Abstract
Obesity predisposes humans to a range of life-threatening comorbidities, including type 2 diabetes and cardiovascular disease. Obesity also aggravates neural pathologies, such as Alzheimer’s disease, but this class of comorbidity is less understood. When Drosophila melanogaster (flies) are exposed to high fat diet (HFD) by supplementing a standard medium with coconut oil, they adopt an obese phenotype of decreased lifespan, increased triglyceride storage, and hindered climbing ability. The latter development has been previously regarded as a potential indicator of neurological decline in fly models of neurodegenerative disease. Our objective was to establish the obesity phenotype in Drosophila and identify a potential correlation, if any, between obesity and neurological decline through behavioral assays and expression microarray. We found that mated female w1118 flies exposed to HFD maintained an obese phenotype throughout adult life starting at seven days, evidenced by increased triglyceride stores, diminished life span, and impeded climbing ability. While climbing ability worsened between seven and fourteen days of exposure to HFD, there was no corresponding alteration in triglyceride content. Microarray analysis of the mated female w1118 fly head revealed HFD-induced changes in expression of genes with functions in memory, metabolism, olfaction, mitosis, cell signaling, and motor function. Meanwhile, an Aversive Phototaxis Suppression assay in mated female flies indicated reduced ability to recall an entrained memory six hours after training. Overall, our results support the suitability of mated female flies for examining connections between diet-induced obesity and nervous or neurobehavioral pathology, and provide many directions for further investigation.
Keywords: Obesity, Disease, High Fat Diet, Neural decline, Drosophila, Memory
Introduction
According to the World Health Organization, obesity is abnormal or excessive fat accumulation that may impair health. The prevalence is staggering, and according to the Centers for Disease Control, 39.8% of the United States adult population is obese (Centers for Disease Control, 2017). Projections based on the current obesity trends estimate that there will be 65 million more obese adults in the US by 2030. Obesity, especially abdominal obesity, is one of the predominant underlying risk factors for metabolic syndrome, which increases the risk of developing insulin resistance, type 2 diabetes, dyslipidemia, cardiovascular diseases, and certain forms of cancer. Unsurprisingly, obesity results in an increased risk of mortality in adults, children and adolescents (Wang et al. 2011; Carr et al. 2004). The currently understood pathogenesis of human obesity includes chronic inflammation (mediated by macrophage activation and abnormal pro-inflammatory signaling) (Haiyan et al. 2003), increased cellular oxidative stress (Shigetada et al. 2004), and abnormal energy homeostasis signaling dynamics, such as leptin or insulin resistance (Montague et al. 1997).
Apart from its previously mentioned comorbidities, associations between obesity and neurocognitive and neurobehavioral function, as well as risk of dementias such as Alzheimer’s disease, have more recently been recognized. Obesity is linked to both atypical and age-related cognitive impairment in humans. There is a correlation between elevated BMI and reduced episodic memory. Mid-life obesity is also a predictor of cognitive impairment at older ages. (Elias et al. 2005; Jeong et al. 2005; Hassing et al. 2010). Rodent models of obesity have shown the effect of high fat diet on neural function, exhibiting cognitive decline, cerebral inflammation, increased oxidative stress, cellular distress and hypothalamic apoptosis (McNay et al. 2010; Valladolid-Acebes et al. 2011; Stranahan and Mattson 2011). At the molecular level, chronic over-nutrition was shown to increase the apoptosis of mature neurons, newborn neurons, dividing cells, or neural stem cells in the hypothalamus, while caloric restriction was able to reverse some of these defects in mice (Li, Tang, and Cai 2012). Conversely, therapeutic interventions in metabolic diseases have often been shown to also protect against neurodegenerative disorders, suggesting that obesity and diabetes contribute to the development of neuro-pathological states (Prickett, Brennan, and Stolwyk 2015). The molecular or physiological mechanisms by which obesity affects nervous function, behavior, or memory have thus far been subject to less investigation relative to obesity’s relationship with diabetes and cardiovascular disease. Whether these mechanisms are specific to the nervous system or indirect through impacts on central energy homeostasis or peripheral signaling is also unclear.
Drosophila can function as a model for various human diseases, a purpose for which it is well suited due to at least 75% of known disease-related genes in humans having functional orthologs in the fly. Overall identity in protein sequence between flies and mammals is approximately 40% between homologs; however, in conserved functional domains, it can be 80 to 90% or higher. Furthermore, the Drosophila model overcomes some of the limitations of mammalian model organisms through rapid generation time, high number of offspring, and the tissue specific control that can be exerted over gene expression by investigators (Reiter et al. 2001). The manipulation of gene expression in flies is achievable via a powerful P-element based system, including the GAL4-UAS system, which allows for tissue-specific patterns of transcriptional activator protein expression to be converted into the expression of any gene or ORF of interest, and the excision-mediated knockouts of genes (Duffy 2002).
A high-fat diet (HFD) causes a phenotype in Drosophila melanogaster that resembles diet-induced obesity and associated pathophysiology in mammals. The phenotype is characterized by increased triglyceride and glucose levels, decreased stress tolerance, and decreased lifespan (Heinrichson and Haddad 2012; Heinrichson et al 2014). It has also been shown that a HFD can induce cardiac fat accumulation and dysfunction in Drosophila (Birse et al. 2010). Meanwhile, murine models of neural pathology experience an impairment in short term memory, anosmia, and neuronal cell death, which are seen as valid indicators of nervous pathology (Pistell et al. 2010). Drosophila models of neurodegeneration have also been established for the study of Alzheimer’s and Parkinson’s disease, which mirror the neurobehavioral pathologies seen in mice. Such neurobehavioral pathologies in flies have been assayed via Aversive Phototaxis Suppression (short term memory), olfactory trap assay and negative geotaxis assays (Feany and Bender 2001; Bouleau and Tricoire 2015; Ali et al. 2011). Of interest, negative geotaxis is noted as being decreased in the HFD model of obesity in Drosophila (Birse et al. 2010).
In this work, we examined the behavioral and transcriptional responses to a high fat diet, with a specific interest in the fly head, utilizing the Drosophila model. The fly head contains the brain, the pericerebral fat body, glia, and neurosecretory cells, all of which are involved in energy homeostasis and conventional obesity pathology (Kühnlein 2010). Flies utilize a system of energy homeostasis that physiologically parallels that of humans. When nutrients are detected in flies, the functional equivalent to human leptin, known as Unpaired 2 (Upd2), is released by an endocrine fat body. Upd2 activates JAK-STAT signaling in GABAergic neurons in the brain. This results in the release of Drosophila Insulin-like Peptide 2 (Ilp2), which is functionally analogous to human insulin. In flies, eight known Insulin-like peptides (Ilps) influence nutrient uptake, growth, fertility, feeding behavior, and lifespan (Rajan and Perrimon 2012; Hombria et al. 2005). As in mammals, there is also some involvement of inflammatory processes in energy homeostasis in flies, as exemplified by the release of Unpaired 3 by macrophages in response to HFD. Unpaired 3 causes increased activity of the JAK-STAT pathway in multiple tissues and results in decreased lifespan and disrupted glucose metabolism (Woodcock et al. 2015). Furthermore, several transcriptomic studies in the fly have indicated upregulation of inflammatory processes in Drosophila after HFD feeding (Hemphill et al., 2018; Henrichson et al., 2014; Jung et al., 2018). This physiological similarity, along with the ability to model and ascertain the extent of both diet-induced obesity and nervous pathology, as well as the aforementioned unique experimental strengths of this invertebrate, make Drosophila a potentially excellent model to study the connection between obesity and nervous decline.
We hypothesized that fly obesity would be accompanied by indicators of nervous system decline, thus confirming the suitability of the fly model for investigating the phenomenon in the future. Our first aim was to emulate obesity in mated female flies and determine the earliest onset of obesity-associated pathophysiology before embarking on our behavioral and gene expression assays. In order to ascertain the earliest possible time-point of onset obesity, we carried out lifespan, triglyceride content, and negative geotaxis assays at 7,14 and 21 days of adult exposure to HFD. This cluster of phenotypes is an affirmed marker of onset-obesity in Drosophila (Birse et al. 2010). Following onset of the obese phenotype, we assessed nervous and neurobehavioral decline through means previously utilized with flies, including olfactory trap assay and Aversive Phototaxic Suppression (APS) assays (Feany and Bender 2001; Zhou et al. 2010; Ali et al. 2011; Bouleau and Tricoire 2015). Finally, to gain initial insight as to the mechanisms by which this decline occurs and suggest genes and pathways for further investigation, we ran a gene expression microarray on whole mated female fly heads (Kühnlein 2010).
Methods
Fly rearing and collection
All stocks were maintained on a Normal Diet (ND) in an incubator at 25°C and 30–50% humidity. Adult virgin female flies were collected at 0–5 days and transferred to separate vials of ND media at approximately equal population density. Virgin females were then mated with isogenic male siblings from the same collection interval for 48 hours, after which mated females were placed on respective experimental media (ND and HFD) and incubated for 7 days at 23°C in most cases, unless otherwise noted.
Drosophila stocks
All fly stocks were obtained from the Bloomington Drosophila Stock Center at Indiana University. Stocks used were as follows: w1118 (Stock 6326), Canton-S (Stock 1) and Oregon-R-C (Stock 5). w1118 females were used for all experiments with the exception of aversive phototaxis suppression, where Canton-S and Oregon-R-C wild-type backgrounds were utilized due to difficulties with reliable phototaxis in white-eyed flies. Mated females were utilized for all studies due to evidence that female mating and egg-laying increases feeding quantity, and because we wanted to encourage consumption of the high fat diet (Carvalho et al., 2006; Wigby et al., 2011).
Experimental diets: normal and high-fat
Our ND consisted of 10% lyophilized yeast, 10% sucrose, 5.2% cornmeal, 1.2% agar, 3.3% tegosept (dissolved in 70% ethanol) and 0.3% propionic acid (w/v). Our High Fat (HFD) diet consisted of the same quantities as our ND, with the addition of 20% Coconut oil (w/v). Coconut oil was added to the regular food as a source of increased saturated fat in the diet, which results from lauric and myristic acid (the main components of coconut oil) (Birse et al. 2010).
Lifespan
Virgin females no older than 3 days post-eclosion were collected and mated with genetically identical male siblings of the same approximate age. Fourteen replicates of 15 flies per experimental condition were placed on respective experimental media (ND and HFD) and incubated at 23°C at regulated humidity on a 12-hour light/dark cycle. Live flies from each condition were counted daily and counts were recorded until no flies remained from a single group and the difference between groups was obvious. Flies were transferred to fresh media every 3–4 days.
Negative Geotaxis
Ten groups of ten flies per experimental condition (ND, HFD) were anesthetized using CO2 and placed in empty vials. The climbing apparatus for each group was prepared such that two empty polystyrene vials are vertically joined by tape with openings facing each other. For the lower vial, a vertical distance of 8 cm was measured above the bottom surface and marked by drawing a circle around the entire circumference of the vial. Flies were then carefully transferred to the bottom vial and allowed 1 hour to recover from CO2 exposure. After acclimation, a vial of flies was gently tapped to the bottom of their vial and the number of flies that crossed the 7-centimeter mark in a time span of 10 seconds was recorded. This assay was repeated for each group 10 times with a 1-minute rest period between each trial to generate an average pass rate per group. This assay was carried out on age-matched, independent collections of mated females at 7, 14, and 21 days of experimental media exposure.
Triglyceride Content Assays
Forty-three individual flies per experimental condition were anesthetized with FlyNap® (Carolina) for ~5 minutes. Flies were then individually weighed on a Cahn 31 Ultra Micro Balance and immediately placed in a 1.5 mL tube with 150 μL of Phosphate Buffered Saline (PBS) with 0.05% TWEEN-20. The tube also contained one scoop of Zirconium Silicate Beads (0.5mm diameter) (Next Advance) for homogenization. Tubes containing flies were then homogenized utilizing a Bullet Blender Blue (Next Advance) at speed 8 for 3 minutes. Following homogenization, tubes were heat inactivated at 70 °C for 5 minutes. Tubes were then centrifuged for 3 minutes at 5000 RPM. Supernatant was transferred to a 96-well plate. Twenty microliters from the master plate were then aliquoted to three separate 96-well plates for a total of 3 technical replicates. The first replicate was blanked in a Synergy HT Multimode Plate Reader (Biotek) to which after, 100 μLs of Infinity Triglyceride Reagent (Thermo Scientific) were synchronously added to all wells within the plate. The plate was then incubated while shaking at 37 °C for 20 minutes and finally read at an absorbance level of 540 nanometers (Hildebrandt et al. 2011). Data was interpolated by standard curve analysis using Triolein (TCI) as a triglyceride standard. This assay was carried out on age-matched, independent cohorts of mated female flies at 7, 14, and 21 days of experimental media exposure.
Aversive Phototaxis Suppression Assay
Over fifty flies per experimental condition were placed in empty individual 15 mL tubes after 7 days of experimental media exposure in preparation for phototaxis assessment. Utilizing a T-maze adopted from Le Bourg and Buecher (Le Bourg and Buecher 2002), flies were assayed for learning and memory by established protocol (Zhou et al. 2010; Ali et al. 2011). Before assaying for short term memory impairment, flies had to be considered phototaxis positive by allowing individual flies to walk towards a light source in a span of ten seconds while in complete darkness. Once considered phototaxis positive, twenty individual flies per experimental condition were trained to avoid the lighted tube by placing a piece of filter paper within the lighted tube containing 0.1% quinine dissolved in distilled water (w/v). Training individual flies to avoid the lighted tube containing quinine was carried out a total of ten times for each individual fly. Individual flies were allowed 1 minute to acclimate in the dark tube after each attempt. Each fly (ND and HFD) was then allowed to walk towards the lighted tube, or avoid it, where avoidance resulted in a positive score and is established as Post Conditioning 0 (PC0). PC0 represents the fly’s ability to learn avoidance of an otherwise attractant. Flies were then placed back on their respective experimental media for 6 hours, after which the same individual flies were re-tested as previously mentioned, with a filter paper saturated in water placed within the lighted tube. This trial (PC6) represents the fly’s ability to recall avoidance of the lighted tube, due to its correlation with an aversive substance. A poor score for PC6 is an indicator of short term memory impairment. This assay was carried out initially with Canton-S due to a lack of reliable, initial phototaxis behavior from the white eyed w1118 flies. A second trial was carried out with Oregon-R-C flies, a different genetic background. Post Conditioning 3 (PC3) and Post Conditioning 24 (PC24) trials were also performed for this assay using an independent cohort of Canton-S mated female flies.
Olfactory Trap Assay
Around 300–400 flies per experimental condition, comprising 4–5 technical replicates per condition, were starved for 24 hours in vials containing water saturated cotton. Flies were then anesthetized with carbon dioxide and 60–80 flies were then transferred to their respective olfactory apparatus (Ditzen et al. 2008). The olfactory apparatus was comprised of two 5 mL bottles placed in a 1L beaker, each containing a respective odorant or a control (water). Both bottles were covered with a flug that was punctured with a 1 mL pipette tip, where the pipette tip was contained within the bottle, allowing for flies to enter each bottle without the possibility of climbing out. The beaker was then covered with aluminum foil that contained small holes which allowed a flow of air into the apparatus. Two known fly attraction odorants were used in two separate experiments; 10% acetoin and 10% yeast paste (Larsson et al. 2004; Becher et al. 2012). Flies were then incubated for 7 hours in 23 °C. Following incubation, flies were placed at −18 °C for 20 minutes and then each replicate was counted to determine the attraction index. Attraction Index: (# flies in odorant trap-# flies in water trap)/ (# total flies in the apparatus), where an attraction index of one corresponds to complete attraction of all flies into the baited odor trap, an index of negative one corresponds to preference for the diluent odor trap, and attraction index of 0 corresponds to no preference for either trap (Ditzen et al. 2008).
Gene Expression Microarray
Drosophila Genome 2.0 Arrays (Affymetrix) were used in this study, with three arrays per experimental diet (w1118 on ND and HFD). Following 7 days on ND or HFD, fly heads were obtained by snap freezing in liquid nitrogen within 15 mL tubes and then vortexing, after which, heads were collected by placing flies on a 710 μm sieve and shaking so that the fly heads fall through to the bottom 425 μm sieve (VWR). Heads were then collected into a 1.5 mL centrifuge tube. Total RNA was extracted from each sample and purified with an RNeasy Mini Kit (Qiagen) as per manufacturer’s instructions. Concentration and purity of RNA (>2.0 260:280 and >1.8 260:230) were determined using a NanoDropTM 2000 (Thermo Scientific). One μg minimum of RNA per sample was then sent to the UT Southwestern Microarray Core Facility where RNA integrity was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies). Following RNA integrity, biotin-labeled cRNA is hybridized to a Drosophila melanogaster probe array and scanned, where fluorescence data was then normalized with Tukey’s Biweight method. Signal log ratio was then utilized on normalized data in order to produce a fold change between experiment versus baseline probes (ND vs HFD). Significance was determined by comparing fold changes between gene pairs of flies exposed to experimental conditions (ND and HFD) using GeneSpring (Agilent Technologies). The Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.8 bioinformatics software was used to identify gene enrichment clusters and functional annotation charts on significant upregulated and downregulated genes (p≤05, fold change of ≥1.5) of flies exposed to a HFD. Gene functional annotation groups considered of interest were selected based on enrichment scores (−Log10(P-value)) of 1.3 or higher (Huang et al. 2009). The gene expression data from the microarrays has been deposited in the NCBI Gene Expression Omnibus (GSE117834).
Quantitative real-time PCR analysis
Following 7 days on ND or HFD, independent samples of mated female fly heads (not utilized for the microarray) were obtained. Total RNA was extracted and checked for purity as previously described. cDNA was produced from total RNA using iScript™ Reverse Transcription (RT) Super Mix (Bio Rad). Quantitative Real-Time PCR was performed using a Bio-Rad MyCyclerTM using iTaq™ Universal SYBR® Green Supermix (Bio Rad). All samples were run at a final cDNA concentration of 8 ng. Two genes identified as upregulated and two identified as downregulated were chosen for microarray validation; Cyp4e3 (fwd: GATGGCATTTTTAGACACCCTGC; rev: CCCGATACAATTTCCTCTGGACA), To (fwd: GTGGATGCCAAATTCCCCGA; rev: ATCCGATCCACCTTCAACGG) (upregulated). Orc3 (fwd: GGCGCCATATACTTCGTGAT; rev; GAGCAGGAAATCGAGGAACA), Hsp27 (fwd: GATGTGTCGCAGTTCAAGCC; rev: GTGGACACTACCTCGTTGGG) (downregulated). RpL32 was utilized as an internal housekeeping gene for relative fold change (fwd: GACGCTTCAAGGGACAGTATCTG; rev: AAACGCGGTTCTGCATGAG) (Zheng et al. 2014). The four differentially expressed genes for validation were randomly chosen from up- and down-regulated genes of potential physiological relevance in energy homeostasis, stress resistance, or neural processes. Equivalent amplification efficiency between experimental genes and housekeeping gene was verified using a standard curve of serially diluted, pooled samples with an r2 at or above .980 and an efficiency between 90 and 100%. Melt curve was also assessed for one peak per reaction to ensure specific priming and lack of primer dimers. Fold change for each gene was determined using the 2−ΔΔCT method (Schmittgen and Livak 2008).
General Statistical Analysis
An unpaired, one-tailed t-test was used to determine significance between ND and HFD results in most assays. Lifespan analysis significance was determined by comparing the survival curves with a log-rank (Mantel-Cox) test. Triglyceride and negative geotaxis assays were additionally analyzed using the Fit Model function in JMP to conduct ANOVA, followed by Tukey’s HSD post-hoc testing to identify differences between groups. In each case, triglyceride and negative geotaxis were modeled on Diet, Time, and Diet*Time. Results for ANOVA are reported as FDf,SS with an accompanying P-value.
RESULTS
Exposure Time Study
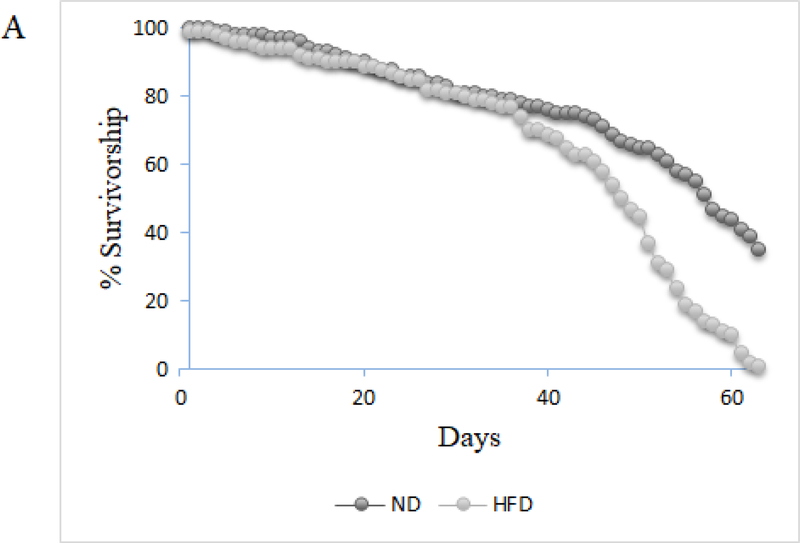
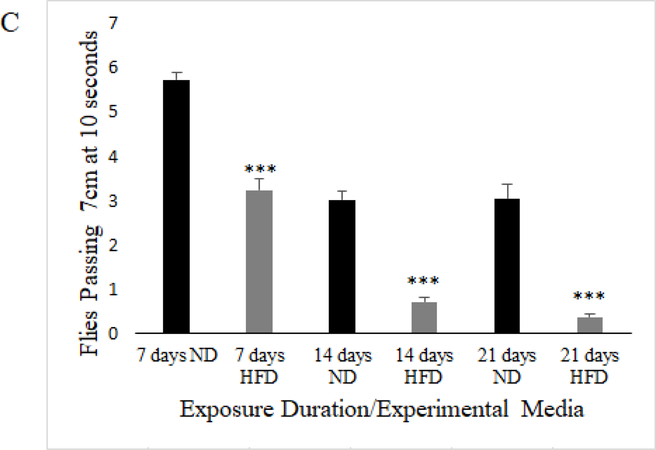
It has previously been established that when flies are exposed to a high-fat diet they experience decreased lifespan, increased triglyceride storage and decreased negative geotaxis (Heinrichson and Haddad 2012; Heinrichson et al. 2014). Because we used our own dietary formula in order to more tightly control its composition, we observed several dietary exposure durations in order to validate that our HFD resulted in the known obesity phenotypes, and to determine the earliest onset of the obese-like phenotype to establish an incubation time for all other assays. We exposed mated female w1118 flies to a HFD, and assayed for lifespan (Figure 1a), triglyceride content (Figure 1b) and negative geotaxis ability (Figure 1c) at regular exposure intervals. We found that HFD flies had significantly shortened lifespans, significantly increased triglyceride content, and significantly decreased negative geotaxis at all time intervals (7, 14, 21 days) (p≤0.005). We found that 7 days of exposure to a HFD was enough to produce an obese-like phenotype in the flies, which established the time of media exposure for all following experiments.
Fig. 1a.
Mated female w1118 flies on a HFD have a significantly decreased lifespan compared to flies on a ND (N=pooled survival across 14 replicates, 15 flies per replicate; lifespan was monitored until one of the two diet exposures had no flies remaining and the difference between groups was clear; Log-rank test of survival curves, p<.005)
Fig. 1b.
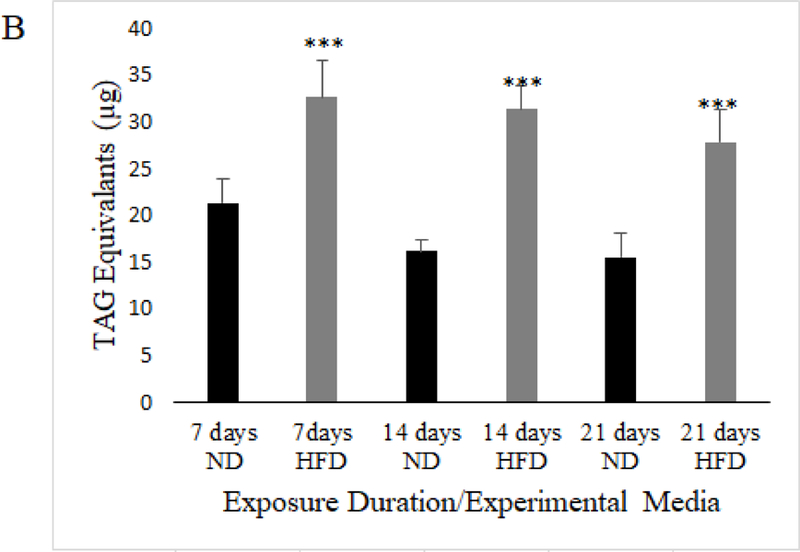
Mated female w1118 flies on a HFD have significantly higher triglyceride content than flies on an ND at 7, 14, and 21 days of exposure to respective media (Data shown represents average TAG equivalents per individual fly (N=43, 43 flies experimental condition; unpaired t-test; p<.005***; error bars represent 95% confidence interval)
Fig. 1c.
Mated female w1118 flies on a HFD have a diminished climbing ability compared to flies on a ND at 7, 14, and 21 days of exposure, represented as an overall average of the average of flies passing the 7 cm mark over ten climbing trials (N=10, 100 flies per experimental condition; unpaired t-test; p<.005***; error bars represent 95% confidence interval)
Utilizing further ANOVA and Tukey’s HSD post-hoc testing, we observe that while HFD significantly increased triglyceride content in mated females after 7 days of exposure, 14 and 21 days of exposure to HFD resulted in no additive increase in triglyceride content (Model: F5,19.71=33.36, p<.0001; Diet: F1,17.89=151.41, p<.0001; Time: F2,1.57=6.648, p=.001; Diet*Time: F2,0.25=1.048, p=.3778; Tukey’s HSD: p≥.05 for all comparisons except between ND and HFD values at each exposure time where p<.001). Meanwhile, negative geotaxis is significantly decreased by HFD after 7 days of exposure, but 14 days of HFD exposure caused even greater deterioration in climbing performance that did not decline additionally after 21 days of exposure (Model: F5,189=85.88, p<.0001; Diet: F1,92.50=210.16, p<.0001; Time: F2,96.15=109.23, p<.0001; Diet*Time: F2,0.34=.3889, p=.6797; Tukey’s HSD p>.05 for all comparisons except between ND and HFD at each time where p<.001, between 7 and 14 days HFD where p<.001, and 7 and 14 days ND where p<.001). We also observe a decline in climbing performance comparing 7 and 14 days of ND exposure, but no further decline when comparing 14 days to 21 days of ND exposure. Therefore, triglyceride content in mated females on a HFD became elevated and was unchanging with exposure duration, but climbing ability declined with HFD and worsened between 7 and 14 days of exposure, identifying a lack of correlation between the triglyceride content and the severity of the decline in climbing performance in this study. All negative geotaxis and triglyceride content data has been made available as Online Resource 1 and Online Resource 2, respectively.
Aversive Phototaxis Suppression Analysis of Flies on a HFD
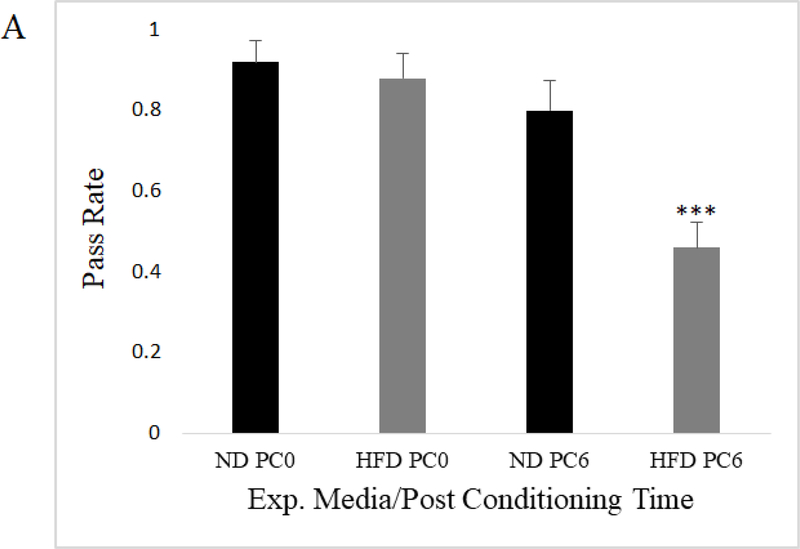
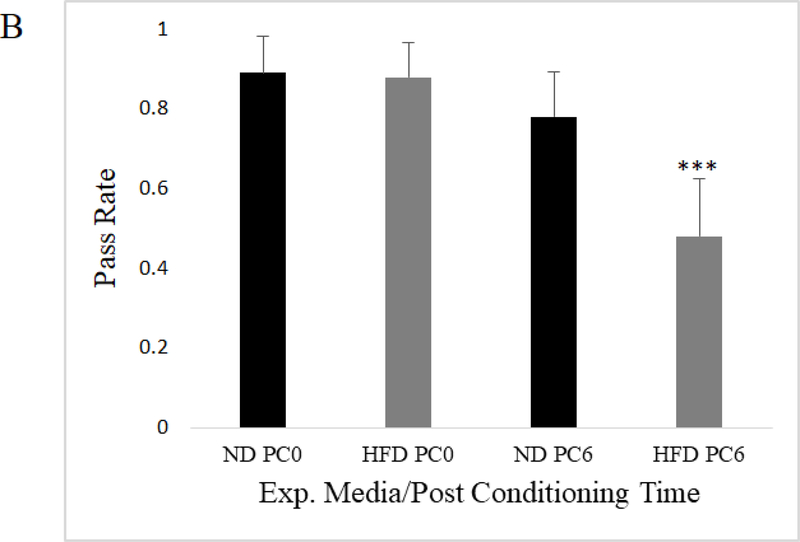
To assess whether flies exposed to a HFD suffer from memory impairment, we utilized aversive phototaxis suppression (AS), which has been previously shown as an indicator of learning and short-term memory function in flies (Feany and Bender 2001; Zhou et al. 2010; Ali et al. 2011; Bouleau and Tricoire 2015). APS refers to the experimental suppression of fly phototaxis behavior via learned aversion (see Methods). In this assay, an individual phototaxic fly is entrained to avoid light by repeatedly forcing exposure to bitter-tasting quinine in association with phototaxis. After they exhibit aversion to light, entrained flies that had been exposed to ND and HFD are given the chance to engage in phototaxis behavior over ten trials at various post conditioning (PC) intervals. If an entrained fly recalls their training and does not engage in phototaxis when given the opportunity, they are considered to have “passed” during that trial. Therefore, “pass rate” as represented below is the average across twenty flies per condition of the percentage of passing performances of ten trials in the APS assay. Mated females from two wild-type fly strains, Canton-S and Oregon-R-C, were utilized for APS as the w1118 flies were not responsive to light during our initial testing. We found that mated females from both fly strains on both experimental media conditions were able to learn avoidance of light, as there was no significant difference in pass rate immediately after aversive training (PC0). Six hours post PC0, at PC6, we found that mated female flies exposed to a HFD had a statistically significant decline in pass rate relative to ND exposed mated female flies. This is indicated by their apparently reduced ability to recall the aversive training 6 hours prior, which could point to impaired short term memory (Figure 2a-b). We also assayed a separate cohort of 17 trained mated female flies of the Canton-S background at PC3 and PC24 and observed that there was no significant difference in pass rate between diet groups at these times (PC3: unpaired t-test p=.272; PC24: unpaired t-test p=..237). There is a general decay in pass rate over time in line with previous aversive phototaxis time course experiments, such that at PC24 pass rate was low and there was effectively no difference between diet groups (Online Resource 3; Perisse et al. 2007).
Fig. 2a.
Mated female Canton-S exposed to a HFD showed competent aversive phototaxic learning behavior with a pass rate that did not differ from ND flies at 0 hours post conditioning (PC0), while showing a significant decline in pass rate at 6 hours post conditioning (PC6) compared to flies on a ND (N=20, 20 individually trained flies per diet; data represents average pass rate per fly tested; error bars represent standard error of the mean; unpaired t-test; p<.005***)
Fig. 2b.
Mated female Oregon-R exposed to a HFD showed competent aversive phototaxic learning behavior with a pass rate that did not differ from ND flies at 0 hours post conditioning (PC0), while showing a significant decline in pass rate at 6 hours post conditioning (PC6) compared to flies on a ND (N=20, 20 individually trained flies per diet; data represents average pass rate per fly tested; error bars represent 95% confidence intervals; unpaired t-test; p<.005***)
Olfaction analysis of flies on a HFD
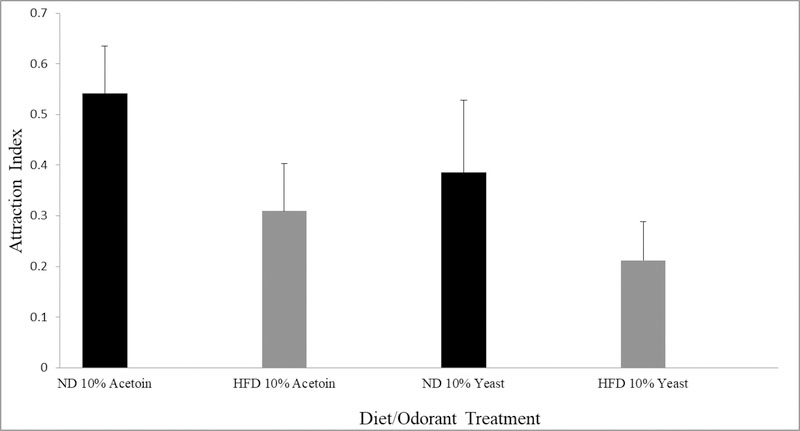
We assayed mated female w1118 fly ability to detect a positive odorant (10% acetoin, or yeast paste) by utilizing a modified olfactory trap assay (Ditzen et al. 2008). We found that although flies exposed to HFD had a lower attraction index for the selected odorants relative to flies exposed to an ND, there was no significant difference in the attraction index (Figure 3; for acetoin: unpaired t-test p=.064 and for yeast paste: unpaired t-test p=.169). Raw counts of flies for each odorant assay are available as Online Resource 4.
Fig. 3.
There was no significant difference in olfaction between mated female w1118 flies on a HFD and those on a ND when using 10% acetoin or 10% yeast paste as an attractant (N=4 experiments, 60–100 flies per experiment; data represents average attraction index; unpaired t-test; error bars represent standard error of the mean).
Transcriptional changes within fly heads on a HFD
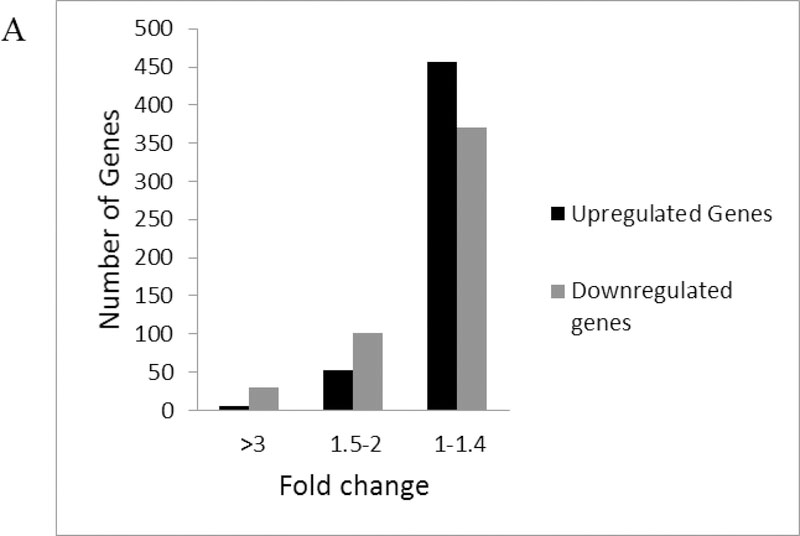
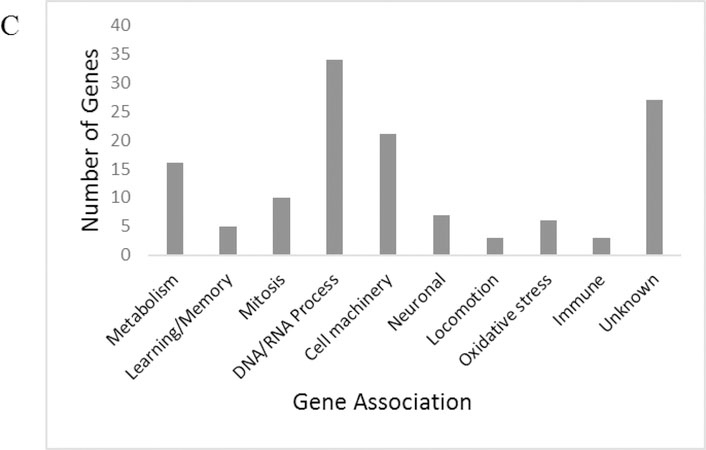
To better characterize brain and nervous function during obesity, we examined gene expression of the isolated mated female fly head. Genes were considered changed in expression for HFD if they had a fold change of 1.5 or greater and the fold change differences were statistically significant when comparing ND to HFD (p ≤0.05) (Jensen K and Sanchez-Garcia J 2013). Microarray analysis of flies exposed to a HFD resulted in 59 upregulated genes, and 132 downregulated genes (Figure 4a). Statistical significance and fold change were analyzed by the University of Texas Southwestern Microarray Facility and are provided (Online Resource 5). Genes were then manually grouped into categories of general function (Figure 4b-c). For significant upregulated genes, DAVID enrichment analysis revealed functional clusters with the highest biological significance ranking (enrichment score ≥1.3) contained gene-annotation groups which consist of Odorant/pheromone proteins, Cytochrome P450, Monooxygenase, and oxidation reduction among others (Table 1a-b). Significant downregulated genes revealed functional clusters with the highest biological significance ranking containing gene annotation groups which consist of Cell cycle, Mitosis, Neurogenesis, RNA binding and DNA replication, among others (Table 2a-b). The full enrichment analysis is available via Online Resource 6. Genes of known physiological significance were then chosen for highlighting based on relevance to our investigation of the effects of a HFD on the central nervous system and central energy homeostasis (Table 3a-b).
Fig. 4a.
Number of genes exhibiting significant expression changes in mated female w1118 flies exposed to a HFD (p≤0.05) with a fold change greater than 1 as indicated by the microarrays by magnitude classification (N=3 biological replicate arrays per dietary treatment)
Fig. 4b.
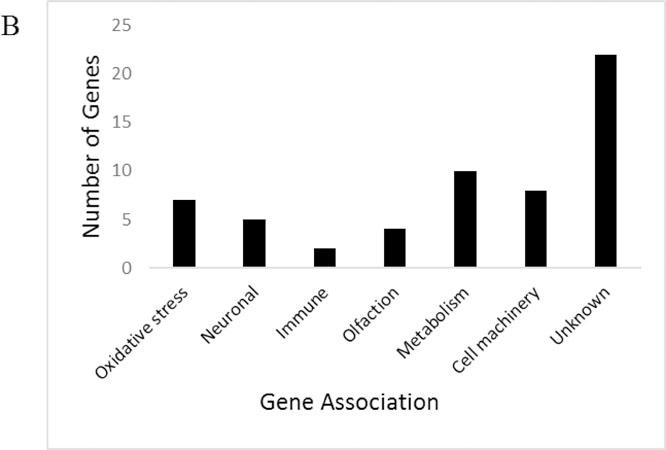
Functional categorization and quantity of significantly upregulated genes based on manual screening of gene ontology (those with p.≤0.05, fold change of ≥1.5)
Fig. 4c.
Functional categorization and quantity of significantly downregulated genes based on manual screening of gene ontology (those with p.≤.0.05, fold change of ≥1.5)
Table 1a.
The top 3 enriched annotation clusters and enrichment scores for the significantly upregulated genes in mated female w1118 fly heads exposed to a HFD using DAVID annotation analysis (Shown due to enrichment scores of ≥1.3; P-value of 0.05 without minus log scale)
| Annotation Cluster | Annotation Terms for Upregulated Genes | Enrichment Score |
|---|---|---|
| Cluster 1 | Odorant binding | 3.48 |
| Pheromone/general odorant binding protein | 3.35 | |
| PhBP | 2.59 | |
| Sensory perception of chemical stimulus | 2.48 | |
| Extracellular region | 2.00 | |
|
Cluster 2 |
Metal ion-binding site:Iron (heme axial ligand) |
1.64 |
| Cytochrome P450, E-class, group I | 1.55 | |
| Monooxygenase activity | 1.54 | |
| Secondary metabolites biosynthesis, transport,
and catabolism |
1.54 | |
| Organelle membrane | 1.54 | |
| Cytochrome P450, conserved site | 1.52 | |
| Oxidoreductase activity | 1.52 | |
| Microsome | 1.51 | |
| Cytochrome P450 | 1.46 | |
| Oxidation-reduction process | 1.36 | |
|
Cluster 3 |
Extracellular region |
2.00 |
Table 1b.
Enrichment scores for the significantly upregulated genes in mated female fly heads exposed to a HFD using DAVID annotation analysis (Shown due to enrichment scores of ≥1.3; P-value of 0.05 without minus log scale)
| Annotation Terms for Upregulated Genes | Enrichment Score |
|---|---|
| Odorant binding | 3.48 |
| Pheromone/general odorant binding protein | 3.35 |
| Signal | 3.29 |
| Sensory perception of smell | 2.77 |
| PhBP | 2.59 |
| Sensory perception of chemical stimulus | 2.48 |
| Extracellular region | 2.00 |
| Metal ion-binding site:Iron | 1.64 |
| Biosynthesis of antibiotics | 1.64 |
| Cytochrome P450, E-class, group I | 1.55 |
| Monooxygenase activity | 1.54 |
| Secondary metabolites biosynthesis | 1.54 |
| Organelle membrane | 1.54 |
| Cytochrome P450 | 1.52 |
| Oxidoreductase activity | 1.52 |
| Microsome | 1.51 |
| Binding site:Heme | 1.40 |
| Oxidation-reduction process | 1.36 |
| Extracellular space | 1.34 |
Table 2a.
The top 8 enriched annotation clusters and enrichment scores for the significantly downregulated genes in mated female w1118 fly heads exposed to a HFD using DAVID annotation analysis (Shown due to enrichment scores of ≥1.3; P-value of 0.05 without minus log scale)
| Annotation Cluster | Annotation Terms for Downregulated Genes | Enrichment Score |
|---|---|---|
| Cluster 1 | Cell cycle | 3.89 |
| Regulation of chromatin binding | 3.62 | |
| Cyclin, N-terminal | 3.42 | |
| Cyclin | 3.40 | |
| CYCLIN | 3.29 | |
| Cyclin-like | 2.96 | |
| Mitosis | 2.66 | |
| SM01332 | 2.62 | |
| Cyclin, C-terminal domain | 2.60 | |
| Cyclin-dependent protein serine kinase regulator activity | 2.19 | |
| Cell division | 2.12 | |
| Syncytial blastoderm mitotic cell cycle | 2.00 | |
| Mitotic nuclear division | 0.89 | |
|
Cluster 2 |
Helicase |
3.48 |
| DNA binding | 1.82 | |
|
Cluster 3 |
Helicase |
3.48 |
| P-loop containing nucleoside triphosphate hydrolase | 1.57 | |
|
Cluster 4 |
Repressor |
1.96 |
| Regulation of synaptic growth at neuromuscular junction | 1.33 | |
|
Cluster 5 |
Helicase |
3.48 |
|
Cluster 6 |
P-loop containing nucleoside triphosphate hydrolase |
1.57 |
|
Cluster 7 |
Zinc-finger |
1.72 |
| Zinc | 1.46 | |
|
Cluster 8 |
Chromatin regulator |
2.31 |
Table 2b.
Enrichment scores for the significantly downregulated genes in mated female fly heads exposed to a HFD using DAVID annotation analysis (Shown due to enrichment scores of ≥1.3; P-value of 0.05 without minus log scale)
| Annotation Terms for Downregulated Genes | Enrichment Score |
|---|---|
| Phosphoprotein | 6.09 |
| Nucleus | 4.92 |
| Positive regulation of translation | 4.74 |
| Cell cycle | 3.89 |
| Neurogenesis | 3.77 |
| RNA binding | 3.64 |
| Regulation of chromatin binding | 3.62 |
| Helicase | 3.48 |
| Protein binding | 3.48 |
| Cyclin, N-terminal | 3.42 |
| Cyclin | 3.40 |
| CYCLIN | 3.29 |
| Oogenesis | 3.24 |
| Cyclin-like | 2.96 |
| Microtubule associated complex | 2.77 |
| Mitosis | 2.66 |
| SM01332 | 2.62 |
| Mitotic sister chromatid segregation | 2.60 |
| Cyclin, C-terminal domain | 2.60 |
| Ribosome biogenesis | 2.48 |
| Nucleolus | 2.33 |
| Chromatin regulator | 2.31 |
| Nucleus | 2.20 |
| Cyclin-dependent protein serine kinase regulator activity | 2.19 |
| Germ cell development | 2.19 |
| Cell division | 2.12 |
| Chaperone-mediated protein folding | 2.10 |
| Syncytial blastoderm mitotic cell cycle | 2.00 |
| Cytoplasm | 2.00 |
| Repressor | 1.96 |
| DNA replication | 1.96 |
| DNA binding | 1.82 |
| DNA replication initiation | 1.77 |
| Zinc-finger | 1.72 |
| Intracellular mRNA localization | 1.70 |
| Nucleic acid binding | 1.68 |
| Mitotic cytokinesis | 1.68 |
| RNA-binding | 1.68 |
| DNA recombination | 1.60 |
| P-loop containing nucleoside triphosphate hydrolase | 1.57 |
| Covalent chromatin modification | 1.55 |
| Regulation of translation | 1.52 |
| Chromosome condensation | 1.52 |
| Zinc | 1.46 |
| OST-HTH/LOTUS domain | 1.42 |
| Transferase activity | 1.37 |
| Translation regulator activity | 1.33 |
| Regulation of synaptic growth at neuromuscular junction | 1.33 |
| Nucleoplasm | 1.30 |
Table 3a.
Select HFD-upregulated genes, highlighted for relevance in neurobehavioral decline, obesity, or energy homeostasis by category in w1118 mated female flies (p≤0.05; fold change greater than 1.5 as indicated by the microarray)
| OlfactionGene Ontology | |
|---|---|
| OS9 | Sensory perception of smell |
| Obp56f | Sensory perception of chemical stimulus |
| Obp69a | Sensory perception of chemical stimulus |
| Obp56a | Sensory perception of chemical stimulus // response to pheromone |
| Orco | G-protein coupled receptor signaling pathway//sensory perception of smell |
| Obp56e | Sensory perception of chemical stimulus |
| Oxidative Stress | |
| Cyp4e3 | Oxidation-reduction process |
| Cyp6a21 | Oxidation-reduction process |
| Cyp4e1 | Oxidation-reduction process |
| l(2)efl | Protein lipidation // response to oxidative stress |
| antdh | Oxidation-reduction process |
| Metabolism | |
| Pepck | Gluconeogenesis |
| Gfat1 | Carbohydrate metabolic process |
| Neural | |
| Cap | Synapse asembly // neurotransmitter secretion |
| Gogo | Axon guidance // photoreceptor cell axon guidance |
Table 3b.
Select HFD-upregulated genes, highlighted for relevance in neurobehavioral decline, obesity, or energy homeostasis by category in w1118 mated female flies (p≤0.05; fold change greater than 1.5 as indicated by the microarray).
| LocomotionGene Ontology | |
|---|---|
| tyf | Defense response to bacterium // locomotor rhythm |
| lola | Startle response// locomotion involved in locomotory behavior |
| Fsn | Neuromuscular synaptic transmission |
| Memory | |
| osk | Long-term memory // visual learning |
| lat | Learning or memory |
| pum | Long-term memory |
| tun | Learning or memory |
| orb | Learning or memory |
| Oxidative stress | |
| dhd | Glycerol ether metabolic process // oxidation-reduction process |
| RnrS | DNA replication // oxidation-reduction process |
| TotC | Response to oxidative stress |
| Hsp27 | Oxidation-reduction process |
| Hsp26 | Oxidation-reduction process |
| Meatabolism | |
| Dob | Triglyceride Lipase |
| Neural | |
| CycA | Neurogenesis |
| Brat | Neurogenesis |
| RpS5b | Neurogenesis |
| Mitosis/DNA Repair | |
| CycB | Regulation of chromatin binding |
| stg | G2/M transition of mitotic cell cycle |
| pim | Mitotic sister chromatid segregation |
| Mcm7 | DNA replication |
| Top1 | DNA replication |
| Orc4 | DNA replication |
Quantitative real-time PCR validation of Microarray
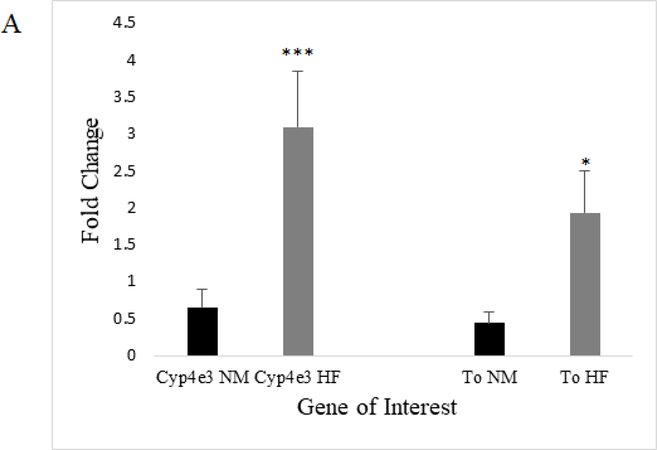
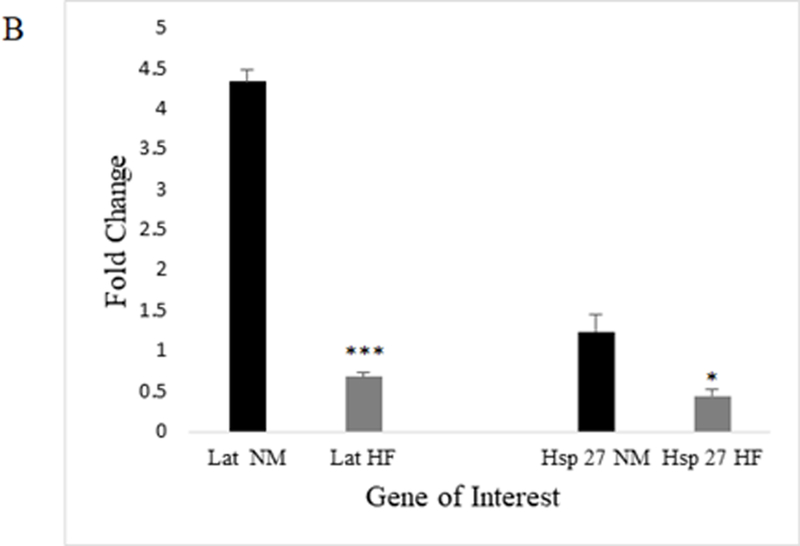
Validation of microarray results was carried out by extracting independent samples of RNA from mated female fly heads that were exposed to experimental diets (ND vs HFD) for 7 days and performing qRT-PCR. We randomly chose 2 upregulated and 2 downregulated genes that had a fold change of 1.5 or greater, were statistically significant (p≤0.05), and were physiologically relevant to our experimental interest. Two upregulated genes Cytochrome P450–4e3 (Cyp4e3) and takeout (To), and two downregulated genes Origin Recognition Complex Subunit 3 (Orc3, also known as and henceforth called Latheo, lat) and Heat shock protein 27 (Hsp27) were chosen (Figure 5a-b). Appropriate directionality and significance of expression change was confirmed for all four genes.
Fig. 5a.
Each figure depicts fold-change in mRNA level including normalization to a housekeeping gene RpL32. There was a significant difference in fold change among HFD-upregulated genes Cyp4e3 and To in mated female flies (N=4 whole head RNA extractions per diet; unpaired t-test; p≤0.005 (***), p≤0.05 (*); error bars represent 95% confidence intervals)
Fig. 5b.
Each figure depicts fold-change in mRNA level including normalization to a housekeeping gene RpL32. There was a significant difference in fold change among HFD-downregulated genes lat and Hsp27 in mated female flies (N=4 whole head RNA extractions per diet; unpaired t-test; p≤0.005 (***), p≤0.05 (*); error bars represent 95% confidence intervals)
DISCUSSION
In this work, we have identified a potential association between diet-induced obesity and nervous decline in mated female Drosophila melanogaster by noting patterns of transcriptional changes in the fly head and confirming behavioral phenotypes which are usually associated with neural impairment. Mated female flies fed our HFD exhibit obesity as early as 1 week of exposure, as well as several phenotypes previously associated with neurodegenerative diseases and rodent neural decline, such as impaired short term memory (as assayed by APS), a decrease in climbing ability, and a trend toward reduced olfactory ability. Microarray analysis revealed changes in the expression of several genes involved in memory, locomotion, olfaction, cell cycle, metabolism, neural function and oxidative stress.
Locomotor decline in flies on a HFD
Locomotion is a physiological process controlled in Drosophila and mammals by the central nervous system via motor neurons. Previous studies with mice have utilized locomotive impairment as a phenotypic indicator of Parkinson’s disease (Aoyama et al. 2000). Negative geotaxis, which gauges the fly’s ability to move against gravity, is a method of assaying activity and locomotion in the fly and has been used as an indicator of Alzheimer’s and Parkinson’s Disease in Drosophila models (Feany and Bender 2001; Ali et al. 2011). In our study, we find that HFD reduces climbing performance after 7 days of exposure for mated female flies, but this climbing performance declines even further between 7 and 14 days of exposure (Figure 1c). Despite this further reduction in climbing ability between 7 and 14 days, we do not see corresponding changes in the triglyceride content of the mated female flies on HFD. In fact, after increasing in triglyceride content at 7 days of exposure to HFD, mated female flies show no change from that elevated content level at 14 and 21 days of exposure (Figure 1b).
Our results may point to a detrimental interaction between aging and obesity in mated female Drosophila. Aged wild-type flies have previously shown decreased climbing ability, and indeed we see a decline in climbing ability on ND between 7 and 14 days of exposure presently (Figure 1c). Because our adult flies were collected over 5–7 days, mated for 2 days, and incubated on HFD for 14 days before the climbing and triglyceride assays at that time point, the decline we observed relative to 7 days exposure to ND is consistent with another report showing age-related climbing decline at 4 weeks of adult age (Rhodenizer et al. 2008). In humans, aging is known to reduce locomotive ability, interaction between aging and obesity in generating locomotive changes is also observed, and pathogenic processes such as inflammation may have differing impacts in muscle by age (Erskine et al., 2017; Laufer, 2005; Maktouf et al., 2018; Rhodenizer et al., 2008; Vincent et al., 2009). Our results may indicate the existence of a similar detrimental interaction between aging and obesity in flies, which will require additional investigation to further define. These findings also imply that the amount of stored triglyceride is not a direct predictor of obesity-induced disease severity, particularly with age, in mated female Drosophila. The connection between triglyceride content alone and physiological detriment is not fully realized in flies. In Drosophila larvae, it was the prevention of the building of triglyceride stores, rather than the building of triglyceride stores, that was detrimental in a high sugar diet capable of inducing obesity (Musselman et al., 2013). Yet, certain pathogenic processes in fly obesity likely require an excess of dietary fat, such as macrophage scavenging of lipids during HFD and their responsive secretion of Unpaired 3 (Upd3). In adult flies Upd3 activates Hopscotch/Stat92E signaling, through this reducing lifespan on a HFD in part via insulin-like peptide resistance, and loss of Upd3 is sufficient to extend lifespan on a HFD (Woodcock et al., 2015). Should the decreasing climbing ability seen during HFD be nervous in origin in mated female flies, this could mean that pathogenic processes in the nervous system are somewhat cumulative from obesity onset (an onset signaled by the initially increased triglyceride content relative to ND at 7 days) and need not be accompanied by further increases in triglyceride to enhance pathology with age.
There is also a potential influence of mated status and oogenesis in our diet exposure studies, though any such potential influence did not seem to differ by diet. Both ND and HFD-exposed mated w1118 females showed decline in negative geotaxis between 7 and 14 days, with a lack of decline in negative geotaxis performance between 14 and 21 days, and significantly reduced negative geotaxis for HFD flies relative to ND flies at all diet exposure times (Figure 1c). Despite this, a trade-off between reproduction and lifespan has been well studied in Drosophila, and mating with subsequent oogenesis can induce profound physiological changes in females, including increased feeding after mating, increased triglyceride storage, altered locomotive patterns, and reduced longevity in laboratory strains (Barnes et al., 2008; Chapman et al., 1993; Isaac et al., 2010; Rush et al. 2007). It is possible that these impacts of mating and post-mating accompany aging as a source of decline in negative geotaxis when comparing 7 and 14 days diet exposure (Figure 1c). It is also possible that by 21 days of diet exposure, the single mating event that fertilized the female flies in our study is no longer sufficient for them to continue generating the same quantity of fertilized eggs. It is known that fecundity declines with age in mated female Drosophila and sperm can be effectively stored for fertilization on the scale of weeks, but not indefinitely (Bloch Qazi et al., 2003; Miller et al., 2014). Although speculative and requiring further investigation, decline in fecundity could factor into the lack of further decline in negative geotaxis performance between 14 and 21 days of diet exposure.
While the cause for impaired locomotion with HFD exposure could be a defect of the nervous system, it is important to note that disturbances of locomotor behavior can also be due to muscle or cardiac pathologies (Kalyani et al. 2014). Our microarray analysis of the mated female w1118 fly head, however, identified 3 significantly downregulated genes involved in locomotion during a HFD: F-box synaptic protein (Fsn), longitudinals lacking (lola) and twenty-four (tyf). Fsn has been shown to be a negative regulator of growth in the synaptic terminal of motor neurons in the fly, loss of which results in overgrowth of synaptic termini, with a large increase in the number of synaptic boutons and branches. In turn, Fsn loss-of-function mutants exhibit impaired synaptic function localized to the neuromuscular junction (Sharma et al. 2014; Wu et al. 2007). The transcription factor lola functions during embryo development for gonad development, programmed cell death in ovaries, guidance of CNS axons and Regulator of Axon–Target Interaction for SNb Motor neurons (Bass et al. 2007; Crowner et al. 2002; Madden et al. 1999; Tripathy et al. 2014). Reduced expression of lola has resulted in impaired startle and locomotion responses in flies (Yamamoto and Zwarts 2008). Tyf is a regulator of circadian rhythm in the fly, but has been implicated in locomotive behavior, where overexpression of tyf in lateral neurons of the fly rescues locomotor function in knockout mutants (Lim et al. 2011). Reduced expression of these genes during obesity could contribute to impaired geotaxis via hindered neuromuscular signaling or a range of other neurophysiological mechanisms.
Memory impairment in flies on a HFD
A hallmark of certain neurodegenerative diseases, such as Alzheimer’s disease, is the impairment of short term memory (Grady et al. 1988; Knight et al. 2014). Elevated BMI in human’s also correlates to short term memory impairment (Elias et al. 2005; Jeong et al. 2005; Hassing et al. 2010). We showed that HFD-induced obese mated female flies from two wild-type strains can learn to avoid a positive stimulus through aversion (aversion to light via quinine), but have significantly decreased ability to remember avoidance of light 6 hours later (at PC6). In Drosophila, studies of appropriate response to entrained memory has led to the observation of gradual decay of performance with time, the extent of which varies by experimental conditions and genetic background (Marguiles et al., 2005; Tully and Quinn, 1985; Perisse et al. 2007). Our results seem to indicate a more rapid loss of entrained memory for HFD exposed mated female flies relative to ND exposed mated female flies, given the lack of significant difference in pass rate between diet groups at PC3, but the appearance of a difference at PC6. However, it is important to note that there are many methods of assaying memory in Drosophila, and our conclusion here is based solely on the assay we deployed, in mated females, and in two genetic backgrounds (Brigui et al. 1990). Our results should warrant further exploration of the impact of obesogenic diets of multiple types on Drosophila memory via alternate methods, ideally those that do not require a locomotive response from the fly. While we do not believe HFD’s depression of locomotion to have played a role in our results (PC0 flies showed no significant difference when comparing ND to HFD, which required similar locomotive performance in the T-maze), broader and definitive claims about an impact on memory require further investigation. We believe it is also important to further explore the influence of sex, mating status, and genetic background on the impact of HFD in memory in Drosophila.
Our findings do correlate with other studies that have similar memory impairment phenotypes with exposure to a HFD. A rodent study showed that consumption of a high fat diet for 3 months caused obesity, insulin resistance, and poor performance in the operant based delayed matching to position task, which assesses spatial working memory in mice and examines short-term information retention and executive function (McNeilly et al. 2011). In humans, a cross-sectional longitudinal study of over 2000 middle aged workers supported the negative linear association between BMI and cognitive function determined by the word-list learning test, which evaluates verbal learning and memory, and Digit-symbol Substitution test (DSST), which assesses attention, response speed, and visuo-motor coordination (Cournot et al. 2006). Impairment of specific cognitive domains such as executive function and short-term memory have been consistently identified in obese individuals when compared to normal weight counterparts (Greenwood and Winocur 2005; Kalmijn et al. 2004; Mond et al. 2007). This being said, the mechanism underlying impairment in cognitive function in rats and humans ingesting a HFD remains elusive.
Our microarray of the mated female fly head during HFD exposure revealed the downregulation of several protein-encoding genes implicated in memory and learning; such as oskar (osk), lat, pumilio (pum), oo18 RNA-binding protein (orb) and tungus (tun). In Drosophila, it has been shown that disruption of lat by mutagenesis results in impaired memory (Boynton and Tully 1992). The genes pum, tun, orb and osk have been associated with learning in Drosophila, as shown by a combination of altered expression on a microarray during a t-maze shock learning assay and subsequent confirmatory mutant and transposon mutagenesis screening (Dubnau et al 2003; Seugnet et al 2009). Reduced expression in these genes could be an indicator of obesity-induced short term memory and possibly long term memory impairment in mated female w1118 Drosophila.
Olfactory seeking in flies on a HFD
Olfactory dysfunction has been known to manifest in neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease in both human and rodent models (Doty 2005). Due to this connection, testing whether flies exposed to an HFD also suffer from impaired olfaction could be a strong indicator of obesity induced neural decline. Rodent models of AD that exhibited amyloid β (Aβ) deposition also exhibited onset olfactory impairment (Wesson et al. 2010). Other studies show a preservation of olfaction ability in neurodegenerative models, such as multiple system atrophy (Krismer et al. 2013). In our olfactory trap assays, we were unable to show a significant difference in attraction index between mated female w1118 flies exposed to ND and those exposed to HFD (Figure 3). However, a trend of reduced attraction index with HFD was observed. Here, we extend the same caution against broad conclusions, in that there are multiple methods of assaying olfactory ability in Drosophila, and there are the influences of sex, mating status, and genetic background to yet investigate. Our microarray data indicated 6 significantly upregulated genes involved in olfaction: Odorant-binding protein 56a, 56f, 69a (Obp), Olfactory-specific 9 (OS9) and Odorant receptor co-receptor (Orco). Gene ontology analysis also showed odorant, pheromone and chemosensory as having high annotation enrichment scores for significant upregulated genes. There are a large number of Obp peptides in Drosophila, and studies show that it takes the suppression of several of these to affect odorant specific olfaction (Swarup et al. 2011). We are unsure as to the reason for the overexpression of olfactory genes, since one would expect a down-regulation of these genes during nervous decline caused by high fat diet. To speculate, the overexpression of these olfactory-related genes may be a compensation mechanism to promote the seeking out of alternative nutrient sources. However, olfactory sensing is a complex process in flies. For example, phosphorylation of Orco appears to be a key factor in its function in olfaction, rather than expression level per se (Guo et al. 2017).
Gene expression analysis of the fly head as a result of a HFD
Although behavioral studies in the fly can provide insight into obesity-induced neurobehavioral changes, a transcriptional profile can provide general evidence of central nervous and energy homeostasis impairment. We were able to detect several genes that changed in expression in w1118 mated female fly heads upon HFD exposure whose ontologies suggest a neural function. These include upregulated genes capricious (caps, cell adhesion and axon guidance; Abrell and Jäckle, 2001; Shinza-Kameda et al. 2006), golden goal (gogo, axon guidance; Tomasi et al. 2008), and downregulated genes involved in neurogenesis, such as Cyclin A (CycA; Lear et al. 1999) and brain tumor (brat; Arbeille and Bashaw 2018) amongst others (Online Resource 5). Because of our interest in the connection between obesity and nervous or neurobehavioral function, we were most intrigued by expression changes in genes affecting memory, olfactory, behavior, or neuromuscular function.
However, there were other interesting and physiologically relevant genes experiencing expression change in the microarray of w1118 mated female fly heads. The most significant HFD-upregulated with greatest fold change (4.43) was CG2837, about which little is known at present. Among the HFD-upregulated genes, Cyp4e3 had one of the greatest fold changes. Cyp4e3 is part of the cytochrome P450s monooxygenases enzymatic superfamily, which is expressed during removal of H2O2 and lipid peroxidation byproducts that can cause increased oxidative stress in the endoplasmic reticulum (Terhzaz et al. 2015). DAVID gene enrichment analysis of significant upregulated genes also shows Cytochrome P450’s and Monooxygenase as having high enrichment scores (1.55, 1.54 respectively). It has been shown that ER stress is increased in obesity (Boden et al. 2008), which could in turn suggest a reason for the overexpression of Cyp4e3, as well as other genes involved in oxidative stress to be mentioned.
Another HFD-upregulated gene was takeout (to). The takeout protein is preferentially expressed in male heads and is similar to small carriers of lipophiles from both insects and mammals, particularly the Juvenile Hormone–binding proteins from other insects (Dauwalder et al. 2002). Takeout function has been shown in Drosophila to be expressed in response to a starvation state and is associated with circadian rhythm, increased locomotive behavior in response to starvation, proper nutrient-responsive feeding behavior, and memory (Meunier et al. 2007; Sarov-Blat et al. 2000). A recent study however, showed that after 24 hours of starvation, to was significantly downregulated in the head of flies (Farhadian et al. 2012). Takeout’s effects are indirectly mediated through its regulatory interaction with juvenile hormone, which in turn is known to mediate fat body development, impact insulin-like peptide release and response, and reduce adult lifespan. Moreover, to overexpression leads to a reduction of JH signaling activity, as genes negatively regulated by JH are disinhibited (Chamseddin et al. 2013). This data correlates with the upregulation of Juvenile hormone esterase duplication (Jhedup) in the fly head in our microarray, whose known function includes the hydrolysis of JH (Crone et al. 2007). To’s function and effects in the context of fly obesity still remain unknown. Further work is needed to better understand the role to and JH may play in the context of fly obesity states.
Several metabolically relevant genes saw expression change upon HFD exposure, including many involved in nucleotide metabolism (Online Resource 5). The upregulated metabolic gene, Glutamine:fructose-6-phosphate aminotransferase 1 (Gfat1) controls the flux of glucose into the hexosamine pathway. The hexosamine pathway has been associated with decreased lifespan and the occurrence of heart dysfunction in flies exposed to an obesogenic diet (as caused by high sugar content) when upregulated. Reduction of hexosamine flux ameliorated both the decreased longevity and heart dysfunction in the high sugar obesity (Na et al. 2013). Although in previous studies suppression of Gfat1 was localized to skeletal muscle, increased expression in the fly head may contribute to pathology largely through altered glucose exposure, insulin resistance, and aberrant glycosylation patterns (Niimi et al. 2001; Patti et al. 1999). Another interesting upregulated gene that we found was Phosphoenolpyruvate carboxykinase 2 (Pepck2), which is the mitochondrial form of a central enzyme in gluconeogenesis (Stark and Guebre-Egziabher 2014). Obesity and type 2 diabetes are known to be associated with an increase in gluconeogenesis as a result of deregulated catabolism and insulin resistance (Magnusson et al. 1992). Interestingly, downregulated gene doppelganger von brummer (dob) is a paralogue to Drosophila TAG lipase brummer (bmm), which is functionally similar to mammalian adipocyte triglyceride lipase (ATGL). In flies, bmm is involved in energy homeostasis through the release of free fatty acids from the fat body. The loss of bmm in flies has resulted in increased fat storage (Grönke et al. 2005; Grönke et al. 2007). The HFD-downregulation of dob, based on the known functions of bmm, is not unexpected given their exposure to a lipid and calorie-rich diet, and the presence of the pericerebral fat body in the fly head. However, its combination with upregulation of Pepck2 and to may be suggestive of a wider pattern of energy homeostasis deregulation.
The majority of significantly downregulated genes during a HFD, both through manual annotation and DAVID gene enrichment analysis, were involved in mitotic functions and DNA replication, which may suggest a protective response in head tissues of HFD-fed mated female w1118 flies induced either by increased oxidative stress or neuronal substrate depletion. Other data shows that downregulation of mitotic genes is correlated with advanced age (Ly et al. 2000). Their downregulation is also implicated in potential early onset familial Alzheimer’s disease through mitotic spindle abnormalities, and sensitivity to reactive oxygen species, where an early response of mitotic cells to oxidative stress is to enter a transient growth-arrested state in which DNA replication is halted (Davies 1999; Fornace et al. 1989; Migliore et al. 2007).
Several significant HFD-downregulated genes were associated with oxidative stress response or reduction such as deadhead (dhd), Turandot C (TotC) and Heat shock protein 26/27 (Hsp). Other downregulated genes with putative redox function as per ontology were also identified, such as Ribonucleoside diphosphate reductase small subunit (RnrS), CG3699 and CG10962, the downregulation of which could be contributing to pathology due to high oxidative stress associated with obesity (Shigeteda et al. 2004). In flies, neuronal overexpression of dhd, a well-known antioxidant homologous to human Thioredoxin-1, was shown to increase lifespan of flies exposed to H2O2 (Floen et al. 2014). Although little is known about TotC function in the adult fly head, all Tot genes are induced under stressful conditions such as bacterial infection, heat shock, paraquat feeding or exposure to ultraviolet light, suggesting that all members of this family play a role in Drosophila stress tolerance (Ekengren and Hultmark 2001). The downregulation of Heat shock protein 27 (Hsp27) in the fly head during HFD was validated through qRT-PCR. Previous studies have shown the neuronal upregulation of Hsp27 in Drosophila leads to an increased lifespan and reduction of oxidative stress (Liao et al. 2008). Interestingly, overexpression of Hsp27 in rodent models of Alzheimer’s Disease resulted in an increase of spatial learning and a significant decrease in Amyloid plaques (Tóth and Szegedi, 2013). This could suggest a mild level of HFD-induced neural impairment as a result of decreased expression of Hsp27 in the mated female w1118 fly head.
Comparison with other gene expression studies of the HFD model
An RNA-Sequencing (RNA-Seq) study of mated female fly heads on a HFD performed by our laboratory, utilizing a different genetic background (Oregon-R-C) and a diet formula with less agar, cornmeal, yeast, and sucrose content showed both similarities and differences in transcriptomic profile relative to the gene ontology enrichments we describe here (Hemphill et al. 2018). There was a prominent enrichment for immune-related functions among HFD-upregulated genes in the RNA-Seq, and presently we find enrichment for biosynthesis of antibiotics (Table 1b). We also presently find isolated expression changes in immune-relevant genes, such as the upregulation of listericin (Online Resource 5; Goto et al. 2010; Hemphill et al. 2018). Likewise, we presently find a functional enrichment among HFD-upregulated genes for odorant binding and sensory perception of smell (Table 1a and 1b). While this enrichment does not exist in the RNA-Seq study, upregulation of the individual olfactory genes Obp56a and Obp56f was confirmed in that study. Among other individual genes showing consistent upregulation between the present study and the RNA-Seq study were to, CG8654, CG5966, and CG3036. Meanwhile, while consistent downregulation between the studies was less frequent, the clearest example was CG3699. Also in the RNA-Seq study, there was upregulation of Hsp26 and Hsp27 during HFD exposure in mated female fly heads, whereas presently we find a downregulation. Still other differentially expressed genes that were in common between the two studies showed opposite direction of effect, including CycA/CycB, dhd, osk, and RnrS (which were downregulated here and upregulated in the RNA-Seq). Noteworthy is that the HFD formula used for the present study exhibited a slowness to lethality relative to the ND for mated female flies (Figure 1, differentiation in mortality rate began after 30 days exposure) relative to the HFD formula utilized in the RNA-Seq study, which consistently caused significant mortality within 7 days and was temperature sensitive in our hands, to the extent it was difficult to sustain large cohorts on the diet (Hemphill et al., 2018).
In another RNA-Seq study of antennal segments from male Canton-S flies exposed to a HFD based on palmitic acid supplementation, the most striking point of comparison with our data is the number of olfactory-related genes that changed in expression. Both studies report upregulation of Obp56a, Obp56f, and Obp69a, while we report opposite directions of expression change for OS9 and Orco (upregulation presently and downregulation in the antennal study). Moreover, this antennal HFD study reports functional enrichment among differentially expressed genes for general immune-related functions, neurological functions (including cognition and general neurological processes), odorant binding, sensory perception, extracellular functions, ribosomal functions, and nucleotide synthesis and handling (Jung et al., 2018). In our results, we report a similar pattern of enrichment among differentially expressed genes, including implications in synthesis of antibiotics, synaptic growth, sensory perception of smell, extracellular function, ribosomal synthesis and regulation, and various interactions with chromatin. Likewise, we point to the HFD-induced differential expression of genes related to cognition, including those involved in learning and memory, in our present results. Finally, in another microarray study of whole w1118 females subjected to a 20% w/v coconut oil-based HFD with a commercially available pre-mixed Drosophila medium as a base, the authors note functional enrichment among differentially expressed genes for immune-related and extracellular functions. A review of differentially expressed genes showed several in common with the present data, including shared downregulation of TotC (stress response), shared upregulation of ImpE1 (Ecdysone-inducible gene E1), and shared upregulation of l(2)efl (involved in oxidative stress). There were also several olfactory-relevant genes increasing in expression in this study, including Obp56d, Obp56a, and Obp49a (Heinrichson et al 2014).
As transcriptomic studies only illustrate gene expression at a moment in time, we believe it would be premature for opposing direction of effect, lack of replication of a specific gene’s differential expression, or lack of replication of a specific functional enrichment category to disqualify the relevance of any given study for this model of obesity, as it may suggest the genes in question are under dynamic regulation (such as when the fly is under metabolic-related stress). Most importantly, multiple variables changed between the transcriptional studies (genetic background, sex, tissue of study, transcriptomic methods, diet formula), making it impossible to say any one variable accounts for the differences observed between the expression profiles. We feel that this underscores a need for standardization of the HFD model across laboratories, or alternatively, a need to contextualize findings involving the HFD model in light of the potential impact of inter-study variability.
Overall, these studies illustrate that a HFD results in changes in the expression of genes involved in stress response, immunity, translation, and DNA interaction, including in the head and antennae of mated female w1118 flies. More surprising, however, is the recurrent change in gene expression for genes involved in sensory perception of smell (particularly Obp56a), as well as other neurological functions, across transcriptomic studies. In Drosophila, an RNAi screen of olfactory binding proteins revealed that knockdown of Obp56a in female flies reduced their attraction to the odorant d-carvone relative to control (Swarup et al., 2011). d-Carvone is a major component of several essential plant oils, including that of Lippia alba, and it has shown neuroactive properties with an ability to immobilize adult flies and reduce larval excitatory post synaptic potential amplitude in neuromuscular junctions (da Silva et al., 2018). Intriguingly, 8-week peritoneal injection of S-carvone in mice seemed to ablate the effects of a HFD feeding regimen, resulting in lower expression of inflammatory genes in white adipose, reduced weight gain, reduction in insulin resistance, and reduction of hepatic fat accumulation (Alsanea and Liu, 2017). Therefore, an interesting question is whether there are physiological implications in the context of HFD that go beyond sensory perception of smell due to the differential expression of these olfactory genes in Drosophila.
CONCLUDING REMARKS
The functional and physiological character of many of these categories of differentially expressed genes have implications in both obese and neurodegenerative pathologies, which when coupled with our phenotypic data regarding memory and locomotive impairment in the context of age, leads us to support a correlation between obesity and neural decline in the mated female fly. We believe this is suggestive that Drosophila melanogaster is a feasible model for the further study of obesity-induced or diet-induced neural and sensory decline. Also, importantly, we believe that this is suggestive that this aspect of obesity pathology deserves further investigation in mammals.
Supplementary Material
ACKNOWLEDGMENTS
This work was made possible via funding through the Pilot Funding for New Research grant mechanism (Contract 387), a program of the Louisiana Board of Regents in partnership with the National Science Foundation’s Experimental Program to Stimulate Competitive Research (LA-EPSCoR). Research reported in this publication was also supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 5 P20 GM103424–15 and 3 P20 GM103424–15S1. Funds were allocated through a Louisiana Biomedical Research Network Summer Research Project and Pilot Grant.
REFERENCES
- 1.Ali YO, Escala W, Ruan K, Zhai RG (2011) Assaying Locomotor, Learning, and Memory Deficits in Drosophila Models of Neurodegeneration. J. Vis. Exp (49), e2504. doi: 10.3791/2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsanea S, Liu D. BITC and S-Carvone Restrain High-Fat Diet-Induced Obesity and Ameliorate Hepatic Steatosis and Insulin Resistance. Pharm Res 2017. November;34(11):2241–2249. doi: 10.1007/s11095-017-2230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbeille E, Bashaw GJ. (2018) Brain Tumor promotes axon growth across the midline through interactions with the microtubule stabilizing protein Apc2. PLoS Genet April 4;14(4):e1007314. doi: 10.1371/journal.pgen.1007314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoyama S, Kase H, Borrelli E (2000) Rescue of locomotor impairment in dopamine D2 receptor-deficient mice by an adenosine A2A receptor antagonist. J Neurosci 1;20(15):5848–52. doi: 10908627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes AI, Wigby S, Boone JM, Partridge L, Chapman T. Feeding, fecundity and lifespan in female Drosophila melanogaster. Proc Biol Sci 2008. July 22;275(1643):1675–83. doi: 10.1098/rspb.2008.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bass P, Cullen K, McCall K (2007). The axon guidance gene lola is required for programmed cell death in the Drosophila ovary. Dev Biol 304: 771–785. doi: 10.1016/j.ydbio.2007.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becher G, Flick G, et al. (2012) Yeast, not fruit volatiles mediate Drosophila melanogasterattraction, oviposition and development. Funct Ecol, 26: 822–828. doi: 10.1111/j.1365-2435.2012.02006.x [DOI] [Google Scholar]
- 8.Birse T, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, Ocorr K, Bodmer R, Oldham S (2010) High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab 12: 533–44. doi: 10.1016/j.cmet.2010.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloch Qazi MC, Heifetz Y, Wolfner MF. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Dev Biol 2003. April 15;256(2):195–211. [DOI] [PubMed] [Google Scholar]
- 10.Boden G, Duan X, Homko C, Molina J, Song W, Perez O, Cheung P, Merali S (2008) Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese insulin-resistant individuals. Diabetes 57:2438–444. doi: 10.2337/db08-0604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouleau S, Tricoire H (2015) Drosophila models of Alzheimer’s disease: advances, limits, and perspectives. J Alzheimers Dis 45(4):1015–38. doi: 10.3233/JAD-142802. [DOI] [PubMed] [Google Scholar]
- 12.Boynton S, & Tully T (1992) Latheo, a New Gene Involved in Associative Learning and Memory in Drosophila Melanogaster, Identified from P Element Mutagenesis. Genetics, 132(1), 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brigui N, Le Bourg E, Médioni J. (1990) Conditioned suppression of the proboscis-extension response in young, middle-aged, and old Drosophila melanogaster flies: acquisition and extinction. J Comp Psychol 104(3):289–96. [DOI] [PubMed] [Google Scholar]
- 14.Carr B, Utzschneider M, Hull L, Kodama K, Retzlaff M, Brunzell D, Shofer B, Fish E, Knopp H, Kahn E (2004) Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 53:2087–094. doi: 10.2337/diabetes.53.8.2087 [DOI] [PubMed] [Google Scholar]
- 15.Carvalho GB, Kapahi P, Anderson DJ, Benzer S (2006) Allocrine modulation of feeding behavior by the sex peptide of Drosophila. Curr. Biol 16, 692–69610.1016/j.cub.2006.02.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control (2017) Prevalence of Obesity Among Adults and Youth: United States, 2015–2016 https://www.cdc.gov/obesity/data/adult.html. Accessed 18 November 2018
- 17.Chamseddin K, Khan S, et al. (2013) takeout-dependent longevity is associated with altered Juvenile Hormone signaling. Mech Ageing Dev 133(11–12): 637–646. doi: 10.1016/j.mad.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman T, Hutchings J, Partridge L. No reduction in the cost of mating for Drosophila melanogaster females mating with spermless males. Proc Biol Sci 1993. September 22;253(1338):211–7. [DOI] [PubMed] [Google Scholar]
- 19.Cournot M, Marquie C, Ansiau D, Martinaud C, Fonds H, Ferrieres J, Ruidavets B (2006) Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology 67:1208–214. doi: 10.1212/01.wnl.0000238082.13860.50 [DOI] [PubMed] [Google Scholar]
- 20.Crone E, Sutherland T, et al. l (2007) Only one esterase of Drosophila melanogaster is likely to degrade juvenile hormone in vivo 37(6): 540–549. doi: 10.1016/j.ibmb.2007.02.010 [DOI] [PubMed] [Google Scholar]
- 21.Crowner D, Madden K, Goeke S, Giniger E (2002). Lola regulates midline crossing of CNS axons in Drosophila. Development 129: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 22.da Silva LVF, Veras Mourão RH, Manimala J, Lnenicka GA. The essential oil of Lippia alba and its components affect Drosophila behavior and synaptic physiology. J Exp Biol 2018. July 26;221(Pt 14). doi: 10.1242/jeb.176909. [DOI] [PubMed] [Google Scholar]
- 23.Dauwalder B, Tsujimoto S, Moss J, Mattox W (2002) The Drosophila takeout gene is regulated by the somatic sex-determination pathway and affects male courtship behavior. Genes Dev 16: 2879–2892. doi: 10.1101/gad.1010302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies A (1999) The broad spectrum of responses to oxidants in proliferating cells: A new padigm for oxidative stress. IUBMB Life 48:41–47. doi: 10.1080/713803463. [DOI] [PubMed] [Google Scholar]
- 25.Ditzen M, Pellegrino M, Vosshall LB (2008) Insect odorant receptors are molecular targets of the insect repellent DEET. Science 319(5871):1838–42. doi: 10.1126/science.1153121 [DOI] [PubMed] [Google Scholar]
- 26.Doty RL (2005) Clinical studies of olfaction. Chem Senses 30 (Suppl. 01) i207–i209. doi: 10.1093/chemse/bjh187 [DOI] [PubMed] [Google Scholar]
- 27.Dubnau J, Chiang A, et al. (2003) The staufen/pumilio Pathway Is Involved in Drosophila Long-Term Memory. Current Biology 13 (4): 286–96. doi: 10.1016/S0960-9822(03)00064-2 [DOI] [PubMed] [Google Scholar]
- 28.Duffy B (2002) Gal4 system in Drosophila: a fly geneticist’s swiss army knife. Genesis 34:1–15. doi: 10.1002/gene.10150 [DOI] [PubMed] [Google Scholar]
- 29.Ekengren S, Hultmark D (2001) A Family of Turandot-Related Genes in the Humoral Stress Response of Drosophila 284(4): 998–1003. doi: 10.1006/bbrc.2001.5067. [DOI] [PubMed] [Google Scholar]
- 30.Elias F, Elias K, Sullivan M, Wolf A, D’Agostino B (2005) Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol Aging 1:11–6. doi: 10.1016/j.neurobiolaging.2005.08.019 [DOI] [PubMed] [Google Scholar]
- 31.Erskine RM, Tomlinson DJ, Morse CI, Winwood K, Hampson P, Lord JM, Onambélé GL. The individual and combined effects of obesity- and ageing-induced systemic inflammation on human skeletal muscle properties. Int J Obes (Lond) 2017. January;41(1):102–111. doi: 10.1038/ijo.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farhadian S, Suárez M, Cho C, Pellegrino M, Vosshall L (2012) Post-fasting olfactory, transcriptional, and feeding responses in Drosophila. Physiol Behav 105(2): 544–553. doi: 10.1016/j.physbeh.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feany M, Bender W (2000) A Drosophila model of Parkinson’s disease. Nature 404, 394–398. doi: 10.1038/35006074 [DOI] [PubMed] [Google Scholar]
- 34.Floen M, Forred B, Bloom E, Vitiello P (2014) Thioredoxin-1 Redox Signaling Regulates Cell Survival in Response to Hyperoxia. Free Radic Biol Med 75:167–177. doi: 10.1016/j.freeradbiomed.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fornace J, Nebert D, et al. (1989) Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol. Cell. Biol 9: 4196–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gargano JW, Martin I, Bhandari P, Grotewiel MS (2005) Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol 40:386–395. doi: 10.1016/j.exger.2005.02.005 [DOI] [PubMed] [Google Scholar]
- 37.Goto A, Yano T, Terashima J, Iwashita S, Oshima Y, Kurata S. (2010) Cooperative regulation of the induction of the novel antibacterial Listericin by peptidoglycan recognition protein LE and the JAK-STAT pathway. J Biol Chem May 21;285(21):15731–8. doi: 10.1074/jbc.M109.082115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grady CL, Haxby V, Horwitz B, et al. (1988) Longitudinal study of the early neuropsychological and cerebral metabolic changes in dementia of the Alzheimer type. J Clin Exp Neuropsychol 10: 576–96. doi: 10.1080/01688638808402796 [DOI] [PubMed] [Google Scholar]
- 39.Greenwood E, Winocur G (2005) High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging 26:42–5. doi: 10.1016/j.neurobiolaging.2005.08.017 [DOI] [PubMed] [Google Scholar]
- 40.Grönke S, Mildner A, et al. (2005) Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila 2(5):323–330. doi: 10.1016/j.cmet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Grönke S, Müller G, Hirsch J, Fellert S, Andreou A, Haase T, Jäckle H, Kühnlein Dual (2007) lipolytic control of body fat storage and mobilization in Drosophila. RP PLoS Biol 5(6):e137. doi: 10.1371/journal.pbio.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo H, Kunwar K, Smith D. (2017) Odorant Receptor Sensitivity Modulation in Drosophila. J Neurosci September 27;37(39):9465–9473. doi: 10.1523/JNEUROSCI.1573-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Haiyan, Barnes Glenn T., et al. (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112(12): 1821–1830. doi: 10.1172/JCI200319451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassing B, Dahl K, Pedersen L, Johansson B (2010) Overweight in midlife is related to lower cognitive function 30 years later: a prospective study with longitudinal assessments. Dement Geriatr Cogn Disord 29(6):543–52. doi: 10.1159/000314874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hedley A, Ogden L, Johnson L, Carroll D, Curtin R., Flegal M (1999–2002) Prevalence of overweight and obesity among US children, adolescents, and adults. JAMA 291:2847–2850. doi: 10.1001/jama.291.23.2847 [DOI] [PubMed] [Google Scholar]
- 46.Heinrichsen ET, Haddad GG (2012) Role of High-Fat Diet in Stress Response of Drosophila. PLoS ONE 7(8): e42587. doi: 10.1371/journal.pone.0042587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinrichsen E, Zhang H, Robinson J, Ngo J, Diop S, Bodmer R, Joiner W, Metallo C, Haddad H. (2014) Metabolic and transcriptional response to a high-fat diet in Drosophila melanogaster. Mol Metab 3(1): 42–54. doi: 10.1016/j.molmet.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemphill W, Rivera O, Talbert M. (2018) RNA-Sequencing of Drosophila melanogaster Head Tissue on High-Sugar and High-Fat Diets. G3 (Bethesda). January 4;8(1):279–290. doi: 10.1534/g3.117.300397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bickmeyer Hildebrandt A, Kühnlein P (2011) Reliable Drosophila body fat quantification by a coupled colorimetric assay. PLoS One 6(9):e23796. doi: 10.1371/journal.pone.0023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hombría J, Brown S, Häder S, Zeidler M (2005) Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev Biol 2:288: 420–433. doi: 10.1016/j.ydbio.2005.09.040 [DOI] [PubMed] [Google Scholar]
- 51.Huang W, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Research, 37, pp. 1–13. doi: 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang da W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols, 4, pp. 44–57. doi: 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 53.Isaac RE, Li C, Leedale AE, Shirras AD. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc Biol Sci 2010. January 7;277(1678):65–70. doi: 10.1098/rspb.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jensen K, Sanchez-Garcia J (2013) Purification of Transcripts and Metabolites from Drosophila Heads. J Vis Exp (73): 50245. doi: 10.3791/50245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeong K, Nam S, Son H, Son J, Cho H (2005) Interactive effect of obesity indexes on cognition. Dement Geriatr Cogn Disord 19(2–3):91–6. doi: 10.1159/000082659 [DOI] [PubMed] [Google Scholar]
- 56.Jung J, Kim DI, Han GY, Kwon HW. The Effects of High Fat Diet-Induced Stress on Olfactory Sensitivity, Behaviors, and Transcriptional Profiling in Drosophila melanogaster. Int J Mol Sci 2018. September 20;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalmijn S, Boxtel P, Ocke M, Verschuren M, Kromhout D, Launer J (2004) Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology 62:275–80. doi: 10.1212/01.WNL.0000103860.75218.A5 [DOI] [PubMed] [Google Scholar]
- 58.Kalyani R, Corriere M, Ferrucci L (2014) Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2(10): 819–829. doi: 10.1016/S2213-8587(14)70034-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knight M, Martins A, Gümüsgöz S, Allan M, Lawrence B (2014) High-fat diet-induced memory impairment in triple-transgenic Alzheimer’s disease (3xTgAD) mice is independent of changes in amyloid and tau pathology. Neurobiology of Aging, 35(8), 1821–1832. doi: 10.1016/j.neurobiolaging.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krismer F, Wenning G, Li Y, Poewe W, Stefanova N (2013) Intact Olfaction in a Mouse Model of Multiple System Atrophy. PLosone 8(5): e64625. doi: 10.1371/journal.pone.0064625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kühnlein R (2010) Energy homeostasis regulation in Drosophila: a lipocentric perspective. Results Probl Cell Differ 52:159–73. doi: 10.1007/978-3-642-14426-4_13 [DOI] [PubMed] [Google Scholar]
- 62.Larsson M, Domingos A, Jones W, Chiappe E, Amrein H, and Vosshall L (2004) Or83b Encodes a Broadly Expressed Odorant Receptor Essential for Drosophila Olfaction. Neuron 2;43(5):703–14. doi: 10.1016/j.neuron.2004.08.019 [DOI] [PubMed] [Google Scholar]
- 63.Laufer Y Effect of age on characteristics of forward and backward gait at preferred and accelerated walking speed. J Gerontol A Biol Sci Med Sci 2005. May;60(5):627–32. [DOI] [PubMed] [Google Scholar]
- 64.Le Bourg E, Buecher C (2002) Learned suppression of photopositive tendencies in Drosophila melanogaster. Anim Learn Behav 30(4):330–41. doi: 10.3758/BF03195958 [DOI] [PubMed] [Google Scholar]
- 65.Lear BC, Skeath JB, Patel NH. (1999) Neural cell fate in rca1 and cycA mutants: the roles of intrinsic and extrinsic factors in asymmetric division in the Drosophila central nervous system. Mech Dev November;88(2):207–19. [DOI] [PubMed] [Google Scholar]
- 66.Li J, Tang Y, Cai D (2012) IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol 10:999–1012. doi: 10.1038/ncb2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao C, Lin Y, Yuh H, Yu K, Wang D (2008) The effect of neuronal expression of heat shock proteins 26 and 27 on lifespan, neurodegeneration, and apoptosis in Drosophila. Biochem Biophys Res Commun 376(4):637–41. doi: 10.1016/j.bbrc.2008.08.161 [DOI] [PubMed] [Google Scholar]
- 68.Lim C, Lee J, Choi C, Kilman VL, Kim J, Park SM, … Choe J (2011) twenty-four defines a critical translational step in the Drosophila clock. Nature 470(7334), 399–403. doi.org: 10.1038/nature09728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ly D, Lockhart D, Lerner R, Schultz P (2000) Mitotic Misregulation and Human Aging. Science 287(5462): 2486–2492. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- 70.Madden K, Crowner D, Giniger E (1999) lola Has the Properties of a Master Regulator of Axon–Target Interaction for SNb Motor Axons of Drosophila. Dev Biol 2(213): 301–313. doi: 10.1006/dbio.1999.9399 [DOI] [PubMed] [Google Scholar]
- 71.Magnusson I, Rothman D, Katz L, Shulman R, Shulman G (1992) Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest 90(4): 1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maktouf W, Durand S, Boyas S, Pouliquen C, Beaune B. (2018) Combined effects of aging and obesity on postural control, muscle activity and maximal voluntary force of muscles mobilizing ankle joint. J Biomech October 5;79:198–206. doi: 10.1016/j.jbiomech.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 73.Margulies C, Tully T, Dubnau J. Deconstructing memory in Drosophila. Curr Biol 2005. September 6;15(17):R700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McNay C, Ong T, McCrimmon J, Cresswell J, Bogan S, Sherwin S (2010) Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem 93:546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McNeilly AD, Williamson R, Sutherland C, Balfour DJ, Stewart CA. (2011) High fat feeding promotes simultaneous decline in insulin sensitivity and cognitive performance in a delayed matching and non-matching to position task. Behav Brain Res February 2;217(1):134–41. doi: 10.1016/j.bbr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 76.Meunier N, Belgacem Y, Martin J (2007) Regulation of feeding behavior and locomotor activity by takeout in Drosophila. Journal of Experimental Biology 210: 1424–1434; doi: 10.1242/jeb.02755. [DOI] [PubMed] [Google Scholar]
- 77.Migliore L, Testa A, Scarpato R. et al. (1997) Spontaneous and induced aneuploidy in peripheral blood lymphocytes of patients with Alzheimer’s disease. Hum Genet 101:299–305. doi: 10.1007/s004390050632. [DOI] [PubMed] [Google Scholar]
- 78.Miller PB, Obrik-Uloho OT, Phan MH, Medrano CL, Renier JS, Thayer JL, Wiessner G, Bloch Qazi MC. The song of the old mother: reproductive senescence in female drosophila. Fly (Austin) 2014;8(3):127–39. doi: 10.4161/19336934.2014.969144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mond M, Rodgers B, Hay J, Darby A, Owen C, Baune T, Kennedy RL (2007) Obesity and impairment in psychosocial functioning in women: the mediating role of eating disorder features. Obesity 15:2769–779. doi: 10.1038/oby.2007.329. [DOI] [PubMed] [Google Scholar]
- 80.Montague T, Farooqi S, Whitehead P, Soos MA, Rau H, Wareham J (1997) Congenital leptin deficiency is associated with severe early-onset obesity in human. Nature 387: 903–908. doi: 10.1507/endocrj.KR-85 [DOI] [PubMed] [Google Scholar]
- 81.Musselman LP, Fink JL, Ramachandran PV, Patterson BW, Okunade AL, Maier E, Brent MR, Turk J, Baranski TJ (2013) Role of fat body lipogenesis in protection against the effects of caloric overload in Drosophila. J Biol Chem March 22;288(12):8028–42. doi: 10.1074/jbc.M112.371047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Na J, Musselman LP, Pendse J, Baranski TJ, Bodmer R, Ocorr K, Cagan R. (2013) A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genet. 2013;9(1):e1003175. doi: 10.1371/journal.pgen.1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niimi M, Ogawara T, et al. l (2001) Identification of GFAT1-L, a novel splice variant of human glutamine: fructose-6-phosphate amidotransferase (GFAT1) that is expressed abundantly in skeletal muscle. J Hum Genet 46: 566 Doi: 10.1007/s100380170022 [DOI] [PubMed] [Google Scholar]
- 84.Patti E, Virkamäki A, Landaker J, Kahn R, Yki-Järvinen H (1999) Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance of early postreceptor insulin signaling events in skeletal muscle. Diabetes 48(8):1562–71. doi: 10.2337/diabetes.48.8.1562 [DOI] [PubMed] [Google Scholar]
- 85.Perisse E, Portelli G, Le Goas S, Teste E, Le Bourg E. (2007) Further characterization of an aversive learning task in Drosophila melanogaster: intensity of the stimulus, relearning, and use of rutabaga mutants. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 193(11):1139–49. doi: 10.1007/s00359-007-0266-2 [DOI] [PubMed] [Google Scholar]
- 86.Pistell J, Morrison D, Gupta S, Knight G, Keller N, Ingram K, Bruce-Keller J (2010) Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol 26;219(1–2):25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prickett C, Brennan L, Stolwyk R (2015) Examining the relationship between obesity and cognitive function: A systematic literature review. Obes. Res. Clin. Pract 9:93–113. doi: 10.1016/j.orcp.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 88.Rajan A, Perrimon N (2012) Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 28;151(1):123–37. doi: 10.1016/j.cell.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reiter T, Potocki L, Chien S, Gribskov M, Bier E. (2001) A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res 1:1114 –125. doi: 10.1101/gr.169101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rhodenizer D, Martin I, Bhandari P, Pletcher SD, Grotewiel M. (2008) Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp Gerontol August;43(8):739–48. doi: 10.1016/j.exger.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rush B, Sandver S, Bruer J, Roche R, Wells M, Giebultowicz J. Mating increases starvation resistance and decreases oxidative stress resistance in Drosophila melanogaster females. Aging Cell 2007. October;6(5):723–6. [DOI] [PubMed] [Google Scholar]
- 92.Sarov-Blat L, So W, Liu L, Rosbash M (2000) The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behavior. Cell 101:647–656. doi: 10.1016/S0092-8674(00)80876-4. [DOI] [PubMed] [Google Scholar]
- 93.Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nature Protocols 3:1101 –1108. doi: 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 94.Seugnet L, Suzuki R, Stidd R, and Shaw P (2009) Aversive phototaxic suppression: evaluation of a short-term memory assay in Drosophila melanogaster. Genes Brain Behav 8(4): 377–89. doi: 0.1111/j.1601-183X.2009.00483.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharma J, Baker S, et al. (2014) Identification of a Peptide Inhibitor of the RPM-1·FSN-1 Ubiquitin Ligase Complex. J Biol Chem 289(50): 34654–34666. doi: 10.1074/jbc.M114.614065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Furukawa Shigetada, Fujita Takuya, et al. (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114(12): 1752–1761. doi: 10.1172/JCI200421625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shinza-Kameda M, Takasu E, Sakurai K, Hayashi S, Nose A. (2006) Regulation of layer-specific targeting by reciprocal expression of a cell adhesion molecule, capricious. Neuron January 19;49(2):205–13. [DOI] [PubMed] [Google Scholar]
- 98.Stark R, Guebre-Egziabher (2014) A role for mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) in the regulation of hepatic gluconeogenesis. J Biol Chem 14;289(11):7257–63. doi: 10.1074/jbc.C113.544759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stranahan AM, Mattson MP (2011) Bidirectional metabolic regulation of neurocognitive function. Neurobiol Learn Mem 96:507–516, doi: 10.1016/j.nlm.2011.01.004, pmid:21236352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Swarup S, Williams T and Anholt R (2011) Functional dissection of Odorant binding protein genes in Drosophila melanogaster. Genes Brain Behav 10(6): 648–57. doi: 10.1111/j.1601-183X.2011.00704.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Terhzaz S, Cabrero P, Brinzer R, Halberg K, Dow J and Davie S ( 2015) A novel role of Drosophila cytochrome P450–4e3 in permethrin insecticide tolerance. Insect Biochem Mol Biol; 67: 38–46. doi: 10.1016/j.ibmb.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tomasi T, Hakeda-Suzuki S, Ohler S, Schleiffer A, Suzuki T. (2008) The transmembrane protein Golden goal regulates R8 photoreceptor axon-axon and axon-target interactions. Neuron March 13;57(5):691–704. doi: 10.1016/j.neuron.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 103.Tóth ME, Szegedi V (2013) Overexpression of Hsp27 ameliorates symptoms of Alzheimer’s disease in APP/PS1 mice. Cell Stress Chaperones 18(6):759–71. doi: 10.1007/s12192-013-0428-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tripathy R, Kunwar S, Sano H, Renault D (2014) Transcriptional regulation of Drosophila gonad formation. Dev Biol 2(392): 193–208. doi: 10.1016/j.ydbio.2014.05.026 [DOI] [PubMed] [Google Scholar]
- 105.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A 1985. September;157(2):263–77. [DOI] [PubMed] [Google Scholar]
- 106.Valladolid-Acebes I, Stucchi P, Cano V, et al. (2011) High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiol Learn Mem 95:80–85. doi: 10.1016/j.nlm.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 107.Vincent HK, Vincent KR, Lamb KM. (2009) Obesity and mobility disability in the older adult. Obes Rev 2010 August;11(8):568–79. doi: 10.1111/j.1467-789X.2009.00703. [DOI] [PubMed] [Google Scholar]
- 108.Wang C, McPherson K, Marsh T, Gortmaker L, Brown M (2011) Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378: 815–25. doi: 10.1016/S0140-6736(11)60814-3 [DOI] [PubMed] [Google Scholar]
- 109.Wesson D, Levy E, Nixon R, and Wilson D (2010) Olfactory Dysfunction Correlates with Amyloid-β Burden in an Alzheimer’s Disease Mouse Model. J Neurosci 13; 30(2): 505–14. doi: 10.1523/JNEUROSCI.4622-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wigby S, Slack C, Grönke S, Martinez P, Calboli FC, Chapman T, & Partridge L (2010) Insulin signalling regulates remating in female Drosophila. Proceedings. Biological sciences, 278(1704), 424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Woodcock K, Kierdorf K, Pouchelon C, Vivancos V, Dionne M, and Geissmann F (2015) Macrophage-Derived upd3 Cytokine Causes Impaired Glucose Homeostasis and Reduced Lifespan in Drosophila Fed a Lipid-Rich Diet. Immunity 20; 42(1): 133–44. doi: 10.1016/j.immuni.2014.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu C, Daniels R, DiAntonio A. (2007) DFsn collaborates with Highwire to down-regulate the Wallenda/DLK kinase and restrain synaptic terminal growth. Neural Development 2:16. doi: 10.1186/1749-8104-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yamamoto A, Zwarts L (2008) Neurogenetic networks for startle-induced locomotion in Drosophila melanogaster 34(105): 12393–12398. doi: 10.1073/pnas.0804889105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zheng Y, Li H, Lu M, Du Y (2014) Evaluation and Validation of Reference Genes for qRT-PCR Normalization in Frankliniella occidentalis. PLoS One 9(10): e111369. doi: 10.1371/journal.pone.0111369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou Xianchong & Escala Wilfredo & Papapetropoulos Spyridon& Zhai Rong. (2010) β-N-Methylamino-L-Alanine Induces Neurological Deficits and Shortened Life Span in Drosophila. Toxins 2 2663–79. doi: 10.3390/toxins2112663 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.