Abstract
In the present study, we have investigated the bioavailability of biotransformed organic zinc enriched dahi in vivo. The results evidence that the rats fed with zinc enriched dahi (ZED and ZEP) significantly increased (p<0.001) body weight and food intake from zero to third weeks. Analysis of zinc by AAS in body parts of different rat groups indicated that zinc content was significantly higher (p<0.001) in serum, femur bone, liver and hair of rats fed ZED/ZEP. Basal diet and inorganic zinc sulphate fed rat group excreted a greater amount of zinc in faeces. The results of in-vivo studies indicated that the bioavailability of organic zinc through dahi/probiotic dahi is high compared to its inorganic form.
Keywords: Bioavailability, Dahi, LAB, Micronutrient deficiency, Probiotic, Zinc
INTRODUCTION
Zinc plays an important function in human nutrition. It is a structural constituent of several enzymes and proteins and is involved in the regulation of appetite, stress level, gene expression, immune system development and cell stability (Roohani et al., 2013). It’s vital role in metabolic pathways, makes zinc as an indispensable element for the human body. Although, required zinc amount varies with the age group, mean daily dietary zinc requirement of populations from several countries is ranged between 4.7 to 18.6 mg (World Health Organization, 2001). To meet the daily dietary requirement, zinc should be supplied regularly through food in adequate amounts, in a bio-available form. Often, food containing zinc does not fulfil the recommended dose for some population groups (Lucia et al., 2014), which has been attributed to poor zinc absorption from the diet. About 17.3% of the world population is at risk of inadequate zinc intake and the risk is highest in children under 5 years of age (Wessells et al., 2012). In particular, zinc deficiency is considered as an emerging public health problem in India and other developing countries (Mogna et al., 2012). Among the various strategies used to control zinc deficiency, fortification of food with presently available inorganic zinc is most common. However, reports suggest that inorganic salts have very little bioavailability as compared to organic form (Siepmann et al., 2005). Organic form is chemically inert, more stable, less prone to mineral and nutrient interactions and sufficient to meet the requirement at lower levels (Swiatkiewicz et al.,2014).
Recently the concept of biotransformation of inorganic minerals into more bioavailable organic form by beneficial organisms such as Lactic Acid Bacteria (LAB) has been reported Mrvcic et al., 2009. LAB may be good vehicle for biomass formation of inorganic zinc into an organic form, in which microelements may bound in the form of protein complexes and are absorbed in the small intestine in a manner typical of peptides and proteins that enable diffusion of microelements into the gut (Mrvcic et al., 2009) (Kaur et al., 2017). Zinc-enriched biomass of certain LAB, particular lactobacilli, could provide the host with a huge amount of bioavailable zinc that would satisfy the necessity of the suggested daily intake.
Dahi, most popular fermented dairy products of India, having a healthy image, consumed almost every day as part of a regular diet may serve as best food to fortify biotransformed organic zinc. Such biotransformed organic zinc coupled with fortification strategy may be used to overcome zinc deficiency in the target population. Further, some studies indicated that incorporation of probiotic lactobacilli can better biotransform inorganic zinc to organic form which renders more bioavailability. Therefore, in the present study, we aim to fortify bacterial transformed organic zinc in dahi/probiotic dahi and evaluate its bioavailability in Wistar rats.
MATERIALS AND METHODS
Procurement and maintenance of cultures
Lactobacillus fermentum SR4, Lactobacillus rhamnosus NCDC 610 and Streptococcus thermophilus NCDC074 were obtained from National Collection of Dairy Cultures (NCDC), ICAR-NDRI, Karnal, Haryana, India. The optimum growth temperature for these cultures was 37°C. They were always maintained in chalk-litmus milk at 4°C and sub-cultured in MRS broth as and when required during the experiment.
Production of organic zinc enriched biomass
Inorganic zinc biotransforming Lactobacillus fermentum SR4, earlier evaluated in our laboratory was used for large-scale production of organic zinc enriched biomass (under previously optimized conditions). Initially, L. fermentum SR4 was inoculated into 10 lit of MRS-cysteine broth containing 1 ppm/mL of zinc sulphate and incubated at 37°C for 48 h using fermenter (New Brunswick™ BioFlo®/CelliGen® 115, Eppendorf, San Francisco, USA) under constant pH 5.5 with a shaking speed of 100 rpm. Zinc-enriched bacterial cells were harvested from the medium by centrifugation (6,000×g for 10 min at 4°C) followed by cell lysis was carried out by sonication. The prepared lysate was lyophilized and stored in −20°C until further use. Zinc content in biomass was analyzed by Flame Atomic Absorption Spectrophotometer (AA-7000 Series, Shimadzu, Kyoto; Japan) with set conditions (gas-helium; weight factor-100 mg; calibration factor-1; volume factor-100; dilution factor-1).
Preparation of organic zinc enriched dahi and probiotic dahi
Fresh whole milk was procured from the experimental dairy of ICAR-NDRI, Karnal and standardized by adding skim milk to attain 13% total solids. The standardized milk was heated to 90°C for 15 min followed by cooling to 37°C. L. fermentum SR4 bacterial lysate was added to milk just before inoculation of the culture so that final product contains 15 ppm of zinc. Based on initial trials, for preparation of zinc-enriched dahi, NCDC074 was inoculated singly at 2% (v/v) while for zinc enriched probiotic dahi NCDC074 and NCDC610 was inoculated into milk in combinations (1:1) at 2 % (v/v) and incubated at 37°C for 4.30 h.
Procurement of animals, experimental design for zinc deficiency and feeding schedule
The animal experimental protocol used in this study was approved by the Institutional Animal Ethics Committee (IAEC) ICAR-NDRI, Karnal (Approval No. IAEC. No.143/16). Male Albino Wistar rats, about 5–6 weeks old used in the study were housed in polycarbonate cages. The animals were randomly divided into six groups (8 rats in each group) and kept in an air-conditioned room at 24±1°C. The basal diets were prepared according to AOAC standards (Table 1). The animals were brought to zinc deficiency by feeding the zinc-deficient basal diet (removal of zinc-containing salt and providing triple distilled drinking water ad libitum for preparation of zinc deficient rats) for 30 days. Subsequently, AAS analysis estimate revealed about 95% reduction of zinc content in stool and sera of treated rats in comparison with untreated control rats. Once zinc deficiency was established, they were given different diets as per the diet plan provided in Table 2. All the treatment groups including controls i.e. Positive controls i.e [positive controli.e [positive control (Basal diet-BD, Milk, Dahi, Zinc Enriched Dahi-ZED, Zinc Enriched Probiotic Dahi-ZEP) and negative control (Zinc Sulfate)] after treatment animals were sacrificed using high concentration of diethyl ether in close chamber and samples were collected for AAS analysis.
TABLE 1.
Composition of diets (AOAC, 1984).
| Diet | Basal diet | Type of diet | Basal diet | Diet | Basal diet |
|---|---|---|---|---|---|
| Component | Quantity (g/100g) | Component | Quantity (g/100g) | Component | Quantity (g/100g) |
| Starch | 53 | Vitamin mixture | 1.0 | Fat | 7.0 |
| Casein | 20 | Mineral mixture | 3.5 | Cellulose | 5.0 |
| Sucrose | 10 | Choline chloride | 0.2 | Methionine | 0.3 |
| Vitamin mixture | Quantity (mg/100 gm) | Vitamin mixture | Quantity (mg/100 gm) | Vitamin mixture | Quantity (mg/100 gm) |
|---|---|---|---|---|---|
| Vitamin A | 2000IU | Ca-D-Pantothenate | 4.0 mg | Mesoinositol | 10 mg |
| Vitamin D | 200 IU | Riboflavin | 0.8 mg | Niacin | 4.0 mg |
| Vitamin K | 0.5 mg | Thiamin-HCl | 0.5 mg | Biotin | 0.04 mg |
| Choline chloride | 200 mg | Pyridoxin-HCl | 0.5 mg | Vitamin B|? | 0.003 mg |
| PABA | 10 mg | Folic acid | 0.2 mg |
| Salt Mixture | Quantity (gm/kg) | Salt Mixture | Quantity (gm/kg) | Salt Mixture | Quantity (gm/kg) |
|---|---|---|---|---|---|
| KH2PO4 | 389gm | ZnSO4.7H2O | 0.548 gm | FeSO4.7H2O | 27gm |
| MgSO4 | 57.3 gm | CuSO4.5H2O | 0.477 gm | MnSO4.H2O | 4.1 gm |
| CaCO3 | 381.4gm | CoCl7.6H7O | 0.023 gm | NaCl | 139.3 gm |
| KI | 0.79 gm |
TABLE 2. Diet plan to remediate zinc deficiency.
*Per kg body weight, #Per 100g of diet. BD: As per the AOAC Supplementary table 1; MILK: Whole milk containing 13% total solids (TS). [TS= Fat 4% + SNF 9%]; DAHI: Culture used for dahi preparation-Streptococcus thermophilus NCDC074 which contains approx.0.9 % of lactic acid as per the titration method; ZED: Culture used for dahi preparation-Streptococcus thermophilus NCDC074 enriched with zinc (please refer material and method section for zinc enrichment); ZEP: Culture used for probiotic dahi preparation Lactobacillus rhamnosus NCDC 610 and Streptococcus thermophilus NCDC074 enriched with zinc (please refer material and method section for zinc enrichment).
| Groups | Basal Diet* | Treatment* | Zinc# | Duration of Treatment |
|---|---|---|---|---|
| BD | 200g | NA | NA | Every day up to 3 weeks after zinc deficiency along with triple distilled drinking water ad libitum |
| MILK | 100g | 100g | NA | Every day up to 3 weeks after zinc deficiency along with triple distilled drinking water ad libitum |
| DAHI | 100g | 100g | NA | Every day up to 3 weeks after zinc deficiency along with triple distilled drinking water ad libitum |
| ZED | 100g | 100g | 15ppm | Every day up to 3 weeks after zinc deficiency along with triple distilled drinking water ad libitum |
| ZEP | 100g | 100g | 15ppm | Every day up to 3 weeks after zinc deficiency along with triple distilled drinking water ad libitum |
| ZS | 200g | NA | 15ppm | Every day up to 3 weeks after zinc deficiency along with triple distilled drinking water ad libitum |
Collection of samples and analysis of parameters
The body weight and feed intake were monitored weekly during the experimental period. Individual rats from different groups were weighed by electronic weighing machine. Blood, liver, femur bone, hair and faeces were collected separately from all six groups. Blood was collected in vacutainer tubes by terminal cardiac puncture and serum was removed carefully. Liver tissues were collected, washed in PBS (pH 7.5) instantly snap frozen and stored at −80°C. The right femur bones were removed and cleaned with adhering tissue and oven-dried (110°C for 24h). For zinc analysis by AAS, initially, samples were dry-ashed at 450°C for 24h in a Muffle furnace. The ash residues gently heated with 5 ml concentrated sulphuric acid, perchloric acid and nitric acid (0.5:1.0:4.0) and evaporated to dryness before a second ashing is performed at 450°C for 4h. The resulting white residue was dissolved in 1M-HCl and diluted in 100 ml ion-free water. The samples were filtered through a Whatman filter paper No. 42. The Hair samples (≈0.5 g) were cut from the hoods of the rats with the help of scissors, placed in a centrifuge-tube and washed with 15 ml sodium lauryl sulphate (20 g/L). Hairs were left for 2 h with intermittent mixing. After centrifugation, the hairs were washed six times with ion-free water. After oven-drying (110°C for 24 h), samples were wetashed in concentrated sulphuric acid, perchloric acid and nitric acid (0.5:1.0:4.0). Fresh fecal samples were collected in the Petri plates. The percent zinc was calculated with the amount of zinc fed with the total amount of zinc excreted in the faeces. The zinc level in femur, liver and faeces were determined according to the method of Lazarte et al., 2015 & Davies et al., 1975. Hair samples were analyzed for zinc content by the method of (Davies et al., 1979) and serum zinc level was estimated by the direct dilution method (Hackley et al., 1968). Zinc content in all the samples was analyzed by AAS.
Statistical analysis
Body weight and diet intake data were analyzed by twoway ANOVA followed by Bonferroni Post-Tests to compare within replicates of different groups. Non-Parametric one-way ANOVA with Friedman test followed by Dunn’s post-test was used for the data obtained from zinc enrichment experiment to compare all pairs of groups using 95% confidence intervals. All the calculations were performed in Prism Graph Pad (Prism version 7.01).
RESULTS AND DISCUSSION
Organic zinc enriched biomass and bacterial lysate containing dahi
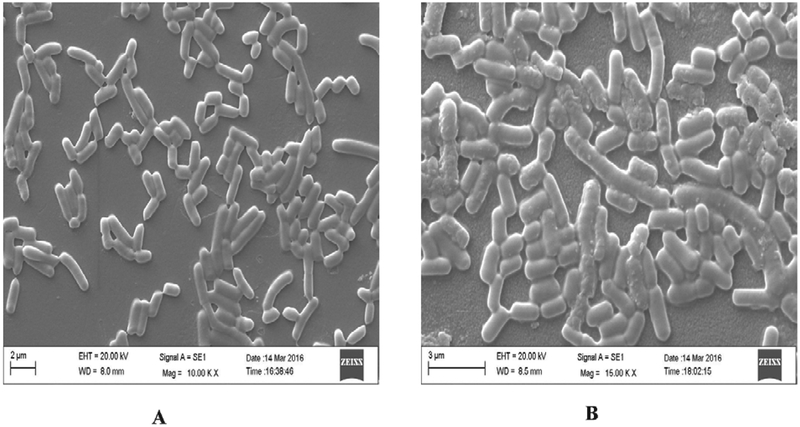
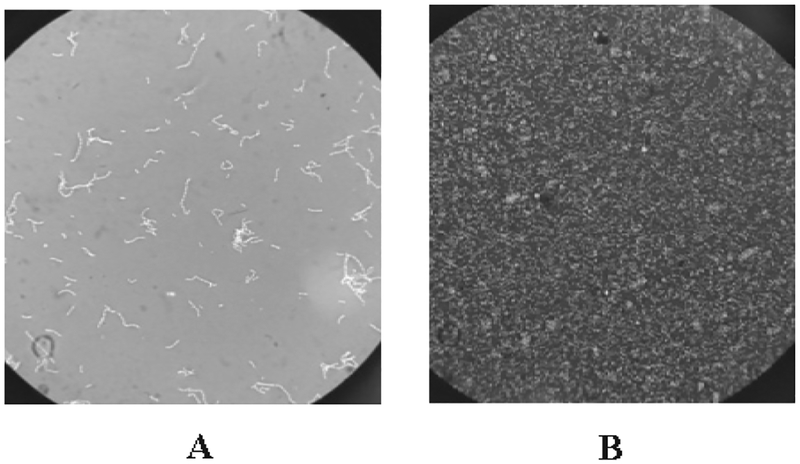
Previously, we have observed that among the Lactobacillus species, L. fermentum SR4 accumulate inorganic zinc on the cell surface (Fig. 1), internalize and biotransform it into organic zinc. In order to carry out in vivo bioavailability experiment in rats, a total of 110 g of zinc-enriched L. fermentum SR4 biomass (Table 3) was produced by the fermentation process, which contained 1690 ppm/g of organic zinc. The binding of zinc and its subsequent biotransformation capabilities varies among the strains and in Lactobacillus species it ranges from 11–135 μmol/g (Leonardi et al., 2013). It was shown that the Leuconostoc mesenteroides could bind 27.10 mg of zinc in aqueous solution (pH 5.0) at 32°C after 24h (Mrvcic et al., 2009). Also, previously selenium/zinc enriched probiotics biomass was prepared which contained total organic selenium and zinc in the concentration of 173.35 μg/g and 4.38 mg/g respectively (Ren et al., 2011). The lysis of bacterial cell (Fig. 2) is essential step to release biotransformed organic zinc, which may facilitate its easy distribution into the product and increases the bioavailability of zinc.
FIGURE 1. Zinc absorption by L. fermentum SR4 after ZnSO4 treatment.
A) Lactobacillus fermentum SR4 without ZnSO4 B) Lactobacillus fermentum SR4 with ZnSO4 observation made by SEM (10,000×)
TABLE 3.
Lactobacillus fermentum SR4 biomass and zinc content.
| Trials | Biomass obtained (g) | Zinc content (mg/g) |
|---|---|---|
| 1 | 36.5 | 1.69 |
| 2 | 36.4 | 1.69 |
| 3 | 37.1 | 1.69 |
| Total | 110 | 1.69 |
FIGURE 2. Ultra-sonication of L. fermentum SR4 biomass.
A) Morphology of intact bacterial cell before cell lysis; B) Image displaying the complete lysis of cell after sonication.
Table 4 represents the microbiological and technological properties of zinc-enriched dahi made by singly or combinations of NCDC 074 and NCDC 610. The identified pH of the dahi and probiotic dahi ranges between 4.02 to 4.89 while titratable acidity from 0.69 to 0.89% LA. Depending on the lactic cultures used, a good quality dahi exhibits pH of 4.0 to 4.9 and titratable acidity of 0.65–1.0% LA (Behare et al., 2013). The lactic count of different dahi found to be in the range of 7.02 to 9.77 log cfu/ml. Addition of organic zinc in the milk did not affect pH, acidity and lactic count of dahi. As per our knowledge, there is no report available indicating the use of bacterial transformed organic zinc in dahi.
TABLE 4. Microbiological and chemical evaluation of dahi and probiotic dahi.
The values are average of three trials
| Days | Dahi (NCDC 074) | Probiotic dahi (NCDC 074: NCDC 610) | ||||
|---|---|---|---|---|---|---|
| pH | Titratable acidity % LA | Viable count (log cfu/ml) | pH | Titratable acidity % LA | Viable count (log cfu/ml) | |
| 1 | 4.21±0.12 | 0.82±0.04 | 9.6±0.6 | 4.64±0.12 | 0.71±0.04 | 7.20±0.4 |
| 2 | 4.22±0.13 | 0.81±0.05 | 9.6±0.2 | 4.57±0.13 | 0.78±0.03 | 7.07±0.2 |
| 3 | 4.58±0.15 | 0.80±0.02 | 9.56±0.4 | 4.62±0.19 | 0.69±0.07 | 7.30±0.5 |
| 4 | 4.57±0.12 | 0.83±0.08 | 9.65±0.6 | 4.02±0.15 | 0.75±0.04 | 7.36±0.2 |
| 5 | 4.50±0.14 | 0.83±0.09 | 9.15±0.9 | 4.56±0.13 | 0.72±0.09 | 7.30±0.6 |
| 6 | 4.60±0.12 | 0.83±0.04 | 9.17±0.1 | 4.37±0.17 | 0.79±0.03 | 7.02±0.4 |
| 7 | 4.67±0.12 | 0.81±0.03 | 9.53±0.4 | 4.21±0.12 | 0.80±0.04 | 7.49±0.8 |
| 8 | 4.69±0.18 | 0.84±0.04 | 9.64±0.3 | 4.20±0.13 | 0.74±0.05 | 7.34±0.3 |
| 9 | 4.65±0.16 | 0.87±0.08 | 9.60±0.9 | 4.24±0.15 | 0.79±0.07 | 7.22±0.4 |
| 10 | 4.69±0.14 | 0.84±0.07 | 9.62±0.2 | 4.56±0.12 | 0.74±0.04 | 7.32±0.4 |
| 11 | 4.69±0.17 | 0.82±0.04 | 9.51±0.6 | 4.76±0.12 | 0.73±0.03 | 7.91±0.2 |
| 12 | 4.60±0.14 | 0.85±0.02 | 9.46±0.8 | 4.58±0.18 | 0.75±0.05 | 7.11±0.4 |
| 13 | 4.67±0.12 | 0.81±0.04 | 9.42±0.3 | 4.11±0.13 | 0.75±0.06 | 7.08±0.6 |
| 14 | 4.62±0.10 | 0.89±0.04 | 9.68±0.8 | 4.37±0.15 | 0.71±0.9 | 7.06±0.2 |
| 15 | 4.62±0.13 | 0.82±0.01 | 9.77±0.2 | 4.89±0.12 | 0.73±0.02 | 7.66±0.1 |
| 16 | 4.72±0.09 | 0.87±0.04 | 9.53±0.6 | 4.27±0.16 | 0.79±0.01 | 7.38±0.3 |
| 17 | 4.64±0.19 | 0.85±0.05 | 9.71±0.8 | 4.76±0.13 | 0.73±0.02 | 7.30±0.2 |
| 18 | 4.65±0.15 | 0.79±0.03 | 9.64±0.1 | 4.56±0.2 | 0.79±0.04 | 7.37±0.4 |
| 19 | 4.60±0.15 | 0.90±0.08 | 9.56±0.3 | 4.67±0.3 | 0.71±0.04 | 7.32±0.6 |
| 20 | 4.62±0.18 | 0.84±0.04 | 9.68±0.3 | 4.21±0.12 | 0.78±0.02 | 7.07±0.4 |
| 21 | 4.61±0.15 | 0.89±0.01 | 9.45±0.6 | 4.57±0.12 | 0.76±0.04 | 7.31±0.2 |
Effect of zinc-enriched bacterial lysate containing dahi on rat
a). Body weight and diet intake
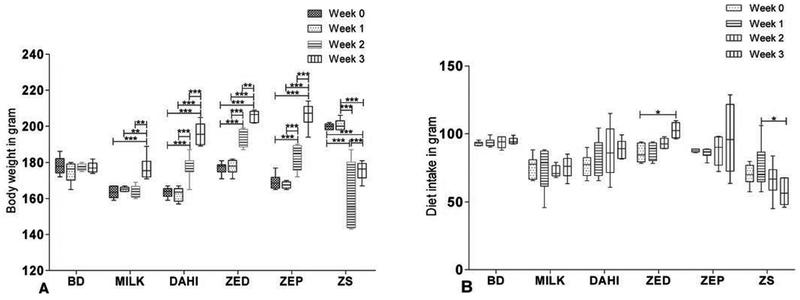
The body weight of rats fed with basal diet was unchanged while significant decrease (p<0.001) was observed in rats fed with zinc sulphate from first to the third week. In case of rats fed with dahi, ZED and ZEP, the body weight was significantly increased (p<0.001) (Fig. 3A), while the increase in body weight was highest in ZEP group. Addition of bacterial transformed organic zinc increased body weight in the rats. Our results are in agreement with previous report i.e, increase in body weight gain in the rats fed with bacterial chelated zinc (El-Husseiny et al., 2012). For optimum growth of rats, 12–15 ppm of zinc was sufficient and dietary zinc level (3 and 15 ppm) affects body weight in rats (Nagalakshmi et al., 2012). Rats fed with inorganic zinc showed decline in feed intake over the period. On the other hand, the feed intake was gradually increased statically (p<0.001) for dahi, ZED and ZEP groups during three weeks (Fig. 3B). The increased zinc absorption in the body may be due to the interaction of the metal ion with proteins or amino acids in the body (Scott et al., 1963). Organic minerals facilitate peptide or amino acid uptake pathway which provides more zinc to target tissue. Milk and milk products contain some amount of zinc and further fermentation of such products helps in easy absorption of zinc in the body. We observed that the body weight or rats fed with milk and dahi was also increased. Further, research is required on the interaction of milk constituents particularly proteins/peptides or carbohydrate with organic zinc, which might influence body weight provided with similar amounts of diet.
FIGURE 3. Determination of changes in body weight and dietary intake in Wistar rats.
A) Box and Whisker plot showing the changes in the body weight of rats fed with different diets B) Changes in the dietary intake of rats. All the readings were recorded after 1 week of the interval, starting from the day zero which is when treatment initiated after Zinc deficiency. Center lines show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, n = 8 animals/group, all reading was taken in triplicates. *p< 0.05, **p<0.01 ***p<0.001.
b). Zinc in serum
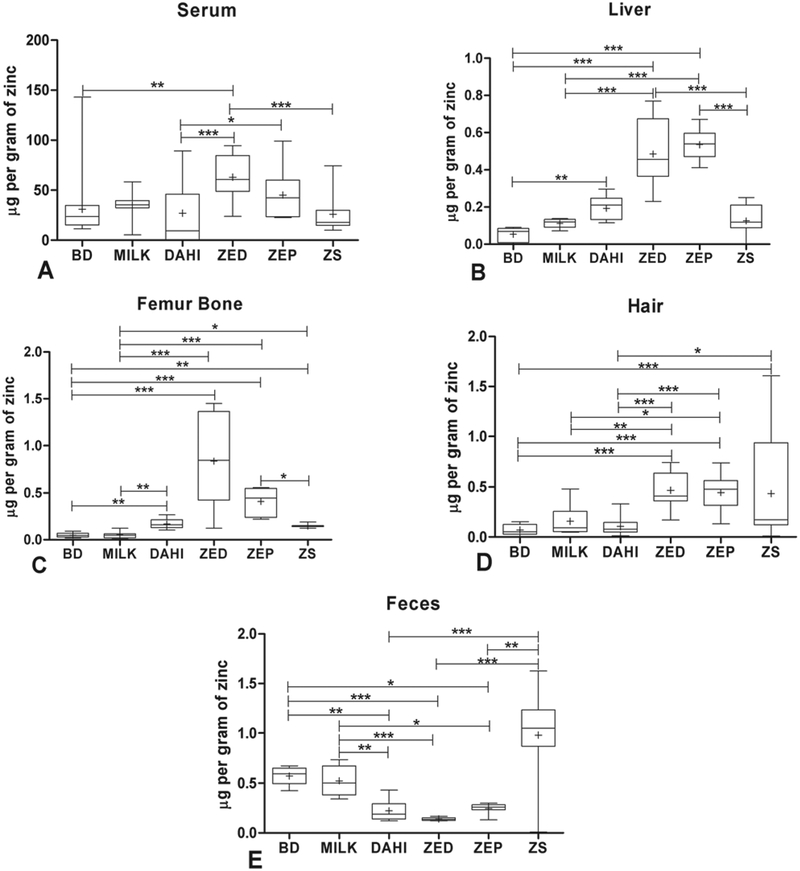
Serum zinc level was increased for the rats fed with organic zinc enriched dahi, with highest concentrations of zinc was present in ZED (Fig. 4A) which did not differ significantly with ZEP group. The inorganic zinc fed to the rats was less bioavailable than organic zinc compared to ZED and ZEP groups. Our results are in agreement with previous reports which indicate that the bioavailability of chelated zinc in serum was between 160 and 250% times to that of inorganic zinc sulphate (Richards et al., 2015). Previously, report showed that supplementation of zinc in the organic form significantly (p<0.001) increased the serum zinc concentrations in animals compared with the inorganic form (Yenice et al., 2015). Oral administration of bound zinc of fungal strain Fusarium oxysporum LZ-1108 to rats at a dose of 10 mg zinc/kg body weight was more bioavailable than zinc gluconate in plasma and blood (Zhang et al., 2014). Probiotic yoghurt fed to rats shown 51–76% higher zinc apparent absorption in serum. Zinc supplementation (30 mg/day for 8 weeks) significantly reduced serum levels of inflammatory markers such as histidine-CRP and IL-6 in obese women (Ibrahim et al., 2006).
FIGURE 4. Estimation of Zinc concentration in different body fluid/organs/parts in Wistar rats.
A. Serum; B. Liver; C. Femur bone; D. Hair; E. Feces. Box and Whisker plots showing the bioavailability of zinc in different body part after three weeks of treatment with six different diets. All the readings were recorded after 3 weeks of 6 different diet treatments after Zinc deficiency. Center lines show the medians; ‘+’ sign shows the mean value; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, n = 24 where 8 animals/group, and all reading were taken in triplicates. *p< 0.05, **p<0.01 ***p<0.001.
c). Zinc in liver
Zinc content in the liver of rats fed with milk and different dahi types was significantly higher than basal diet and zinc sulphate groups (Fig. 4B). Highest zinc content was observed in the ZED group which did not differ significantly with ZEP. An increase in the content of the zinc in the liver implies more accumulation of organic zinc. Fermentation of milk leads to the production of various peptides/products that facilitate absorption of zinc and implicated the more biosorption of zinc in ZEP and ZED groups. ZnO Nanoparticles and organic forms showed a greater zinc retention as compared to the other mineral sources (Liao et al., 2010). This may be due to the better performance of nanoparticles attributed to their size, larger surface area, and increased mucosal permeability that results in better improved intestinal absorption and tissue depositions. Zinc concentration in liver was highest in organic zinc and Nano zinc (93.63 ± 2.06 ppm), while lowest zinc concentration was found to be present in the basal diet group (55.49 ± 3.33 ppm) (Sahoo et al., 2014).
d). Zinc in femur bone
The zinc content in ZED was significantly (p<0.01) higher compared to other groups. However, no significant difference was observed in ZED and ZEP group. Lower femur zinc level was observed in the rats fed with basal diet followed by zinc sulphate (Fig. 4C). The result shows that in case of femur bone the inorganic zinc was less bioavailable than ZED/ZEP group. Zinc is predominant mineral in bone metabolism and its level in the femur is a good indicator of zinc bioavailability in rats. Sufficient amount of zinc is required for bone growth, development, and mineralization (Bao et al., 2010). The zinc concentration in tibia bone of chicks was highest in organic zinc (135.38 ± 3.18 ppm) and lowest in the basal diet group (80.15 ± 1.55 ppm) (Sahoo et al., 2014). In agreement with other study (Star et al., 2012), we found bacterial transformed organic zinc had more bioavailability as compared to zinc sulphate.
e). Zinc in hair
The highest hair zinc levels were found in bacterial zinc fed with ZED group. No significant difference was observed among ZED and ZEP groups. The lowest zinc concentration was seen in the rats fed with basal diet followed by a diet containing zinc sulphate. The inorganic zinc fed to the rats was less bioavailable compared to the rats fed with dahi and ZED/ZEP groups (Fig. 4D). The acute zinc deficiency increases the rate of hair loss in experimental animals as well as in humans too. This may be due to a lack of growth at the hair follicle. Addition of zinc to the diet of Texel sheep increases the wool zinc level significantly (Ryan et al., 2002). It was also previously determined that addition of chelated zinc with amino acids to the diet enables longer hair growth in dogs and calves respectively in comparison to zinc oxide (Lowe et al., 1994).
f). Zinc in faeces
Significantly higher (p<0.001) amount of zinc was found in the faeces of rats fed with ZS group (Fig. 4E) and lower in the ZED and ZEP group. These results are in accordance with the previous reports, that the supplementation of trace elements in the organic form reduced the excretion of its concentration in faeces compared with the inorganic source (Yenice et al., 2015). The inorganic zinc fed to the rats was less bioavailable as more of the zinc is excreted in the form of faeces whereas the bacterial transformed zinc fed to the rats showed more bioavailability.
CONCLUSION
The study provides a new perspective on the specific use of Lactobacillus fermentum SR4 for biotransformation of inorganic zinc. Bacterial transformed zinc enriched dahi and probiotic dahi was more bioavailable in rats as compared to inorganic zinc sulphate. Biotransformed organic zinc coupled with fortification strategy may be used to overcome zinc deficiency in the target population.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the contribution of Director, ICAR-NDRI, Karnal, for the financial support and providing a necessary facility for carrying out this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations Used:
- AAS
Atomic Absorption Spectroscopy
- BD
Basal diet
- LA
Lactic acid
- LAB
Lactic acid bacteria
- ZED
Zinc enriched dahi
- ZEP
Zinc enriched probiotic dahi
- ZS
Zinc sulphate
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- Bao Y, Choct MM, Iji PA and Bruerton K (2010). Trace mineral interactions in broiler chicken diets. British poultry science 51: 109–117. [DOI] [PubMed] [Google Scholar]
- Behare PV, Singh R, Nagpal R and Rao KH (2013). Exopolysaccharides producing Lactobacillus fermentum strain for enhancing rheological and sensory attributes of low-fat dahi. Journal of Food Science and Technology 50: 1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NT and Nightingale R (1975). The effects of phytate on intestinal absorption and secretion of zinc, and whole-body retention of Zn, copper, iron and manganese in rats. British Journal of Nutrition 34: 243–258. [DOI] [PubMed] [Google Scholar]
- Davies NT and Olpin SE (1979). Studies on the phytate: zinc molar contents in diets as a determinant of Zn availability to young rats. British Journal of Nutrition 41: 591–603. [PubMed] [Google Scholar]
- El-Husseiny M, Hashish SM, Ali RA, Arafa SA, El-Samee LDA and Olemy AA (2012). Effects of feeding organic zinc, manganese and copper on broiler growth, carcass characteristics, bone quality and mineral content in bone, liver and excreta. International Journal of Poultry Science 11: 368–377. DOI: 10.3923/ijps.2012.368.377. [DOI] [Google Scholar]
- Hackley M, Smith JC and Halsted JA (1968). A simplified method for plasma zinc determination by atomic absorption spectrophotometry. Clinical chemistry 14: 1–5. [PubMed] [Google Scholar]
- Ibrahim, Halttunen T, Tahvonen R and Salminen S (2006). Probiotic bacteria as potential detoxification tools: assessing their heavy metal binding isotherms. Canadian Journal of Microbiology 52:877–85. https://www.ncbi.nlm.nih.gov/pubmed/17110980 [DOI] [PubMed] [Google Scholar]
- Kaur G, Ali SA, Kumar S, Mohanty AK and Behare P (2017). Label-Free Quantitative Proteomic Analysis of Lactobacillus fermentum NCDC 400 during Bile Salt Exposure. Journal of Proteomics 7: 36–45. [DOI] [PubMed] [Google Scholar]
- Lazarte E, Vargas M and Granfeldt Y (2015). Zinc bioavailability in rats fed a plant-based diet: a study of fermentation and zinc supplementation. Food & Nutrition Research 59: 27796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi Zanoni S, Lucia M, Amaretti A, Raimondi S and Rossi M (2013). Zinc Uptake by Lactic Acid Bacteria. ISRN Biotechnology 312917, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Hung WL, Jan KC, Yeh AI, Ho CT and Hwang LS (2010). Nano/sub-microsized lignan glycosides from sesame meal exhibit higher transport and absorption efficiency in Caco-2 cell monolayer. Food Chemistry 119: 896–902. [Google Scholar]
- Lowe A, Wiseman J and Cole DJA (1994). Absorption and retention of zinc when administered as an amino-acid chelate in the dog. The Journal of Nutrition 124: 2572S–2574S. [DOI] [PubMed] [Google Scholar]
- Lucia M, Santos LL, Rodrigues VC, Martino HS and Sant’Ana HM (2014). Bioavailability of zinc in Wistar rats fed with rice fortified with zinc oxide. Nutrients 6: 2279–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogna Nicola S, Pane M, Lorenzini P, Strozzi G and Mogna G (2012). Selenium and zinc internalized by Lactobacillus buchneri Lb26 (DSM 16341) and Bifidobacterium lactis Bb1 (DSM 17850): Improved bioavailability using new biological approach. Journal of Clinical Gastroenterology 46 (P): S41–S45. [DOI] [PubMed] [Google Scholar]
- Mrvcic Prebeg T, Barisic L, Stanzer D, Bacun-Druzina V and Stehlik-Tomas V (2009). Zinc Binding by Lactic Acid Bacteria. Food Technology & Biotechnology 47: 381–388. [Google Scholar]
- Nagalakshmi, Ramulu SP and Rani MU (2012). Effect of graded levels of zinc supplementation on growth performance and oxidative defence mechanism in rats. IOSR Journal of Pharmacy 2: 36–41. [Google Scholar]
- Ren Z, Zhao Z, Wang Y and Huang K (2011). Preparation of selenium/zinc-enriched probiotics and their effect on blood selenium and zinc concentrations, antioxidant capacities, and intestinal microflora in canine. Biological Trace Element Research 141: 170–183. [DOI] [PubMed] [Google Scholar]
- Richards M, Levans FJ and Wedekind KJ (2015). Greater bioavailability of chelated compared with inorganic zinc in broiler chicks in the presence or absence of elevated calcium and phosphorus. Open Access Animal Physiology 7:97110. [Google Scholar]
- Roohani R, Hurrell R, Kelishadi, Schulin R (2013). Zinc and its importance for human health: an integrative review. Journal of Research in Medical Sciences 18: 144–157. [PMC free article] [PubMed] [Google Scholar]
- Ryan JP, Kearns P and Quinn T (2002). Bioavailability of dietary copper and zinc in adult Texel sheep: A comparative study of the effects of sulphate and Bioplex supplementation. Irish Veterinary Journal 55: 221–224. [Google Scholar]
- Sahoo, Swain R and Mishra SK (2014). Effect of inorganic, organic and nano zinc supplemented diets on bioavailability and immunity status of broilers. International Journal of Advanced Research 2: 828–837. [Google Scholar]
- Scott L and Zeigler TR (1963). Chelation in Nutrition, Evidence for Natural Chelates Which Aid in the Utilization of Zinc by Chicks. Journal of Agricultural and Food Chemistry 11:123–125. DOI: 10.1021/jf60126a006 [DOI] [Google Scholar]
- Siepmann M, Spank S, Kluge A, Schappach A and Kirch W (2005). The pharmacokinetics of zinc from zinc gluconate: a comparison with zinc oxide in healthy men. International Journal of Clinical Pharmacology & Therapeutics 43: 562–565. [DOI] [PubMed] [Google Scholar]
- Star L, van der Klis JD, Rapp C and Ward TL (2012). Biotransformed zinc enriched Dahi Bioavailability of organic and inorganic zinc sources in male broilers. Poultry Science 91: 3115–3120. [DOI] [PubMed] [Google Scholar]
- Swiatkiewicz A, Arczewska-wosek, and Jozefiak D (2014). The efficacy of organic minerals in poultry nutrition: review and implications of recent studies. World’s Poultry Science Journal 70: 475–486. [Google Scholar]
- Wessells R and Brown KH (2012). Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 7: e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Environmental Health Criteria, (2001). 221: Zinc, Geneva: P. 360. [Google Scholar]
- Yenice Mızrak C, Gültekin M, Atik Z and Tunca M (2015). Effects of Organic and Inorganic Forms of Manganese, Zinc, Copper, and Chromium on Bioavailability of These Minerals and Calcium in Late-Phase Laying Hens. Biological Trace Element Research 167: 300–307. [DOI] [PubMed] [Google Scholar]
- Zhang X, Peng Y, Li X, Ma G and Wang M (2014). Higher bioavailability of organically bound zinc from high zinc enriched fungi. International Journal of Vitamin and Nutrition Research 84: 277–285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.