Abstract
This study assessed the feasibility of studying animal-assisted activities (AAA) in inpatient pediatric oncology and collected preliminary data on potential benefits of AAA for this population. Patients at a large pediatric hospital were identified using electronic medical records and approached with physician approval. Patients completed surveys before and after a therapy dog visit in their private hospital room. Data on infections were ascertained by electronic medical record review. Provider surveys were placed in provider common areas and distributed through a link in an e-mail. We summarized resultsusing descriptive statistics and estimated mean changes in pre- and postintervention distress and conducted hypothesis tests using the paired t test. The study population (mean age = 12.9 years) consisted of 9 females and 10 males. Following the therapy dog visit, patients had lower distress and significant decreases in worry, tiredness, fear, sadness, and pain. Providers were generally supportive of the intervention. Eight patients developed infections during the 14 days after the dog visit but none could be clearly attributed to the therapy dog visit. The study’s primary limitation was that there was no control group. However, results support the feasibility of and need for future studies on AAA in pediatric oncology.
Keywords: cancer, animal-human bonding, pet therapy, distress
Introduction
Animal-assisted activities (AAA) “provide opportunities for motivational, educational and/or recreational benefits to enhance quality of life . . . delivered by a specially trained professional, paraprofessional and/or volunteer, in partnership with an animal that meets specific criteria for suitability” (Pet Partners, 2016). AAA (eg, therapy dog visits) are very common in pediatric hospital settings (Chubak & Hawkes, 2016). However, the use of AAA varies widely among institutions that provide pediatric oncology care (Chubak & Hawkes, 2016) and there has been little research on the effectiveness or safety of AAA in this setting (American Humane Association, 2013; Bouchard, Landry, Belles-Isles, & Gagnon, 2004; Gagnon et al., 2004; Urbanski & Lazenby, 2012). A 2012 systematic review on animal-facilitated therapy in pediatric oncology found evidence of benefits in other pediatric hospital settings (Urbanski & Lazenby, 2012), but found only 1 published study of AAA focused on pediatric oncology inpatients, which did not collect any patient-reported outcomes (Bouchard et al., 2004; Gagnon et al., 2004). In that study, 16 pediatric oncology patients (3-13 years old) at a Canadian hospital cared for a dog for an entire day (8-16 hours) in a room designated for this activity (Gagnon et al., 2004)—an approach that differs markedly from usual AAA in pediatric oncology units (Chubak & Hawkes, 2016). Our recent review of the literature identified 1 additional study. A small study in Brazil suggested—based on reports from guardians and nursing staff—that therapy dog visits could distract pediatric oncology patients and increase happiness (Moreira et al., 2016). However, no patient-reported outcomes were collected in this study either.
Thus, in preparation for larger observational studies and randomized controlled trials to fill gaps on effectiveness and safety in this setting, we conducted a pilot study of AAA in an inpatient pediatric oncology unit. Our aims were to (a) assess the feasibility of studying AAA in pediatric oncology and (b) to collect preliminary data on potential benefits.
Materials and Methods
Setting
This study was conducted at Seattle Children’s Hospital in Seattle, Washington between November 2015 and March 2016. Seattle Children’s Hospital is an academic medical center in the Pacific Northwest, serving Washington, Alaska, Montana, and Idaho. At the time of this study, Seattle Children’s Hospital had a therapy dog program but teams did not visit the hematology/oncology unit.
All study procedures were approved by the Seattle Children’s Hospital Institutional Review Board. Providers were e-mailed a “FAQ sheet” to introduce the study (see the appendix).
Population
Inpatients aged 7 to 25 years (inclusive) on the hematology/oncology unit who were present on at least 1 of the 25 potential therapy dog visit days were eligible for this study. Patients were ineligible if they had a recent bone marrow transplant; viral, respiratory, contact, or enteric precautions in place; or an allergy to dogs documented in the electronic medical record (EMR). On the day before a study visit, the research assistant screened patient EMRs for eligibility. The attending physician was then contacted to ask whether there was any reason not to approach potentially eligible patients. Following approval from the attending physician, the research assistant went to the patients’ bedsides to describe the study to families and seek consent/assent. Patients 7 to 17 years old were enrolled if they gave assent and a parent provided written consent. Patients 18 through 25 years old provided written informed consent for themselves. Regardless of patient age, parents who were present during the therapy dog visit provided written informed consent for researchers to observe their actions during the intervention.
Intervention
Seattle Children’s Hospital’s Therapy Dog Program requires each therapy team (1 handler and 1 dog) be registered with a therapy team-registering organization that is independent of the hospital. The registering organization is required to meet the hospital’s therapy training, evaluation, and renewal criteria. Once this requirement is met, the team proceedes with the on-boarding process, which ensures the hospital population and therapy dog team are a good fit. The dogs’ owners are responsible for the dogs’ safety, health, care, and overall well-being at all times while visiting at the hospital.
The study intervention consisted of a one-time visit between a therapy dog team and each patient; a single handler-dog team conducted all visits. The visit occurred in each patient’s private hospital room. The therapy dog team was instructed to conduct their visit as they would on other hospital units, that is, no allowable activities were specifically mandated or discouraged. The team followed the standard safety practices employed for therapy dog visits throughout the hospital, including hand sanitization before and after contact with the dog and no licking. The only difference for patients in this study was that Infection Prevention requested that patients (if ambulatory) be encouraged to use soap and water or an alcohol-based hand wipe after petting the dog, rather than alcohol-based gel. The rationale was that soap and water or wipes—as opposed to gel—would be more likely to remove particulate matter. Visits were limited to approximately 20 minutes, but no minimum duration was required. We implemented this time restriction to allow for multiple visits per day; this duration was consistent with usual visit lengths at Seattle Children’s Hospital.
Visits began with the handler introducing herself and her dog and asking permission to visit with the patient. Generally, the handler sat in a chair next to the bed, provided hand sanitizer to the patient, and invited him/her to pet the dog. She talked with the patient and family and often invited the dog to show the patient a trick. At the end of the visit, she provided the patient with her dog’s “business card,” which included a photo, to provide children with a keepsake.
Data Collection
To assess feasibility of future studies, we collected data on study recruitment rates, return of patient surveys, and factors that might affect the willingness of hospitals to participate in future studies (eg, staff views and incidence of infection). To assess whether the intervention showed promise for reducing distress, we collected details of how the intervention was delivered and information on patient experiences (self-reported) and behaviors (observed).
Data sources consisted of (a) patient self-report surveys completed before and after the therapy dog visit, (b) direct observation of the visit by the research assistant, (c) EMR review, (d) case review by Infection Prevention staff, and (e) surveys open to any hematology/oncology staff (eg, physicians, nurses, child life professionals). In addition, adverse event forms were provided to patients (along with self-addressed stamped envelopes) and were made available to staff in staff work and break areas to ensure we captured any events that may not have been observed during the visit or noted in the EMR.
Patient Surveys
The research assistant handed each patient a printed copy of the preintervention self-report survey immediately following consent. In most cases, completed forms were collected prior to the therapy dog visit. Patients completed age-appropriate versions of the Distress Thermometer (Patel et al., 2011), Patient Reported Outcomes Measurement Information System (PROMIS) short forms (depressive symptoms, anxiety, and peer relationships; Hinds et al., 2013), and PedsQL Present Functioning Scales (afraid/scared, sad/blue, angry, worried, tired, hurting/discomfort/pain; Sherman, Eisen, Burwinkle, & Varni, 2006). For children who needed assistance completing the forms, a parent generally read them the questions and/or marked forms per the child’s instructions. The Distress Thermometer and PROMIS forms refer to the past week and past 7 days, respectively, whereas the PedsQL Present Functioning Scales instruct those completing it to share “how you feel now.” The Distress Thermometer and PedsQL instruments are visual analog scales; the PROMIS forms use a standard set of 5 responses (never, almost never, sometimes, often, almost always). The Distress Thermometer consists of a thermometer-like image on which respondents mark their level of distress, as well as a checklist of physical, practical, emotional, spiritual, and family/social problems. These forms were administered to characterize our study population psychosocially and to facilitate comparisons with other populations.
Immediately after the therapy dog visit, the research assistant handed patients the postintervention forms to complete the PedsQL Present Functioning Scales and an open-ended feedback form. The other surveys were not repeated because they referred to the prior week and responses were therefore not expected to have changed over the course of a single therapy dog visit.Patients were encouraged to complete the forms while the research assistant was present to hand forms back directly, but this was not always possible due to patient medical needs. If necessary, the research assistant returned to retrieve the forms later in the day or the following day.
Surveys were scored using published or supplied guidelines. Early in the study, we noticed that the PedsQL Present Functioning Scale had printed incorrectly, so the scale was not exactly 10 cm. For 7 patients, the scale length ranged from 94 to 99 mm. We converted scores to what they would be if the scale had been 100 mm as intended (ie, by dividing the rating by the scale length and multiplying by 100). The total PedsQL Present Functioning Scales score was computed as an average of the 6 scales; the emotional distress summary score was the average of fear, worry, sadness, and anger scores (Sherman et al., 2006).
Direct Observation
During the therapy dog visit, the research assistant used a semistructured form developed for this study with expert input to note interactions such as petting and playing between patient and dog, as well as parent and dog. Patient and parent behaviors (eg, smiling, crying) were noted and direct quotes were collected. The research assistant recorded the visit from start to finish and who was present, including other study team members who observed the visits.
Medical Record Review
At the end of the study, the research assistant reviewed the EMR of each enrolled participant. Data were collected on paper abstraction forms and included information on: patient’s medical history (type of malignancy, history of nondog allergies, history of asthma, or reactive airway disease); any reported allergic reaction to the therapy dog during or after the visit; infections or illnesses diagnosed in the 14 days after the dog visit and whether the new illness/infection was suspected by providers to be from the therapy dog visit; and whether the patient was taking antimicrobial agents in the 14 days after the therapy dog visit. The 14-day period was selected with the assumption that any infection caused by the dog visit would manifest itself clinically by then.
Case Review by Infection Prevention Staff
As an additional way to assess the safety of the visits, at the conclusion of the study, the research team provided Infection Prevention staff with the list of study participants and dates of therapy dog visits. The infection preventionist noted any infections documented in the EMR between each patient’s therapy dog visit and the end of the study—not restricted to the 14 days after the visit—and returned this information to the research team.
The research team analyzed only those infections that were newly documented during the 14 days after the therapy dog visit. Both the chart review and infection prevention review data were used to determine when the patient developed a new infection. We did not consider an infection to be new if similar symptoms were documented on the previous day. In the case where multiple symptoms appeared on the same day (eg, fever, vomiting, runny nose), we counted these clusters of new symptoms as a single infection.
Provider/Staff Surveys
We designed a brief feedback form to collect information on provider/staff perceptions on the effects of therapy dog visits on patients and providers/staff, their concerns, and their overall assessment of allowing therapy dog visits in pediatric oncology. Over the entire study period, the research team made paper surveys available (along with information sheets and stamped return envelopes) in staff common areas in the inpatient pediatric oncology unit. In addition, we recruited providers/staff to complete the survey via the Internet. Three times toward the end of the study, a department administrator forwarded an e-mail on behalf of the study team to a listserv comprising 206 inpatient hematology/oncology/bone marrow transplant physicians and midlevel providers, inpatient nurses and nursing assistants, and the leadership team. The e-mail invited providers—regardless of whether or not they were present on a pilot study day—to complete the survey anonymously through the weblink provided in the e-mail. We were interested in views from all staff, even those not working on the day of visits, thus we asked about the acceptability of AAA visits in pediatric oncology in general and we asked about the effects of visits on staff and patients,which may have occurred on days following therapy dog visits.
Analysis
We computed descriptive statistics (including counts, means, standard deviations, medians, ranges, and interquartile ranges) as appropriate, on patient demographic characteristics as well as both patient and provider/staff surveys. We computed standard errors of the estimated mean changes in pre- and postintervention PedsQL Present Functioning scales and conducted hypothesis tests using the paired t test. Because our sample size was small and the change scores displayed notable left skewness, we conducted exploratory analyses testing the similarity of the distributions of the pre- and postintervention PedsQL Present Functioning scales using the Wilcoxon signed rank test.
In post hoc analyses, we stratified changes in PedsQL Present Functioning scores by sex and age (<13 vs ≥13 years). We did not formally test differences between subgroups due to small sample sizes. We also conducted an analysis restricted to patients with no/low distress at baseline to explore whether AAA visits may benefit even those patients without moderate/high distress. Qualitative information (written responses to open-ended survey questions) were not formally analyzed but were reviewed to provide examples of the range of attitudes expressed by patients and providers, and for use in developing future studies.
Results
Patient Characteristics
Nineteen patients participated in this research study. Sex was evenly distributed among participants (Table 1) and the mean age was 12.9 years (SD = 3.6). No patients between the ages of 19 and 25 years enrolled. A slightly greater proportion of female patients (55%) were younger than 13 years compared with male patients (45%). Though patients could be included regardless of diagnosis, all participants had diagnoses of leukemia/lymphoma, sarcoma, or brain cancer. More than half of patients had a documented history of some nondog allergy (eg, cats, foods, medications).
Table 1.
Characteristics of Pediatric Oncology Inpatients Participating in Therapy Dog Pilot Study.
| Characteristic | No. of patients (%) |
|---|---|
| Sex | |
| Female | 9 (47) |
| Male | 10 (53) |
| Age group (years) | |
| 7-13 | 11 (58) |
| 13-17 | 7 (37) |
| 18-25 | 1 (5) |
| Cancer type | |
| Leukemia/lymphoma | 8 (42) |
| Sarcoma | 7 (37) |
| Brain | 4 (21) |
| History of asthma or reactive airway disease | 4 (21) |
| History of nondog allergies | 12 (63) |
Aim 1: Feasibility of Studying AAA
Recruitment and Return of Surveys
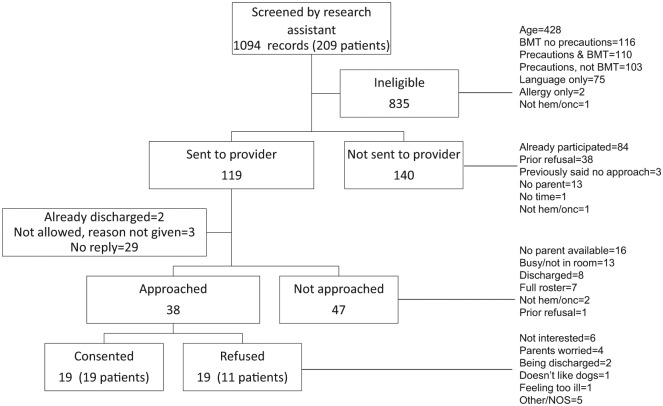
Between November 2015 and March 2016, we recruited 19 (of 20 planned) patients to participate in our pilot study of therapy dog visits for pediatric oncology inpatients. The research assistant screened a total of 1094 records. (Because patients were often in the hospital for many days, there were often multiple records for a single patient, therefore, 1 record = 1 patient screened before a study visit day.) Figure 1 shows the recruitment process in detail. In brief, most records were excluded due to age, bone marrow transplant, or presence of isolation precautions. A total of 38 patient approaches were made to 30 patients, yielding a total of 19 unique participants. The main reason for nonparticipation among eligible patients was lack of interest, followed by parental worry.
Figure 1.
Recruitment of patients into pilot study of animal-assisted activities in inpatient pediatric oncology. BMT, Bone marrow transplant; hem/onc, hematology/oncology; NOS, not otherwise specified.
We received pre- and postvisit forms from 18 of 19 patients. With the exception of the Distress Thermometer (4 patients did not complete), no more than 2 respondents left any single question blank. All PedsQL items were complete on both pre- and postvisit surveys for the 18 patients who returned their forms.
Staff and Provider Surveys
Forty-eight providers/staff responded to our survey, all via the Web interface. Nearly half of participants were nurses (n = 23, 48%), one-quarter were physicians (n = 12, 25%), and the remainder were primarily physician assistants and medical assistants. Fourteen (29%) were working on the hematology/oncology unit on one of the pilot days. Among these 14 providers/staff, 12 (86%) reported that they thought the therapy dog visit had an effect on patients; all described the effect as positive in response to an open-ended question. Seven of 14 providers/staff (50%) reported that the therapy dog visit had no effect on them or their work. Of the 6 who reported an effect, only 1 reported a negative effect (disruption). When asked about their concerns, 1 wrote disruption to work flow and 2 wrote of concerns related to risk of infections. Among all 48 respondents, 35 (73%) thought having therapy dog visits on the hematology/oncology unit was a good idea, 1 (2%) thought it was not, 4 (8%) were unsure, and 8 (17%) did not respond.
Infections and Adverse Events
Eight of 19 patients (42%) experienced at least one new infection during the 14 days after their therapy dog visit. The most common type of infection was febrile neutropenia (n = 4, 21%), followed by upper respiratory infection (n = 3, 16%), Clostridium difficile (n = 2, 11%), and other fever (n = 2, 11%). None of the infections could be clearly attributed to the therapy dog visit, nor could we definitively rule out that possibility since we had no control group. No adverse events (eg, bites, allergic reactions) were reported by patients, staff, or providers on adverse event report forms.
Aim 2: Potential Benefits of AAA
Direct Observation
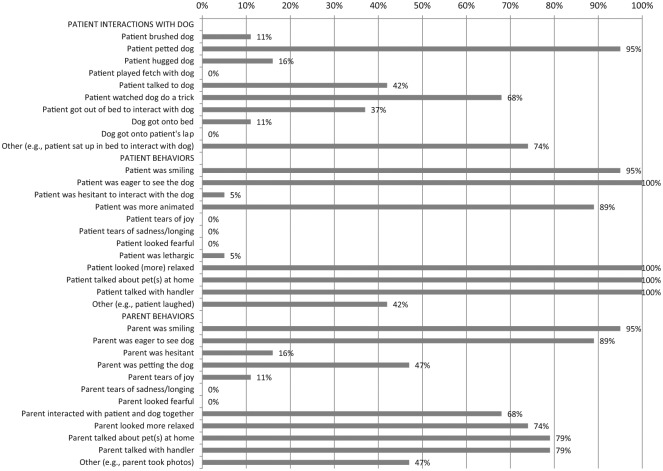
Patients and parents always or almost always were eager to see the dog (patients, 100%; parents, 89%), smiled (patients and parents, 95%), became animated (patients, 89%; parents, not measured), looked more relaxed (patients, 100%; parents, 74%), talked about pets at home (patients, 100%; parents, 79%), and talked with the handler (patients, 100%; parents, 79%). The most common interactions during visits were petting the dog and watching the dog do a trick. Almost all visits (79%) concluded because the 20-minute limit had been reached, rather than due to patient or handler preference at an earlier time point.
Patient Surveys
At baseline, 3 of 19 patients (16%) reported no distress, 7 (37%) reported mild distress (rating >0 to <5), 5 (26%) had moderate-to-high distress (rating ≥5), and 4 did not complete the Distress Thermometer rating (n = 4, 21%). The most common problems endorsed on the Distress Thermometer (≥30%) were feeling worried/anxious, feeling nervous, feeling sad/depressed, feeling irritable/annoyed, feeling bored/not wanting to do anything, pain, nausea, feeling tired, and problems with school/tutoring (not shown). Similarly, on the PROMIS scales, nearly half of patients reported at least sometimes feeling worried and feeling sad or unhappy (Figure 2). Worry and tiredness were rated the highest on the PedsQL Present Functioning scales with mean scores of 31.4 (SD = 25.2) and 36.2 (SD = 29.8) out of 100, respectively (Table 2).
Figure 2.
Interactions and behaviors observed during therapy dog visits (n = 19).
Table 2.
Differences in PedsQL Present Functioning Scales Before and After Therapy Dog Visit (N = 18).
| Item | Previsit |
Postvisit |
Mean Score Change (95% Confidence Interval) | P Value (t Test) | ||
|---|---|---|---|---|---|---|
| Mean Scorea (SD) | Median Score (IQR) | Mean Scorea (SD) | Median Score (IQR) | |||
| Fear | 11.9 (20.6) | 2.6 (0.0-10.0) | 1.7 (2.2) | 1.0 (0.0-3.2) | −10.1 (−19.8, −0.4) | .04 |
| Sadness | 15.5 (22.7) | 5.0 (0.0-22.0) | 3.2 (6.5) | 0.5 (0.0-2.1) | −12.4 (−22.4, −2.3) | .02 |
| Anger | 5.2 (11.2) | 0.5 (0.0-4.0) | 2.9 (6.3) | 0.5 (0.0-2.1) | −2.4 (−5.0, 0.3) | .07 |
| Worry | 31.4 (25.2) | 32.0 (8.0-50.0) | 4.5 (7.2) | 1.5 (0.0-5.0) | −26.9 (−39.7, −14.1) | <.01 |
| Tiredness | 36.2 (29.8) | 30.0 (11.7-51.0) | 11.6 (14.8) | 3.5 (1.1-20.2) | −24.6 (−37.6, −11.6) | <.01 |
| Pain | 13.2 (19.9) | 4.0 (0.0-16.0) | 4.5 (7.9) | 2.0 (0.0-4.0) | −8.7 (−15.9, −1.4) | .02 |
| Total scorea | 18.9 (14.7) | 14.3 (8.5-30.0) | 4.7 (4.8) | 3.1 (1.2-7.6) | −14.2 (−20.6, −7.7) | .002 |
| Emotional distress summary scoreb | 16.0 (14.1) | 11.6 (4.5-29.8) | 3.1 (3.8) | 1.4 (0.0-5.2) | −12.9 (−19.3, −6.5) | .005 |
Abbreviations: IQR, interquartile range; SD, standard deviation.
Total score is an average of the fear, sadness, anger, worry, tiredness, and pain scales.
Emotional distress summary score is an average of the fear, sadness, anger, and worry scales.
We received postintervention forms from 18 patients. Reductions in the total score and emotional distress summary score were significant (Table 2). We observed large and statistically significant decreases in worry and tiredness following therapy dog visits, and smaller but significant changes in fear, sadness, and pain (Table 2). The reduction in anger was small and not statistically significant. P values from Wilcoxon signed rank tests (not shown) in exploratory analyses were similar to those from the t test, reflecting the robustness of findings to distributional assumptions. Our exploratory analyses suggested that changes in the PedsQL Present Functioning Scales scores were similar in males and females (not shown), with a few exceptions. Compared to females, males experienced smaller decreases in sadness and pain, but larger decreases in tiredness after the therapy dog visit. Results appeared to differ by age group, with children younger than 13 years—who started with much worse scores—experiencing greater improvements, in general, than older children (Table 3).
Table 3.
Differences in PedsQL Present Functioning Scales Before and After Therapy Dog Visit, by Age.
| Item | <13 Years (n = 11) |
≥ 13 Years (n = 7) |
||||
|---|---|---|---|---|---|---|
| Previsit Mean Score (SD) | Postvisit Mean Score (SD) | Mean Score Change (95% CI) | Previsit Mean Score (SD) | Postvisit Mean Score (SD) | Mean Score Change (95% CI) | |
| Fear | 16.6 (25.2) | 2.3 (2.6) | −14.3 (−30.4, 1.8) | 4.4 (6.3) | 0.9 (1.2) | −3.6 (−8.6, 1.5) |
| Sadness | 21.0 (27.4) | 4.3 (8.1) | −16.7 (−33.2, −0.2) | 7.1 (8.4) | 1.5 (2.3) | −5.6 (−13.0, 1.8) |
| Anger | 2.4 (3.5) | 1.5 (1.8) | −1.0 (−3.0, 1.1) | 9.6 (17.3) | 5.0 (10.0) | −4.6 (−11.5, 2.2) |
| Worry | 41.8 (25.6) | 2.1 (2.5) | −39.7 (−56.3, −23.1) | 14.9 (13.8) | 8.2 (10.5) | −6.7 (−15.1, 1.6) |
| Tiredness | 44.1 (33.4) | 10.4 (10.9) | −33.7 (−52.5, −14.9) | 23.9 (18.9) | 13.6 (20.4) | −10.3 (−24.7, 4.1) |
| Pain | 18.5 (23.8) | 6.7 (9.6) | −11.8 (−23.7, 0.1) | 4.7 (6.3) | 1.0 (1.3) | −3.7 (−8.7, 1.3) |
| Total scorea | 24.1 (15.3) | 4.6 (4.1) | −19.5 (−28.4, −10.6) | 10.8 (9.7) | 5.0 (6.2) | −5.8 (−12.0, 0.5) |
| Emotional distress summary scoreb | 20.4 (14.9) | 2.5 (3.3) | −17.9 (−27.0, −8.8) | 9.0 (10.0) | 3.9 (4.6) | −5.1 (−11.3, 1.1) |
Abbreviations: SD, standard deviation; CI, confidence interval.
Total score is an average of the fear, sadness, anger, worry, tiredness, and pain scales.
Emotional distress summary score is an average of the fear, sadness, anger, and worry scales.
In response to the question “Did you like having the dog visit you?,” 17 of 18 respondents (94%) checked “Yes, a lot” and 1 respondent checked “Yes, a little.” None reported not liking the dog visit. In response to an open-ended question on what they liked, 8 (44%) reported some variation of the dog being calm or calming/relaxing. The most common responses to what patients did not like about the visits were that they wished they could have interacted more or for longer with the dog. In response to an open-ended question on how visits made patients feel, 10 of 18 (56%) wrote some variation of “happy.” All other comments were positive with the exception of 1 patient who wrote “no different.”
Discussion
Our results support the feasibility of and need for future large-scale studies on the effects of AAA in pediatric oncology. We demonstrated that it is possible to recruit patients for and collect data on therapy dog visits in an inpatient pediatric oncology setting at an institution where AAA is not part of usual supportive care for children with cancer. Recruitment and data collection were feasible. Positive feedback from staff and providers also support the feasibility of future research in AAA for pediatric oncology patients. In general, providers/staff endorsed the opinion that AAA are a good idea in pediatric oncology settings and believed that AAA had positive effects on patients. Some providers/staff expressed concerns about risk for infection in patients with severely compromised immune systems, however, we did not observe any adverse events. During the 14 days after the therapy dog visit, 8 patients developed infections, including febrile neutropenia in 4 of 19 (21%), on par with previously published data that febrile neutropenia occurs in 31% of neutropenic periods among children with cancer (excluding hematopoietic stem cell transplant patients) (Castagnola et al., 2007). If therapy dog visits do not increase the risk of infection, we would expect the incidence of febrile neutropenia in our study to be lower than the published estimate because not all patients in our study were neutropenic. None of the infections in our study could be clearly attributed to the therapy dog visit; however, our assessment was based only on EMR review and we had no control group. Thus, additional research—with control groups including patients admitted during periods without therapy dog visits, as well as patients admitted to the unit during the time a therapy dog was visiting, but who did not personally visit with the therapy dog—is needed to rigorously assess risks of infection in this population. Of note, this pilot study required a strict protocol to assess eligibility, ensuring that patients with higher risks for infection were not approached. Consequently, it is important to note that the generalizability of our findings may not extend to cancer patients who are profoundly immunocompromised.
Distress in general and levels of worry and fatigue, in particular, appeared to decrease immediately following the therapy dog intervention. These observations were consistent with patient and provider survey feedback that the intervention affected patients positively. Several parents and staff members told our team informally that the dog visit was the first time the child had smiled or been happy in a long time.
In a prior pilot study, Gagnon et al. (2004) studied 16 pediatric oncology patients (3-13 years old) at a Canadian hospital. In this study, pediatric oncology inpatients cared for a dog for an entire day (8-16 hours) in a room designated for this activity. The researchers surveyed parents (n = 16) and nurses (n = 12), but not the patients. Parents and nurses reported positive changes associated with dog visits. All the nurses and 79% of parents agreed with the statement: “By being responsible for a dog, my child was able to relieve or reduce anxiety.” Other outcomes even more frequently endorsed were developing a sense of “being essential to someone,” pride/accomplishment, better acceptance of hospitalization, being more receptive to and compliant with treatment, bonding with the dog, and seeming happier (Gagnon et al., 2004). A recent, small study of therapy dog visits was conducted in a Brazilian hospital to inform whether nurses could incorporate animal-assisted therapy in care for pediatric oncology patients. Patient guardians (n = 10) generally reported distraction and increased happiness in their children, and nursing staff (n = 6) found it easier to communicate with patients after therapy dog interactions (Moreira et al., 2016). Our findings that most providers/staff were in favor of AAA are consistent with the Gagnon study (Gagnon et al., 2004), a study of providers at an adult outpatient cancer center in California (Bibbo, 2013), medical ward staff at a children’s hospital in Australia (Moody, King, & O’Rourke, 2002), nursing staff in the pediatric oncology study in Brazil (Moreira et al., 2016), and medical staff at an Italian pediatric hospital (Caprilli & Messeri, 2006).
Our study’s primarily limitation is that there was no control group. Changes in distress before and after the intervention suggest a positive effect of therapy dog visits but do not prove that the association is causal. Our findings—supported by patient and provider comments—suggest that therapy dog visits are feasible in the pediatric oncology inpatient setting, are associated with positive changes in distress and symptoms in children admitted to the hospital for cancer, and do not substantially increase the risk of infection in patients who are not severely immunocompromised.
In summary, we found that following the therapy dog visit, pediatric oncology patients had lower distress and significant decreases in worry, tiredness, fear, sadness, and pain. Providers were generally supportive of the intervention. While there were several infections in the weeks following the dog visits, none could be clearly attributed to the therapy dog visit. To our knowledge, ours is the first study of patient-reported outcomes and infection incidence following AAA in a pediatric oncology inpatient setting. Larger, controlled studies are necessary to assess both whether AAA interventions increase risk for infection and whether AAA visits are associated with lasting improvements in quality of life and other patient-reported outcomes in children with cancer.
Acknowledgments
The authors would like to thank study participants as well as the following staff at Seattle Children’s Hospital who helped make this research possible: Dr Douglas Hawkins, Ms Joan Heath, Ms Karin Rogers, Ms Deborah Kruse, and Mr Andrew Mullenix. We also thank Dr Rebecca Hubbard for statistical advice and comments on the article and Ms Rebecca Ziebell and Ms. Julia Anderson for assistance with data management.
Author Biographies
Jessica Chubak, PhD is an Associate Scientific Inves-tigator at Kaiser Permanente Washington Health Research Institute and an Affiliate Associate Professor in the Depart-ment of Epidemiology at the University of Washington. Her training is in molecular biology, bioethics, and epidemiology.
Rene Hawkes, BS is a Research Project Manager II at Kaiser Permanente Washington Health Research Institute and an Affiliate Professor in the Department of Education at Seattle Pacific University. Her training and areas of expertise are in psychology, early childhood education, and empathy.
Christi Dudzik, LMHC, MC is a mental health counselor and animal assisted therapy specialist who co-manages the visiting therapy dog program at Seattle Children’s Hospital. She consults with hospital therapy dog programs, teaches classes for professionals and volunteers who want to work with their animals in the field of animal assisted interactions, and works with individuals experiencing dog fear.
Jessica Foose-Foster, BS is a Clinical Research Associate at Seattle Children’s Research Institute. Her experience includes several years of bench level research and two years of coordinating studies in several departments.
Lauren Eaton, MA, LMHCA is a Clinical Research Associate at Seattle Children’s Research Institute. Her training is in psychology and behavioral health. She holds a Masters degree in Counseling from Seattle University.
Rebecca H. Johnson, MD is a Pediatric Oncologist and the Medical Director of the Adolescent and Young Adult Oncology Program at Mary Bridge Hospital in Tacoma, WA.
Catherine Fiona Macpherson, RN, PhD, CPON is a staff nurse on the Cancer Care Unit at Seattle Children’s Hospital.
Appendix
Footnotes
Authors’ Note: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R03CA169576. This research also received support from Kaiser Permanente Washington Health Research Institute (formerly Group Health Research Institute) and from crowdfunding via experiment.com.
References
- American Humane Association. (2013). Canines and childhood cancer (CCC): Examining the effects of therapy dogs with childhood cancer patients and their families. Retrieved from http://www.americanhumane.org/interaction/programs/animal-assisted-therapy/canines-and-childhood-cancer.html
- Bibbo J. (2013). Staff members’ perceptions of an animal-assisted activity. Oncology Nursing Forum, 40, E320-E326. doi: 10.1188/13.ONF.E320-E326 [DOI] [PubMed] [Google Scholar]
- Bouchard F., Landry M., Belles-Isles M., Gagnon J. (2004). A magical dream: A pilot project in animal-assisted therapy in pediatric oncology. Canadian Oncology Nursing Journal, 14, 14-17. [DOI] [PubMed] [Google Scholar]
- Caprilli S., Messeri A. (2006). Animal-assisted activity at A. Meyer Children’s Hospital: A pilot study. Evidence-Based Complementary and Alternative Medicine, 3, 379-383. doi: 10.1093/ecam/nel029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnola E., Fontana V., Caviglia I., Caruso S., Faraci M., Fioredda F., . . . Haupt R. (2007). A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hemopoietic stem cell transplantation. Clinical Infectious Diseases, 45, 1296-1304. doi: 10.1086/522533 [DOI] [PubMed] [Google Scholar]
- Chubak J., Hawkes R. (2016). Animal-assisted activities: Results from a survey of top-ranked pediatric oncology hospitals. Journal of Pediatric Oncology Nursing, 33, 289-296. doi: 10.1177/1043454215614961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon J., Bouchard F., Landry M., Belles-Isles M., Fortier M., Fillion L. (2004). Implementing a hospital-based animal therapy program for children with cancer: A descriptive study. Canadian Oncology Nursing Journal, 14, 217-222. [DOI] [PubMed] [Google Scholar]
- Hinds P. S., Nuss S. L., Ruccione K. S., Withycombe J. S., Jacobs S., DeLuca H., . . . DeWalt D. A. (2013). PROMIS pediatric measures in pediatric oncology: Valid and clinically feasible indicators of patient-reported outcomes. Pediatric Blood & Cancer, 60, 402-408. doi: 10.1002/pbc.24233 [DOI] [PubMed] [Google Scholar]
- Moody W. J., King R., O’Rourke S. (2002). Attitudes of paediatric medical ward staff to a dog visitation programme. Journal of Clinical Nursing, 11, 537-544. doi: 10.1046/j.1365-2702.2002.00618.x [DOI] [PubMed] [Google Scholar]
- Moreira R. L., Gubert F. d. A., Sabino L. M. M. d., Benevides J. L., Tomé M. A. B. G., Martins M. C., Brito M. d. A. (2016). Assisted therapy with dogs in pediatric oncology: Relatives’ and nurses’ perceptions. Revista Brasileira de Enfermagem, 69, 1188-1194. [DOI] [PubMed] [Google Scholar]
- Patel S. K., Mullins W., Turk A., Dekel N., Kinjo C., Sato J. K. (2011). Distress screening, rater agreement, and services in pediatric oncology. Psycho-Oncology, 20, 1324-1333. doi: 10.1002/pon.1859 [DOI] [PubMed] [Google Scholar]
- Pet Partners. (2016). Terminology. Retrieved from https://petpartners.org/learn/terminology/
- Sherman S. A., Eisen S., Burwinkle T. M., Varni J. W. (2006). The PedsQL™ Present Functioning Visual Analogue Scales: Preliminary reliability and validity. Health and Quality of Life Outcomes, 4, 75. doi: 10.1186/1477-7525-4-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski B. L., Lazenby M. (2012). Distress among hospitalized pediatric cancer patients modified by pet-therapy intervention to improve quality of life. Journal of Pediatric Oncology Nursing, 29, 272-282. doi: 10.1177/1043454212455697 [DOI] [PubMed] [Google Scholar]