Software (Biosignal Analysis and Medical Imagine Group, University of Kuopio, Kuopio, Finland).
Sexual Function
Sexual function was assessed with the Female Sexual Function Index (FSFI; Rosen et al. 2000), an empirically validated, 19-item questionnaire. The FSFI assesses six domains of sexual function: desire (two items), arousal (four items), vaginal lubrication (four items), orgasm (three items), satisfaction (three items), and sexual pain (three items). The clinical cutoff that reliably discriminates between women with and without a sexual dysfunction diagnosis is 26.55 (Rosen et al. 2000). In other words, women whose scores are above that cutoff are considered sexually functional, and women whose scores fall below that cutoff are considered sexually dysfunctional (Rosen et al. 2000).
Data Analysis
Regression analyses were used to test the relationship between sexual function and SDNN. Separate linear regression analyses were conducted for the sexual arousal function domain of the FSFI as well as for the FSFI total score, controlling for age as a covariate. Finally, FSFI total scores were used to dichotomize participants as either sexually functional or sexually dysfunction. A logistic regression analysis was then conducted to assess the ability of SDNN to predict sexual function status (functional or dysfunctional).
Analysis of variance (ANOVA) tests were used to test the relationship between SDNN groups and sexual function. Participants were categorized into one of three HRV groups (below average, average, and above average) based on previously published normative SDNN values (Umetani et al. 1998). Umetani et al. (1998) defined the effects of age and gender on the normal range of time domain HRV over nine decades in healthy subjects. They found that HRV declined with age, and HRV was lower in women than in men until age 30, when gender differences decreased. In the current study, SDNN was calculated from the neutral film segments in order to match the HRV normative values (Umetani et al. 1998), which apply to resting HRV only. Among the three groups, there were no significant differences with respect to sexual orientation and age. There were, however, significant differences in relationship status among the three groups, F(1,71) = 4.535, p < .02. Significantly more single women were in the average HRV group than in the below average and above average groups. There were also significant differences in race, F(1,71) = 4.138, p < .02. Significantly more Asian American women and women of other/unspecified racial identities were in the average HRV group than in the below average and above average groups.
All analyses were conducted with SPSS statistical software version 22.0 (SPSS Inc., Chicago, IL, USA). In all analyses, a two-tailed p < .05 was considered statistically significant.
Results
Sample Characteristics
The final sample included 72 women, aged 18–39 (M = 22.7, SD = 4.3; see Table 1). With respect to relationship status, 55.6 % of the participants were single or dating, and 41.7 % reported being married or in a committed relationship. The sample was 62.5 % Caucasian, 11.1 % Asian American, and 2.8 % African American. Based on the FSFI, 70.8 % of the participants in this sample were considered sexually functional (M = 28, SD = 5.2), and 29.2 % were considered sexually dysfunctional. Please see Table 3.
Table 3.
| Below average HRV n (%) |
Average HRV n (%) |
Above average HRV n (%) |
|
|---|---|---|---|
| Sexual function status | |||
| Dysfunctional | 8(11.1) | 12 (16.7) | 1 (1.4) |
| Functional | 6 (8.3) | 31 (43.1) | 14 (19.4) |
Normative resting HRV values established by Umetani et al. (1998)
Sexual function status determined by empirically validated FSFI clinical cutoff score (Rosen et al. 2000)
Resting HRV and Sexual Arousal Function
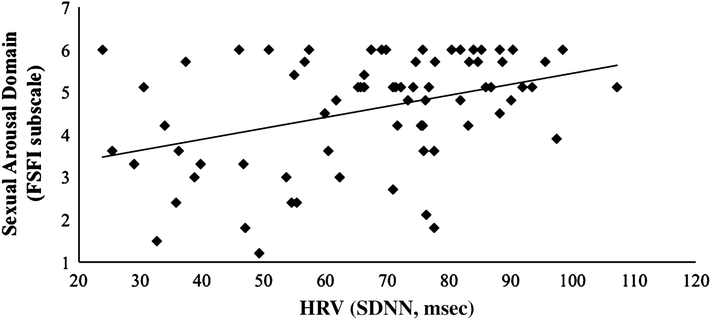
To determine if resting SDNN was associated with sexual arousal function, we performed a linear regression with FSFI arousal domain scores regressed on SDNN. The overall model was significant, R2 = .15, F(1, 71) = 12.395, p < .001 (see Fig. 1), with SDNN significantly correlating with sexual arousal function. As SDNN increased, sexual arousal function also increased.
Fig. 1.
Linear relationship between resting HRV and sexual arousal function
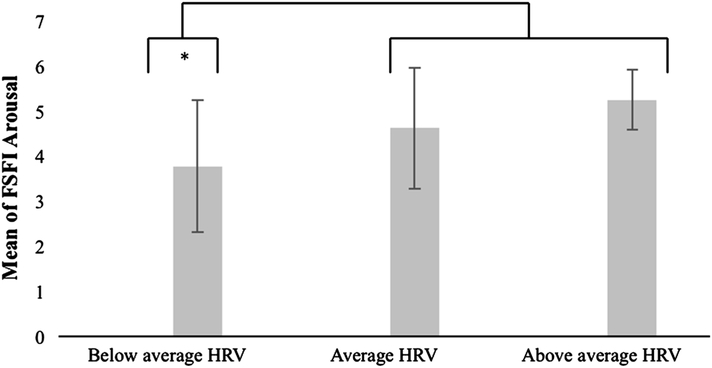
A one-way ANOVA revealed a significant effect of HRV group (below average, average, above average) on sexual arousal function, F(2, 69) = 7.113, p = .003. As a Levene statistic of 3.973, p = .023 revealed that the homogeneity of variance assumption was violated among the three groups, we used Welch’s F test to correct this violation. Planned specific contrasts indicated that women with below average HRV had significantly lower scores on the FSFI sexual arousal scale (indicative of more severe arousal dysfunction) than women with average HRV and women with above average HRV combined, t(16.109) = 2.819, p = .012. This difference had a moderate effect size, r = .57.
Resting HRV and Overall Sexual Function
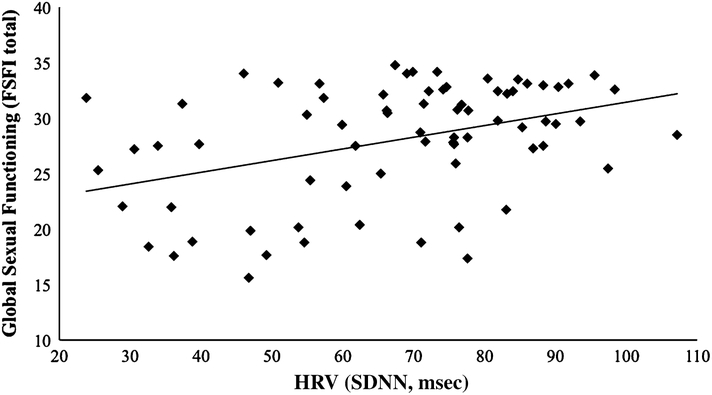
To determine whether resting SDNN was associated with overall sexual function (that is, the combined domains of desire, arousal, lubrication, orgasm, satisfaction, and pain), we performed a linear regression with FSFI total scores regressed on SDNN. The overall model was significant, R2 = .16, F(1, 71) = 13.359, p<.001 (see Fig. 2), with SDNN significantly correlating with overall sexual function.
Fig. 2.
Linear relationship between resting HRV and overall sexual function
A one-way ANOVA indicated that there was a significant effect of HRV group (below average, average, above average) on overall sexual function, F(2, 69) = 6.257, p = .003. As above, a Levene statistic of 4.469, p = .015 revealed that the homogeneity of variance assumption was violated among the three groups, and thus we used Welch’s F test. Planned contrasts revealed that women with below average HRV had significantly lower total FSFI scores (indicative of poorer overall sexual function) than both women with average HRV and above average HRV combined, t(16.015) = 3.145, p = .006. This difference had a moderate to high moderate effect size, r = .62. Further, compared to women with average HRV, women with below average HRV had significantly lower total FSFI scores, t(20.234) = 2.380, p = .027. As with sexual arousal function, the effect size was moderate, r = .47 (Fig. 3).
Fig. 3.
Mean FSFI arousal domain scores by HRV group
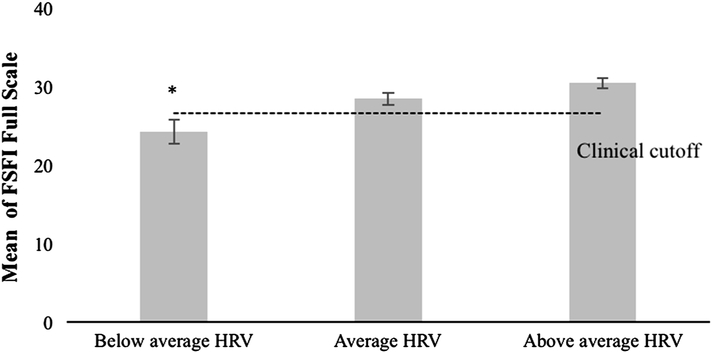
A logistic regression indicated that resting SDNN significantly predicted sexual function status, B = .045, Wald χ2(1) = 9.091, p = .003. Participants were dichotomized into two groups, a clinical sexual dysfunction group and a non-clinical sexual function group, using Rosen et al. (2000) empirically validated FSFI clinical cutoff score. The lower the SDNN measured during the neutral film, the more likely a participant’s FSFI total score fell in the dysfunctional range. During the neutral film, each additional unit change in SDNN was associated with a 1.046 % higher likelihood of being categorized as sexually functional (Fig. 4).
Fig. 4.
Mean FSFI full-scale scores by HRV group
HRV During Erotic Film and Overall Sexual Function
There are currently no established normative values for SDNN during sexual arousal; however, we present summary statistics from our data in Table 4. A logistic regression analysis indicated that SDNN during the erotic film segment significantly predicted sexual function status, B = .049, Wald χ2(1) = 11.287, p = .001. Again, the lower the SDNN measured during the erotic film, the more likely the participants’ FSFI total scores placed them in the dysfunctional range. During the erotic film, each additional unit change in SDNN was associated with a 1.050 % higher likelihood of being categorized as sexually functional.
Table 4.
HRV during neutral and erotic films by sexual function (FSFI)a status
| Average HRV during neutral film |
Average HRV during erotic film |
|||
|---|---|---|---|---|
| SDNN | sd | SDNN | sd | |
| Sexual function status | ||||
| Dysfunctional | 55.87 | 19.52 | 51.01 | 19.07 |
| Functional | 72.28 | 18.02 | 71.63 | 20.47 |
Sexual function status determined by empirically validated FSFI clinical cutoff score (Rosen et al. 2000)
Discussion
This study examined the relationships between SDNN, an index of resting HRV, and sexual arousal function and overall sexual function in women. Results indicated that low resting HRV, which is indicative of a highly SNS-dominant autonomic balance, significantly predicted scores on a self-report measure of sexual arousal dysfunction and overall sexual dysfunction. Furthermore, sexual function status (functional or dysfunctional) was significantly associated with HRV group. That is, women who had below average resting HRV (relatively high SNS) had significantly lower FSFI scores compared to women who had average resting HRV (moderate SNS dominance) or above average resting HRV (low SNS dominance). These findings are consistent with both recent clinical literature on HRV and research on the effect of autonomic balance on female genital arousal. Given that low resting HRV has been associated with depression, anxiety, and alcohol dependence (Kemp et al. 2010; Kemp et al. 2012; Quintana et al. 2013), it is not surprising that low HRV may also predict female sexual dysfunction. These findings are also consistent with laboratory studies, which indicated that high levels of SNS activation inhibit (Meston and Gorzalka 1996b) while moderate and low SNS levels facilitate genital sexual arousal (Meston and Gorzalka 1996a; Meston and Gorzalka 1995a, b).
An examination of SDNN during the erotic film clip revealed that HRV during this segment was also a significant predictor of sexual function as measured by the FSFI. It is possible that physiological responses during the erotic film clip, rather than those that occurred during the neutral film clip, may better approximate physiological responses in real world situations. However, this measure may be limited in clinical utility, given the difficulty in assessing HRV during sexual arousal; as such, it is important to note that resting HRV was also a significant and robust predictor of sexual dysfunction.
The results of this study have implications for research on the relationship between HRV and sexual function in women. Although this study is the first to link resting HRV to female sexual arousal function and overall sexual function, there is already an established relationship between resting HRV and erectile dysfunction (ED) in men. The present study indicated that low HRV, which is often linked with SNS dominance, might place women at risk for sexual arousal problems and overall sexual difficulties. Similarly, research on men has shown that sympathetic pathways play an anti-erectile role while parasympathetic pathways play a pro-erectile role (Giuliano and Rampin 2004). In men without sexual arousal problems, an increase in PNS activity signals endothelial cells in the penis to release relaxing agents (Solomon et al. 2003), which cause smooth muscle relaxation in the arteries supplying the erectile tissue. This process leads to an increase in blood flow to the penis as well as a decrease in outflow from the penis. In men with sexual arousal problems, the elevation of SNS activity negatively affects vascular functioning and disturbs the sympathovagal balance, which inhibits blood flow (McVary 2006; Simpson et al. 2006). Because SNS activity tends to be elevated in men with ED (McVary 2006; Simpson et al. 2006; Pagani 2000), HRV has been used as a marker of ED (Pagani 2000). HRV has been shown to be significantly lower in men with ED relative to healthy controls (Fernandez et al. 2010; Lee et al. 2011).
In women, genital arousal is mediated by some of the same mechanisms as genital arousal in men (Levin 2002). Both male sexual function and female sexual function rely on smooth muscle relaxation and vasodilation to allow for increased blood flow to the genitals. In women, smooth muscle relaxation leads to increased blood flow into the cavernosal tissues present in the clitoral bulbs. During sexual arousal, the clitoral bulbs fill with blood and cuff the vaginal opening, which leads to an expansion of the vulva. If smooth muscle relaxation does not occur, blood flow to the cavernosal tissues is hindered and genital arousal is impaired (Levin 2002). Given that SNS activity is elevated in both men and women with sexual arousal problems, insights from the literature on HRV and ED may apply to female sexual dysfunction.
One such insight that may be relevant to female sexual dysfunction is the relationship between ED and cardiovascular disease (CVD). ED has been shown to be a robust early indicator of CVD (Billups et al. 2005; Thompson et al. 2005). Impeded blood flow resulting from atherosclerosis initially manifests in small arteries (Roose 2003), so the small penile artery is particularly vulnerable to blockage (Montorsi et al. 2005). Males who present with erectile dysfunction of vascular rather than psychological origin who are asymptomatic for ischemic heart disease have been shown to be at increased risk for future negative cardiovascular events (Greenstein et al. 1997; Montorsi et al. 2005). It is important to note that CVD is the leading cause of death for both men and women in the United States (Coulter 2011). More women die each year of CVD than men, and the lifetime risk of developing CVD in women by age 50 is 39 % (Coulter 2011). Because the vascular mechanisms governing male sexual function are similar to those involved in female sexual function, it is possible that arousal dysfunction could be an early prognostic factor for CVD in women.
HRV has been associated with anxiety (Kemp et al. 2012), depression (Kemp et al. 2010), and now with female sexual dysfunction. Female sexual dysfunction is also associated with anxiety and depression (Laurent and Simons 2009). Heart rate variability may be a marker of some third variable, potentially automatic imbalance, which then leads to depression, anxiety, and female sexual dysfunction. Further research in this area should investigate common variables among the three disorders.
To our knowledge, these results provide the first empirical evidence for low HRV as a potential risk factor of sexual dysfunction in women. If this finding is replicated in future studies, HRV may prove to be a cost effective, easy to administer, and non-intrusive index for both assessing potential sexual dysfunction and for monitoring treatment progress. This could be of interest to clinicians treating patients with female sexual arousal dysfunction, particularly in patients with co-morbid conditions relevant to cardiovascular function.
It is worth noting several limitations of this study. Given the difficulties inherent in assessing heart rate variability outside the laboratory, and the necessity of using self-report data to determine participant’s sexual function, the generalizability of these findings is uncertain. Future research may benefit from a portable device that calculates HRV, which allows participants to measure their own HRV from home (Bloemers et al. 2010). Also, although the vaginal photoplethysmography is generally considered non-invasive (Janssen et al. 2007) and participants were given an adequate habituation period, the insertion of a vaginal probe may have been an unusual experience which may have affected our measure of HRV, particularly in women with sexual dysfunction. Some participants in this study (n = 12) were not screened for conditions and medications that could alter sexual response and SNS activity. Specifically, because we used archival data across studies with varied sampling strategies and procedures as well as different aims, we did not have data on mental health variables such as depression or anxiety, which may be a mediating pathway between HRV and sexual function. Future research on the relationship between HRV and sexual function should control for such factors and others that could impact SNS activity, such as smoking, athleticism, and antidepressant medication use.
Despite these limitations, however, the results of this study reveal a significant relationship between HRV and sexual function, thus establishing low HRV as a potential risk factor of sexual dysfunction in women. Furthermore, HRV may also be a useful marker of treatment-related improvements in sexual arousal function, and it may be used as an index of sexual arousal function in clinical trials of medications developed to treat female sexual arousal disorders.
Acknowledgments
This research was supported by a grant from the National Institute of Child Health and Human Development (NICHD, RO1 HD051676) to Cindy M. Meston. Tierney A. Lorenz was supported by a grant from the NICHD (T32HD049336). The views presented here are solely those of the authors and do necessarily not represent the official views of the National Institutes of Health.
References
- Appelhans BM, & Luecken LJ (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10(3), 229–240. doi: 10.1037/1089-2680.10.3.229. [DOI] [Google Scholar]
- Billups KL, Bank AJ, Padma-Nathan H, Katz S, & Williams R (2005). Erectile dysfunction is a marker for cardiovascular disease: results of the minority health institute expert advisory panel. The Journal of Sexual Medicine, 2(1), 40–50; discussion 50–52. doi: 10.1111/j.1743-6109.2005.20104_1.x. [DOI] [PubMed] [Google Scholar]
- Bloemers J, Gerritsen J, Bults R, Koppeschaar H, Everaerd W, Olivier B, & Tuiten A (2010). Induction of sexual arousal in women under conditions of institutional and ambulatory laboratory circumstances: a comparative study. The Journal of Sexual Medicine, 7(3), 1160–1176. doi: 10.1111/j.1743-6109.2009.01660.x. [DOI] [PubMed] [Google Scholar]
- Coulter SA (2011). Epidemiology of cardiovascular disease in women. Texas Heart Institute Journal, 38(2), 145–147. [PMC free article] [PubMed] [Google Scholar]
- Fernandez EA, Neto EPS, Abry P, Macchiavelli R, Balzarini M, Cuzin B, et al. (2010). Assessing erectile neurogenic dysfunction from heart rate variability through a generalized linear mixed model framework. Computer Methods and Programs in Biomedicine, 99(1), 49–56. doi: 10.1016/j.cmpb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Giuliano F, & Rampin O (2004). Neural control of erection. Physiology and Behavior, 83(2), 189–201. doi: 10.1016/j.physbeh.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Greenstein A, Chen J, Miller H, Matzkin H, Villa Y, & Braf Z (1997). Does severity of ischemic coronary disease correlate with erectile function? International Journal of Impotence Research, 9(3), 123–126. [DOI] [PubMed] [Google Scholar]
- Heiman JR (1980). Female sexual response patterns: Interactions of physiological, affective, and contextual cues. Archives of General Psychiatry, 37, 1311–1316. [DOI] [PubMed] [Google Scholar]
- Hejjel L, & Roth E (2004). What is the adequate sampling interval of the ECG signal for heart rate variability analysis in the time domain? Physiological Measurement, 25(6), 1405–1411. doi: 10.1088/0967-3334/25/6/006. [DOI] [PubMed] [Google Scholar]
- Janssen E, Prause N, & Geer JH (2007). The sexual response In Cacioppo JT (Ed.), Handbook of psychophysiology (3rd ed., pp. 245–266). Cambridge, England: Cambridge University Press. [Google Scholar]
- Kemp AH, & Quintana DS (2013). The relationship between mental and physical health: Insights from the study of heart rate variability. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 89(3), 288–296. doi: 10.1016/j.ijpsycho.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Felmingham KL, Matthews S, & Jelinek HF (2012). Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: Implications for cardiovascular risk. PLoS One, 7(2), e30777. doi: 10.1371/journal.pone.0030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, & Gatt JM (2010). Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biological Psychiatry, 67(11), 1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Laurent SM, & Simons AD (2009). Sexual dysfunction in depression and anxiety: Conceptualizing sexual dysfunction as part of an internalizing dimension. Clinical Psychology Review, 29(7), 573–585. doi: 10.1016/j.cpr.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Lee JY, Joo K-J, Kim JT, Cho ST, Cho DS, Won Y-Y, & Choi JB (2011). Heart rate variability in men with erectile dysfunction. International Neurourology Journal, 15(2), 87–91. doi: 10.5213/inj.2011.15.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin RJ (2002). The physiology of sexual arousal in the human female: a recreational and procreational synthesis. Archives of Sexual Behavior, 31(5), 405–411. [DOI] [PubMed] [Google Scholar]
- Lorenz TA, Harte CB, Hamilton LD, & Meston CM (2012). Evidence for a curvilinear relationship between sympathetic nervous system activation and women’s physiological sexual arousal. Psychophysiology, 49(1), 111–117. doi: 10.1111/j.1469-8986.2011.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVary K (2006). Lower urinary tract symptoms and sexual dysfunction: Epidemiology and pathophysiology. BJU International, 97 (Suppl 2), 23–28; discussion 44–45. doi: 10.1111/j.1464-410X.2006.06102.x. [DOI] [PubMed] [Google Scholar]
- Meston CM, & Gorzalka BB (1995a). The effects of sympathetic activation on physiological and subjective sexual arousal in women. Behaviour Research and Therapy, 33(6), 651–664. [DOI] [PubMed] [Google Scholar]
- Meston CM, & Gorzalka BB (1995b). The effects of sympathetic activation on physiological and subjective sexual arousal in women. Behavior Research and Therapy, 33(6), 651–664. [DOI] [PubMed] [Google Scholar]
- Meston CM, & Gorzalka BB (1996a). Differential effects of sympathetic activation on sexual arousal in sexually dysfunctional and functional women. Journal of Abnormal Psychology, 105(4), 582–591. [DOI] [PubMed] [Google Scholar]
- Meston CM, & Gorzalka BB (1996b). The effects of immediate, delayed, and residual sympathetic activation on sexual arousal in women. Behavior Research and Therapy, 34(2), 143–148. [DOI] [PubMed] [Google Scholar]
- Meston CM, Gorzalka BB, & Wright JM (1997). Inhibition of subjective and physiological sexual arousal in women by clonidine. Psychosomatic Medicine, 59, 339–407. [DOI] [PubMed] [Google Scholar]
- Meston CM, & Heiman JR (1998). Ephedrine-activated physiological sexual arousal in women. Archives of General Psychiatry, 55(7), 652–656. [DOI] [PubMed] [Google Scholar]
- Montorsi P, Ravagnani PM, Galli S, Rotatori F, Briganti A, Salonia A, et al. (2005). The artery size hypothesis: A macrovascular link between erectile dysfunction and coronary artery disease. The American Journal of Cardiology, 96(12B), 19M–23M. doi: 10.1016/j.amjcard.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Pagani M, & Malliani A (2000). Interpreting oscillations of muscle sympathetic nerve activity and heart rate variability. Journal of Hypertension, 18(12), 1709–1719. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ, McGregor IS, Hickie IB, & Kemp AH (2013). Heart rate variability predicts alcohol craving in alcohol dependent outpatients: Further evidence for HRV as a psychophysiological marker of self-regulation. Drug and Alcohol Dependence, 132(1–2), 395–398. doi: 10.1016/j.drugalcdep.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim CM, & Suri JS (2006). Heart rate variability: A review. Medical and Biological Engineering and Computing, 44(12), 1031–1051. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- Rellini A, & Meston CM (2007). Sexual function and satisfaction in adults based on the definition of child sexual abuse. The Journal of Sexual Medicine, 4(5), 1312–1321. doi: 10.1111/j.1743-6109.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose SP (2003). Depression: Links with ischemic heart disease and erectile dysfunction. The Journal of Clinical Psychiatry, 64(suppl 10), 26–30. [PubMed] [Google Scholar]
- Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. (2000). The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. Journal of Sex and Marital Therapy, 26(2), 191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- Simpson JD, Doux JD, Lee PY, & Yun AJ (2006). Peripheral arterial disease: A manifestation of evolutionary dislocation and feed-forward dysfunction. Medical Hypotheses, 67(4), 947–950. doi: 10.1016/j.mehy.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Solomon H, Man JW, & Jackson G (2003). Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominator. Heart (British Cardiac Society), 89(3), 251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, & Coltman CA (2005). Erectile dysfunction and subsequent cardiovascular disease. JAMA, the Journal of the American Medical Association, 294(23), 2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- Umetani K, Singer DH, McCraty R, & Atkinson M (1998). Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. Journal of the American College of Cardiology, 31(3), 593–601. [DOI] [PubMed] [Google Scholar]
- Xhyheri B, Manfrini O, Mazzolini M, Pizzi C, & Bugiardini R (2012). Heart rate variability today. Progress in Cardiovascular Diseases, 55(3), 321–331. doi: 10.1016/j.pcad.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Ziemssen T, Gasch J, & Ruediger H (2008). Influence of ECG sampling frequency on spectral analysis of RR intervals and baroreflex sensitivity using the EUROBAVAR data set. Journal of Clinical Monitoring and Computing, 22(2), 159–168. doi: 10.1007/s10877-008-9117-0. [DOI] [PubMed] [Google Scholar]