Abstract
Patient: Male, 61
Final Diagnosis: Left ventricular hypertrophy
Symptoms: Chest pain
Medication: —
Clinical Procedure: Coronary angiography • echocardiogram
Specialty: Cardiology
Objective:
Challenging differential diagnosis
Background:
Wellens syndrome is a form of unstable angina that warrants a timely intervention to prevent extensive myocardial infarction. A few conditions can lead to electrocardiogram (EKG) changes mimicking Wellens syndrome.
Case Report:
A 61-year-old African American man with no significant medical history was admitted for chest pain and new biphasic EKG changes in leads V2 through V6 concerning for Wellens’ syndrome. He was found to have hypertension during his hospitalization and had left ventricular hypertrophy by echocardiogram. He was urgently evaluated with a cardiac catheterization, which demonstrated a normal coronary artery anatomy. The patient was diagnosed with pseudo-Wellens syndrome.
Conclusions:
LVH secondary to hypertension could mimic Wellens syndrome and should be considered when evaluating patients with anterior T wave abnormalities on electrocardiograms. In patients who do not have acute coronary syndrome and in whom the T wave abnormalities are not classic for Wellens-type changes, non-invasive imaging instead of cardiac catheterization may be indicated initially.
MeSH Keywords: Acute Coronary Syndrome; Anterior Wall Myocardial Infarction; Hypertrophy, Left Ventricular; Hypertension
Background
Wellens syndrome is a harbinger of an impending myocardial infarction that warrants an urgent therapeutic intervention. The classic presentation of the syndrome includes biphasic or symmetrically inverted T waves in the precordial leads preceded by a recent history of angina [1]. Different mimics of Wellens syndrome have shown unremarkable coronary angiograms. We present an interesting case of a middle-aged man with chest pain and biphasic T waves on his electrocardiogram (EKG) who had a normal coronary anatomy during cardiac catheterization.
Case Report
A 61-year-old African American man with no significant past medical history presented to our emergency department for evaluation of atypical left sided chest pain of 3 days duration. He had no history of coronary artery disease and was not on any medications. There was no history of recreational drug use. At the time of admission, his vital signs were remarkable for elevated blood pressure of 150/104 mmHg. The heart rhythm was regular and there was no murmur. The jugular venous pressure was not elevated.
Blood chemistries, including serum potassium, were within normal limits. His 12-lead electrocardiogram (EKG) showed normal sinus rhythm with interval development of new Wellens-type biphasic T waves in leads V2 to V6, as well as inverted T waves in the inferior leads (Figure 1). His previous EKG was normal (Figure 2). Serial cardiac troponin T assays were within normal limits. Resting transthoracic echocardiogram (TTE) was remarkable for concentric left ventricular hypertrophy with no regional wall abnormality (Figure 3). His chest pain improved with sublingual nitroglycerin. Given this clinical picture, we questioned whether cardiac catheterization was necessary. At the same time, there was a concern for unstable angina in view of dynamic EKG changes and response to sublingual nitroglycerin. This reasoning prompted a coronary angiography, which demonstrated normal coronary arteries with no significant stenosis. No further EKG was done after cardiac catheterization. The following day, the patient’s blood pressure remained persistently elevated above 180/100 mmHg, consistent with a diagnosis of systemic hypertension. Since his coronary angiography was normal, his biphasic T wave changes and LVH on TTE were believed to be related to untreated hypertension. He was followed up in clinic with no further symptoms.
Figure 1.
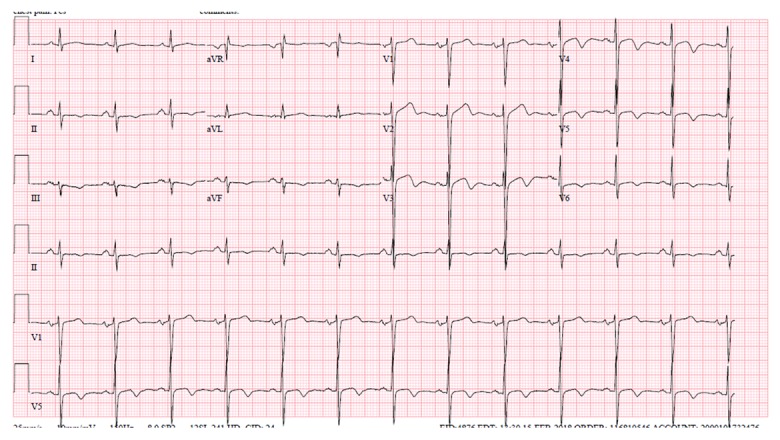
Demonstrates biphasic T waves in leads V2 through V6.
Figure 2.
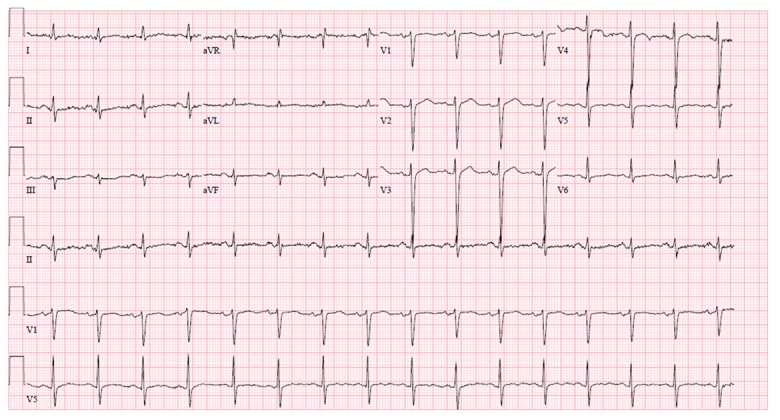
Shows a previously normal EKG.
Figure 3.
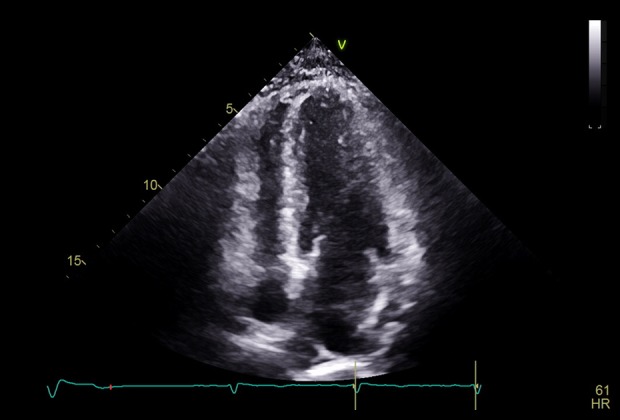
Transthoracic echocardiography (apical 4-chamber view) showing concentric LVH.
Discussion
In 1982, de Zwaan et al. identified a subgroup of high-risk patients hospitalized for unstable angina [1]. These subjects had a characteristic biphasic T wave pattern in the precordial leads of their EKGs associated with critical stenosis of the proximal left anterior descending artery (LAD) [1]. The authors demonstrated that 75% of these patients who received medical therapy without coronary revascularization later suffered an extensive anterior wall myocardial infarction within a mean period of 9 days [1]. In a follow-up study in 1988, it was observed that all the patients admitted with unstable angina and had precordial biphasic T wave pattern on EKG had significant occlusion in the LAD by coronary angiography [2]. It was concluded that Wellens syndrome (or LAD coronary T wave syndrome) indicates an impending myocardial infarction [2].
Diagnostic criteria for Wellens syndrome include: symmetric and deeply inverted T waves (type B pattern) in leads V2 and V3, sometimes in leads V1, V4–V6 or biphasic T waves (type A pattern) in leads V2 and V3, isoelectric or minimal ST segment elevation less than 1 mm, no pathologic Q waves, no loss of R wave progression, a history of angina, presence of typical EKG pattern in pain-free period, and normal or minimally elevated cardiac enzymes [2,3]. These criteria could easily distinguish Wellens syndrome from other etiologies of inverted T waves. Other causes of inverted T waves include early repolarization, cerebral T waves, post-tachycardia T wave pattern, digitalis effect, hypokalemia, bundle branch block, Wolff-Parkinson-White pre-excitation, acute pericarditis, acute pulmonary embolism, and left ventricular hypertrophy [4,5].
While identifying EKG alterations meeting Wellens criteria is crucial, it should be noted that the sensitivity of inverted T waves significant for LAD stenosis is 69%, specificity is 89% and positivity predictive value is 86% [6]. This means that EKG changes suggestive of Wellens syndrome do not always indicate its presence. A clinical entity in which Wellens EKG pattern is present but with normal coronary anatomy has been defined as pseudo-Wellens syndrome. This syndrome has been described in patients with myocardial bridge due to external coronary artery compression [7] as well as coronary spasm from cocaine use [8]. Heavy marijuana use [9] and acute cholecystitis [10] have also been implicated in pseudo-Wellens syndrome, but their pathophysiologic mechanisms are unclear. In our case, the biphasic pseudo-Wellens EKG manifestation was likely due to left ventricular hypertrophy from systemic hypertension. Our patient’s EKG was positive for LVH by Romhilt-Estes criteria based on left atrial involvement and QRS duration >0.09 s in the absence of digitalis effect [11]. The new ST changes in our patient are likely permanent, as sometimes also seen in patients of African descent [12].
The ST-T segment changes seen in LVH are due to repolarization abnormalities of the hypertrophied muscle of the left ventricle [13]. Typical EKG alterations associated with LVH include early repolarization, asymmetric T wave inversion, and poor R wave progression [13]. Others findings include concave ST elevation in leads V1–V3 and down-sloping diffuse ST depression with negative T waves in the inferior and anterolateral leads [14]. Another distinguishing feature in favor of LVH as opposed to Wellens syndrome is that ST–T changes usually involve all the precordia leads. In light of this, clinicians should exercise caution before rushing such patients to the cardiac catheterization laboratory. In general, patients presenting with chest pain need to be risk-stratified, and diagnostic testing for coronary artery disease should be based on pretest probability. Low-risk patients can undergo treadmill stress testing if baseline EKG is normal. Intermediate-risk patients should be evaluated with stress testing with imaging, while those in the high-risk category should be investigated with coronary angiography. Our patient’s clinical presentation and EKG findings were worrisome for unstable angina; on this basis, we elected to proceed with coronary angiography.
We propose that detection of LVH on echocardiogram in the face of a new diagnosis of systemic hypertension and biphasic T waves with negative cardiac biomarkers should raise a question about pseudo-Wellens syndrome. Furthermore, the presence of T wave changes in V3 to V6 should make one suspicious for LVH. If unstable angina is ruled out, such patients may be considered for non-invasive workup such as stress testing or coronary CT angiography. High-resolution coronary CT angiography has been shown to have good diagnostic accuracy [15]. Additionally, contrast-enhanced cardiac magnetic resonance imaging has been used in patients with pseudo-Wellens T waves to rule out obstructive coronary artery disease, thus obviating the need for invasive procedures [16]. Further research in this area is ongoing.
Conclusions
This case presentation highlights left ventricular hypertrophy masquerading as Wellens syndrome. Differential diagnosis of biphasic T waves in V2–V4 and negative T waves in V5–V6 in the precordial leads should include the possibility of pseudo-Wellens syndrome. Depending on the clinical scenario, non-invasive imaging instead of cardiac catheterization may be considered and appropriate.
Footnotes
Department and Institution where work was done
Department of Hospital Medicine, Mayo Clinic Health System, La Crosse, WI, U.S.A.
Conflict of interest
None.
References:
- 1.de Zwaan C, Bär FWHM, Wellens HJJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J. 1982;103(4 Pt 2):730–36. doi: 10.1016/0002-8703(82)90480-x. [DOI] [PubMed] [Google Scholar]
- 2.de Zwaan C, Bär FW, Janssen JHA, et al. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowing of the proximal LAD coronary artery. Am Heart J. 1989;117(3):657–65. doi: 10.1016/0002-8703(89)90742-4. [DOI] [PubMed] [Google Scholar]
- 3.Rhinehardt J, Brady WJ, Perron AD, Mattu A. Electrocardiographic manifestations of Wellens’ syndrome. Am J Emerg Med. 2002;20(7):638–43. doi: 10.1053/ajem.2002.34800. [DOI] [PubMed] [Google Scholar]
- 4.Hayden GE, Brady WJ, Perron AD, et al. Electrocardiographic T-wave inversion: Differential diagnosis in the chest pain patient. Am J Emerg Med. 2002;20(3):252–62. doi: 10.1053/ajem.2002.32629. [DOI] [PubMed] [Google Scholar]
- 5.Hanna EB, Glancy DL. ST-segment depression and T-wave inversion: Classification, differential diagnosis, and caveats. Cleve Clin J Med. 2011;78(6):404–14. doi: 10.3949/ccjm.78a.10077. [DOI] [PubMed] [Google Scholar]
- 6.Haines DE, Raabe DS, Gundel WD, Wackers FJT. Anatomic and prognostic significance of new T-wave inversion in unstable angina. Am J Cardiol. 1983;52(1):14–18. doi: 10.1016/0002-9149(83)90061-9. [DOI] [PubMed] [Google Scholar]
- 7.Kaplanis I, Michas G, Arapi S, et al. Myocardial bridge as a cause of pseudo-Wellens’ syndrome. Hell J Cardiol. 2017;58(6):453–55. doi: 10.1016/j.hjc.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Lin AN, Lin S, Gokhroo R, Misra D. Cocaine-induced pseudo-Wellens’ syndrome: A Wellens’ phenocopy. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-222835. pii: bcr-2017222835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreras ML, Das A, Okwuosa T. Pseudo-Wellens syndrome after heavy marijuana use. Cleve Clin J Med. 2017;84(8):590–91. doi: 10.3949/ccjm.84a.16133. [DOI] [PubMed] [Google Scholar]
- 10.Grautoff S, Balog M, Winde G. Pseudo-Wellens’ syndrome and intermittent left bundle branch block in a case of acute cholecystitis. Am J Emerg Med. 2018;36(7):1323.e1–e6. doi: 10.1016/j.ajem.2018.03.081. [DOI] [PubMed] [Google Scholar]
- 11.Romhilt DW, Bove KE, Norris RJ, et al. A critical appraisal of the electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. Circulation. 1969;40(2):185–95. doi: 10.1161/01.cir.40.2.185. [DOI] [PubMed] [Google Scholar]
- 12.Roukoz H, Wang K. ST elevation and inverted T wave as another normal variant mimicking acute myocardial infarction: The prevalence, age, gender, and racial distribution. Ann Noninvasive Electrocardiol. 2011;16(1):64–69. doi: 10.1111/j.1542-474X.2010.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brady WJ. ST segment and T wave abnormalities not caused by acute coronary syndromes. Emerg Med Clin North Am. 2006;24(1):911–111. vi. doi: 10.1016/j.emc.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Birnbaum Y, Alam M. LVH and the diagnosis of STEMI – How should we apply the current guidelines? J Electrocardiol. 2014;47(5):655–60. doi: 10.1016/j.jelectrocard.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Pontone G, Bertella E, Mushtaq S, et al. Coronary artery disease: diagnostic accuracy of CT coronary angiography – a comparison of high and standard spatial resolution scanning. Radiology. 2014;271(3):688–94. doi: 10.1148/radiol.13130909. [DOI] [PubMed] [Google Scholar]
- 16.Bucciarelli-Ducci C, Denes P, Holly TA, Wu E. Pseudo Wellens T-waves in patients with suspected myocardial infarction: How cardiac magnetic resonance imaging can help the diagnosis. Int J Cardiol. 2008;128(2):e68–71. doi: 10.1016/j.ijcard.2007.05.062. [DOI] [PubMed] [Google Scholar]


