Abstract
Objective
To evaluate patients with stable COPD for the presence of potentially pathogenic microorganisms (PPM), systemic inflammation and the effects of short-term antibiotic therapy in PPM positive patients.
Methods
From January 2016 to June 2017, we enrolled 96 stable COPD patients. Bacterial cultures from sputum collections were quantitated, along with markers for systemic inflammation including serum C-reactive protein (CRP), interleukin-8 (IL-8) and plasma fibrinogen (FIB) in all patients. All enrolled patients were followed for 12 months. Forty patients were identified as PPM positive and were randomly divided into an antibiotic group and a control group. The antibiotic group was treated with moxifloxacin orally for 6 days. Lung function and markers for systemic inflammation were repeatedly measured at 30 days and 6 months in PPM positive subjects.
Results
Binary logistic regression analysis showed that risk factors for PPM positive are bronchiectasis (OR 4.18, 95% CI 1.20–14.59; P=0.025), COPD assessment test (CAT) ≥20 (OR 17.55, 95% CI 2.82–109.18; P=0.002), spontaneous sputum (OR 15.09, 95% CI 1.36–168.02; P=0.027) and sputum purulence (OR 38.43, 95% CI 5.39–274.21; P=0.000). CRP and IL-8 were higher in PPM positive group than those in PPM negative group (P=0.001, P=0.007, respectively), but there were no differences of FIB between the two groups (P=0.086). Compared to the PPM negative group, the rate of acute exacerbation of COPD was higher (P=0.029) and time to next acute exacerbation was shorter (P=0.030) in PPM positive group. There were no differences in lung function and systemic inflammatory markers either in the control group or the antibiotic group at different time points of follow-up.
Conclusion
PPM exists in stable COPD patients and can cause systemic inflammation and is associated with acute exacerbation of COPD. Short-term antibiotic therapy had no effect on systemic inflammation nor on acute exacerbation of COPD.
China Clinical Trials Registry: ChiCTR-IOR-15006769
Keywords: COPD, potentially pathogenic microorganisms, systemic inflammation, antibiotics, C-reactive protein, interleukin-8
Introduction
COPD has become the fourth leading cause of death in the world and is expected to become the third by 2020. Potential pathogenic microorganisms (PPM) exist in the lower respiratory tract of patients with stable COPD.1–3 PPM causes local airway inflammation2,4–10 and systemic inflammation.5,8,11 PPM is associated with acute exacerbation of COPD,7,9,12 accelerated decline of forced expiratory volume in one second (FEV1)13,14 and poor health-related quality of life.8,15
Researchers have investigated whether antibiotic treatment can alleviate local or systemic inflammation, reduce the rate of acute exacerbation COPD, delay the decline of lung function, and improve the health-related quality of life by reducing the bacterial load or even eradicating PPM. Some studies have shown that antibiotics can reduce local16,17 and systemic inflammation.17 Other studies have not observed these effects on bacterial load,18 airway18,19 or systemic inflammation.19 Nevertheless, long-term macrolide therapy has been found to reduce the times of acute exacerbations of COPD,16,20–23 improve health-related quality of life.22,23 However, long-term antibiotic use has also been shown to lead to an increase in bacterial resistance18 and hearing loss in some patients.23 Short-term antibiotic therapy can reduce the risk of bacterial resistance and drug-related side effects, but results demonstrating whether it can reduce bacterial load or even eradicate PPM, reduce local or systemic inflammation, and decrease acute exacerbation have been inconsistent.18,19,24–26
There are many inflammatory markers that have been used in previous studies to measure systemic inflammation from PPM in stable COPD. The most commonly used markers are serum C-reactive protein (CRP), interleukin-8 (IL-8) and plasma fibrinogen (FIB), but the conclusions from these markers have also been inconsistent. Some studies have found that PPM lead to elevated serum CRP,5 IL-85 and plasma FIB.8,11 One study found that PPM leads to elevated plasma FIB but not CRP.11 In most previous studies examining the effects of antibiotics on inflammation and acute exacerbation of COPD, all patients in the antibiotics groups received antibiotics regardless of the presence or absence of PPM in the lower respiratory tract.16,18–23 However, PPM negative patients may not benefit from antibiotic therapy. If antibiotics are used indiscriminately, it will increase antibiotic exposure and the risk of antimicrobial resistance. Therefore, the systemic inflammatory markers of PPM and the efficacy of short-term antibiotic therapy need to be further studied. The aim of this study was to further clarify the levels of systemic inflammatory markers in patients with stable COPD and PPM and the impact of short-term antibiotic therapy on these patients.
Methods
Study design and objectives
From January 2016 to June 2017, patients with a history of COPD were screened. Subjects enrolled were mainly composed of patients who regularly participated in the health education activities of COPD in our department. A diagnosis of COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014 criteria was required for eligibility.27 Asthma-COPD overlap syndrome (ACOS) was excluded according to diagnostic criteria. The diagnosis of ACOS needs to meet 2 major criteria and 2 minor criteria. The major criteria included very positive bronchodilator test (increase in FEV1 (% predicted) ≥15% and ≥400 ml), eosinophilia in sputum and personal history of asthma before the age of 40. Minor criteria included high total IgE, personal history of atopy and positive bronchodilator test (increase in FEV1 (% predicted) ≥12% and ≥200 ml) on two or more occasions.28 Stable COPD was defined as the absence of symptoms of lower respiratory tract infections (increase in dyspnea, cough and/or sputum purulence) within three months prior to inclusion in the study.2 Acute exacerbation of was defined as an acute change of symptoms that were beyond normal day-to-day variation and required a change in daily therapeutic drug regimens.27 All patients had contact numbers registered for both land-line and mobile phone for follow-up interviews. The study protocol was approved by the Ethics Committee of Luhe Hospital, and all patients provided written informed consent. We confirm that this study was based on the Helsinki Declaration.
Inclusion criteria: All patients included in the study met the diagnostic criteria of COPD based on GOLD 2014 criteria (i.e. FEV1/FVC <70% after inhalation of bronchodilator).
Exclusion criteria: Patients were excluded from the study if they had treatment with antibiotics in the past three months; quinolone allergy; immunosuppressive therapy; long-term systemic steroid treatment; history of malignant tumors; or limited activity due to illness.
Primary outcome was systemic inflammation in PPM positive patients with stable COPD.
Secondary outcomes included the effects of PPM on stable COPD patients and the effects of short-term antibiotic therapy on systemic inflammatory markers and acute exacerbation of COPD.
The baseline characteristics of the stable COPD patients were collected on the day of enrollment, including age, sex, smoking status, COPD assessment test (CAT), frequent hospitalization due to acute exacerbation of COPD in the previous year (≥2 times), inhalation drug therapy, domiciliary oxygen therapy, home mechanical ventilation, bronchiectasis, diabetes, coronary heart disease, hypertension, chronic heart failure, chronic renal failure and history of cerebral infarction.
Patients positive for PPM (40) were randomly divided into either the antibiotic group or the control group. The antibiotic group was treated with moxifloxacin 400 mg orally once a day for 6 days, whereas the control group maintained the original treatment with no antibiotic intervention. Lung function and markers of systemic inflammation were measured repeatedly at 30 days and 6 months in PPM positive subjects. The patients enrolled were subject to a follow-up interview by telephone for 12 months. The data collected included the patient’s daily treatment, acute exacerbation, and admissions due to acute exacerbation.
Measurements
The sputum specimens were collected for PPM detection on the day of enrollment. For those patients without existing sputum specimens, 0.9% saline, or 3%, 4% and 5% hypertonic saline were inhaled successively at 7 mins intervals until a qualified sputum specimen was obtained. A volume of 1 mL of sputum volume was required and was immediately sent for examination. Qualified sputum specimens are defined as those specimens with less than 10 epithelial cells and more than 25 white blood cells in the field of low power microscopy.29,30
Quantitative bacterial cultures from sputum were carried out following accepted laboratory methods.31 PPM is recognized as agent causing respiratory infections included: Haemophilus spp., Moraxella catarrhalis, Streptococcus pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, enterobacteria, Staphylococcus aureus and others.1,32,33 PPM was considered significant only when the growth was greater than 106 cfu, except from Streptococcus pneumonia, where growth greater than 105 cfu was considered adequate.34 Lung function and measurements of CRP, IL-8 and FBI were examined on the day of enrollment. Serum levels of CRP, IL-8, and plasma FIB were determined by latex turbidimetry, chemiluminescence, and immunoturbidimetry, respectively.
Statistical analysis
SPSS version 17.0 for Windows software (SPSS Inc., Chicago, IL, USA) was used for data management and statistical analysis. Continuous variables data were expressed as the mean (SD) or median (range), where as categorical data were presented as a number or percentage. Continuous variables with normal distributions were compared using the parametric unpaired two-independent-group Student's t-test, whereas those data not normally distributed were compared using the nonparametric Mann-Whitney U-test. Kruskai-Wallis ANOVA and one-way ANOVA were applied to compare the differences of systemic inflammation markers and lung function at the time of enrollment, 30 days and 6 months of follow-up, respectively. Binary logistic regression analysis was used to assess the following risk factors for patients with PPM positive: gender, smoking status, frequent hospitalization in the previous year (≥2 times), age ≥75 ys, bronchiectasis, coronary heart disease, hypertension, diabetes, chronic congestive heart failure, cerebral infarction history, inhaled corticosteroid treatment, domiciliary oxygen therapy, home noninvasive mechanical ventilation treatment, spontaneous sputum, sputum purulence, CAT ≥20 and FEV1 (% predicted) <50%. Calculate the risk factors for odds ratio (OR) and 95% for confidence interval (CI). A Chi-square test was used to compare the counting data. P<0.05 was considered statistically significant.
Sample size estimation: According to Banerjee’s study, plasma FIB in 27 PPM positive patients were significantly higher than 40 PPM negative patients.8 In the study of Marin, et al, serum CRP levels in 39 PPM positive patients were higher than those in PPM negative patients.5 Therefore, in order to observe systemic inflammation by these markers, we planned to include 40 PPM positive patients with stable COPD.
Results
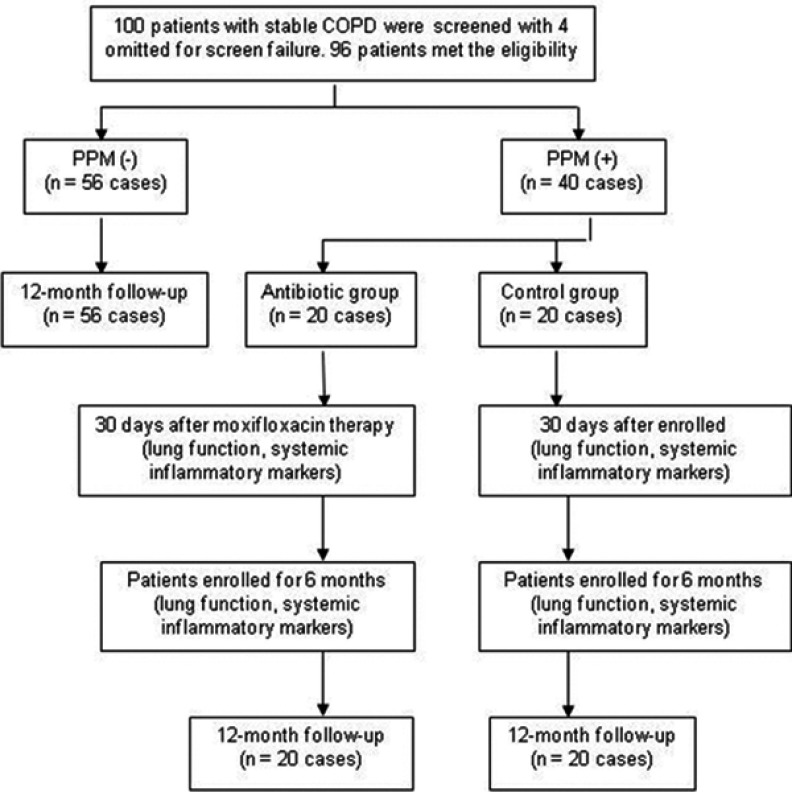
A total of 100 stable patients with a history of COPD were screened. Lung function of 96 patients met the diagnostic criteria of COPD for inclusion in our study. Of the 96 patients, 56 were PPM negative and 40 were PPM positive. Forty PPM positive patients were randomly divided into either the antibiotic group (n=20) or the control group (n=20). All 96 patients completed the 12-month follow-up telephone interview; there were no deaths during the follow-up period. (Figure 1)
Figure 1.
Screening, grouping, randomization, and follow-up.
Abbreviations: COPD, chronic obstructive pulmonary disease; PPM, potentially pathogenic microorganism.
A total of 19 sputum specimens were obtained by saline atomization, 17 cases in PPM negative group and 2 cases in PPM positive group, respectively. Among these 19 cases, only one case had purulent sputum, and the sputum was PPM negative. The other 18 cases had mucous sputum, and only 2 sputum specimens were PPM positive.
We observed no differences between the PPM positive and PPM negative patients when examing for age, sex, smoking status, frequent admissions in the previous year due to acute exacerbation of COPD (≥2 times), comorbidities, domiciliary oxygen therapy, home mechanical ventilation and inhalation drug therapy. We also found that 78.1% (75/96) of the enrolled patients adhered to long-term inhalation therapy. (Table 1)
Table 1.
Demographic characteristics and general clinical data of 96 patients with stable COPD
| Characteristics | PPM (-) (n=56) |
PPM (+) (n=40) |
P-value |
|---|---|---|---|
| Age (years) | 65.5 (6.7) | 65.5 (67.5) | 0.998 |
| Male gender (%) | 48 (85.7) | 35 (87.5) | 0.801 |
| Ex and current smoking, n (%) | 50 (89.3) | 32 (80.0) | 0.204 |
| Frequent admissions in the previous year (≥2), n (%) | 3 (5.4) | 3 (7.5) | 1.000 |
| Purulent sputum, n (%) | 4 (7.3) | 17 (42.5) | 0.000 |
| Bronchiectasis, n (%) | 11 (19.6) | 17 (42.5) | 0.015 |
| Comorbidities | |||
| Chronic renal failure, n (%) | 4 (7.1) | 1 (2.5) | 0.587 |
| Coronary heart disease, n (%) | 9 (16.1) | 8 (20.0) | 0.619 |
| Hypertension, n (%) | 29 (52.7) | 23 (57.5) | 0.644 |
| Diabetes, n (%) | 6 (10.7) | 2 (5.0) | 0.553 |
| Chronic congestive heart failure, n (%) | 3 (5.4) | 7 (17.5) | 0.114 |
| History of cerebral infarction, n (%) | 1 (1.8) | 3 (4.2) | 0.388 |
| CAT score | 13.8 (5.1) | 17.8 (7.7) | 0.033 |
| Lung function | |||
| FEV1 (% pred) | 50.9 (18.8) | 44.6 (19.6) | 0.019 |
| FVC (% pred) | 64.2 (19.3) | 60.2 (17.3) | 0.029 |
| FEV1/FVC | 60.7 (10.7) | 56.3 (12.3) | 0.196 |
| Respiratory rate (beat/min) | 19.6 (1.1) | 20.0 (1.4) | 0.374 |
| Heart rate (beat/min) | 82.6 (6.0) | 81.4 (7.8) | 0.141 |
| Blood pressure | |||
| Systolic (mmHg) | 124.7 (10.6) | 129.1 (19.2) | 0.822 |
| Diastolic (mmHg) | 82.5 (12.7) | 83.6 (13.3) | 0.324 |
| Domiciliary oxygen therapy, n (%) | 12 (26.0) | 13 (32.5) | 0.223 |
| Home mechanical ventilation, n (%) | 1 (1.8) | 3 (4.2) | 0.388 |
| Inhalation drug therapy | |||
| ICS ± LABA, n (%) | 20 (32.5) | 13 (35.7) | 0.744 |
| ICS ± LABA ± LAMA, n (%) | 16 (28.6) | 16 (40.0) | 0.242 |
| LAMA, n (%) | 6 (10.4) | 4 (10.7) | 1.000 |
| No inhalation therapy, n (%) | 14 (25.0) | 7 (17.5) | 0.381 |
| Systemic inflammation | |||
| CRP (mg/L) | 2.8 (1.6–1.6) | 8.2 (1.6–13.8) | 0.001 |
| FIB (g/L) | 7.3 (3.2–11.2) | 5.8 (3.2–6.6) | 0.086 |
| IL-8 (pg/mL) | 19.0 (7.1–13.4) | 43.2 (9.2–59.6) | 0.007 |
Note: Data are presented as mean (SD) or n (%) or median (range).
Abbreviations: PPM, potentially pathogenic microorganisms; CAT, chronic obstructive pulmonary disease assessment test; FEV1 (% pre), forced expiratory volume in one second (percentage of predicted); FVC (% pre), forced vital capacity (percentage of predicted); FEV1/FVC, ratio of forced expiratory volume in one second to forced vital capacity. ICS, inhaled corticosteroid; LABA, long-acting beta2-adrenergic agonist; LAMA, long-acting muscarinic antagonist; CRP, C-reactive protein; FIB, fibrinogen; IL-8, interleukin-8.
Binary logistic regression analysis showed that risk factors for PPM positive are bronchiectasis (OR 4.18, 95% CI 1.20–14.59; P=0.025), CAT ≥20 (OR 17.55, 95% CI 2.82–109.18; P=0.002), spontaneous sputum (OR 15.09, 95% CI 1.36–168.02; P=0.027) and sputum purulence (OR 38.43, 95% CI 5.39–274.21; P=0.000).
CRP and IL-8 were higher in the PPM population than those in the PPM negative population (P=0.001, P=0.007, respectively), but there were no differences of FIB between the two groups (P=0.086). (Table 1)
PPM was mainly composed of Klebsiella pneumoniae (21 cases) and Pseudomonas aeruginosa mucosa (6 cases). Etiology in the antibiotic group consisted of Klebsiella pneumoniae (8 cases); Pseudomonasaeruginosa (3 cases); Acinetobacter baumannii (2 cases); Pseudomonas oryzae, Pseudomonas putida, Pseudomonas fluorescens, Klebsiella acidogenicus, Pseudomonas maltophilia, Acinetobacter phenanthrene and Streptococcus pneumonia (7 cases, each having one strain). Etiology in control group consisted of Klebsiella pneumonia (12 cases) and Pseudomonas aeruginosa (3 cases), Klebsiella acidogenicus, Pseudomonas aeruginosa, Acinetobacter baumannii and Enterobacter agglomerates (4 cases, each having one strain), and one case contained a mixed infection of Acinetobacter baumannii and Klebsiella pneumoniae.
Follow-up at 12 months: The rate of acute exacerbation of COPD was higher (52.5% vs 30.4%, P=0.029), and time to next acute exacerbation was shorter (P=0.030), in the PPM positive patients compared to PPM negative patients. There were no differences in the rates of hospitalization and the time to next hospitalization due to acute exacerbation between the PPM negative and PPM positive patients. (Table 2)
Table 2.
Comparison of outcomes between the groups of PPM negative and PPM positive
| 12-month telephone follow-up | PPM (-) (n=56) |
PPM (+) (n=40) |
P-value |
|---|---|---|---|
| Acute exacerbation, n (%) | 17 (30.4) | 21 (52.5) | 0.029 |
| Hospitalization due to acute exacerbation, n (%) | 14 (25.0) | 16 (40.0) | 0.118 |
| Time to next acute exacerbation (days) | 191.3 (68.5) | 135.7 (73.3) | 0.030 |
| Time to next hospitalization (days) | 192.6 (78.8) | 148.6 (100.8) | 0.228 |
Note: Data are presented as mean (SD) or n (%).
Abbreviation: PPM, potentially pathogenic microorganisms.
There were also no differences observed between the antibiotic group and the control group when examined for age, sex, smoking status, frequent hospitalization in the previous year due to acute exacerbation of COPD, bronchiectasis, comorbidities, lung function, inhalation drug therapy, domiciliary oxygen therapy, home mechanical ventilation, CAT score, lung function and systemic inflammatory markers. (Table 3)
Table 3.
Demographic characteristics and general clinical data of randomized PPM positive patients with stable COPD
| Characteristics | Antibiotic group (n=20) |
Control group (n=20) |
P-value |
|---|---|---|---|
| Age (years) | 66.9 (7.1) | 65.2 (6.8) | 0.431 |
| Male, n (%) | 2 (85.0) | 3 (87.5) | 1.000 |
| Ex and current smoking, n (%) | 16 (80.0) | 16 (80.0) | 1.000 |
| Frequent admissions in the previous year (≥2), n (%) | 1 (5) | 3 (15) | 0.605 |
| Purulent sputum, n (%) | 5 (25) | 3 (15) | 0.693 |
| Domiciliary oxygen therapy, n (%) | 7 (35) | 7 (35) | 1.000 |
| Home mechanical ventilation, n (%) | 2 (10) | 1 (5) | 1.000 |
| Bronchiectasis, n (%) | 10 (50.0) | 7 (35.0) | 0.337 |
| CAT score | 19.5 (7.2) | 15.8 (8.1) | 0.277 |
| Lung function | |||
| FEV1 (% pred) | 43.8 (15.7) | 45.6 (24.6) | 0.844 |
| FVC (% pred) | 59.1 (13.8) | 61.6 (21.8) | 0.750 |
| FEV1/FVC | 57.2 (13.2) | 55.2 (15.6) | 0.699 |
| Respiratory rate (beat/min) | 19.5 (1.6) | 20.1 (1.0) | 0.314 |
| Heart rate (beat/min) | 82.0 (4.0) | 84.2 (6.6) | 0.371 |
| Blood pressure | |||
| Systolic (mmHg) | 126.0 (17.0) | 132.0 (21.1) | 0.309 |
| Diastolic (mmHg) | 81.9 (8.9) | 85.1 (16.6) | 0.454 |
| Inhalation drug therapy | |||
| ICS ± LABA, n (%) | 8 (40.0) | 4 (20.0) | 0.301 |
| ICS ± LABA ± LAMA, n (%) | 8 (40.0) | 8 (40.0) | 1.000 |
| LAMA, n (%) | 2 (10.0) | 2 (10.0) | 1.000 |
| No inhalation therapy, n (%) | 2 (10.0) | 6 (30.0) | 0.236 |
| Systemic inflammation | |||
| CRP (mg/L) | 6.1 (1.6–9.5) | 10.8 (1.6–19.3) | 0.059 |
| FIB (g/L) | 5.7 (3.1–8.2) | 5.9 (3.4–5.4) | 0.156 |
| IL-8 (pg/mL) | 37.8 (9.0–23.9) | 50.2 (9.5–74.6) | 0.204 |
Note: Data are presented as mean (SD), n (%) or median (range).
Abbreviations: PPM, potentially pathogenic microorganisms; CAT, chronic obstructive pulmonary disease assessment test; FEV1 (% pre), forced expiratory volume in one second (percentage of predicted); FVC (% pre), forced vital capacity (percentage of predicted); FEV1/FVC, ratio of forced expiratory volume in one second to forced vital capacity. ICS, inhaled corticosteroid; LABA, long-acting beta2-adrenergic agonist; LAMA, long-acting muscarinic antagonist; CRP, C-reactive protein; fibrinogen; IL-8, interleukin-8.
Follow-up for 12 months: there were no differences between the antibiotic group and the control group in the rate of acute exacerbation of COPD, the time to the next acute exacerbation and the rate of hospitalization due to acute exacerbation. (Table 4)
Table 4.
Comparison of clinical outcomes between patients in PPM negative group and PPM positive group
| 12-month telephone follow-up | PPM (-) (n=56) |
PPM (+) (n=40) |
P-value |
|---|---|---|---|
| Acute exacerbation, n (%) | 11(55.0) | 7(35.0) | 0.341 |
| Hospitalization due to acute exacerbation, n (%) | 10(50.0) | 7(35.0) | 0.523 |
| Time to next acute exacerbation (days) | 181.2 (85.1) | 199.3 (55.4) | 0.614 |
| Time to next hospitalization (days) | 172.3 (98.3) | 210.0 (60.0) | 0.438 |
Note: Data are presented as mean (SD) or n (%).
Abbreviation: PPM, potentially pathogenic microorganisms.
There were also no differences in lung function and systemic inflammatory markers between the control group and the antibiotic group at the time of enrollment, 30 days and 6 months of follow-up. (Tables 5 and 6)
Table 5.
Follow-up of CAT, markers for systemic inflammation and lung function in control group
| Variables | Baseline | 30 days follow-up | 6 months follow-up | P-value |
|---|---|---|---|---|
| CAT | 15.8 (8.1) | 15.7 (4.3) | 0.973 | |
| Systemic inflammation | ||||
| CRP (mg/L) | 10.8 (1.6–19.3) | 5.2 (1.6–22.2) | 10.0 (1.6–30.0) | 0.583 |
| FIB (g/L) | 3.8 (3.3–9.1) | 7.3 (4.1–10.1) | 9.4 (5.4–11.0) | 0.087 |
| IL-8 (pg/mL) | 50.2 (9.5–74.6) | 18.0 (9.8–74.3) | 17.1 (7.8–72.1] | 0.842 |
| Lung function | ||||
| FEV1 (% pred) | 45.6 (24.6) | 44.2 (22.9) | 44.9 (22.5) | 0.991 |
| FVC (% pred) | 61.6 (21.8) | 61.7 (20.6) | 61.9 (19.2) | 1.000 |
| FEV1/FVC | 55.2 (15.6) | 52.7 (10.9) | 55.1 (12.9) | 0.868 |
Note: Data are presented as mean (SD) or median (range).
Abbreviations: CAT, chronic obstructive pulmonary disease assessment test; PPM, potentially pathogenic microorganisms; CRP, C-reactive protein; FIB, fibrinogen; IL-8, interleukin-8; FEV1 (% pre), forced expiratory volume in one second (percentage of predicted); FVC (% pre), forced vital capacity (percentage of predicted); FEV1/FVC, ratio of forced expiratory volume in one second to forced vital capacity.
Table 6.
Follow-up of CAT, markers for systemic inflammation and lung function in the antibiotic group
| Variables | Baseline | 30 days follow-up | 6 months follow-up | P-value |
|---|---|---|---|---|
| CAT | 19.5 (7.2) | 15.5 (5.9) | 0.139 | |
| Systemic inflammation | ||||
| CRP (mg/L) | 6.1 (1.6–9.5) | 3.0 (1.6–1.6) | 5.5 (1.6–5.8) | 0.306 |
| FIB (g/L) | 5.7 (3.1–8.2) | 4.1 (3.1–3.9) | 4.9 (3.1–8.8) | 0.583 |
| IL-8 (pg/mL) | 37.8 (9.0–23.9) | 10.1 (5.1–14.6) | 8.5 (3.5–10.7) | 0.057 |
| Lung function | ||||
| FEV1 (% pred) | 43.8 (15.7) | 43.0 (15.5) | 44.8 (14.9) | 0.951 |
| FVC (% pred) | 59.1 (13.8) | 59.7 (14.4) | 63.2 (11.3) | 0.711 |
| FEV1/FVC | 57.2 (13.2) | 57.8 (12.6) | 57.0 (13.5) | 0.986 |
Note: Data are presented as mean (SD) or median (range).
Abbreviations: CAT, chronic obstructive pulmonary disease assessment test; CRP, C-reactive protein; FIB, fibrinogen; IL-8, interleukin-8; FEV1 (% pre), forced expiratory volume in one second (percentage of predicted); FVC (% pre), forced vital capacity (percentage of predicted); FEV1/FVC, ratio of forced expiratory volume in one second to forced vital capacity.
Discussion
In most studies, the PPM positive cutoff values of quantitative bacteria culture for sputum, Bronchoalveolar lavage fluid (BALF) and protected specimen brush (PSB) were ≥106 cfu,34 ≥103 cfu1,2,9 and ≥102 cfu,1,2,12 respectively. Other studies arbitrary defined the cutoff values of PPM positive for sputum, BALF and PSB as ≥102 cfu,3,5,14,35 ≥102 cfu,6 ≥103 cfu,3 respectively. In some studies, PPM was detected by PCR4,10,15,24,36 or 16S rRNA gene amplification and pyrosequencing.37 Quantitative detection of PPM showed that the rate of PPM positive in stable COPD was 29%~68%.3,5,6,8,9,11,14,35 The different detection rates of PPM may be related to specimen type, pathogenic detection method, cutoff values of PPM and severity of airflow restriction. Because BALF and PSB are invasive methods of sample collection, and qPCR and 16S rRNA pyrophosphate sequencing are expensive and need high technical expertise, these are not suitable for widespread application in clinical practice. In our study, qualified sputum was spontaneous expectorated or induced by saline inhalation atomization. Quantitative bacterial culture shave been carried out widely in hospitals at different levels. Therefore, our method is easy to implement and disseminate.
Our results showed that the PPM positive rate in stable COPD was 41.7% (40/96), with Klebsiella pneumoniae and Pseudomonas aeruginosa being the main pathogens present. The composition of the PPM was similar to that of another study in China.10 PPM in European studies is mainly composed of Haemophilus influenza4,14,15,35 and Moraxella catarrhalis.4,15 This suggests that there are geographical differences in PPM composition in stable COPD, and that there may be differences in inflammatory responses. Some studies found that the decrease of FEV1 is related to the increase of airway bacterial load.13 Severe airflow restriction is an independent risk factor for PPM,3 and color of sputum is associated with the presence of PPM.35 In our study, FEV1 and FVC were lower in PPM positive group than those in PPM negative group, binary logistic regression analysis showed that bronchiectasis, CAT ≥20, spontaneous sputum and sputum purulence are the risk factors for PPM positive. This indicated that the severity of COPD, sputum purulence and bronchiectasis were closely related to PPM positivity.
Whether CRP can reflect systemic inflammation caused by PPM is inconsistent. Some studies have found that PPM leads to elevated serum CRP, IL-85 and plasma CRP levels.8,11 In contrast, another study did not find an increase in CRP.11 Our results showed that serum CRP and IL-8 levels were higher in the PPM positive patients than PPM negative patients, whereas no differences in FIB were evident between the two patient populations. These results indicate that CRP and IL-8 were more sensitive indicators of systemic inflammation for stable COPD, PPM positive patients in our study.
Long-term antibiotic therapy can reduce the acute exacerbation of COPD, but can also increase the risk of bacterial resistance. The results of short-term antibiotic therapy for stable COPD have been inconsistent. A few studies have shown that short-term oral antibiotics can reduce airway bacterial load and reduce airway inflammation,26 and even eradicate PPM in a short time, but bacteria quickly re-colonized.24 However, most studies have found that short-term antibiotic therapy does not reduce airway inflammation18,19,25 nor acute exacerbation of COPD,18,25,26 and can even lead to an increase in bacterial resistance.18 In our study, the 12-month follow-up results showed that the rate of acute exacerbation of COPD in the PPM positive patients was higher than that in PPM negative patients. Also, the time to the next acute exacerbation was shorter in PPM positive patients. These results indicate that PPM can cause more acute exacerbation. In order to observe the efficacy of short-term antibiotic treatment in our study, patients with PPM were randomly divided into an antibiotic group and a control group and were followed for 12 months. We found no differences in the rate of acute exacerbation of COPD nor in the time to the next acute exacerbation of COPD between the two groups. The systemic inflammation markers of the two groups also did not change during the follow-up at 6 months. The study results indicate that short-term antibiotic therapy cannot alleviate systemic inflammation and reduce acute exacerbation of COPD.
Our study has some limitations. First, we did not assess airway local airway inflammation and explore the relationship between local and systemic inflammation. Second, during the follow-up period, the number of PPM positive patients who were repeatedly tested for PPM was relatively small. We did not observe dynamic changes of PPM. Finally, future studies will also be needed to detect virus in lower respiratory airways because chronic virus infection can also lead to airway inflammation.38
Conclusion
PPM exists in the lower respiratory tract in patients with stable COPD that can cause systemic inflammation and lead to an increase in serum CRP and IL-8. PPM is associated with acute exacerbation of COPD. Short-term antibiotic therapy had no effect on systemic inflammation nor acute exacerbation of COPD.
Data sharing statement
Authors allow sharing personal identification participant data and specific data related to the paper. Furthermore, data related to the paper can be accessed freely from the date of publication at the Clinical Trial Management Public Platform: http://www.medresman.org/uc/sindex.aspx.
Disclosure
Jin-Xiang Wang and Hui-Qiao Li are co-first authors for this study. The authors report no conflicts of interest in this work.
References
- 1.Cabello H, Torres A, Celis R, et al. Bacterial colonization of distal airways in healthy subjects and chronic lung disease: a bronchoscopic study. Eur Respir J. 1997;10(5):1137–1144. [DOI] [PubMed] [Google Scholar]
- 2.Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14(5):1015–1022. [DOI] [PubMed] [Google Scholar]
- 3.Zalacain R, Sobradillo V, Amilibia J, et al. Predisposing factors to bacterial colonization in chronic obstructive pulmonary disease. Eur Respir J. 1999;13(2):343–348. [DOI] [PubMed] [Google Scholar]
- 4.Barker BL, Haldar K, Patel H, et al. Association between pathogens detected using quantitative polymerase chain reaction with airway inflammation in COPD at stable state and exacerbations. Chest. 2015;147(1):46–55. doi: 10.1378/chest.14-0764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marin A, Garcia-Aymerich J, Sauleda J, et al. Effect of bronchial colonisation on airway and systemic inflammation in stable COPD. COPD. 2012;9(2):121–130. doi: 10.3109/15412555.2011.636407 [DOI] [PubMed] [Google Scholar]
- 6.Sethi S, Maloney J, Grove L, Wrona C, Berenson CS. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(9):991–998. doi: 10.1164/rccm.200509-1525OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57(9):759–764. doi: 10.1136/thorax.57.9.759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee D, Khair OA, Honeybourne D. Impact of sputum bacteria on airway inflammation and health status in clinical stable COPD. Eur Respir J. 2004;23(5):685–691. [DOI] [PubMed] [Google Scholar]
- 9.Tumkaya M, Atis S, Ozge C, Delialioglu N, Polat G, Kanik A. Relationship between airway colonization, inflammation and exacerbation frequency in COPD. Respir Med. 2007;101(4):729–737. doi: 10.1016/j.rmed.2006.08.020 [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Gu X, Weng Y, et al. Quantitative analysis of pathogens in the lower respiratory tract of patients with chronic obstructive pulmonary disease. BMC Pulm Med. 2015;15:94. doi: 10.1186/s12890-015-0094-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh R, Mackay AJ, Patel AR, et al. Inflammatory thresholds and the species-specific effects of colonising bacteria in stable chronic obstructive pulmonary disease. Respir Res. 2014;15:114. doi: 10.1186/s12931-014-0114-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosell A, Monsó E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005;165(8):891–897. doi: 10.1001/archinte.165.8.891 [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(8):1090–1095. doi: 10.1164/rccm.200210-1179OC [DOI] [PubMed] [Google Scholar]
- 14.Marin A, Monsó E, Garcia-Nuñez M, et al. Variability and effects of bronchial colonisation in patients with moderate COPD. Eur Respir J. 2010;35(2):295–302. doi: 10.1183/09031936.00126808 [DOI] [PubMed] [Google Scholar]
- 15.Bafadhel M, Haldar K, Barker B, et al. Airway bacteria measured by quantitative polymerase chain reaction and culture in patients with stable COPD: relationship with neutrophilic airway inflammation, exacerbation frequency, and lung function. Int J Chron Obstruct Pulmon Dis. 2015;10:1075–1083. doi: 10.2147/COPD.S80091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He ZY, Ou LM, Zhang JQ, et al. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respiration. 2010;80(6):445–452. doi: 10.1159/000321374 [DOI] [PubMed] [Google Scholar]
- 17.Tan C, Huang H, Zhang J, He Z, Zhong X, Bai J. Effects of low-dose and long-term treatment with erythromycin on interleukin-17 and interleukin-23 in peripheral blood and induced sputum in patients with stable chronic obstructive pulmonary disease. Mediators Inflamm. 2016;2016:4173962. doi: 10.1155/2016/4173962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brill SE, Law M, El-Emir E, et al. Effects of different antibiotic classes on airway bacteria in stable COPD using culture and molecular techniques: a randomised controlled trial. Thorax. 2015;70(10):930–938. doi: 10.1136/thoraxjnl-2015-207194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prins HJ, Daniels JM, Lindeman JH, Lutter R, Boersma WG. Effects of doxycycline on local and systemic inflammation in stable COPD patients, a randomized clinical trial. Respir Med. 2016;110:46–52. doi: 10.1016/j.rmed.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 20.Suzuki T, Yanai M, Yamaya M, et al. Erythromycin and common cold in COPD. Chest. 2001;120(3):730–733. doi: 10.1378/chest.120.3.730 [DOI] [PubMed] [Google Scholar]
- 21.Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178(11):1139–1147. doi: 10.1164/rccm.200801-145OC [DOI] [PubMed] [Google Scholar]
- 22.Blasi F, Bonardi D, Aliberti S, et al. Long-term azithromycin use in patients with chronic obstructive pulmonary disease and tracheostomy. Pulm Pharmacol Ther. 2010;23(3):200–207. doi: 10.1016/j.pupt.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 23.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. doi: 10.1056/NEJMoa1104623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miravitlles M, Marín A, Monsó E, et al. Efficacy of moxifloxacin in the treatment of bronchial colonisation in COPD. Eur Respir J. 2009;34(5):1066–1071. doi: 10.1183/09031936.00195608 [DOI] [PubMed] [Google Scholar]
- 25.Simpson JL, Powell H, Baines KJ, et al. The effect of azithromycin in adults with stable neutrophilic COPD: a double blind randomised, placebo controlled trial. PLoS One. 2014;9(8):e105609. doi: 10.1371/journal.pone.0105609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siva R, Bafadhel M, Monteiro W, Brightling CE, Pavord ID. Effect of levofloxacin on neutrophilic airway inflammation in stable COPD: a randomized, double-blind, placebo-controlled trial. Int J Chron Obstruct Pulmon Dis. 2014;9:179–186. doi: 10.2147/COPD.S55419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD; 2014. Available from: http://www.goldcopd.org/ Accessed May14, 2015).
- 28.Soler-Cataluña JJ, Cosío B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012;48(9):331–337. doi: 10.1016/j.arbres.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 29.Murray PR, Washington JA. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin Proc. 1975;50(6):339–344. [PubMed] [Google Scholar]
- 30.Heineman HS, Chawla JK, Lopton WM. Misinformation from sputum cultures without microscopic examination. J Clin Microbiol. 1977;6(5):518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pye A, Stockley RA, Hill SL. Simple method for quantifying viable bacterial numbers in sputum. J Clin Pathol. 1995;48(8):719–724. doi: 10.1136/jcp.48.8.719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sethi S, Sethi R, Eschberger K, et al. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(4):356–361. doi: 10.1164/rccm.200703-417OC [DOI] [PubMed] [Google Scholar]
- 33.Matkovic Z, Miravitlles M. Chronic bronchial infection in COPD. Is there an infective phenotype. Respir Med. 2013;107(1):10–22. doi: 10.1016/j.rmed.2012.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miravitlles M, Espinosa C, Fernández-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPD. Chest. 1999;116(1):40–46. doi: 10.1378/chest.116.1.40 [DOI] [PubMed] [Google Scholar]
- 35.Miravitlles M, Marín A, Monsó E, et al. Colour of sputum is a marker for bacterial colonisation in chronic obstructive pulmonary disease. Respir Res. 2010;11:58. doi: 10.1186/1465-9921-11-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miravitlles M, Anzueto A. Antibiotics for acute and chronic respiratory infection in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(9):1052–1057. doi: 10.1164/rccm.201302-0289PP [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Nuñez M, Millares L, Pomares X, et al. Severity-related changes of bronchial microbiome in chronic obstructive pulmonary disease. J Clin Microbiol. 2014;52(12):4217–4223. doi: 10.1128/JCM.01967-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson TM, Donaldson GC, Johnston SL, Openshaw PJ, Wedzicha JA. Respiratory syncytial virus, airway inflammation, and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(8):871–876. doi: 10.1164/rccm.200509-1489OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD; 2014. Available from: http://www.goldcopd.org/ Accessed May14, 2015).