Abstract
Providing timely palliative and end-of-life care (PC/EOL) information to parents of children with a serious illness is a national health care priority. The goals of this study were to determine feasibility, acceptability, and parent responses related to a PC/EOL communication intervention, titled “Communication Plan: Early through End of Life (COMPLETE)” to parents of children with a brain tumor. The study was a 2-site prospective, single-group pilot study targeting parents’ stress and coping outcomes. The sample included 13 parents of 11 children (ie, 11 families). During the first 6 months postdiagnosis, we evaluated parent outcomes at 4 time points (baseline and 3 post-sessions). Our findings included significant decline in decision regret (P = .0089); strong, significantly increased hope (P ≤ .0001); and significantly decreased uncertainty (P = .04). Over time, more than half of the parents (61.5%) preferred to receive information about their child’s current condition and PC/EOL options. Our findings provide evidence to suggest that the COMPLETE intervention is feasible and acceptable and produces promising effects on 3 parent outcomes (ie, decision regret, hope, and uncertainty) in parents of children with a brain tumor. Further research is indicated to evaluate COMPLETE with a larger sample of parents of children with cancer and with a control group.
Keywords: palliative care communication, parents, children with brain tumors
The National Institute of Nursing Research launched the campaign “Palliative Care Conversations Matter” to promote awareness of palliative care (PC) benefits to support family members and to foster early PC discussions by providers with patients and family members (National Institute of Nursing Research, 2014). Research indicates that PC contributes to effective symptom management in children with a poor prognosis and may minimize emotional distress experienced by affected family members (Grady, 2014). Studies also indicate that integration of early PC, advanced-care planning and end-of-life care (EOL) support may minimize negative responses among parents (Broom, Kirby, Good, Wootton, & Adams, 2014).
Research on PC/EOL Communication and Support
Pediatric providers often struggle with initiating early discussions about PC/EOL options and prognosis with parents due to having limited or no PC/EOL communication training and perceptions about parents’ preferences and readiness to receive difficult information about their child’s condition. Providers have reported their fear that early PC/EOL discussions with parents may take away hope (Almack, Cox, Moghaddam, Pollock, & Seymour, 2012; Granek, Krzyzanowska, Tozer, & Mazzotta, 2013) and increase emotional distress among parents (Mack & Joffe, 2014). Also, a gap exists in prospective studies to evaluate parental PC/EOL information preferences and coping responses after receiving PC/EOL information during the child’s early diagnosis and prognosis period.
Conflicting philosophies exist about the purpose of PC/EOL and timing of PC consultation for children with cancer (Dalberg et al., 2013). In one study, 44.2% of pediatric providers indicated children with cancer are typically referred to PC when EOL is inevitable and cure is no longer a goal (Thompson, Knapp, Madden, & Shenkman, 2009). Analysis of PC consultation patterns prior to the death of children with cancer found that 40% of PC referrals were offered after the first relapse and only 16% of PC referrals occurred during the first 30 days after diagnosis (Johnson & Vadeboncoeur, 2012). Thus, pediatric providers often delay discussions with parents at diagnosis about their child’s poor prognosis and PC/EOL support until after standard neuro-oncology treatments (ie, radiation therapy with or without chemotherapy) have failed. Delayed parental discussions about a child’s poor prognosis may contribute to increased emotional distress, impede formation of alternate forms of hope for a child, and delay PC referrals. Sharing of PC/EOL information is usually based on providers’ beliefs about receptivity of PC/EOL information (Mack & Joffe, 2014) rather than on parent reactions to early PC/EOL information (Durall, Zurokowski, & Wolfe, 2012).
Parent/Child PC/EOL Communication Outcomes
Investigators have primarily evaluated hope and uncertainty among adult oncology patients with a poor prognosis. Responses included no difference in worry among patients who received PC/EOL information compared with those who did not receive PC/EOL information (Wright, Zhang, & Ray, 2008); increased hope among patients who perceived receiving clear PC/EOL information (Hagerty, Butow, & Ellis, 2005); and maintained a hopeful attitude, after receiving news of no possible cure (Smith et al., 2011). In comparison, bereaved parents’ preferences for PC/EOL information (Hendricks-Ferguson, 2007; Mack, Wolfe, Cook, Cleary, & Weeks, 2006) has provided evidence of parents’ perceptions of having decision regret about decisions made about their child’s care because of not receiving PC/EOL information earlier.
A nurse-physician (RN/MD) team approach to manage patient care is associated with positive patient care outcomes (Levetown, 2008). Absence of clear RN/MD communication is associated with less satisfaction in medical care (Tang, Chan, Zhou, & Liaw, 2013). A review of studies on bereaved parents’ perspectives about their child’s EOL revealed dissatisfaction with provider communication, clarity of information, and level of concern for the child (Aschenbrenner, Winters, & Belknap, 2012). No published studies evaluated prospective PC/EOL communication interventions delivered to caregivers of patients with cancer, using an RN/MD team.
Study Purpose
The purpose of this article is to report feasibility, acceptability, and outcome data from a 2-site prospective, single-group pilot study of the Communication Plan: Early through End of Life intervention (COMPLETE), which targets parents of children with a brain tumor and a poor prognosis. Our reports of the parent and RN/MD qualitative responses, related to a quality assurance review for protocol fidelity, will be presented in a subsequent article. The study aims were to evaluate COMPLETE for parent-related feasibility and receptivity (ie, % of eligible parents consenting and completing measures at each time point); describe parental responses after receiving COMPLETE (ie, parent appraisals of information preferences, RN/MD-delivered information, emotional needs/resources, symptom management and of emotional distress, uncertainty, hope, satisfaction with RN/MD communication, decision regret, advanced care planning); and evaluate COMPLETE for MD/RN-related feasibility and fidelity (ie, % of delivered intervention activities).
Theoretical Framework
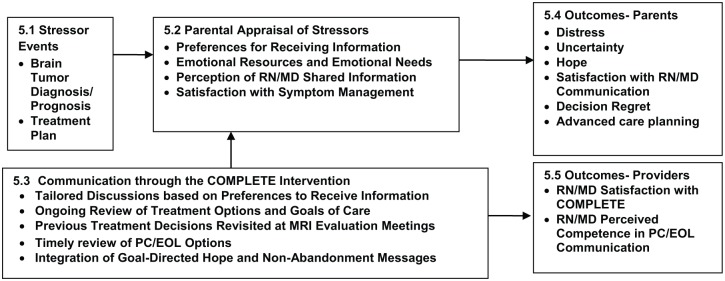
COMPLETE was guided by 2 theories: Stress, Appraisal, and Coping Theory (Lazarus & Folkman, 1984) and the Double ABCX Model (McCubbin & Patterson, 1983). Concepts from both theories were incorporated into our investigator-developed model (Hendricks-Ferguson & Haase, 2009; see Figure 1). Tenets of the Stress, Appraisal, and Coping Theory include coping responses occur in response to perceived threats and emotional resources may foster positive coping. Double ABCX model components include the following: “A,” stressor event (eg, cancer diagnosis with a poor prognosis); “B,” parents’ perceived support (ie, provider’s use of empathy and clear responses); “C,” perception of stressor (ie, understanding of prognosis); and “X,” demand for change (ie, increased need to cope with stressors). Parental distress occurs if uncertainty about child’s prognosis exceeds existing emotional resources. COMPLETE was designed to help reduce parental distress through RN/MD providing timely prognosis information, information about PC/EOL options based on information preferences, and integration of hope and nonabandonment messages (details of the COMPETE intervention are provided in Supplemental Figure 1, available online at http://jpo.sagepub.com/content/by/supplemental-data).
Figure 1.
COMPLETE conceptual framework.
Design
The design was a prospective, longitudinal single-group pilot study.
Setting and Sample
We recruited eligible parents from 2 sites, Cardinal Glennon Children’s Hospital in St Louis, Missouri, and Riley Hospital for Children in Indianapolis, Indiana. Both sites are members of the Children’s Oncology Group and offer similar pediatric cancer treatment protocols. COMPLETE was delivered to parents during scheduled parent meetings by our trained RN/MD teams at both sites. Our sample goal was to recruit 21 to 24 parents (12 families) of 12 children diagnosed with a chart documented brain tumor diagnosis with a poor prognosis (ie, overall survival rate of less than 50% at 3 years after diagnosis). Parent inclusion were (a) aged 18 years or older; (b) biological parent, step-parent, or legal guardian; (c) able to read and speak English; and (d) informed that child (aged infant to 18 years) had a brain tumor diagnosis and a poor prognosis. Parent exclusion criteria were, if (a) child’s brain tumor was chart documented as a good prognosis; (b) parents have chart documented neurological and/or cognitive impairments; (c) either parent in a decision-making couple (ie, dyad) declined consent. Inclusion criteria for the parents’ child were the following: aged infant to 18 years and chart documented brain tumor diagnosis and a poor prognosis.
Study Measures
An evaluator was present during parents’ completion of measures to answer questions and ensure completion of items. Each of the used study measures are presented in the order that corresponds with variables listed in each box of the COMPLETE Conceptual Framework (see Figure 1). Following is a description of the demographic form used at baseline (T1) and the study measures that were used to evaluate parental responses across 4 time points (ie, T 1 = baseline and T2, T3, and T4 after receiving COMPLETE).
Demographic Information Form
This investigator-developed form was used to record and evaluate baseline information related to stressor variables at diagnosis (brain tumor diagnosis, prognosis, and treatment plan) in our model (see Figure 1, 5.1) and demographic information for enrolled parents (gender, marital status, and race) and their children (age, gender, and race).
PedsQL Brain Tumor Module (Version 1.0)
The 24-item PedsQL Brain Tumor Module measures satisfaction with symptom management (see Figure 1, 5.2). Each item is rated on a 5-point scale ranging from 0 (never) to 4 (always). Higher scores indicate fewer observed/reported symptoms during the previous 7 days. The PedsQL includes items specific for ages 2 to 4 years (toddler), 5 to 7 years (young child), 8 to 12 years (child), and 13 to 18 years (adolescent). For this article, only pain and hurt subscale items were analyzed. This module is reliable and valid and the reliability coefficients range from .78 to .92 (Bhat et al., 2005).
Pediatric Health Care Satisfaction Hematology/Oncology Module (PedsQL-HCS)
The 24-item PedsQL-HCS measures emotional needs, perception of RN/MD shared information, satisfaction with symptom management (see Figure 1, 5.2), and satisfaction with RN/MD communication (see Figure 1, 5.4). Each item is rated on a 5-point Likert-type scale ranging from 0 (very dissatisfied) to 4 (very satisfied). Higher scores indicate higher satisfaction. The PedsQL-HCS includes 5 subscales that assess parent satisfaction regarding: emotional needs by the amount of time providers spent talking to the child and parent (4 items assessed parents’ emotional needs); general satisfaction (3 items assessed communication received); information provided on child’s diagnosis and treatments (5 items assessed information received about child’s diagnosis, treatments, and test results); inclusion of family and answering their questions (4 items assessed sensitivity to parental concerns); and technical skills in responding to the child’s needs (4 items assessed efforts attending to child’s symptoms). The PedsQL-HCS is reliable and valid, and the reliability coefficients range from .82 to .95 (Varni, Quiggins, & Ayala, 2000).
Parent Experience of Child Illness (PECI)–Short Form
The 25-item PECI (Bonner et al., 2006) measures parents’ emotional resources (see Figure 1, 5.2), emotional distress, and uncertainty (see Figure 1, 5.4). Items related to emotional resources on the PECI are focused on guilt–worry and unresolved sorrow and anger. Each item is rated on a 4-point scale ranging from 1 (never) to 4 (always). Scores indicate fewer perceived resources for self-efficacy and competency. Higher distress scores indicate more anxiety, higher burden of responsibility for treatment decisions and symptom management, and greater sense of loss about the child’s illness. Higher uncertainty scores indicate providers should offer supportive therapy. The PECI is reliable and valid, and the reliability coefficients range from .72 to .89 (Bonner et al., 2006).
Decision Regret Scale (DRS)
The 5-item DRS is a unidimensional scale. The DRS measures parents’ health care decision regret, defined as remorse or distress over a decision at a given point in time (see Figure 1, 5.4). Participants were directed to respond based on any regrets about health care decisions during the past 7 days. Items are rated on a 5-point scale ranging from 1 (strongly agree) to 5 (strongly disagree). Higher regret scores are associated with lower satisfaction with treatment-related decisions. The DRS is reliable and valid, and the reliability coefficients range from .81 to .92 (Brehaut et al., 2003).
Herth Hope Index (HHI)
The 12-item HHI is a multidimensional measure of hope during a chronic or serious illness (see Figure 1, 5.4). The HHI was used in this study to measure parental hope. Items are rated on a 4-point scale ranging from 1 (strongly disagree) to 5 (strongly agree). Higher scores indicate higher hope. The HHI is reliable and valid, and the reliability coefficients range from .92 to .93 (Herth, 1992).
Investigator-Developed Protocol Tracking Forms
Parent Preferences for Receiving Information (PPRI) form
This form was used to assess parental preferences to receive information about a child’s prognosis, cancer treatments, and PC/EOL support (Option 1) or to only receive current prognosis and cancer treatment information (Option 2; see Figure 1, 5.2). Parents’ responses were used by RN/MD teams to guide the amount of shared PC/EOL information with parents.
Advanced Care Planning (ACP) form
This form was used to assess parent preferences about life-support treatment options, in the event their child may need EOL care in the future in an intensive care unit (ICU). We chose to develop our own ACP form to provide a check list of items to assess parental preferences about common life-support treatment options for their child. Our ACP form includes 7 life-support treatment options (ie, cardiopulmonary resuscitation, do not resuscitate, cancer-directed therapies, breathing machine, antibiotics, blood transfusions, and artificial hydration and nutrition). Each option included a yes/no option for care preferences of a child in the ICU (see Figure 1, 5.4). Also, our ACP form focused solely on life-support treatment options that our RN/MD dyads could easily normalize planned ACP discussions with parents during the initial portion of scheduled parent sessions.
RN/MD Provider Outcomes: COMPLETE Quality Assurance (QA) Form
This checklist was used to evaluate COMPLETE intervention fidelity as delivered by RN/MD dyads. The QA form has yes/no response options for each component of the intervention: Getting to Know You (eg, parents’ concerns, hopes, and understanding of child’s condition); Establish Therapeutic Alliance to foster parental trust; Establish Prognosis and Communicate Effectively used for prognosis discussion with percentages; Establish Goals of Care to provide initial nonabandonment “We” messages; Establish Goal-Directed Treatment Options to record ACP preferences; and Parents’ Communication Preferences to learn parent preferences for receiving information about prognosis and PC/EOL options.
Study Procedures
After receiving institutional review board approvals, eligible and consented parents were enrolled. A neuro-oncology trained MD or RN at each site notified the principal investigator of all eligible families. An institutional review board–approved team member then introduced the study to eligible parents during routine scheduled clinic visits. Next, the team member explained the study and assessed parents’ interest to participate. Parental consent was then obtained in a private room, and we also obtained age-appropriate assent for parents’ participation and collection of the child’s demographic data. Following consent and assent, a trained evaluator was present: at baseline (T1) and 3 subsequent measurement time points (T2, T3, and T4) that occurred after each session (S1, S2, S3). Evaluators monitored parents’ completion of measures, answered questions, and minimized incomplete data.
Intervention Description
We conducted this study in 2 phases. In Phase I, we implemented and evaluated training procedures to prepare neuro-oncology experienced RN/MD teams to deliver COMPLETE according to protocol. As described in our Phase I training article (Hendricks-Ferguson et al., 2015), 4 RN/MD teams received 2 days of training that included a review of best PC/EOL communication practices and coached role-playing scenarios of new diagnosis and tumor progression situations with bereaved parents to ensure the COMPLETE intervention would be delivered well and as planned. In Phase II, COMPLETE was delivered during scheduled clinic appointments by RN/MD teams, who were also the primary neuro-oncology providers, to parents of children newly diagnosed with a brain tumor. Session times varied based on the tumor type, prognosis, and planned treatments, and sessions occurred within 4 weeks after the child’s magnetic resonance imaging (MRI) evaluations. S1 was conducted during a parent meeting to discuss the child’s disease status, prognosis, and treatment options following diagnosis. S2 and S3 followed shortly after the next parent meetings to provide information about the child’s response to cancer treatments as evaluated by the child’s MRI evaluation reports (ie, approximately 10 to 16 weeks for S2 and from 22 to 26 weeks for S3). Results of MRI scans were shared with parents by RN/MD teams during these sessions as part of the protocol.
COMPLETE sessions included an RN/MD approach to engage parents in early PC/EOL discussions that included hope and nonabandonment dialogue, tailored to individual team member’s communication style and parents’ information preferences. Also, the RN/MD providers used the investigator-developed COMPLETE intervention visual forms during delivery of the intervention (Hendricks-Ferguson, Haase, & Kane, 2010) to enhance hope and nonabandonment messages. In addition, the parent forms were used to guide discussions of recommended treatment and PC/EOL options and to normalize ACP discussions early, and across all sessions.
Analysis
Descriptive statistics were computed for all variables to evaluate data quality and assumptions of statistical tests. All tests were 2-sided, and the significance level was set at .05. The SAS Version 9.3 (Cary, NC) was used to perform all statistical analysis. Differences in appraisal of stressors and outcomes across the baseline (before Session 1) and 3 post–baseline sessions were evaluated by calculating the trend of means across the 4 time points. To account for correlations among repeated measures from the same parent, the longitudinal data were analyzed using a “mixed model” to examine the change in the parents’ responses on each study measure (HHI, PECI, PedsQL, & DRS) over time according to 4 time points (T1, T2, T3, and T4). The effect sizes were computed as the change in means between time points, divided by the pooled standard deviation calculated from the model. In the “mixed model,” time was treated as a set of dummy variables (with baseline as reference category) to avoid the restrictive assumption that the trend across time was linear. The ACP scores were calculated by adding all ACP responses options, collected at 3 sessions. Similar statistical mixed model was used to analyze the trend of ACP scores across sessions. The least square mean and standard deviation are shown in Table 2. This pilot study was designed to evaluate feasibility and usefulness of COMPLETE; it was not powered for effect size estimation. Although we calculated effect sizes to obtain preliminary estimates for our grant application, the study was not powered to have confidence in these estimates. Therefore, because of our small sample size and insufficient power, we could not conduct stratified analysis according to the children’s age.
Table 2.
Parent Appraisal of Outcome Variables.
| Parent Outcome—Measurement Tool | T1,a Mean ± SEb | T2, Mean ± SEb | T3, Mean ± SEb | T4, Mean ± SEb | P c |
|---|---|---|---|---|---|
| Distress—PECI, Guilt & Worry | 2.75 ± 0.14 | 2.49 ± 0.10 | 2.38 ± 0.16 | 2.49 ± 0.15 | .0671 |
| Distress—PECI, Unresolved Sorry & Anger | 2.51 ± 0.15 | 2.35 ± 0.13 | 2.19 ± 0.16 | 2.21 ± 0.16 | .1652 |
| Uncertainty—PECI, Long-term uncertainty | 2.48 ± 0.22 | 2.07 ± 0.16 | 1.86 ± 0.19 | 1.96 ± 0.23 | .0432 |
| Hope—HHI | 36.38 ± 1.64 | 38.24 ± 1.08 | 38.59 ± 1.18 | 37.51 ± 1.24 | <.0001 |
| Satisfaction with MD/RN communication—PedsQL-HCS, Communication | 21.73 ± 0.74 | 20.81 ± 1.05 | 21.61 ± 1.40 | 22.56 ± 0.75 | .6432 |
| Satisfaction with MD/RN communication—PedsQL-HCS, General Satisfaction | 14.28 ± 0.36 | 14.45 ± 0.31 | 14.09 ± 0.36 | 14.37 ± 0.38 | .1704 |
| Decision Regret—DRS | 14.87 ± 3.93 | 12.28 ± 3.07 | 14.04 ± 4.75 | 7.34 ± 1.88 | .0089 |
| S1 (Session 1), Mean ± SE | S2 (Session 2), Mean ± SE | S3 (Session 3), Mean ± SE | P c | ||
| Advance Care Planning: ACPd | 6.58 ± 0.71 | 5.67 ± 0.82 | 4.93 ± 1.07 | .5560 |
Abbreviations: PECI, Parent Experience of Child Illness Scale (included 4 factors: 1 = Guilt and Worry, 2 = Unresolved Sorrow and Anger, 3 = Uncertainty, 4 = Emotional Resources); HHI, Herth Hope Index; PedsQL-HCS, Peds Quality-of-Life Healthcare Satisfaction Oncology & Hematology Module (includes 1 subscale score: 1 = General Satisfaction); DRS, Decision Regret Scale; ACP, Advance Care Planning Checklist.
Time points = T1 (baseline), T2, T3, and T4.
Least squares means.
From mixed model.
The ACP was completed during Sessions 1, 2, and 3.
Results
Sample
We enrolled 13 parents of 11 children using convenience sampling. Parent characteristics are (a) gender: mothers (85.7%) and fathers (14.3.%); (b) ethnicity: Caucasian (69.2%), African American (15.4%), and Hispanic (15.4%); and (c) marital status: single parents (38.5%), married couples (30.8%), parent living with a partner (23.1%), and divorced parents (7.7%). Characteristics of the 11 children were the following: (a) gender: 5 girls (45.5%) and 6 boys (54.6%); (b) age: 0.4 to 14.5 years (mean = 6 years); and (c) ethnicity: Caucasian (63.6%), African American (18.2%), and Hispanic (18.2%). All the children had a brain tumor and a poor prognosis.
Aim 1: Parent Feasibility and Receptivity
The CONSORT diagram (Figure 2) provides information on recruitment after initial screening as well as retention.
Figure 2.
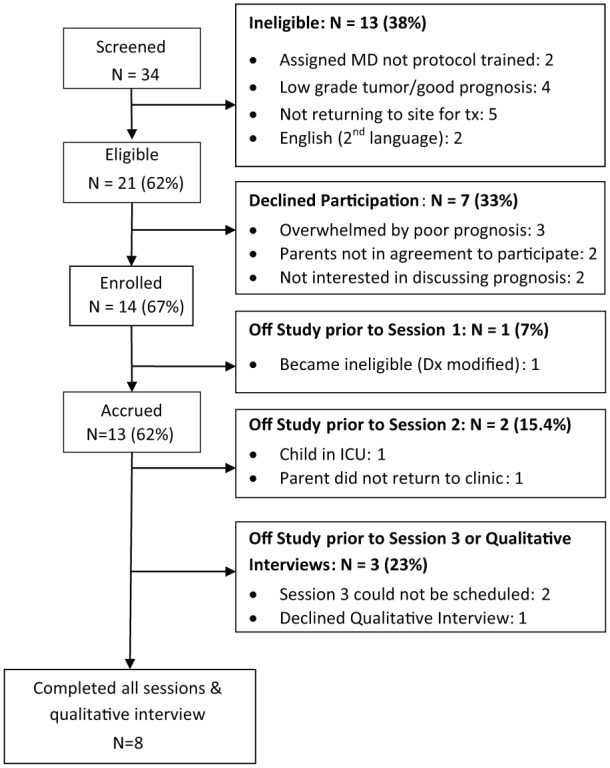
CONSORT diagram for accrual, intervention delivery, and data collection.
Percentage of Eligible Parents Consenting
A total of 34 parents were initially screened for child eligibility across 2 sites. Thirteen of the 34 parents were subsequently not consented for the following reasons: 1 child (2 parents) was assigned to an oncologist who was not protocol trained; 2 children (4 parents) had prognosis upgraded to good; 3 children (5 parents) went elsewhere for treatment; and parents of 1 child (2 parents) could not read the measures because English was their second language. Among our final screening for eligibility 21 parents (62%) were eligible to consent to enroll in the study. Among the 21 eligible parents, 7 (33%) declined participation for the following reasons: being overwhelmed with child’s diagnosis (3 single parents), parents not in agreement to participate (2 parents), and parents did not want to discuss their child’s potential future care needs (2 parents).
Percentage of Parent Completers
Measures were completed as follows: (a) 13 parents completed baseline measures before Session 1, (b) 12 parents completed measures after Session 1, (c) 12 parents completed measures after Session 2, and (d) 10 parents completed measures after Session 3. Factors influencing parents completing sessions were the following: (a) 1 child became ineligible when the diagnosis was modified prior to Session 1; (b) 1 child was admitted to the ICU, preventing Session 2 scheduling for 2 parents; and (c) 1 child’s parents did not return to the clinic. Among the 10 parents completing all measures, 8 completed a poststudy qualitative interview. Also, among the 10 parents completing all measures, 1 single mother declined to participate in a follow-up qualitative interview and another single mother did not return to complete the qualitative interview.
Aim 2: Parental Responses After Receiving the COMPLETE Intervention
Parent Appraisal of Stressors
Parent appraisal of stress-related variables is reported in Table 1. Across the 3 sessions, we evaluated parental appraisal of (a) preference for receiving information, (b) emotional needs/resources, (c) perception of RN/MD-shared information, and (d) child symptoms and satisfaction with symptom management. Evaluation of parents’ information preferences was as follows: 6 mothers (46.2%) preferred to receive all information about the child’s condition and 5 mothers (46.2%) preferred to receive only current information about the child’s condition. Among fathers, 1 father (7.7%) preferred all information and 1 father (7.7%) preferred only current information.
Table 1.
Parent Appraisal of Stressor Variables.
| Stressors—Measurement Tools | T1,a Mean ± SEb | T2, Mean ± SEb | T3, Mean ± SEb | T4, Mean ± SEb | P c |
|---|---|---|---|---|---|
| Emotional resources—PECI | 2.20 ± 0.17 | 2.36 ± 0.14 | 2.32 ± 0.13 | 14.48 ± 1.29 | .1481 |
| Emotional needs—PedsQL | 14.48 ± 1.29 | 13.27 ± 1.34 | 15.18 ± 1.12 | 15.78 ± 1.37 | .3102 |
| Perception of MD/RN shared information—PedsQL-HCS, Information Subscale | 21.96 ± 0.83 | 23.30 ± 0.50 | 22.70 ± 0.95 | 22.36 ± 0.88 | .2423 |
| Perception of MD/RN shared information—PedsQL-HCS, Inclusion of Family Subscale | 19.14 ± 0.18 | 19.24 ± 0.28 | 19.14 ± 0.45 | 18.62 ± 0.36 | .2436 |
| Satisfaction with symptom management—PedsQL-HCS, Technical Skills Subscale | 17.56 ± 0.68 | 17.69 ± 0.59 | 18.24 ± 0.58 | 17.95 ± 0.49 | .8281 |
| Stressor: satisfaction with symptom management—PedsQL-HCS, General Satisfaction Subscale | 14.28 ± 0.36 | 14.45 ± 0.31 | 14.09 ± 0.36 | 14.37 ± 0.38 | .1704 |
| Stressor: satisfaction with symptom management—PedsQL-BTM, Pain and Hurt Subscale | 7.62 ± 0.82 | 7.69 ± 1.02 | 8.37 ± 0.92 | 5.91 ± 0.66 | .0245 |
Abbreviations: PECI, Parent Experience of Child Illness Scale (PECI Subscale: Emotional Resources); PedsQL-HCS, Peds Quality-of-Life Healthcare Satisfaction Oncology & Hematology Module (PedsQL Subscales: Emotional Needs, Information, Inclusion of Family, Technical Skills, & General Care); PedsQL-BTM, Peds Quality-of-Life Brain Tumor Module (PedsQL-BTM Subscale: Pain & Hurt).
Time points = T1 (baseline), T2, T3, and T4.
Least-squares means.
From mixed model.
The following variables did not show significant differences: (a) parents’ emotional resources, defined as self-efficacy and competence; (b) emotional needs, referring to time staff spent attending to emotional needs; (c) parents’ appraisal of information shared by RN/MDs about their child’s diagnosis and treatments, inclusion of family, and answering parents’ questions; and (d) parents’ appraisal of stressors related to child’s brain-tumor symptom management, based on their evaluation of the provider’s technical skills to manage symptoms and their child’s pain. However, parents’ appraisal of their child’s pain symptoms significantly changed (P = .0245) over time, in that parents’ perceptions of their child’s pain showed the highest perceived pain scores at T3 measurement (after Session 2) and the lowest pain scores at T4 measurement (after Session 3).
Parents’ Appraisal of Outcomes
Parental outcomes for distress (ie, guilt and worry; unresolved sorrow and anger), long-term uncertainty and hope, satisfaction with RN/MD communication, decision regret, and advanced-care planning are shown in Table 2. Parental responses for distress were mixed, showing a trend of decreased parental guilt and worry over time at marginal level of significance (P = .0671) while parents’ unresolved sorrow and anger showed no significant difference over time. Parents’ self-reported long-term uncertainty significantly decreased (P = .0432) and hope significantly increased (P ≤ .0001) over time.
Parents’ satisfaction with RN/MD communication (eg, time to explain, listen, and prepare parents for diagnostic tests and satisfaction with child’s care) showed no significant differences over time. However, parents’ scores were consistently high, indicating high satisfaction with received RN/MD communication. Also, a significant difference in parents’ decision regret responses (P = .0089) appeared over time, with a greater decrease in decision regret from the first to the last time point after receiving sessions. All ACP Checklist Form responses related to future preferences for the child’s care were marked “Yes” on the form at Session 1. Although no significant changes in parent preferences were observed during the 2 subsequent sessions, the mean differences scores displayed a trend toward a decreasing number of “yes” options over time (see Table 2).
Aim 3: Provider Outcomes for Protocol Fidelity
We evaluated the percentage of activities delivered according to protocol and fidelity evaluation at each COMPLETE session. Our QA evaluation found that 86% to 92% of the planned communication activities and messages were delivered by RN/MD teams across the intervention sessions. Specifically, 92% of planned communication activities were delivered during Session 1, and 86% were delivered during Sessions 2 and 3.
Discussion
This is the first study to report the feasibility of delivering an early PC/EOL communication intervention to parents of children with a brain tumor at diagnosis and during cancer-directed therapy, using an RN/MD team approach. Our results provide several promising findings about the feasibility of implementing the COMPLETE intervention. Our study also provides encouraging evidence supporting further evaluation of COMPLETE in a randomized controlled trial with parents of children with any cancer diagnoses associated with a poor prognosis.
Evaluation of parent data provided 4 key findings. First, our findings offer preliminary support that COMPLETE had a positive impact on parents’ hope, uncertainty, and decision regret. With a larger sample size the intervention might also have demonstrated a positive impact on the parental emotional resources of guilt and worry. Second, the data provided evidence that slightly more parents prefer to be given all treatment and PC/EOL information during the early-diagnosis period of their child’s brain tumor and over time. Third, our findings provided evidence that parents of a child with a brain tumor and a poor prognosis are receptive to a communication intervention that provides information about PC/EOL during the first 6 months after diagnosis. Fourth, parents were accepting of early discussions regarding ACP options for their child’s care.
Regarding parent receptivity to participating in this study, our rate of accrual (62% of eligible parents) is similar to the Seattle Pediatric Palliative Care Project (Hays et al., 2006) and reasons for declining participation reflect some of the stressors this study aims to address. Similar to other parent studies, we enrolled primarily the child’s mother. Given the limited data on current divorce rates among parents of children with cancer in the United States (Seyse, 2010), further research is needed to evaluate perspectives and needs of nontraditional parent caregivers (eg, single fathers, divorced parents). Also, parents completed all items on the study measures they attempted. Perhaps this is due to the inclusion in our data collection plan of a trained evaluator who was present to answer questions and review the completed forms before parents departed. Also, in follow-up interviews with parents, none commented that the number of items or time to complete them had been a burden.
The finding that parental hope significantly increased over time offers tentative evidence that, by receiving early and sensitively delivered information about a child’s prognosis and PC/EOL options, parents may adjust their hope instead of sustaining unrealistic hope or losing hope. COMPLETE may have helped parents to acquire a hopeful attitude after receiving clear PC/EOL information from our RN/MD dyads. This finding may be attributed to (a) providers using communication skills to develop a therapeutic alliance with parents, (b) parents having protected time in a safe environment to process information about their child’s condition and ask questions, and (c) parents receiving sustained-hope and nonabandonment messages by the RN/MD dyads. If supported by further research in a larger and randomized controlled clinical trial, our finding of increased parental hope over time could address a frequently documented provider fear that delivering PC/EOL information to parents of children with a life-threatening illness may take hope away (Mack & Joffe, 2014). Questions still exist about what the parents’ hoped-for outcomes may be for a child with cancer at diagnosis and over time and what factors may influence parental hope.
Parents’ self-reported guilt and worry approached near significance and their uncertainty significantly decreased over time. Parents may have reported decreased guilt, worry, and uncertainty because they received clear information about their child’s prognosis or because COMPLETE provided protected time for RN/MD to listen to their concerns. Our findings provide support to assertions of other investigators that if providers convey timely and clear information about a loved one’s treatments and prognosis, caregivers may experience a decreased level of uncertainty and worry (Bonner et al., 2006). Still, given the small sample and single group design of this study, more research is needed to further evaluate parental guilt, worry, and uncertainty relative to parents simply having protected time with providers to discuss the child’s treatment responses and all treatment options, including PC/EOL support.
Parents’ responses showed significantly decreased decision regret over time related to decisions about the child’s condition and treatments. After receiving COMPLETE, parents may have felt more equipped to make informed decisions about their child’s condition. This explanation is partially supported by the finding that the parental satisfaction with RN/MD communication remained high over time. Another explanation may be that we recruited mostly mothers, and women are more receptive to social support when confronted with the stressors of having a child receiving oncology treatments (Altay, Kilicarslan, Sari, & Kisecik, 2014) and social support for family caregivers of patients with cancer when making important health-care decisions (Hudson, Aranda, & Kristjanson, 2004). Still questions exist whether protected time with providers during will decrease decision regret with future parents.
To date, no published studies have reported parental preferences regarding receiving information from RNs/MDs about their child’s oncology treatments, PC/EOL, and ACP options. Our findings showed no significant difference in parents’ preferences about receiving PC/EOL and ACP information over time. Most parents were receptive to early discussions about their child’s prognosis, treatment options, and PC/EOL. Factors contributing to this finding are (a) only 1 of the 11 children showed a poor response to oncology treatments that required admission to an ICU during our study, (b) COMPLETE was delivered during the first 6 months after diagnosis when most children with brain tumors do not show signs of tumor progression, and (c) early discussions about PC/EOL and ACP may have helped parents consider possible outcomes in a noncrisis situation. Supporting our explanations are tenets of family coping and crisis theory that suggest early discussions of difficult topics and possible solutions in a noncrisis situation (McCubbin & Patterson, 1983).
Regarding parent perceptions about their child’s symptoms, parents perceived that their child’s pain significantly increased over time. One explanation for this finding may be that their child experienced increased headache and intracranial pressure because of the inoperable brain tumor. However, future research should include evaluation of MRI changes before definite conclusions can be made.
Evaluation of the RN/MD dyad activities delivered according to protocol showed variations (inconsistent organization of communication) and omissions (lack of review of prognosis and use of percentages). To evaluate effects of communication interventions in future studies, we recommend ongoing PC/EOL communication training of RN/MD dyads to maintain skills and minimize intervention drift.
This study had several limitations. Generalizability of findings and cause-and-effect conclusions are not possible because the study design did not include a control group, randomization, or a large sample. Also, we do not know which components of the protocol influenced observed changes or which components were not helpful to parents. Also, our findings are based on a convenience sample and our investigator developed protocol tracking forms (ie, PPRI, ACP, QI) were first tested in this small pilot study. Because our grant funding only allowed for data collection during the first 6 months after diagnosis, all the parents’ children were only receiving standard neuro-oncology treatments and had not been referred exclusively to receive EOL. Also, our sample included primarily mothers and non-Hispanic White parents. Because of these limitations, our findings cannot be generalized to parents of children with brain tumors in other settings.
Surprisingly, our single-group unpowered pilot study that was designed to evaluate feasibility and acceptability of COMPLETE did provide significant findings that support further testing of our early PC/EOL focused-communication intervention that was delivered by RN/MD teams according to the information preferences of parents of children with a brain tumor and a poor prognosis. The especially promising findings of this small feasibility/acceptability study regarding parental hope, uncertainty, and decision regret provide support for an efficacy study of COMPLETE in a randomized controlled trial with a larger sample of children with other types of cancer. We recommend including additional communication training skills for the RN/MD teams who will deliver COMPLETE and the study of mechanisms by which parental hope may change over time and bereaved parents’ perspectives about any benefits of receiving COMPLETE prior to a child’s death.
Supplementary Material
Acknowledgments
The authors also wish to convey their sincere gratitude to the nurses and parents who participated in our delivery of the COMPLETE intervention. Also, we wish to convey our sincere gratitude to the following experts that provided expert input and guidance during the planning and preparation of the grant (R21NRO11071-O1A1) that supported this study: (a) our PC/EOL nurse consultant, Pamela S. Hinds, PhD, RN, FAAN, at Children’s National Medical Center in Washington, DC; (b) our communication expert, Richard Frankel, PhD at Indiana University; and (c) our ethics expert, Paul R. Helft, MD, at Indiana University.
Author Biographies
Verna L. Hendricks-Ferguson, PhD, RN, CHPPN, FPCN, FAAN, is an Associate Professor at St. Louis University, School of Nursing in St. Louis, MO
Kamnesh Pradhan, MD, is an Assistant Professor at Indiana University School of Medicine in Indianapolis, IN 46202. He is also a pediatric hematologist-oncologist at Riley Hospital for Children in Indianapolis, IN
Chie-Schin Shih, MD is an Assistant Professor at Indiana University School of Medicine in Indianapolis, IN 46202. He is also a pediatric hematologist-oncologist at Riley Hospital for Children in Indianapolis, IN
Karen M. Gauvain, MD, is an Assistant Professor at Washington University School of Medicine in Indianapolis, IN 46202. She is also a pediatric hematologist-oncologist at St. Louis Children’s Hospital in St. Louis, MO. At the time this study was conducted, Dr. Gauvain held the position as an Assistant Professor at Saint Louis University, School of Medicine and a pediatric hematologist-oncologist at Cardinal Glennon Children’s Hospital in St. Louis, MO
Javier R. Kane, MD, is a Professor at Texas A & M College of Medicine in Temple Texas. At the time of this study was conducted Dr. Kane held the position as the Director of the Division of Palliative and End-of-Life Care at St. Jude Children’s Research Hospital in Memphis, TN
Jingxia Liu, PhD, is an Assistant Professor and Statistician in the Division of Public Health Sciences at Washington University School of Medicine in St. Louis, MO
Joan E. Haase, PhD, RN, FAAN, is the Endowed Holmquist Professor in Pediatric Oncology Nursing at Indiana University School of Nursing and Co-Director of the IUPUI Signature Center for Research in Palliative and End-of-Life Communication and Training in Indianapolis, IN. At the time the grant for this study was prepared, Dr. Haase was the mentor for Dr. Verna Hendricks-Ferguson during her 3-year NINR T32 (NRO7066) post-doctoral fellowship at Indiana University.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by an exploratory/development grant, awarded under the American Recovery Act of 2009 at the National Institutes of Health and National Institute of Nursing Research (R21NRO11071-O1A1) to Dr Verna Hendricks-Ferguson (2009-2012). The content is the sole responsibility of the authors and does not represent official views of the National Institute s of Health. Dr Hendricks-Ferguson also wishes to acknowledge that this grant was prepared during the last year of her 3-year postdoctoral fellowship sponsored by the NINR T32 (NR07066) Training in Behavioral Nursing Grant at Indiana University, School of Nursing, under the mentorship of Dr Joan E. Haase. The authors wish to also thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St Louis, MO, for the use of the Biostatistics Core, which provided biostatistical consultation and analysis service by Dr Jingxia Liu. The Siteman Cancer Center is supported in part by NCI Cancer Center Support Grant #P30 CA91842.
References
- Almack K., Cox K., Moghaddam N., Pollock K., Seymour J. (2012). After you: Conversations between patients and healthcare professionals in planning for end of life care. BMC Palliative Care, 11, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altay N., Kilicarslan E., Sari C., Kisecik Z. (2014). Determination of social support needs and expectations of mothers of children with cancer. Journal of Pediatric Oncology Nursing, 31, 147-153. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner A. P., Winters J. M., Belknap R. A. (2012). Integrative review: Parent perspectives on care of their child at the end of life. Journal of Pediatric Nursing, 27, 514-522. [DOI] [PubMed] [Google Scholar]
- Bhat S. R., Goodwin T. L., Burwinkle T. M., Lansdale M. F., Dahl G. V., Huhn S. L., . . . Fisher P. F. (2005). Profile of daily life in children with brain tumors: An assessment of health-related quality of life. Journal of Clinical Oncology Nursing, 23, 5493-5550. [DOI] [PubMed] [Google Scholar]
- Bonner M. J., Hardy K. K., Guill A. B., McLaughlin C., Schweitzer H., Carter K. (2006). Development and validation of the parent experience of child illness. Journal of Pediatric Psychology, 31, 310-321. [DOI] [PubMed] [Google Scholar]
- Brehaut J. C., O’Connor A. M., Wood T. J., Hack T. F., Siminoff L., Gordan E., Feldman-Stewart D. (2003). Validation of the decision regret scale. Medical Decision Making, 23, 281-292. [DOI] [PubMed] [Google Scholar]
- Broom A., Kirby E., Good P., Wootton J., Adams J. (2014). The troubles of telling: Managing communication about the end of life. Qualitative Health Research, 24, 151-162. [DOI] [PubMed] [Google Scholar]
- Dalberg T., Jacob-Files E., Carney P. A., Meyrowitz J., Fromme E., Thomas G. (2013). Pediatric oncology providers perceptions and barriers and facilitators to early integration of pediatric palliative care. Pediatric Blood Cancer, 60, 1875-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durall A., Zurokowski D., Wolfe J. (2012). Barriers to conducting advance care discussions for children with life-threatening conditions. Pediatrics, 129, e975-e982. [DOI] [PubMed] [Google Scholar]
- Grady P. A. (2014). NIH makes palliative care more attainable for pediatric patients and their families. Retrieved from http://www.nih.gov/news/videos/2014/0220-palliative.htm
- Granek L., Krzyzanowska M. K., Tozer R., Mazzotta P. (2013). Oncologists’ strategies and barriers to effective communication about the end of life. Journal of Oncology Practice, 9, e129-e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerty R. G., Butow P. N., Ellis P. M. (2005). Communicating with realism and hope: Incurable cancer patient’s views on disclosure of prognosis. Journal of Clinical Oncology, 23, 1278-1288. [DOI] [PubMed] [Google Scholar]
- Hays R. M., Valentine J., Haynes G., Geyer J. R., Villareale N., McKinstry B., Varni J.W., Churchill S.S. (2006). The Seattle pediatric palliative care project: Effects on family satisfaction and health-related quality of life. Journal of Palliative Medicine, 9(3), 716-728. [DOI] [PubMed] [Google Scholar]
- Hendricks-Ferguson V. L. (2007). Parental perspectives of initial end-of-life communication. International Journal of Palliative and Hospice Care, 13(11), 1-10. [DOI] [PubMed] [Google Scholar]
- Hendricks-Ferguson V. L., Haase J. (2009). COMPLETE conceptual framework (Unpublished manuscript).
- Hendricks-Ferguson V. L., Haase J., Kane J. (2010). COMPLETE intervention visual forms (Unpublished manuscript).
- Hendricks-Ferguson V. L., Kane J. R., Pradhan K. R., Shih C. S., Gauvain K., Haase J. (2015). Evaluation of physician and nurse dyad training procedures to deliver a palliative and end-of-life communication intervention to parents of children with a brain tumor. Journal of Pediatric Oncology Nurses, 32, 337-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herth K. (1992). Abbreviated instrument to measure hope: Development and psychometric evaluation. Journal of Advanced Nursing, 17, 1251-1259. [DOI] [PubMed] [Google Scholar]
- Hudson P. L., Aranda S., Kristjanson L. J. (2004). Meeting the Supportive Needs of Family Caregivers in Palliative Care: Challenges for Health Professionals. Journal of Palliative Medicine, 7(1), 19-25. [DOI] [PubMed] [Google Scholar]
- Johnson D., Vadeboncoeur C. (2012). Palliative care consultation in pediatric oncology. Supportive Care in Cancer, 20, 799-803. [DOI] [PubMed] [Google Scholar]
- Lazarus R. S., Folkman S. (1984). Stress, appraisal, and coping. New York, NY: Springer. [Google Scholar]
- Levetown M. (2008). Communicating with children and families: From everyday interactions to skill in conveying distressing information. Pediatrics, 121, e1441-e1460. [DOI] [PubMed] [Google Scholar]
- Mack J. W., Joffe S. (2014). Communicating about prognosis: Ethical responsibilities of pediatricians and parents. Pediatrics, 133(Suppl. 1), S24-S30. [DOI] [PubMed] [Google Scholar]
- Mack J. W., Wolfe J., Cook E. F., Cleary P. D., Weeks J. C. (2006). Communication about prognosis between parents and physicians of children with cancer: Parent preferences and the impact of prognostic information. Journal of Clinical Oncology, 24, 5265-5270. [DOI] [PubMed] [Google Scholar]
- McCubbin H. I., Patterson J. M. (1983). The family stress process: The double ABCX model of adjustment and adaptation. In McCubbin H. I., Susman M. B., Patterson J. M. (Eds.), Social stress and the family: Advances in family stress theory and research (pp. 7-37). New York, NY: Haworth. [Google Scholar]
- National Institute of Nursing Research. (2014). Palliative Care Conversations Matter. Retrieved from https://www.ninr.nih.gov/newsandinformation/conversationsmatter/conversations-matter-newportal#.WAdEwSTzKZg
- Smith T. J., Lindsay A. D., Virago E.A., Khatcheressian J., Matsuyama R., Lyckholm L. J. (2011). A pilot trial of decision aids to give truthful prognostic and treatment information to chemotherapy patients with advanced cancer. Journal of Supportive Oncology, 9(2), 79-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syse Z, Longi J., Lyngstad T. H. (2010). Does childhood cancer affect parental divorce rates? A population-based study. Journal of Clinical Oncology, 28(5), 872-877. [DOI] [PubMed] [Google Scholar]
- Tang C. J., Chan S.W., Zhou W. T., Liaw S. Y. (2013). Collaboration between hospital physicians and nurses: an integrated literature review. International Nursing Research, 60(3), 291-302. [DOI] [PubMed] [Google Scholar]
- Thompson L., Knapp C., Madden V., Shenkman E. (2009). Pediatrician’s perceptions of and preferred timing for palliative care. Pediatrics, 123, 282-288. [DOI] [PubMed] [Google Scholar]
- Varni J. W., Quiggins D. J., Ayala G. X. (2000). Development of the pediatric hematology/oncology parent satisfaction survey. Children’s Health Care, 29, 243-255. [Google Scholar]
- Wright A., Zhang B., Ray A. (2008). Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. Journal of the American Medical Association, 300, 1665-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.