Abstract
Objective
Cardiovascular disorders in diabetes condition arise from increased oxidative stress. Both regular mild exercise and testosterone influence on body’s antioxidant system in diabetes. In this study, we evaluated treatment of testosterone and voluntary exercise, alone or together on oxidative stress in the heart and blood of diabetic rats.
Methods
Type 1 diabetes was induced by intraperitoneal injection of 50 mg/kg of streptozotocin in rats. Sixty three rats have been divided into eight groups as follows: Diabetes, diabetes+ testosterone, diabetes+ exercise, diabetes+ testosterone+ exercise, diabetes+ castration, diabetes+ castration+ testosterone, Diabetes+ castration+ exercise, Diabetes+ castration+ exercise+ testosterone. Type 1 diabetes was induced by intraperitoneal injection of 50 mg/kg of streptozotocin in the male Wistar rats and after a week, castration was performed. After 42 days of treatment with testosterone (2 mg/kg/day) or voluntary exercise alone or in combination, SOD, GPX and CAT activities and MDA levels were measured in the blood and heart tissue samples in the groups of study. In the end of study, SOD, GPX and CAT activities and MDA levels were measured in blood and heart tissue samples in the groups of study.
Results
SOD, GPX and CAT activities significantly (p<0.05) increased in groups that treated either testosterone or exercise and MDA level significantly (p<0.01) decreased in the blood and heart tissue of diabetic and castrated diabetic rats. Simultaneously, treatment with testosterone and exercise had a synergistic effect on antioxidant enzymes level in diabetic and diabetic castrated rats. In the castrated animals with diabetes, SOD, GPX and CAT activities significantly decreased (p<0.05) and MDA levels significantly increased (p<0.05) in blood and heart tissue.
Conclusion
Voluntary exercise and testosterone alone or together heightened body’s antioxidant system and were able to reduce the MDA levels in blood and heart of diabetic and castrated diabetic rats.
Keywords: testosterone, rats, diabetes mellitus, oxidative stress, exercise
INTRODUCTION
Diabetes mellitus (DM) as a highest healthcare concern worldwide is a multifaceted endocrine and metabolic syndrome (1). The causes of high mortality in diabetic subjects are cardiovascular problems with increased oxidative stress in cardiac tissue that can worsen this condition (2).
Hyperglycemia in diabetes causes diabetes’s pathophysiology that is mediated by oxidative stress (3). Increased oxygen/nitrogen free radicals levels lead to increased oxidative stress, that plays an important role in diabetic cardiovascular complications (4).
Exercise has beneficial effects on both types of diabetes. Exercise performance improves blood glucose metabolism, increases insulin sensitivity in the whole body and diminishes cardiovascular risk factors in diabetes condition (5). In exhaustive exercise, oxidative stress induces through generation of reactive oxygen species (ROS) but voluntary exercise is beneficial to our health that considered mild to moderate exercise (6). Our previous studies in male wistar rats showed that voluntary exercise is an efficient therapeutic approach to diabetes complications (7, 8).
Testosterone is the main androgen produced by the testes in men. There are numerous known interactions between the endocrine system and metabolism (9, 10). Testosterone directly affects energy metabolism and, correspondingly, oxidative balance (11, 12). It is shown that testosterone decreases in the blood of diabetic men and male diabetic rats (13). Also, it is reported that testosterone deficiency contributes to many diabetes complications (14). It is assumed that treatment with testosterone in diabetic men probably reduces diabetes complications by diminishing oxidative stress (15). In this study, we aimed to evaluate the effects of concurrent use of testosterone and exercise, on oxidative stress in blood and heart tissue in diabetic rats and castrated rats with diabetes.
METHODS
Animals
Sixty three wistar male rats (230-250g, four months old) were obtained from the animal house of University of Medical Sciences, Tabriz, Iran, and were designed in eight groups. Normal conditions including temperature 22°C, 12 hours of brightness and, 12 hours of darkness were observed for all animals. Herein, all animal care and experimental procedures were done referring to the Principles of Laboratory Animal Care (NIH publication, no. 85–23, revised 1985). The groups are as follows:
1 - Diabetes: diabetic rats treated with placebo (Dia).
2 - Diabetes - Testosterone: diabetic rats treated with testosterone (Dia -T).
3 - Diabetes– Exercise: diabetic rats treated with placebo and exercise (Dia-E).
4 - Diabetes -Exercise - Testosterone: diabetic rats treated with testosterone and exercise (Dia-T-E).
5 - Diabetes - castrated: diabetic rats treated with placebo and sham operated without removing the testicles (Dia-Cas).
6 - Diabetes - castrated - Testosterone: diabetic-castrated rats treated with testosterone (Dia-Cas-T).
7 - Diabetes - castrated - Exercise: diabetic-castrated rats treated with placebo and exercise (Dia-Cas-E).
8 - Diabetes - castrated – Testosterone- Exercise: diabetic-castrated rats have been treated with testosterone and exercise (Dia-Cas-T-E).
During 6 weeks, animals treated with testosterone and exercise that is explained with details in the following lines.
At the end, pentobarbital sodium (35 mg/kg, i.p.) has been used for anaesthesia. Blood samples were collected from the inferior vena cava and were stored in tubes. Then the thorax was opened and the heart was removed, washed three times in ice cold saline and blotted individually on ash-free filter paper, used for preparation of tissue homogenates. The determination of MDA, CAT, GPx and SOD in the blood and heart are described in detail in the following paragraphs (16). Sham data are not shown because there was no significant difference with Diabetes group.
Gonadectomy and testosterone therapy
At first, ketamine hydrochloride (80 mg/kg) and xylazine hydrochloride (5 mg/ kg) were used for anaesthesia. At that time, small cuts were made on the abdominal skin and testes were removed. It should be noted that testosterone therapy was done instantly after surgery to escape from hormonal effect. Testosterone propionate was purchased from UNIGEN, Life Science and injected intraperitoneally (2 mg/ kg) for six weeks. Rats not treated with testosterone, received the equal volume of DMSO vehicle.
Creating diabetes model
Type 1 diabetes model was created by intraperitoneal injection 50 mg/kg of streptozotocin (Sigma, St. Louis, Missouri, USA) in 0.05 M citrate buffer (17). To confirm diabetes, after 72 hours, blood glucose levels were assessed by a glucometer and rats with blood glucose levels ≥300 mg/dL (16.67 mmol/L) were considered to have diabetes (17). It should be noted that surgery was initially performed, then seven days after, diabetes was induced.
Voluntary exercise
In this study, rats in Dia-E and Dia-Cas-E groups had free access to vertical running the wheel that attached to their cages for 6 weeks. Running distance was monitored daily. Animals with a run distance <2000 m/day would go out of the study.
On the last day of study, heart tissue was excised and blood samples were collected. Then, left heart samples were homogenized and antioxidant activities levels were measured in 1.15% KCl solution. The resulted substance was centrifuged for 1 min at the speed of 1000 rpm at 4°C. The tissue homogenate was then stored at -20°C for Glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT) activities and malondialdehyde (MDA) measurements (16).
Determination of Antioxidant Enzymes
Superoxide dismutase (SOD) activity was determined by a RANSOD kit (Randox Crumlin, UK) according to Delmas-Beauvieux et al. (18). This method uses xanthine and xanthine oxidase to generate superoxide radicals which react with 2-(4-iodophenyl)-3 (4-nitrophenol)-5-phenyl tetrazolium chloride (ITN) to form a red formazan dye which was evaluated at 505 nm by a spectrophotometer (Pharmacia Biotech; England) and the levels of inhibition of this reaction considered to be SOD activity and evaluated by using associated formulation and SOD levels was calculated comparing to the standard curve and was shown as U/mg protein. Glutathione peroxidase (GPX) activity was evaluted according to the method described by Paglia and Valentine with a RANSEL kit (Randox Crumlin, UK) (19).
Malondialdehyde, Catalase Measurement
Thiobarbituric acid reactive substances (TBARSs) in homogenates were used for evaluation of Malondialdehyde (MDA) levels (20). Catalase activity was assessed by Aebi method (21). The principle of measurement is breakdown of H2O2 in 240 nm at 25°C. The supernatant obtained by myocardial homogenate was mixed to 0.002% Triton X-100, 0.1 mM EDTA, 0.5 M potassium phosphate buffer, and 15 mM H2O2 in 1 mL final volume at pH 7.0. Activity was considered within the initial 15s breakdown levels. The first absorbance was confirmed at 240 nm by a spectrophotometer. Then, it mixed and the absorbance records again (A240 at t = 15) and levels of MDA were considered as nM H2O2 consumed/min/mg of tissue protein.
Statistical analysis
Results are expressed as means ± S.E.M. Statistical analysis was performed using SPSS 17 (SPSS/PC-17, SPSS Inc., USA). The statistical differences between the groups were tested by conducting one or two-way ANOVA and also Tukey’s test was used to compare quantitative data. Statistical significance was defined as p<0.05.
RESULTS
Effects of testosterone and voluntary exercise on lipid peroxidation in the heart tissue and blood samples
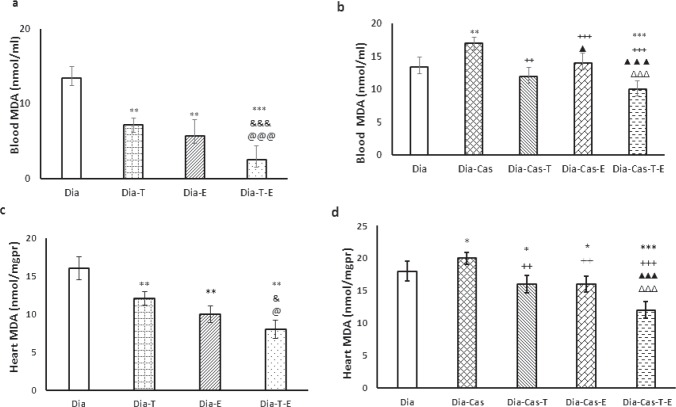
Figure 1a and 1c shows that, 6 weeks of testosterone treatment or exercise performance, meaningfully (p<0.01) reduced the level of MDA in the heart and blood samples in Dia-T and Dia-E groups compared to the Dia group. Also, a significant difference can be seen in the level of MDA of Dia-E-T group compared to the Dia-Tes in the heart tissue (p<0.05) and blood samples (p<0.001). Also, 6 weeks treatment of Dia group with both interventions had a noticeable reducing effect on MDA levels in the blood (p<0.001) and heart tissue (p<0.05) compared to each Dia -T or Dia -E groups.
Figure 1.
a) Effect of 6 weeks testosterone and exercise treatment, alone or together, on MDA levels in the blood of rats with diabetes b) Effect of 6 weeks testosterone and exercise treatment, alone or together, on MDA levels in the blood of castrated rats with diabetes. c) Effect of 6 weeks testosterone and exercise treatment, alone or together, on MDA levels in the heart tissue of rats with diabetes d) Effect of 6 weeks testosterone and exercise treatment, alone or together, on MDA levels in the heart tissue of castrated rats with diabetes. Data are expressed as mean± SEM for 7 animals. *p<0.05, **p<0.01, *** p<0.001 vs the Dia group. & p<0.05, &&& p<0.001 vs the Dia-T group. @ p<0.05, @@@ p<0.001 vs the Dia-E group. ++ p<0.01, +++ p<0.001 vs the Dia-Cas group. ΔΔΔ p<0.001 vs the Dia-Cas-E group. ▲▲▲ p<0.001 vs the Dia-Cas-T group.
Figure 1b and 1d show that castration significantly increased the level of MDA in the heart tissue (p<0.05) and blood samples (p<0.01) in the diabetic castration groups relative to Dia group. After 6 weeks of treatment with testosterone, we saw significant setback of MDA levels in the heart tissue (p<0.01) and blood samples (p<0.01) in Dia-Cas-T group comparing with Dia-Cas group. One-way ANOVA showed that the MDA levels were significantly lower in the diabetes-castration-exercise group in the heart tissue (p<0.01) and blood samples (p<0.001) compared to the Dia-Cas group. Treatment of the Dia-Cas group with both interventions at the same time significantly (p<0.001) decreased the MDA levels in the heart and blood compared to the Dia-Cas-T and Dia-Cas-E (Fig. 1b, d).
Effects of testosterone and voluntary exercise on antioxidant enzymes in the heart tissue and blood samples
SOD
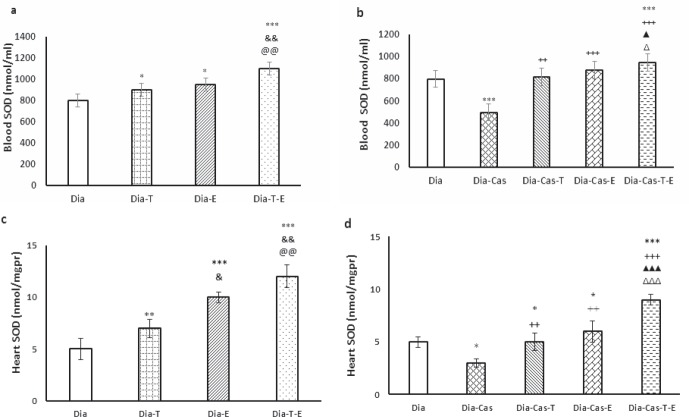
Each one of testosterone or exercise in the groups with diabetes markedly (p<0.05) improved SOD activity in blood samples relative to Dia group (Fig. 2a). Also, the treatment with both interventions at the same time in Dia group had a marked effect (p<0.01) on the SOD protein levels in blood samples compared to the Dia-T and Dia-E. Gonadectomy markedly (p<0.001) diminished SOD activity in blood samples compared to the Dia group. Also, testosterone therapy of Dia-Cas groups (p<0.01) or exercise (p<0.001) significantly improved SOD protein levels in blood samples when compared to the Dia-Cas. Treatment with testosterone and exercise at the same time in diabetic castrated rats had a marked (p<0.05) addictive result on SOD activity in blood samples compared to the Dia-Cas-T or Dia-Cas-E groups (Fig. 2b).
Figure 2.
a) Effect of 6 weeks testosterone and exercise treatment, alone or together, on SOD protein levels in the blood of rats with diabetes b) Effect of 6 weeks testosterone and exercise treatment, alone or together, on SOD protein levels in the blood of castrated rats with diabetes. c)Effect of 6 weeks testosterone and exercise treatment, alone or together, on SOD protein levels in the heart tissue of rats with diabetes d) Effect of 6 weeks testosterone and exercise treatment, alone or together, on SOD protein levels in the heart tissue of castrated rats with diabetes. Data are expressed as mean± SEM for 7 animals. *p<0.05, **p<0.01, *** p<0.001 vs the Dia group. & p<0.05, && p<0.01 vs the Dia-T group. @@ p<0.01 vs the Dia-E group. ++ p<0.01, +++ p<0.001 vs the Dia-Cas group. Δ p<0.05, ΔΔΔ p<0.001 vs the Dia-Cas-E group. ▲p<0.05, ▲▲▲ p<0.001 vs the Dia-Cas-T group.
After 6 weeks of testosterone (p<0.01) treatment or exercise (p<0.001) in the groups with diabetes markedly increased SOD protein levels in heart relative to the Dia group. Also, combination therapy with both interventions in Dia group resulted in a marked effect (p<0.01) on the SOD protein levels in the heart tissue were compared to the Dia- T and Dia- E (Fig. 2c). Castration significantly (p<0.05) decreased SOD protein levels in the heart tissue compared to the Dia group (Fig. 2d). The one-way ANOVA showed that treatment of Dia-Cas groups with testosterone or exercise significantly (p<0.01) increased SOD protein levels in the heart tissue compared to the Dia-Cas in heart tissue. Also, the Dia-Cas-T-E group had a significantly (p<0.001) higher SOD activity in the heart tissue relative to the Dia-T and Dia-E groups.
CAT
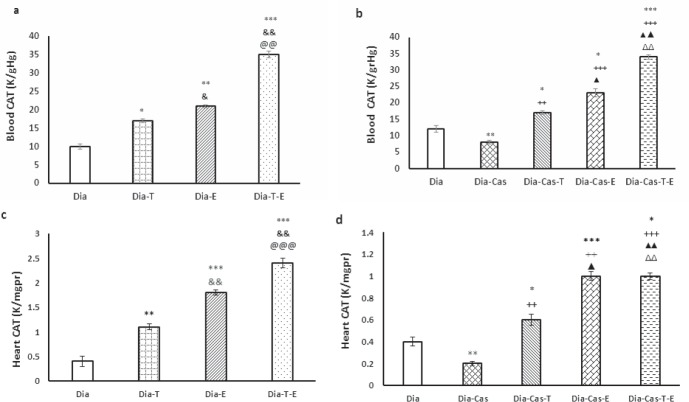
CAT protein levels in the blood samples significantly enhanced in diabetic-testosterone (p<0.05) and diabetic-exercise (p<0.01) groups versus Dia group. Also, testosterone and exercise treatment at the same time in Dia group had a significant effect (p<0.01) on the CAT protein levels in blood samples compared to the Dia-T and Dia-E (Fig. 3a).
Figure 3.
a) Effect of 6 weeks testosterone and exercise treatment, alone or together, on CAT protein levels in the blood of rats with diabetes b) Effect of 6 weeks testosterone and exercise treatment, alone or together, on CAT protein levels in the blood of castrated rats with diabetes. c)Effect of 6 weeks testosterone and exercise treatment, alone or together, on CAT protein levels in the heart tissue of rats with diabetes d) Effect of 6 weeks testosterone and exercise treatment, alone or together, on CAT protein levels in the heart tissue of castrated rats with diabetes. Data are expressed as mean± SEM for 7 animals. *p<0.05, **p<0.01, *** p<0.001 vs the Dia group. & p<0.05, && p<0.01 vs the Dia-T group. @@@ p<0.001 vs the Dia-E group. ++ p<0.01, +++ p<0.001 vs the Dia-Cas group. Δ p<0.05, ΔΔ p<0.01 vs the Dia-Cas-E group. ▲▲ p<0.01 vs the Dia-Cas-T group.
Gonadectomy markedly (p<0.01) diminished CAT activity in the blood samples compared to the Dia group. Also, testosterone (p<0.01) or exercise (p<0.001) meaningfully improved CAT activity in blood samples when compared to the Dia-Cas. Applying simultaneously both interventions on diabetic castrated rats had a marked increasing effect (p<0.01) on CAT protein levels compared to the Dia-Cas-T or Dia-Cas-E groups (Fig. 3b).
After 6 weeks of testosterone treatment (p<0.01) or exercise (p<0.001) in the groups with diabetes CAT activity markedly increased in the heart compared to the Dia group. Also, combined testosterone and exercise had a marked effect on the CAT activity in the heart of Dia group compared to the Dia-T (p<0.01) and Dia- E (p<0.001) (Fig. 3c). Gonadectomy meaningfully (p<0.01) reduced CAT activity in the heart tissue compared to the Dia group. The two-way ANOVA showed that, treatment of Dia-Cas groups with testosterone or exercise markedly (p<0.001) improved CAT activity in the heart tissue compared to the Dia-Cas. Also, the Dia-Cas-T-E group had significantly (p<0.01) higher CAT activity in the heart tissue relative to the Dia-T and Dia-E groups (Fig. 3d).
GPX
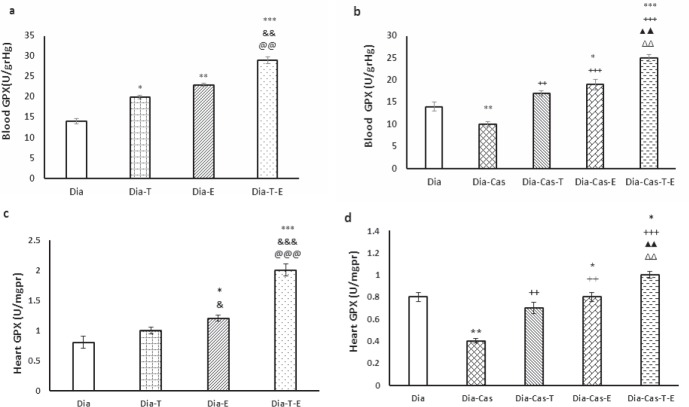
The testosterone treatment for 6 weeks (p<0.05) or exercise (p<0.01) in the groups with diabetes significantly improved GPX protein levels in the blood samples compared to Dia group. Also, applying both interventions on Dia group resulted in a marked effect (p<0.01) on the GPX activity in blood samples compared to the Dia-T and Dia-E (Fig. 4a). Gonadectomy markedly (p<0.01) declined GPX activity in blood samples compared to the Dia group. Also, testosterone therapy of Dia-Cas (p<0.01) or exercise (p<0.001) meaningfully increased GPX activity in blood samples compared to the Dia-Cas (Fig. 4b). Intervention with testosterone and exercise at the same time in diabetic castrated rats showed a significant growing effect (p<0.01) on GPX protein levels compared to the Dia-Cas-T or Dia-Cas-E groups.
Figure 4.
a) Effect of 6 weeks testosterone and exercise treatment, alone or together, on GPX protein levels in the blood of rats with diabetes b) Effect of 6 weeks testosterone and exercise treatment, alone or together, on GPX protein levels in the blood of castrated rats with diabetes. c) Effect of 6 weeks testosterone and exercise treatment, alone or together, on GPX protein levels in the heart tissue of rats with diabetes d) Effect of 6 weeks testosterone and exercise treatment, alone or together, on GPX protein levels in the heart tissue of castrated rats with diabetes. Data are expressed as mean± SEM for 7 animals. *p<0.05, **p<0.01, *** p<0.001 vs the Dia group. & p<0.05, && p<0.01 vs the Dia-T group. @@ p<0. 01, @@@ p<0. 001 vs the Dia-E group. ++ p<0.01, +++ p<0.001 vs the Dia-Cas group. ΔΔp<0. 01 vs the Dia-Cas-E group. ▲▲ p<0.01 vs the Dia-Cas-T group.
Six weeks of exercise training in the group with diabetes markedly (p<0.05) improved GPX activity in the heart tissue relative to the Dia group. Also, concurrently treatment with both interventions in Dia group presented a marked effect (p<0.001) on the GPX activity in the heart tissue compared to the Dia- T and Dia- E (Fig. 4c). Castration significantly (p<0.01) decreased GPX protein levels in the heart tissue compared to the Dia group. The one -way ANOVA showed that treatment of Dia-Cas groups with testosterone or exercise markedly (p<0.01) improved GPX activity in the heart tissue compared to the Dia-Cas. Also, the Dia-Cas-T-E group had markedly (p<0. 01) higher GPX activity in the heart relative to the Dia-T and Dia-E groups (Fig. 4d).
DISCUSSION
The present study showed that oxidative stress increases with castration in the diabetic rats, represented by increased lipid peroxidation products (MDA) and decreased antioxidant defenses (SOD,GPx,CAT). Eight weeks after induction of diabetes, depletion of endogenous testosterone in rats significantly reduced SOD, GPx, and CAT and elevated MDA in the blood and cardiomyocytes. Remarkably, testosterone therapy with physiological dose inverted the decrease of antioxidant defenses and enhanced lipid peroxidation products due to gonadectomy. Furthermore, this study presented that testosterone treatment of rats with diabetes decreased the lipid peroxidation product MDA and increased superoxide scavenger SOD, GPX, CAT. An extensive study confirms that many of heart complications are mediated by oxidative stress. Studies have previously demonstrated the role of increased oxidative stress and decreased antioxidant capacity in diabetic heart (22-24). Testosterone exerts a variety of anabolic effects on many organs (25, 26). It is confirmed that cardiac tissue in all mammalian has many androgen receptors (AR) that characterized the bold physiological effects of androgens on cardiac tissue (9). Newly, research has uncovered that diabetic male rats have decreased testosterone levels compared to normal rats (27). According to other studies, lower free testosterone levels in diabetic men have been proven (28). Few studies have been conducted about the antioxidant effect of exogenous testosterone on the cardiac tissue, and inconsistent data observed in different tissues. It can be assumed that the inconsistent reports are related to variety of experimental model used (29, 30). Nordeen et al., and Kujawa et al., in two independent studies showed that testosterone has protective effect on the recovery of motoneurons (31, 32), while Ahlbom et al., defined that testosterone protected cerebellar granular cells from increased oxidative stress condition via a receptor-mediated mechanism (29). Gonadectomy studies indicated that testosterone can be a protector against oxidative injury in rats, and this claim is confirmed by the neuroprotective effect of other androgens (33, 34). Marin et al. found that testosterone treatment had not any effect on SOD and GPX levels (35). For patients with cardiovascular risk factor that are suffering from diabetes (36) modest intensity exercise is recommended.
The present study also showed that exercise elevated antioxidant enzyme (SOD, CAT, GPx) activities and declined levels of MAD in the blood and cardiomyocytes in trained groups compared with sedentary groups. In line with previous studies, our outcomes showed that exercise rises activities of antioxidant enzymes and shows protective effects against oxidative damage in almost all tissues (37). Contrarily, Judge et al. presents that voluntary exercise decreases MnSOD activity and has no significant effect on enzymes measured (GPX, CAT) in the heart. Moreover, SOD, GPX, or CAT activities do not change in the heart by voluntary exercise. Also, MDA levels had not any significant change after 78 weeks exercise in rats (38). This contradiction may be due to differences of age at the time of death and duration of exercise. However, exercise can exert different bioactivities on diabetic heart but exactly their mechanisms of action are unknown. Several possible mechanisms can mediate the role of exercise in the oxidative stress shown by us.
The molecular mechanisms of the increasing results of exercise on SOD, GPX and CAT enzyme activities are perhaps strictly linked to increase in phosphorylation and activation of Nrf-2 (nuclear factor erythroid 2–related factor 2, an emerging regulator of cellular resistance to oxidants) (39). So, Activated Nrf-2 binds to the antioxidant response elements (ARE) to activate gene transcription (40). Interestingly, each testosterone and exercise increased SIRT1 (silent mating type information regulation 2 homolog) activity that can moderate the cellular stress response directly. Also it improved antioxidants capacity and arrest of cell cycle for helping DNA repair related to oxidative stress (41, 42). It has been suggested that increased activity of NO can be induced by testosterone and exercise (42, 43). In addition, according to this study, treatment of animals with testosterone and exercise at the same time had a synergistic increasing effect on SOD, GPX and CAT activities and a synergistic decreasing effect on MAD levels. In this study, testosterone and exercise elicited protective role against oxidative stress, as it seems that testosterone and exercise reinforce each other’s effects mutually. Fry and Lohnes’ study demonstrated that exercise through an unknown pathway increases performance of testosterone. So, this claim can partially be confirmed by the large muscle mass of sportsmen who take testosterone(44). It is also described that men with testosterone supplementation and exercise program have structural or biochemical changes in the cardiac tissue that develops more efficiently (45).
Study limitation
We did not measure other factors involving oxidative stress and we refer to previous studies.
In conclusion, this study confirms the hypothesis that increased oxidative stress in diabetes condition can be due to testosterone deficiency. Probably, heart malfunction in diabetes condition is mediated by testosterone deficiency through the induction of oxidative stress. The present study also revealed that each exercise training and testosterone therapy improve diabetes-related increased oxidative stress. Also, according to our results combination of both of these interventions have a more powerful effect on the decrease of oxidative stress.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Soares R. Angiogenesis in diabetes. Unraveling the angiogenic paradox. Open Circ Vasc J. 2010;3:3–9. [Google Scholar]
- 2.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radical Biology and Medicine. 2011;50(5):567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moussa S. Oxidative stress in diabetes mellitus. Romanian J Biophys. 2008;18(3):225–236. [Google Scholar]
- 4.Bondor C, Potra A, Rusu C, Moldovan D, Bolboacă S, Kacso I. Relationship of oxidative stress to urinary angiotensin converting enzyme 2 in type 2 diabetes mellitus patients. Acta Endocrinologica (Buchar) 2016;12(2):150–156. doi: 10.4183/aeb.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howarth FC, Al-Ali S, Al-Sheryani S, Al-Dhaheri H, Al-Junaibi S, Almugaddum FA, Qureshi MA, Ljubisavijevic M. Effects of voluntary exercise on heart function in streptozotocin (STZ)–induced diabetic rat. Int J Diabetes & Metabolism. 2007;15:32–37. [Google Scholar]
- 6.Huang K-C, Wu W-T, Yang F-L, Chiu Y-H, Peng T-C, Hsu B-G, Liao K-W, Lee R-P. Effects of freshwater clam extract supplementation on time to exhaustion, muscle damage, pro/anti-inflammatory cytokines, and liver injury in rats after exhaustive exercise. Molecules. 2013;18(4):3825–3838. doi: 10.3390/molecules18043825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chodari L, Mohammadi M, Ghorbanzadeh V, Dariushnejad H, Mohaddes G. Testosterone and voluntary exercise promote angiogenesis in hearts of rats with diabetes by enhancing expression of VEGF-A and SDF-1a. Canadian J diab. 2016;40(5):436–441. doi: 10.1016/j.jcjd.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Chodari L, Mohammadi M, Mohaddes G, Alipour MR, Ghorbanzade V, Dariushnejad H, Mohammadi S. Testosterone and Voluntary Exercise, Alone or Together Increase Cardiac Activation of AKT and ERK1/2 in Diabetic Rats. Arq Bras Cardiol. 2016;107(6):532–541. doi: 10.5935/abc.20160174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Wu S, Ruan Y, Hong L, Xing X, Lai W. Testosterone suppresses oxidative stress via androgen receptor-independent pathway in murine cardiomyocytes. Mol Med Rep. 2011;4(6):1183–1188. doi: 10.3892/mmr.2011.539. [DOI] [PubMed] [Google Scholar]
- 10.Túnez I, Feijóo M, Collado JA, Medina FJ, Peña J, Muñoz MdC, Jimena I, Franco F, Rueda I, Muntané J. Effect of testosterone on oxidative stress and cell damage induced by 3-nitropropionic acid in striatum of ovariectomized rats. Life sciences. 2007;80(13):1221–1227. doi: 10.1016/j.lfs.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Norris DO, Hobbs SL. The HPA axis and functions of corticosteroids in fishes. Science Publishers. 2006:721–765. Fish Endocrinology (2 Vols): [Google Scholar]
- 12.Sangiao-Alvarellos S, Polakof S, Arjona FJ, García-López A, del Río MPM, Martínez-Rodríguez G, Míguez JM, Mancera JM, Soengas JL. Influence of testosterone administration on osmoregulation and energy metabolism of gilthead sea bream Sparus auratus. Gen comparative endocrinol. 2006;149(1):30–41. doi: 10.1016/j.ygcen.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Khaneshi F, Nasrolahi O, Azizi S, Nejati V. Sesame effects on testicular damage in streptozotocin-induced diabetes rats. Avicenna Journal of Phytomedicine. 2013;3(4):347–355. [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett-Connor E, Khaw K-T, Yen S. Endogenous sex hormone levels in older adult men with diabetes mellitus. American Journal of Epidemiology. 1990;132(5):895–901. doi: 10.1093/oxfordjournals.aje.a115732. [DOI] [PubMed] [Google Scholar]
- 15.Chodari L, Mohammadi M, Mohaddes G, Ghorbanzadeh V, Dariushnejad H. The effect of testosterone and voluntary exercise, alone or together, on miRNA-126 expression changes in heart of diabetic rats. Acta Endocrinol (Buchar) 2017;13(3):266–271. doi: 10.4183/aeb.2017.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noeman SA, Hamooda HE, Baalash AA. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetology & Metabolic Syndrome. 2011;3(1):17. doi: 10.1186/1758-5996-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tripathi V, Verma J. Different models used to induce diabetes: a comprehensive review. Int J Pharm Sci. 2014;6(6):29–32. [Google Scholar]
- 18.Delmas-Beauvieux M, Peuchant E, Dumon M, Receveur M, Le Bras M, Clerc M. Relationship between red blood cell antioxidant enzymatic system status and lipoperoxidation during the acute phase of malaria. Clinical biochemistry. 1995;28(2):163–169. doi: 10.1016/0009-9120(94)00071-3. [DOI] [PubMed] [Google Scholar]
- 19.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of laboratory and clinical medicine. 1967;70(1):158–169. [PubMed] [Google Scholar]
- 20.Somi MH, Hajipour B, Asl NA, Estakhri R, Azare AN, Zade MN, Haghjou AG, Vatankhah AM. Pioglitazone Attenuates Ischemia/ Reperfusion-Induced Liver Injury in Rats. Transplantation Proceedings. 2009;41(10):4105–4109. doi: 10.1016/j.transproceed.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 21.Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 22.Montilla PL, Vargas JF, Túnez IF, Carmen M, Agueda M, Valdelvira M, Cabrera ES. Oxidative stress in diabetic rats induced by streptozotocin: protective effects of melatonin. J Pineal Res. 1998;25(2):94–100. doi: 10.1111/j.1600-079x.1998.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang G-g, Li W, Lu X-h, Zhao X, Xu L. Taurine attenuates oxidative stress and alleviates cardiac failure in type I diabetic rats. Croatian medical journal. 2013;54(2):171–179. doi: 10.3325/cmj.2013.54.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao H, Chen L. Icariin reduces mitochondrial oxidative stress injury in diabetic rat hearts. Zhongguo Zhong yao za zhi- Zhongguo zhongyao zazhi. China Journal of Chinese Materia Medica. 2011;36(11):1503–1507. [PubMed] [Google Scholar]
- 25.Marsh JD, Lehmann MH, Ritchie RH, Gwathmey JK, Green GE, Schiebinger RJ. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation. 1998;98(3):256–261. doi: 10.1161/01.cir.98.3.256. [DOI] [PubMed] [Google Scholar]
- 26.Golden KL, Marsh JD, Jiang Y, Moulden J. Gonadectomy alters myosin heavy chain composition in isolated cardiac myocytes. Endocrine. 2004;24(2):137–140. doi: 10.1385/ENDO:24:2:137. [DOI] [PubMed] [Google Scholar]
- 27.Khaneshi F, Nasrolahi O, Azizi S, Nejati V. Sesame effects on testicular damage in streptozotocin-induced diabetes rats. Avicenna J phytomedicine. 2013;3(4):347–355. [PMC free article] [PubMed] [Google Scholar]
- 28.Van Dam EW, Dekker JM, Lentjes EG, Romijn FP, Smulders YM, Post WJ, Romijn JA, Krans HMJ. Steroids in adult men with type 1 diabetes. Diabetes Care. 2003;26(6):1812–1818. doi: 10.2337/diacare.26.6.1812. [DOI] [PubMed] [Google Scholar]
- 29.Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Research. 2001;892(2):255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- 30.Nishino H, Nakajima K, Kumazaki M, Fukuda A, Muramatsu K, Deshpande SB, Inubushi T, Morikawa S, Borlongan CV, Sanberg PR. Estrogen protects against while testosterone exacerbates vulnerability of the lateral striatal artery to chemical hypoxia by 3-nitropropionic acid. Neuroscience research. 1998;30(4):303–312. doi: 10.1016/s0168-0102(98)00010-8. [DOI] [PubMed] [Google Scholar]
- 31.Nordeen E, Nordeen K, Sengelaub D, Arnold A. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–674. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- 32.Kujawa KA, Kinderman NB, Jones KJ. Testosterone-induced acceleration of recovery from facial paralysis following crush axotomy of the facial nerve in male hamsters. Experimental Neurology. 1989;105(1):80–85. doi: 10.1016/0014-4886(89)90174-x. [DOI] [PubMed] [Google Scholar]
- 33.Túnez I, Montilla P, Del Carmen Munoz M, Feijóo M, Salcedo M. Protective effect of melatonin on 3-nitropropionic acid-induced oxidative stress in synaptosomes in an animal model of Huntington’s disease. J Pineal Research. 2004;37(4):252–256. doi: 10.1111/j.1600-079X.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 34.Veiga S, Garcia-Segura LM, Azcoitia I. Neuroprotection by the steroids pregnenolone and dehydroepiandrosterone is mediated by the enzyme aromatase. J Neurobiology. 2003;56(4):398–406. doi: 10.1002/neu.10249. [DOI] [PubMed] [Google Scholar]
- 35.Marin DP, Bolin AP, de Cassia Macedo dos Santos R, Curi R, Otton R. Testosterone suppresses oxidative stress in human neutrophils. Cell biochemistry and function. 2010;28(5):394–402. doi: 10.1002/cbf.1669. [DOI] [PubMed] [Google Scholar]
- 36.Naderi R, Mohaddes G, Mohammadi M, Ghaznavi R, Ghyasi R, Vatankhah AM. Voluntary exercise protects heart from oxidative stress in diabetic rats. Advanced pharmaceutical bulletin. 2015;5(2):231. doi: 10.15171/apb.2015.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J, McCarter R, Yu B. Influence of age, exercise, and dietary restriction on oxidative stress in rats. Aging Clinical and Experimental Research. 1996;8(2):123–129. doi: 10.1007/BF03339566. [DOI] [PubMed] [Google Scholar]
- 38.Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2005;289(6):R1564–R72. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- 39.Toborek M, Seelbach MJ, Rashid CS, András IE, Chen L, Park M, Esser KA. Voluntary exercise protects against methamphetamine-induced oxidative stress in brain microvasculature and disruption of the blood–brain barrier. Molecular neurodegeneration. 2013;8(1):22. doi: 10.1186/1750-1326-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. Journal of Biological Chemistry. 2009;284(20):13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corbi G, Conti V, Russomanno G, Rengo G, Vitulli P, Ciccarelli AL, Filippelli A, Ferrara N. Is physical activity able to modify oxidative damage in cardiovascular aging? Oxidative medicine and cellular longevity. 2012 doi: 10.1155/2012/728547. Article ID 728547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ota H, Akishita M, Akiyoshi T, Kahyo T, Setou M, Ogawa S, Iijima K, Eto M, Ouchi Y. Testosterone deficiency accelerates neuronal and vascular aging of SAMP8 mice: protective role of eNOS and SIRT1. PloS one. 2012;7(1) doi: 10.1371/journal.pone.0029598. e29598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eksakulkla S, Suksom D, Siriviriyakul P, Patumraj S. Increased NO bioavailability in aging male rats by genistein and exercise training: using 4, 5-diaminofluorescein diacetate. Reproductive Biology and Endocrinology. 2009;7(1):93. doi: 10.1186/1477-7827-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fry A, Lohnes C. Acute testosterone and cortisol responses to high power resistance exercise. Human Physiology. 2010;36(4):457–461. [PubMed] [Google Scholar]
- 45.Gonçalves L, de Souza RR, Maifrino LBM, Caperuto ÉC, Carbone PO, Rodrigues B, Gama EF. Resistance exercise and testosterone treatment alters the proportion of numerical density of capillaries of the left ventricle of aging Wistar rats. The Aging Male. 2014;17(4):243–247. doi: 10.3109/13685538.2014.919252. [DOI] [PubMed] [Google Scholar]