Abstract
Purpose
Lung cancer is the most common malignant tumor in the world, and its incidence and mortality are very high. This study focuses on the mechanism of non-small cell lung cancer to find new therapeutic targets.
Methods
We used RT-PCR and Western blot to verify the linear relationship between E2F1 and IRF5 in normal lung tissue and lung cancer tissues. Secondly, we used overexpression and knock down E2F1 in cell lines to detect the expression of IRF5. The prime enzyme reporter plasmid verified that E2F1 binds to the core promoter region of IRF5; finally, CHIP experiments demonstrated that E2F1 binds directly to IRF5.
Results
We verified that E2F1 and IRF5 are decreased in patient tissues, and there is a strong linear relationship between E2F1 and IRF5. Secondly, we used overexpression of E2F1 or E2F1 siRNA transfected into HCC827 cells and found that E2F1 positively regulates the activity of the IRF5 promoter and the mRNA level of IRF5. Finally, the results of a chromatin immunoprecipitation assay demonstrated that E2F1 bound to the promoter region of IRF5 in vitro. These results suggested that the E2F1 transcription factor is the primary determinant for activating the basal transcription of the IRF5.
Conclusion
The transcription factor E2F1 positively regulates IRF5 in non-small cell lung cancer.
Keywords: interferon regulatory factor 5, E2F transcription factor 1, non-small cell lung cancer
Introduction
Lung cancer remains the leading cause of cancer worldwide, and its incidence and mortality have been significantly increased, closing to 1 in 5 (18.4%) cancer deaths in 2018.1 Non-small cell lung cancer (NSCLC), accounts for approximately 85% of all lung cancers. Despite the continued decline in smoking rates and early detection and treatment, the 5-year relative survival rate for lung cancer is currently only 18%.2 Therefore, there is an urgent need to develop new biomarkers to accurately detect the metastasis and recurrence of lung cancer. It is critical to study the molecular mechanisms of NSCLC progression and new targeted drugs to improve patient survival.
Interferon regulatory factor 5 (IRF5), a member of the interferon regulatory factor (IRF) family with diverse roles, is commonly found in malignant tumors.3 However, the expression of IRF5 in tumor is inconsistent and even the opposite. In some types of human cancers, the expression of IRF5 is upregulated, and it can promote its development, leading to a poor prognosis. On the contrary, it is a different story in some other types of cancers.4 It is reported that the expression of IRF5 is reduced in gastric cancer, renal cancer, and is associated with the progression and metastasis of breast cancer.5–7 Massimino M suggested that IRF5 is a target of BCR-ABL kinase activity and reduces CML cell proliferation.8,9 Cevik O reported that IRF5 inhibits hepatitis C virus (HCV) replication and HCV-associated hepatocellular carcinoma.10 In contrast, tumor-promoting effects of IRF5 have also been reported. IRF5 is highly expressed in primary and immortalized thyroid carcinoma, but there is no expression in normal thyroid cells, whereas ectopic IRF5 expression increases the proliferation rate and colony formation potential of malignant thyroid cells.11 IRF5 is up-regulated in Hodgkin’s lymphoma (HL) and is a key regulator of the abnormal transcriptome characteristics of the disease.12 The opposite functions of IRF5 may be due to the existence of multiple alternative splice variants, which with different cell type-specific expression and functions.13 Conclusively, IRF5 is a key factor in the regulation of cancer. However, few studies reported if IRF5 is differentially expressed in lung cancer, its role in lung cancer remains undefined. Therefore, a better understanding of IRF5 may provide additional therapeutic goals for disease management and requires further attention.
E2F transcription factor 1 (E2F1) belongs to the E2F transcription factor family and is involved in cell cycle control and DNA damage response. It also promotes apoptosis and inhibits cancer production.14 E2F1 is closely related to diffuse large B-cell lymphoma, bladder cancer, tongue cancer and gastric cancer.15–18 And E2F1 is considered to be highly expressed in SCLC. Wang T reported E2F1 promotes EMT by regulating ZEB2 gene expression in SCLC.19 Park S.M suggested LncRNA EPEL promotes lung cancer cell proliferation through E2F target activation.20 While there are few literature reports about E2F1 expression in NSCLC.
Despite the important roles of E2F1 and IRF5 in cancers, few studies have investigated the relationship between E2F1 and IRF5 in human NSCLC. Nevertheless, we predicted that E2F1 may be a pivotal factor for IRF5. In this study, we first identified the roles of E2F1 and IRF5 in NSCLC samples. Then we verified that the change of E2F1 expression in NSCLC cell lines resulted in a significant increase in the expression and promoter activity of IRF5. Furthermore, we verified that E2F1 can bind to the promoter region of IRF5. These findings indicated that E2F1 positively regulates the transcription of IRF5 by binding to the minimal promoter region of IRF5 in NSCLC.
Materials and methods
Subjects and sample collection
Lung cancer and adjacent normal tissues were obtained after surgical resection from patients and stored at −70 °C in the First Affiliated Hospital of Nanjing Medical University. This study was approved by the Institutional Research Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. All patients have signed the written informed consent. We declared the research was carried out according to the World Medical Association Declaration of Helsinki.
Cell culture and reagents
Human type II alveolar lung epithelium cells (A549) cells and Hela cell lines (Hela) were obtained from the American Type Culture Collection (ATCC) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum, 1% penicillin and streptomycin. Human lung adenocarcinoma cell line (HCC827) were obtained from the American Type Culture Collection (ATCC) and maintained in RPMI 1640 with 10% FBS, 1% penicillin and streptomycin. Cells were incubated at 37 °C in 5% CO2.
Plasmids and small interfering RNA (siRNA)
Transcriptional start site (TSS) of human IRF5 promoter was set as + 1 according to Shu J reported [21] and the IRF5 genomic DNA fragment − 179 to + 62 was inserted into the pGL3-Basic vector (Promega) named as pGL3-241. The software JASPAR database version 5.0 (jaspar.genereg.net) was used for the prediction of potential transcriptional binding sites of IRF5. According to the site-directed mutagenesis kit (Takara) protocol, the E2F1 binding site mutation promoter (pGL3-mut) was created by PCR from the cloned pGL3-241 plasmid. The site-special mutagenized plasmids were named as mut-E2F1-A, mut-E2F1-B, mut-E2F1-C, mut-E2F1-A+B, mut-E2F1-A+C, mut-E2F1-B+C, mut-E2F1-A+B+C according to the binding sites. The site-special mutagenized primers were listed in Table 1. The overexpression plasmids pENTER-E2F1 and the corresponding control plasmid pENTER are kept by our laboratory. The double-stranded siRNAs were synthesised and high performance purified (GenePharma). The targeted in the E2F1 mRNA and negative control sequences used were as follows: E2F1: 5ʹ-CACTGAATCTGACCACCAATT-3ʹ (sense); 5ʹ-TTGGTGGTCAGATTCAGTGTT-3ʹ(antisense);
Table 1.
Clinical characteristic of subjects
| Tumor type | Differentiation stage | Age | Sex | Tumor size (cm) | |
|---|---|---|---|---|---|
| NSCLC | T2N0M0 | III | 63 | M | 4*3*2 |
| T1N0M0 | IA | 51 | M | 2*2*1 | |
| T3N0M0 | IIB | 58 | M | 7*6*2 | |
| T2N1M0 | IIB | 68 | F | 5*4.5*4 | |
| T1N0M0 | II | 59 | M | 2.5*1.5*1.2 | |
| T2N0M0 | IB | 48 | M | 3.5*3*3 | |
| T2N0MO | IB | 52 | M | 1.6*1.6*1.5 | |
| T2N0M0 | IB | 64 | M | 3.5*3*2.5 | |
| T2N0M0 | IIA | 49 | F | 4.5*5*3 | |
| SCLC | T2N2M0 | IIIA | 54 | F | 4*2*2 |
Notes: Classification of the lung tumor samples used in the current studies. Total RNA and protein samples from lung tumors and their matching non-tumor control were obtained from The First Affiliated Hospital, Nanjing Medical University.
Control: 5ʹ-UUCUCCGAACGUGUCACGU-3ʹ(sense), 5ʹ-ACGUGACACGUUCGGAGAATT-3ʹ(antisense)
Quantitative real-time PCR (qRT-PCR)
Total RNA from tissue and cells was extracted using TRIzol reagent (Invitrogen) and cDNA was reverse-transcribed using Primescript RT Reagent (Takara). qRT-PCR was performed in the LightCycler480II (Roche) using SYBR Green technology (Takara). Each sample was assessed with the specification of amplification by analyzing the melting curve. The primers were used for qRT-PCR as follows:
IRF5: 5ʹ-GGGCTTCAATGGGTCAACG-3ʹ (sense); 5ʹ-GCCTTCGGTGTATTTCCCTG-3ʹ(antisense);
E2F1: 5ʹ-AGCGGCGCATCTATGACATC-3ʹ (sense); 5ʹ-GTCAACCCCTCAAGCCGTC −3ʹ(antisense);
GAPDH: 5ʹ-TGGTATCGTGGAAGGACTCATGAC-3ʹ (sense); 5ʹ-TGCCAGTGAGCTTCCCGTTCAGC-3ʹ(antisense).
The GAPDH gene was used as a normalized standard and relative expression level was calculated with the comparative CT method.
Cell transfection and luciferase assays
According to the manufacturer’s protocol, A549 and HCC827 cells were conducted using Lipofectamine™ 3000 (Invitrogen). The cells were seeded into 96-well plates (1.5 × 104/well). After 24 hrs, 100 ng luciferase promoter plasmids and 4 ng pRL-TK plasmids were transfected into cells using Lipofectamine™ 3000. For siRNA and overexpression assay, pGL3-241 or pGL3-mut plasmid was co-transfected with siE2F1 or pENTER-E2F1 into cells. Promoter activity was detected by a Dual-luciferase Reporter Assay System (Promega) and normalized to the activity of pRL-TK. Results were representative of at least three independent experiments performed in triplicate.
Western blot
Total protein from frozen tissues or cells was extracted using a Total Protein Extraction Kit ((Beyotime). Protein concentrations were measured using the BCA Protein Assay Kit (Beyotime). Equal amount of protein was run by 10% SDS-PAGE and blotted on a PVDF membrane (Millipore). To block non-specific sites, the membranes were incubated in 5% dry milk in TBS-T saline (0.25 M Tris-HCl; pH 7.6, 0.19 M NaCl, 0.1% Tween 20) for 2 h, the membrane immunodetected with anti-IRF5 (Abcam), anti-E2F1 (Abcam), and anti-GAPDH antibody (Proteintech) at 4 °C overnight at a dilution of 1:2000–1:4000. Then membranes were washed three times with TBS-T and treated with goat anti-rabbit IgG (Proteintech). Signals of membranes were measured by chemiluminescence (ECL) system, scanned and analyzed by Image Lab Software (Bio-Rad)
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed with the EZ-Magna ChipTM A kit (Millipore) according to the manufacturer’s instructions. A total of 1×107 Hela cells were fixed in 1% formaldehyde at room temperature for 10 min. The cell lysates were sonicated to generate 200–1,000 bp DNA fragments. Then immunoprecipitated with anti-IgG antibody (Millipore), anti-E2F1 antibody (Abcam) and anti-acetyl histone H3 antibody (Millipore). After reverse cross-linking and DNA purification, DNA from input or immunoprecipitated samples were assayed by qRT-PCR using SYBR Green (Takara) with the following primer: 5ʹ-GGGCTTCAATGGGTCAAGG-3ʹ (sense); 5ʹ-GCCTTCGGTGTATTTCCCTG-3ʹ (antisense).
Statistical analysis
The results were presented as the mean ± standard error and experiments were repeated at least three independent experiments. Statistical analysis was performed using GraphPad Prism 7 and SPSS 22.0. Results were considered statistically significant at *p<0.05.
Results
IRF5 and E2F1 are reduced in human NSCLC tissues
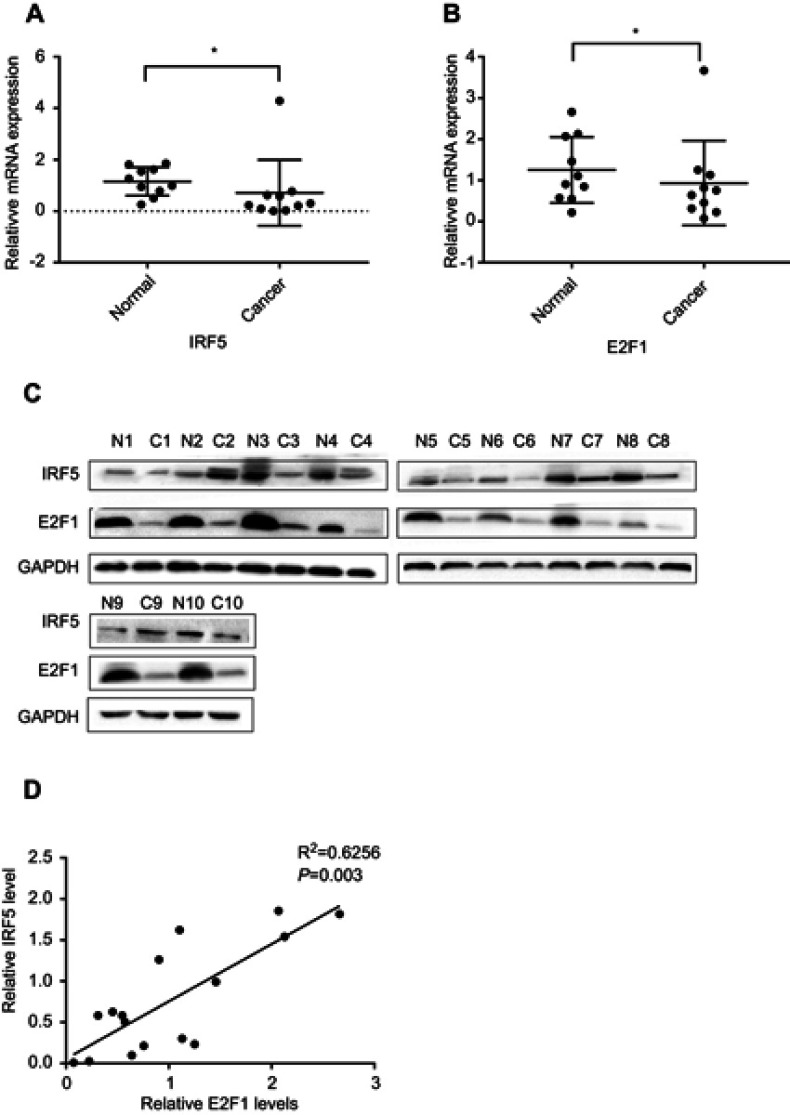
IRF5 and E2F1 expression levels were analyzed by qRT-PCR and Western blot in 10 pairs of tumor tissues and matched adjacent normal tissues. Our results showed a significant decrease in IRF5 and E2F1 expression in NSCLC tissues compared to adjacent normal tissues (Figure 1A–C). The IRF5 expression showed a strong linear correlation with the expression of E2F1 in tumor tissues and normal subjects in Figure 1D.
Figure 1.
IRF5 and E2F1 are reduced in human NSCLC tissues. (A and B) The expression of E2F1 and IRF5 was analyzed by qRT-PCR (*p < 0.05). (C) Protein levels of E2F1 and IRF5 between normal and cancer in NSCLC. (D) The correlation between E2F1 and IRF5 was tested with the Pearson correlation test. The differences in expression levels of E2F1 and IRF5 between normal tissues and cancers were determined using a paired t-test. The mRNA and protein levels of E2F1 and IRF5 were compared with GAPDH.
E2F1 upregulates IRF5 expression
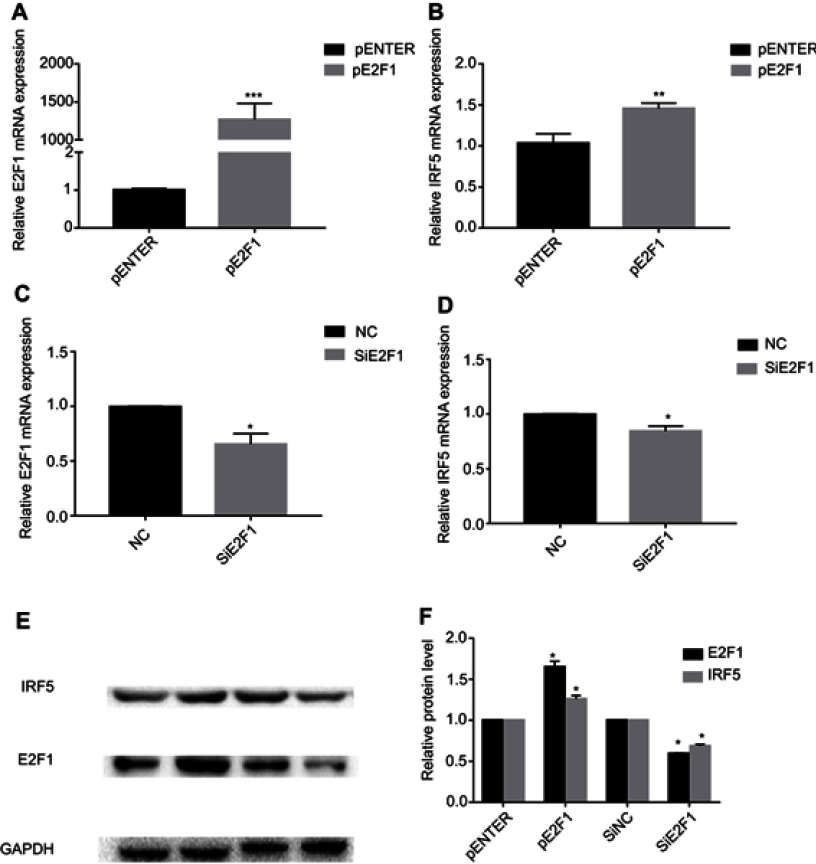
We measured the mRNA levels of E2F1 and IRF5 in the HCC827 cells by qRT-PCR to determine whether E2F1 affects IRF5 expression. Compared with the empty plasmid controls, the overexpression of E2F1 enhanced endogenous IRF5 mRNA levels (50%) (Figure 2A and B). Meanwhile, the RNA expression of IRF5 decreased by 20% in siE2F1-treated (Figure 2C and D). To investigate the effect of E2F1 on the expression of IRF5 protein levels, total protein was extracted from HCC827 cells and subjected to Western blot. As shown in Figure 2E and F, IRF5 protein expression increased in E2F1-overexpression treated group compared with controls in HCC827 cells, which was in consistent with the mRNA expression. And siE2F1 treated group reduced the IRF5 protein expression.
Figure 2.
E2F1 upregulates IRF5 expression. (A–B) Quantification of IRF5 mRNA level after pE2F1-mediated overexpression of E2F1 in HCC827 cells for 48h. (C–D) Quantification of IRF5 mRNA level after E2F1 siRNA-mediated knockdown in HCC827cells for 48h. (E–F) IFR5 and E2F1 protein levels were detected by Western blot analysis in HCC827 cells after transfected with different doses E2F1 siRNA orE2F1 overexpression plasmid for 48 h. *p<0.05, **p<0.01, ***p<0.001.
E2F1 decreases IRF5 transcriptional promoter activity
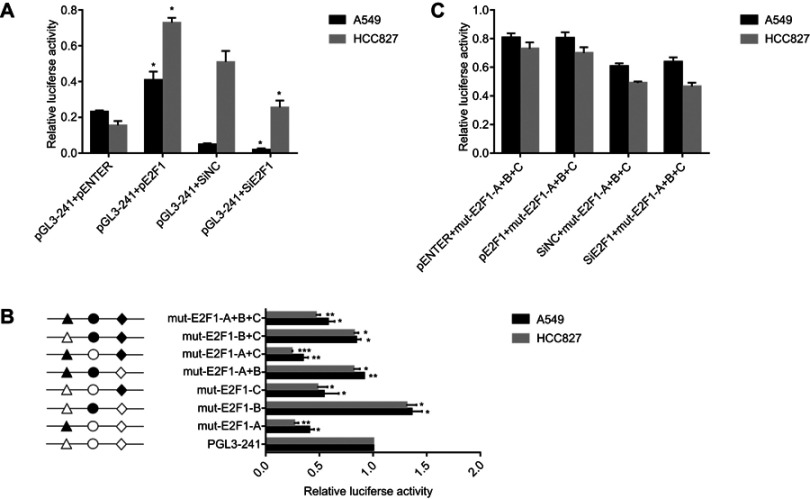
As Shu J reported that, Sp1 transcription factors directly binds to IRF5 gene promoter and regulates its transcriptional activity. we cloned the sequence between −179 and +62 upstream of human IRF5 promoter. To further investigate transcription factors of this region, we used the JASPAR database version 5.0 found that this region has three E2F1 transcription factor binding sites. To determine whether E2F1 directly regulates IRF5 transcription, we transfected E2F1-overexpression plasmid and siE2F1 into A549 and HCC827 cells. As shown in Figure 3A, the overexpression of E2F1 increased the luciferase activity and siE2F1 decreased it. To determine the function of E2F1 binding site in IRF5 promoter, a series of plasmids with 2-3bp point mutations of E2F1 binding sites were constructed and transiently transfected into A549 and HCC827 cells. As shown in Figure 3B, mutations of E2F1-A and E2F1-C in the A549 and HCC827 cells reduced the promoter activity. However, mutation of E2F1-B in A549 and HCC827 cells increased the promoter activity respectively. And mutations of E2F1-A+C had an obvious reduction effect, E2F1-A+B and E2F1-B+C has a little influence compared with E2F1-A+C.The triple mutation of E2F1-A+B+C also decreased the promoter activity. We co-transfected the E2F1-overexpression plasmid and siE2F1 with the mutated IRF5 core promoter into A549 and HCC827 cells, respectively. As shown in Figure 3C, changes in promoter activity were not statistically significant. We conclude that these three E2F1 binding sites play an important role in the activity of the IRF5 core promoter. Mutations of E2F1-A and E2F1-C positively regulate IRF5 core promote activity, while mutation of E2F1-B is reversed, and mutation of E2F1-A has a greater effect than mutation of E2F1-C.
Figure 3.
E2F1 decreases IRF5 transcriptional promoter activity. (A) A549 and HCC827 cells were cotransfected with E2F1 overexpression plasmid or siE2F1 and pGL-241 for 24 h, respectively. (B) A549 and HCC827 cells were cotransfected with E2F1 overexpression plasmid or siE2F1 and mut-E2F1-A+B+C for 24 h, respectively. (C) The schematic structure of the reporter construct is shown on the left. Three different binding sites for the transcription factor E2F1 are represented by different open shapes. Mutations are shown in bold above the histogram. Various constructs fused to the firefly luciferase reporter vector were co-transfected into A549 and HCC827 cells with the Renilla luciferase expression vector. The level of firefly luciferase activity was normalized to Renilla luciferase activity (*p<0.05, **p<0.01, ***p<0.001). The measures represented three independent experiments and are shown as the mean ± S.D. of the normalized luciferase activity.
E2F1 binds to the minimal promoter of IRF5 in vitro
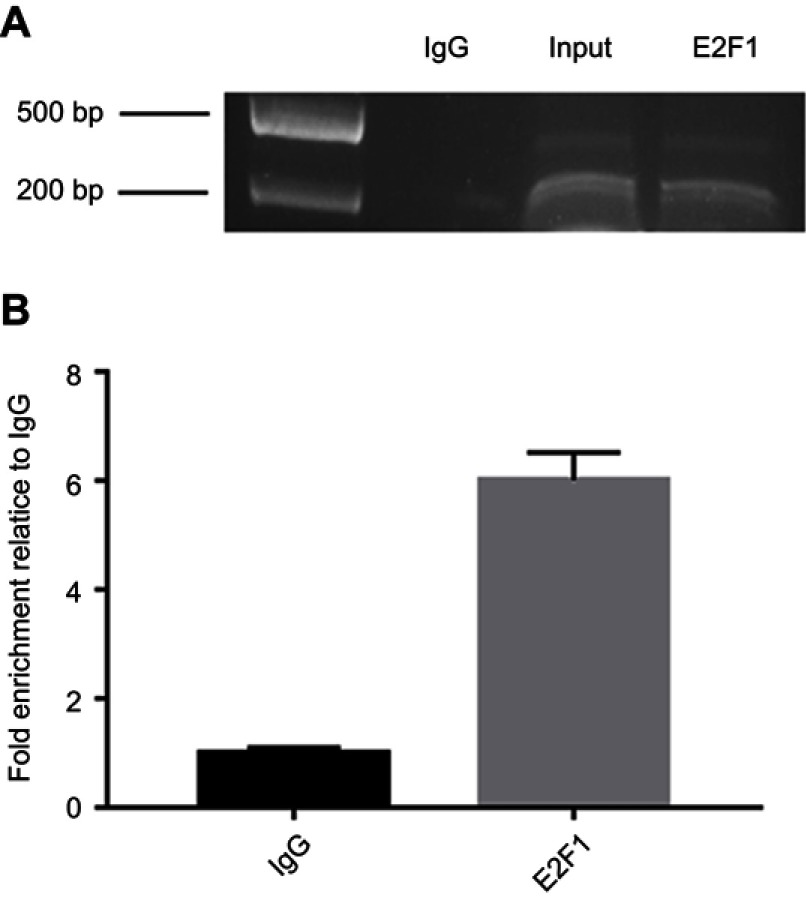
To examine whether E2F1 binds to the promoter region of IRF5 in vitro, a ChIP assay was performed in Hela cells. HeLa cells were cross-linked with formaldehyde, then the cells were lysed and chromatin were sonicated. The chromatin was immunoprecipitated with an anti-E2F1, anti-IgG antibody, and the DNA precipitated in the complexes was subjected to PCR using primers flanking the region containing the E2F1-A binding site. As shown in Figure 4, the sequence of the IRF5 promoter was immunoprecipitated by the anti-E2F1 antibody, and the non-specific IgG antibody could not immunoprecipitated the sequence in vitro, and then the precipitated DNA was amplified by PCR and RT-PCR. As shown in Figure 4A and B, binding of E2F1 was detected at −179 to +62 region, where binding of non-specific IgG failed to be found, it was revealed that E2F1 can bind to the IRF5 minimal promoter in vitro.
Figure 4.
E2F1 binds to the minimal promoter of IRF5 in vitro. (A) CHIP for detecting binding of E2F1 to the IRF5 promoter in vitro. The protein precipitated by the anti-E2F1 antibody binds in vitro to the amplified sequence of the IRF5 promoter, whereas the anti-IgG antibody cannot precipitate the bound protein in the sequence. (B) The immunoprecipitated chromatin fragments were analyzed by quantitative PCR using primer pairs spanning the putative E2F1 binding site.
Discussion
The aim of this study was to determine the relationship between E2F1 and IRF5 in NSCLC and to investigate its underlying mechanisms. For this purpose, we examined their expression profiles and showed increased expression of both IRF5 and E2F1, which suggesting a strong linear correlation between IRF5 and E2F1 expression in NSCLC. Little is known about the reasons for this correlation. Further study unequivocally showed that overexpression of E2F1 increased promoter activity, mRNA and protein levels of the IRF5 gene, while repression of E2F1 reduced IRF5 expression. These observations suggest that E2F1 is a positive regulator of IRF5.
IRF5 is a transcription factor which belongs to the family of interferon regulatory factor (IRF), and was originally identified as a regulator of type I IFN gene expression of which encodes a 60–63-kDa polypeptide.3,21 Subsequent studies reported that IRF5 can be involved in host defense pathogens, tumorigenesis and autoimmunity.22,23 IRF5 is transcribed into nine distinct alternatively spliced informs. The existence of multiple IRF5 spliced isoforms with distinct cell type-specific expression, cellular localization, differential regulation, and dissimilar functions in virus-mediated type I IFN gene induction. V1-V4 were transcribed in human primary plasmacytoid dendritic cells (PDC), whereas in human primary peripheral blood mononuclear cells V5 and V6 were identified. And V7-V9 were detected only in human cancer.3,4 However, few studies have reported the function of IRF5 in lung cancer. Li reported that IRF5 and IRF7 were key transcription factors in IFN pathway that determined viral sensitivity of lung cancer cells.24 And our study indicated a decrease in the expression of IRF5 in non-small cell lung cancer, consistently.
The E2F transcription factor family plays an important role in cell proliferation, differentiation and apoptosis.14,25 At present, the E2F transcription factor family is known to be composed of 8 genes, E2F1 to E2F8, which are usually divided into two categories, E2F1, E2F2 and E2F3a are effective transcriptional activators, E2F3b and E2F4 to 8 are considered to inhibit transcription.25 Zhou reported that E2F1 was upregulated in NSCLC, its expression was negatively correlated with miR-936 expression.26 Liu reported that miR-433 inhibit the progression of NSCLC by targeting E2F3.27 Park indicated that E2F8 is overexpression in LC and is necessary for the growth of LC cells.28 E2F1 is a transcription factor involved in the regulation of a variety of biological activities, including cell cycle, apoptosis, proliferation, angiogenesis, tumor resistance, infiltration and metastasis.18–20 Previous study reported that E2F1 promotes tumor proliferation in small cell lung cancer, and this study demonstrates that in non-small cell lung cancer, E2F1 expression is inconsistent with small cell lung cancer.19,20 Our group previously showed that E2F1 positively regulates the transcription of IRF3 through binding to the E2F1 binding site.29–31 In this study, we observed a significant decrease of E2F1 in NSCLC tissues. Based on this result, we indicate that E2F1, as an oncogene, may inhibit the transcriptional activity of IRF5.
In conclusion, we demonstrated that the transcription factor E2F1 positively regulates the IRF5 expression by binding to the promoter specific region in NSCLC. To our knowledge, this is the first study to report the reduction of E2F1 and IRF5 both in human NSCLC tissues and various cell lines. With anti-tumor effect, E2F1 and IRF5 may represent potential therapeutic targets for NSCLC in future.
Acknowledgments
This work was supported by grants from Jiangsu Province Science and Education Enhancing Health Project Innovation Team (Leading Talent) Program (CXTDA2017018 to G.Z), Nanjing Science and Technology Development Program (201503003 to G.Z) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. The authors are grateful to all study participants.
Abbreviations
IRF5, Interferon regulatory factor 5; E2F1, E2F transcription factor 1; NSCLC, Non-small cell lung cancer; LC, lung cancer; IRF, interferon regulatory factors; HCV, Hepatitis C virus; HL, Hodgkin’s lymphoma; IRF3, interferon regulatory factor 3; A549, Human type II alveolar lung epithelium cells; HCC827, Human lung adenocarcinoma cell line; DMEM, Dulbecco’s Modified Eagle Medium; RPMI, Hyclone rpmi medium modified; FBS, fetal bovine serum; siRNA, small interfering RNA; TSS, Transcriptional start site; qRT-PCR, Quantitative real-time PCR; BSA, bovine serum albumin; PBS, phosphate-buffered saline; TBS, Tris-buffered saline.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R.L., Torre L.A., Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Siegel, R.L., Miller, K.D., Jemal, A. Cancer Statistics. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Yanai, H., Chen, H.M., Inuzuka, T., Kondo, S., Mak, T.W., Takaoka, A., Honda, K., Taniguchi, T. Role of IFN regulatory factor 5 transcription factor in antiviral immunity and tumor suppression. Proc Natl Acad Sci U S A. 2007;104(9):3402–3407. doi: 10.1073/pnas.0611559104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savitsky, D., Tamura, T., Yanai, H., Taniguchi, T. Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol Immunother. 2010;59(4):489–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong, S.M., Lee, H.G., Cho, S.G., Kwon, S.H., Yoon, H., Kwon, H.J., Lee, J.H., Kim, H., Park, P.G., Kim, H., Hayward, S.D., Park, J.H., Lee, J.M. Hypermethylation of the interferon regulatory factor 5 promoter in Epstein-Barr virus-associated gastric carcinoma. J Microbiol. 2015;53(1):70–76. [DOI] [PubMed] [Google Scholar]
- 6.Bai, Q., Liu, L., Xia, Y., Wang, J., Xi, W., Qu, Y., Xiong, Y., Long, Q., Xu, J., Guo, J. IRF5 is associated with adverse postoperative prognosis of patients with non-metastatic clear cell renal cell carcinoma. Oncotarget. 2017;8(27):44186–44194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garaud, S., Willard-gallo, K. IRF5: a rheostat for tumor-infiltrating lymphocyte trafficking in breast cancer. Immunol Cell Biol. 2015;93(5):425–426. [DOI] [PubMed] [Google Scholar]
- 8.Massimino, M., Consoli, M.L., Mesuraca, M., Stagno, F., Tirro, E., Stella, S., Pennisi, M.S., Romano, C., Buffa, P., Bond, H.M., Morrone, G., Sciacca, L., Di Raimondo, F., Manzella, L., Vigneri, P. IRF5 is a target of BCR-ABL kinase activity and reduces CML cell proliferation. Carcinogenesis. 2014;35(5):1132–1143. [DOI] [PubMed] [Google Scholar]
- 9.Manzella, L., Tirro, E., Pennisi, M.S., Massimino, M., Stella, S., Romano, C., Vitale, S.R., Vigneri, P. Roles of interferon regulatory factors in chronic myeloid leukemia. Curr Cancer Drug Targets. 2016;16(7):594–605. [DOI] [PubMed] [Google Scholar]
- 10.Cevik, O., Li, D., Baljinnyam, E., Manvar, D., Pimenta, E.M., Waris, G., Barnes, B.J., Kaushik-Basu, N. Interferon regulatory factor 5 (IRF5) suppresses hepatitis C virus (HCV) replication and HCV-associated hepatocellular carcinoma. J Biol Chem. 2017;292(52):21676–21689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masimino, M., Vigneri, P., Fallica, M., et al IRF5 promotes the proliferation of human thyroid cancer cells. Mol Cancer. 2012;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babaian, A., Romanish, M.T., Gaqnier, L., Kuo, L.Y., Karimi, M.M., Steidl, C., Mager, D.L. Onco-exaptation of an endogenous retroviral LTR drives IRF5 expression in Hodgkin lymphoma. Oncogene. 2016;35(19):2542–2546. [DOI] [PubMed] [Google Scholar]
- 13.Mancl, M.E., Hu, G., Sangster-Guity, N., Olshalsky, S.L., Hoops, K., Fitzgerald-Bocarsly, P., Pitha, P.M., Pinder, K., Barnes, B.J. Two discrete promoters regulate the alternatively spliced human interferon regulatory factor-5 isoforms. Multiple isoforms with distinct cell type-specific expression, localization, regulation, and function. J Biol Chem. 2005;280(22):21078–21090. [DOI] [PubMed] [Google Scholar]
- 14.Engelmann, D., Putzer, B.M. The dark side of E2F1: in transit beyond apoptosis. Cancer Res. 2012;72(3):571–575. [DOI] [PubMed] [Google Scholar]
- 15.Samaka, R.M., Aiad, H.A., Kandil, M.A., Asaad, N.Y., Holah, N.S. The prognostic role and relationship between E2F1 and SV40 in diffuse large B-cell lymphomaof Egyptian patients. Anal Cell Pathol (Amst). 2015;2015:919834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelrahman, A.E., Rashed, H.E., Elkady, E., Elsebai, E.A., El-Azony, A., Matar, I. Fatty acid synthase, Her2/neu, and E2F1 as prognostic markers of progression in non-muscle invasive bladder cancer. Ann Diagn Pathol. 2019;39:42–52. [DOI] [PubMed] [Google Scholar]
- 17.Kwong, R.A., Nguyen, T.V., Bova, R.J., Kench, J.G., Cole, I.E., Musgrove, E.A., Henshall, S.M., Sutherland, R.L. Overexpression of E2F-1 is associated with increased disease-free survival in squamous cell carcinoma of the anterior tongue. Clin Cancer Res. 2003;9(10 Pt 1):3705–3711. [PubMed] [Google Scholar]
- 18.Manicum, T., Ni, F., Ye, Y., Fan, X., Chen, B.C. Prognostic values of E2F mRNA expression in human gastric cancer. Biosci Rep. 2018;38(6):20181264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, T., Chen, X., Qiao, W., Kong, L., Sun, D., Li, Z. Transcription factor E2F1 promotes EMT by regulating ZEB2 in small cell lung cancer. BMC Cancer. 2017;17(1):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park, S.M., Choi, E.Y., Bae, D.H., Sohn, H.A.,Kim, S.Y., Kim, Y.J. The LncRNA EPEL promotes lung cancer cell prolifeation through E2F target activation. Cell Physiol Biochem. 2018;45(3):1270–1283. [DOI] [PubMed] [Google Scholar]
- 21.Shu, J., Wang, X.H., Zhou, L.B., Jiang, C.M., Yang, W.X., Jin, R., Wang, L.L., Zhou, G.P. Expression of interferon regulatory factor 5 is regulated by the Sp1 transcription factor. Mol Med Rep. 2016;14(3):2815–2822. [DOI] [PubMed] [Google Scholar]
- 22.Alsamman, K., El-Masry, O.S. Interferon regulatory factor 1 inactivation in human cancer. Biosci Rep. 2018;38(3). pii: BSR20171672. doi: 10.1042/BSR20171672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvin, P., Thean, H.T., Siew, K.N. Interferon regulatory factor 9 structure and regulation. Front Immunol. 2018;9:1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, Q., Tainsky, M.A. Epigenetic silencing of IRF7 and/or IRF5 in lung cancer cells leads to increased sensitivity to oncolytic viruses. PLoS One. 2011;6(12):e28683. doi: 10.1371/journal.pone.0028683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claire, A., Eros, L.D., Kristian, H. The E2F family: specific functions and overlapping interests. Embo J. 2004;23(24):4709–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou, X., Tao, H. Overexpression of mircoRNA-936 suppresses non-small cell lung cell proliferation and invasion via targeting E2F1. Exp Ther Med. 2018;16(3):2696–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, N., Liu, Z., Zhang, W., Li, Y., Cao, J., Yang, H., Li, X. MircroRNA-433 reduces cell proliferation and invasion in non-small cell lung cancer via directly targeting E2F transcription factor 3. Mol Med Rep. 2018;18(1):1155–1164. [DOI] [PubMed] [Google Scholar]
- 28.Park, S.A., Platt, J., Lee, J.W., Lopez-Giraldez, F., Herbst, R.S., Koo, J.S. E2F8 as a novel therapeutic target for lung cancer. J Natl Cancer Inst. 2015;107(9). pii:djv151. doi: 10.1093/jnci/djv151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu, L.H., Gao, S., Jin, R., Zhuang, L.L., Jiang, L., Qiu, L.Z., Xu, H.G., Zhou, G.P. Repression of interferon regulatory factor 3 by the Epstein-Barr virus immediate-early protein Rta is mediated through E2F1 in the Hela cells. Mol Med Rep. 2014;9(4):1453–1459. doi: 10.3892/mmr.2013.1812 [DOI] [PubMed] [Google Scholar]
- 30.Xu, H.G., Ren, W., Zou, L., Wang, Y., Jin, R., Zhou, G.P. Direct repression of the human IRF-3 promoter by E2F1. Immunogenetics. 2011;63(4):189–196. [DOI] [PubMed] [Google Scholar]
- 31.Xu, H.G., Ren, W., Lu, C., Zhou, G.P. Characterization of the human IRF-3 promoter and its regulation by the transcription factor E2F1. Mol Biol Rep. 2010;37(7):3073–3080. [DOI] [PubMed] [Google Scholar]