Abstract
Context
Endothelial dysfunction and diabetic cardiomyopathy are critical complications of diabetes. Gallic acid (GA) plays a significant role in cardiovascular disorders resulted from diabetes. In addition, increased plasma miR-24, miR-126 associated with endothelial dysfunction.
Aim
The current study was designed to assess the effects of GA on plasma miR-24, miR-126 levels in the diabetic rats.
Animals and Methods
Adult male Sprague-Dawley rats were divided into three groups (n=8): control (C), diabetic (D) and diabetic group treated with GA (D+G, 25 mg/kg, by gavage) for eight weeks. The blood glucose level, body weight, lipid profile, blood pressure, plasma miR-24 and miR-126 levels, antioxidant and inflammatory biomarkers were measured.
Results
The plasma levels of miR-24, miR-126, body weight, high-density lipoprotein cholesterol (HDL-c), total anti-oxidant capacity (TAC) and the systolic blood pressure significantly reduced and blood glucose, total cholesterol (TC), triglycerides (TG), very low-density lipoprotein cholesterol (VLDL-c), malondialdehyde (MDA), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α) and low-density lipoprotein cholesterol (LDL-c) significantly elevated among the diabetic rats compared with the control group. However, GA restored body weight, blood pressure, TC, TG, VLDL-c, TNF-α, miR-126, blood glucose, HDL-c, MDA, TAC, miR-24 and IL-6 among the GA treated rats compared with the diabetic group.
Conclusion
GA improves inflammation, oxidative stress and hypotension result from diabetes. These protective effects are probably mediated via increasing plasma miR-24 and miR-126 levels.
Keywords: Diabetes, Endothelium, MicroRNA, Gallic acid
INTRODUCTION
Diabetes mellitus is the most common metabolic multifactorial disease in which endothelial dysfunction, dyslipidemia, cardiomyopathy and micro- and macro-vascular complications are the major causes of death in diabetic patients (1, 2).
MicroRNAs (miRNAs) are a class of short (21–25 nucleotides long) non-protein encoding RNAs that modulate gene expression via targeting mRNAs for degradation or translational repression (3). In the cardiovascular system, different miRNAs are implicated in regulation of diabetes and diabetic complications including atherosclerosis and angiogenesis. Particularly, miR-24 and miR-126 decrease in diabetic patients (2, 4). In addition, the high level of miR-126 is expressed in endothelial cells and is involved in vascular repair, angiogenesis and has anti-inflammatory effects by endothelial vascular adhesion molecule (VCAM-1) expression (4). Recent studies have demonstrated that miR-24 is involved in the modulation of endothelial cells’ function including apoptosis, inflammation and proliferation (5-8). In addition, a recent study has reported that reduction of miR-24 level in heart endothelial cells is associated with endothelial dysfunction in diabetic mice (9). It also has been indicated that miR-24 over-expression reduced atherosclerosis by decreasing vascular smooth muscle cells (VSMCs) inflammation (10). Thus, miR-24 and miR-126 describe novel therapeutic targets to prevent against vascular complications of diabetic patients. The main oxidizing factors are reactive oxygen species (ROS), including superoxide anions and hydroxyl radicals. ROS and its associated DNA injury regulate miRNAs processing and maturation, thus is effective on miRNAs targets (11).
GA is a natural component that belongs to the larger family of polyphenols and is specifically found in gallnuts, grapes, tea leaves, oak bark, blackberry and pomegranates (12). A previous investigation has shown that GA could restore vasodilator response of mesenteric arterial bed to histamine using the endothelium H1 receptor in diabetic rats (13). On the other hand, antioxidant agents acting on ROS and ionic pumps dysfunction in the heart in high glucose conditions provide protection against the QT interval prolongation (14). Taking into consideration all above points, this study aimed to evaluate the efficacy of chronic GA treatment on plasma level of miR-24 and miR-126 that both microRNAs regulate oxidative stress and inflammation as well as vascular complications induced by diabetes in the alloxan-induced diabetic rats.
MATERIALS AND METHODS
Drugs
GA (3, 4, 5-Trihydroxybenzoic acid), heparin and alloxan monohydrate were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.) and Ketamine (10%) and xylazine (2%) were obtained from Alfasan Co (Woderen- Holland).
Animal groups
Adult male Sprague-Dawley rats (250 ± 20g, obtained from Ahvaz Jundishapur University of Medical Sciences Animal House Center, Ahwaz, Iran) were housed in cages at room temperature (22-25°C) with a 12 h light/dark cycle and free access to food and water throughout the experiment. After seven days of adaptation, the rats were assigned randomly into three groups (8 rats each): control (C), diabetic (D) and diabetic treated with GA (D+G). After verification of diabetes in the alloxan injected animals, GA 25 mg/kg/day (15-17, 27) was administered by gavage for eight weeks. The experimental protocol and procedures were approved by the Institutional Animal Care and Ethics Committee of the Ahvaz Jundishapur University of Medical Sciences based on the guidelines for the care and use of laboratory animals (grant No. APRC-94-25).
Induction of diabetes
Diabetes was induced in the rats by a single intraperitoneal (IP) injection of alloxan (120 mg/kg). Six h after alloxan administration, the rats have received 10 % glucose solution for the next 24 h to prevent against the lethal hypoglycemic effect induced by alloxan. After 4 days, the rats that exhibited blood glucose higher than 250 mg/dL considered as the diabetic rats. At the beginning of experiment and eight weeks after induction of diabetes blood samples were collected from tail vein and the blood glucose level was measured using a glucometer (19-21).
Blood samples from abdominal vein were poured into EDTA containing tubes and centrifuged at 4000 g for 10 min, then the plasma was stored at -80°C for later measurement of lipid profile, total anti-oxidant capacity (TAC), malondialdehyde (MDA), TNF-α, IL-6 and molecular assessments.
Plasma lipid profile, TAC, MDA, TNF-a and IL-6
Total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c) were measured using appropriate commercial kits (Pars Azmun, Tehran, Iran). In addition, the very low-density lipoprotein cholesterol (VLDL-c) was computed using the following equation: VLDL-c = Total serum triglycerides / 5. Furthermore, plasma levels of TAC, MDA and IL-6 were determined by commercial assay kits (Zell Bio, Ulm, Germany). Finally, the TNF-α levels were measured by appropriate ELISA kit (Diaclone, Besancon, France). The measurements were carried out according to the manufacturer’s protocols.
Total RNA extraction, cDNA synthesis and quantitative real time PCR
Mature miRNAs were extracted from 250 µl plasma using Trizol reagent (Qiagen, USA) according to the manufacturer’s protocol and rapidly stored at -80°C. RNA concentration was measured by Nanodrop (Nanodrop thermo scientific S.N:D015). Reverse transcription into cDNA was performed by TaqMan miRNA reverse transcription kit (Qiagen, USA). Real time PCR was done with Light Cycler® 96 real time PCR system by these conditions: 95°C for 15 min, then 45 cycles at 94°C for 15 sec, 55°C for 30 sec and 70°C for 30 sec. The miRNAs were amplified using specific miRNAs primers and a universal primer (Qiagen, USA). Moreover, RNase-free water was used as negative control in each step. The U6 (MS00033740) was used as internal controls for the normalization of the miR-24 (MS00005537) and miR-126 (MS00000329) templates. The threshold cycle (Ct) was acquired from amplification of all miRNAs. Ct was organized in the exponential phase for each target PCR and the relative expressions of miR-24 and miR-126 were analyzed by the 2-(DDCt) method (22). Sequences of primers used were as follows:
U6: 5’-CTCGCTTCGGCAGCACA-3´
MiR-24: 5’-UGGCUCAGUUCAGCAGGAACAG-3´
MiR-126: 5’-UCGUACCGUGAGUAAUAAUGCG-3´
Systolic blood pressure recording
The blood pressure was measured by tail plethysmography coupled to a recorder system (Powerlab, AD Instrument, Australia) coupled to a computer. The systolic blood pressure recorded 3-4 times and the average was considered as animal systolic blood pressure.
Statistical analysis
The data were presented as mean ± SEM. In this study Kolmogorov-Smirnov analysis was used to determine the normal distribution of the data. We concluded that there was no significant difference between the experimental data and normally distributed data. Thus, the data were analyzed by one way analysis of variance (ANOVA) followed by LSD post hoc test or pair t-test as appropriate. P<0.05 was regarded statistically significant.
RESULTS
Body weight and blood glucose
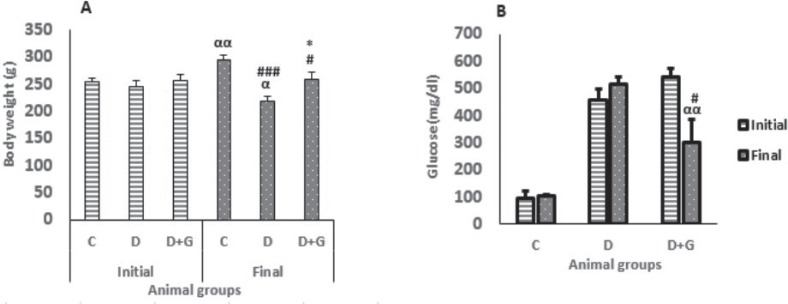
Eight weeks after induction of diabetes the body weight of diabetic group was decreased significantly compared with the control group (218.12 ± 8.38 vs. 294.12 ± 9.32, P<0.001) and initial weight (218.12 ± 8.38 vs. 245.75 ± 11.24, P<0.05). On the other hand, GA administration for eight weeks in the diabetic group significantly prevented the body weight reduction compared with the untreated diabetic rats (258.25 ± 13.5 vs. 218.12 ± 8.38, P<0.05, Fig. 1a).
Figure 1.
Body weight (a) and blood glucose level (b) (mean ± SEM, n = 8) in control (C), diabetic (D) and diabetic treated with GA (25 mg/kg, D+G) before and after eight weeks of treatment.
Furthermore, eight weeks after induction of diabetes the plasma glucose was not decreased in the diabetic group. However, treatment with GA in the diabetic animals reduced significantly plasma glucose compared with the untreated diabetic rats (300.25 ± 85.8 vs. 514 ± 27.7, P<0.05) and initial glucose level (300.25 ± 85.8 vs. 543.75 ± 27.8, P<0.01, Fig. 1b).
Plasma lipid profile
The lipid profile of different groups was shown in Table 1. As indicated, TC, TG, VLDL-c (P<0.001) and LDL-c (P<0.05) were increased but HDL-c (P<0.001) was decreased significantly in the diabetic animals when compared with the control group. Nevertheless, GA administration for eight weeks resulted in a significant reduction of TC, TG and VLDL-c (P<0.05) and a significant increase in HDL-c (P < 0.01) compared with the untreated diabetic animals.
Table 1.
Lipid profile in animal groups
| Groups | C | D | D+G |
| TC (mg/ dL) | 44.87±1.24 | 61.22±2.11### | 50.11±1.69* |
| TG (mg/ dL) | 29.57±2.7 | 56.55±4.3### | 38.37±3.9* |
| HDL-c (mg/ dL) | 35.37±0.59 | 25.57±0.64### | 31.62±0.84** |
| LDL-c (mg/ dL) | 23.85±0.4 | 27.71±0.47# | 24.25±0.94 |
| VLDL-c (mg/ dL) | 5.91±0.5 | 11.21±0.86### | 7.6±0.79* |
TC, total cholesterol; TG, triglycerides; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; VLDL-c, very low-density lipoprotein cholesterol. (Mean ± SEM, n = 8), # P < 0.05, ### P < 0.001 versus control group, * P < 0.05, ** P < 0.001 versus untreated diabetic group. (One-way ANOVA followed by LSD post hoc test).
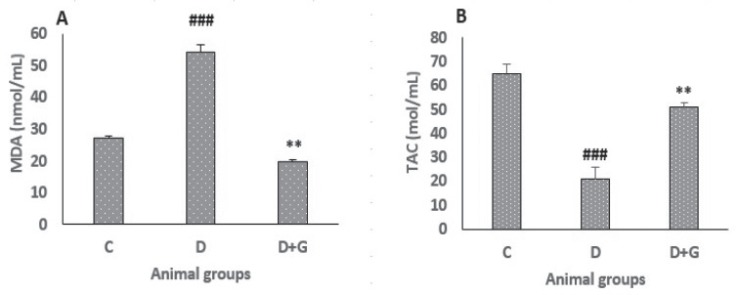
Plasma MDA and TAC
As shown in Fig. 2a, the plasma level of MDA was increased significantly in the diabetic animals compared with the control group (54.07 ± 2.6 vs. 27.06 ± 0.7, P < 0.001). However, oral administration of GA for eight weeks in the diabetic rats resulted in a significant reduction of MDA plasma level when compared with the untreated diabetic group (19.7 ± 0.8 vs. 54.07 ± 2.6, P<0.01). TAC level was measured to assess the non-enzymatic defense activity in the plasma against the oxidative damage. As shown in Fig. 2b, TAC plasma level in the diabetic rats was decreased significantly compared with control group (21 ± 5 vs. 65 ± 4, P<0.001). However, administration of GA in the diabetic rats increased significantly TAC level when compared with the untreated diabetic rats (51 ± 2 vs. 21 ± 5, P<0.01).
Figure 2.
Data were analyzed by one-way ANOVA followed by LSD post hoc test. ###p<0.001 versus control group, **p<0.01 versus untreated diabetic group.
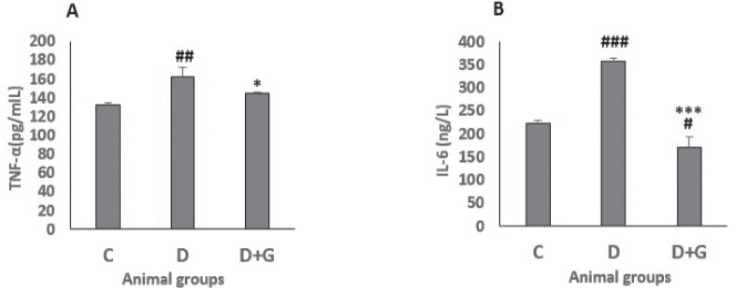
Plasma TNF-α and IL-6
The diabetic rats showed a significant higher TNF-α plasma level than the control rats (162.12 ± 10.5 vs. 132.5 ± 1.82, P<0.01). However, the TNF-α level in the diabetic rats treated with GA was significantly lower than that of untreated diabetic animals (144.5 ± 1 vs. 162.12 ± 10.5, P<0.05, Fig. 3a). In addition, the plasma level of IL-6 of diabetic animals was significantly higher than of control group (357.5 ± 6.8 vs. 223.27 ± 7, P<0.001). Nevertheless, IL-6 level was decreased significantly in the diabetic rats administered with GA compared with both control and untreated diabetic rats (171.09 ± 23.6 vs. 223.27 ± 7 and 357.5 ± 6.8, P<0.05, P<0.001, respectively, Fig. 3b).
Figure 3.
Significance was analyzed by one-way ANOVA followed by LSD post hoc test. #p<0.05, ##p<0.01, ###p<0.001, versus control group. *p<0.05, ***p<0.001 versus untreated diabetic group.
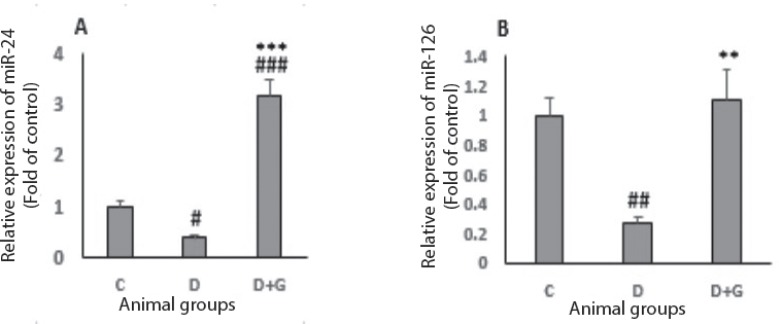
Plasma miR-24 and miR-126
The results indicated that a significant reduction observed in plasma level of miR-24 in the diabetic animals compared with control group (0.37 ± 0.04 vs. 1 ± 0.1, P<0.05). Nevertheless, administration of GA in the diabetic animals induced a significant increase in plasma level of miR-24 compared with the control and untreated diabetic groups (3.16 ± 0.31 vs. 1 ± 0.1 and 0.37 ± 0.04 respectively, P<0.001, Fig. 4a). The plasma level of miR-126 decreased significantly in the diabetic group when compared with control rats (0.27 ± 0.07 vs. 1 ± 0.2, P<0.01). However, treatment with GA in the diabetic animals induced a significant increase in plasma level of miR-126 compared with the untreated diabetic group (1.1 ± 0.33 vs. 0.27 ± 0.07, P<0.01, Fig. 4b).
Figure 4.
Data were analyzed by one-way ANOVA followed by LSD post hoc test. #p<0.05, ##p<0.01, ###p<0.001, versus control group, **p<0.01, ***p<0.001 versus untreated diabetic group.
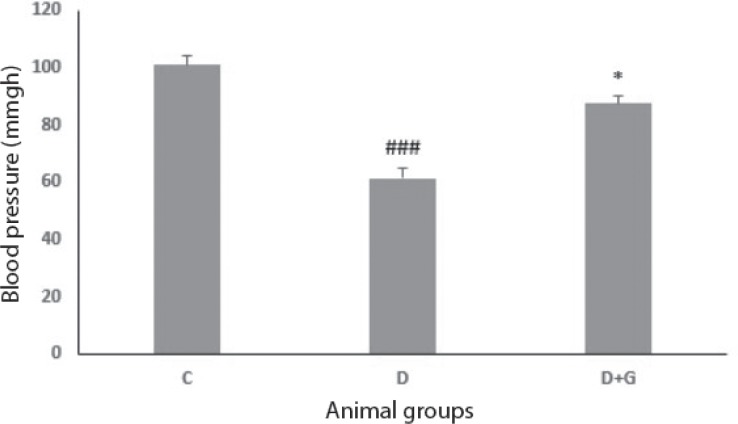
Systolic blood pressure
The systolic blood pressure was reduced significantly in the diabetic rats in comparison with the control group (61.32 ± 3.4 vs. 101 ± 3, P<0.001). On the other hand, treatment of diabetic animals with GA inhibited this reduction in blood pressure significantly compared with the untreated diabetic group (87.5 ± 2.6 vs. 61.32 ± 3.4, P<0.05, Fig. 5).
Figure 5.
Data were analyzed by one-way ANOVA followed by LSD post hoc test. ###p<0.001 versus control group, *p<0.05 versus untreated diabetic group.
DISCUSSION
The present study was aimed to assess the effects of GA in diabetic rats, which was associated with significant reduction in miR-24, miR-126, TAC, systolic blood pressure, and elevation in plasma MDA, TNF-α, IL-6 levels. This study showed that administration of GA in the diabetic rats improved the plasma level of miR-24 and miR-126 involved in important pathways in endothelial dysfunction and possibly hypotension.
In this study the triglyceride, cholesterol, LDL-c and VLDL-c levels were increased and the HDL-c level was decreased in the diabetic animals. However, GA treatment restored these parameters. Therefore, it is possible that improved glucose and lipid profile levels via GA administration have a main function in the improvement of body weight.
Recently, hyperglycemia is regarded to be a primary factor for the change of lipid profile. Dyslipidemia is generally well demonstrated in diabetes mellitus; it is determined as an index for the assessment of type I diabetes and beta-cell dysfunction (23). Dyslipidemia is associated to atherosclerosis and cardiovascular disorders (23). Moreover, it is associated to reduced plasma miR-126 levels (24). Therefore, this negative relationship between plasma miR-126 and dyslipidemia may indicate a possible role of miR-126 in the metabolism of lipids in diabetes.
A previous study indicated that patients with coronary artery disease and type 2 diabetes mellitus had lower plasma miR-126 levels. It was proposed that hyperglycemia can cause a reduction in the miR-126 level in the endothelial particles (3). Decreased miR-126 level which may modulate vascular integrity and angiogenesis contributes in the complications and pathogenesis of diabetes (25).
Li et al. has indicated that hyperglycemia and advanced glycation end products increased TNF-α, IL-6 and ROS and reduced miR-126 expression in endothelial progenitor cells, also increased miR-126 expression, decreased ROS and inflammatory factors (26).
Vascular smooth muscle cells (VSMCs) dysfunction plays an important role in the pathogenesis of vascular disease induced by diabetes. Recent studies have demonstrated that miR-24 may contribute to atherosclerotic vascular diseases and diabetes (27, 28). It has been reported that miR-24 is a key modulator of migration and proliferation induced by high glucose in VSMCs, and proposed that increased miR-24 in vascular system may lead to prevent the progression of diabetic atherosclerosis (10).
A recent study has revealed that GA had protective effects in various pathological diseases such as cardiovascular diseases linked with diabetes (29). Polyphenols from red wine have been also reported for anti-inflammatory properties by increasing miR-126 in human colon-derived CCD-18Co myofibroblast cells (30). Regarding the increased level of miR-126 and miR-24 in plasma following GA treatment in diabetic rats, protective effects of GA on inflammation, oxidative stress and vascular complications induced by diabetes is possibly related to GA-induced miR-126 and miR-24 expression.
In the present study, hypotension was observed in the alloxan-induced diabetic rats after eight weeks. The administration of GA improved the alloxan-induced hypotension toward controls. This protective effect of GA may be due to the improvement of oxidant/anti-oxidant ratio and glucose homeostasis that observed in this study and/or ventricular contractility, peripheral resistance and arterial compliance induced by diabetes (13). It has been reported that GA improved ventricular contractility and peripheral resistance in patients with cardiovascular disorders (31).
Dicer is abundantly expressed in VSMCs and has a critical role in supporting vascular function (32). The structure and function of vascular system is impaired in mouse model of VSMC deletion of Dicer. In this model, the vessels are dilated and reduced medial thickness due to decreased cellular proliferation and contractile response in vascular system resulted in hypotension (33). Recently, Albinsson et al. have demonstrated that miRNA turnover modulated important genes levels in VSMCs that were involved in the regulation of contraction (30). Moreover, it has been shown that miR-24, a VSMC-enriched miRNA, possibly involved in the induction of hyperplasia in neointimal and vascular resistance via controlling VSMC function(10). Thus, protective effect of GA on hypotension induced by diabetes may result from the improvement of miR-24 plasma level in the diabetic rats although more investigations are needed to be done.
A previous investigation has shown that ROS and inflammation resulted in cell death in the heart by increasing inflammatory cytokines levels and activating apoptotic pathway in diabetic animals (34). According to this study it seems that apoptosis increases in the alloxan-induced diabetic rats as evidenced via increasing MDA, IL-6 and TNF-α and decreasing TAC. These findings could be improved by GA, indicating that oxidative stress and inflammation trigger cardiovascular injury in diabetes.
In conclusion, the results propose that GA attenuates plasma miR-24 and miR-126 levels, hypotension result from diabetes. These protective effects are probably mediated via increasing plasma miR-24 and miR-126 levels and decreasing inflammation and oxidative stress. Thus, the miR-24 and miR-126 could be a promising target for GA in the improvement of cardiovascular disorders and suggest it as a complementary compound for patients with diabetes. However, more investigations are needed to confirm GA effects and to find out the mechanisms by which these two miRs mediate the effects of GA.
Conflict of interest
The author declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgement
The authors gratefully thank the assistance and funding of the Ahvaz Physiology Research Center and Research Affairs of the Ahvaz Jundishapur University of Medical Sciences (grant No. APRC-9425). This paper was extracted from data of the Ph.D. thesis of Miss. Fatemeh Ramezani Aliakbari, Ph.D. student of physiology.
References
- 1.Barutta F, Bruno G, Matullo G, Chaturvedi N, Grimaldi S, Schalkwijk C, Stehouwer CD, Fuller JH, Gruden G. MicroRNA-126 and micro-/macrovascular complications of type 1 diabetes in the EURODIAB Prospective Complications Study. Acta Diabetol. 2017;54(2):133–139. doi: 10.1007/s00592-016-0915-4. [DOI] [PubMed] [Google Scholar]
- 2.Xiang Y, Cheng J, Wang D, Hu X, Xie Y, Stitham J, Atteya G, Du J, Tang WH, Lee SH, Leslie K, Spollett G, Liu Z, Herzog E, Herzog RI, Lu J. Hyperglycemia repression of miR-24 coordinately upregulates endothelial cell expression and secretion of von Willebrand factor. Blood. 2015;125(22):3377–3387. doi: 10.1182/blood-2015-01-620278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107(6):810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Gao G, Yang C, Zhou K, Shen B, Liang H, Jiang X. The role of circulating microRNA-126 (miR-126): a novel biomarker for screening prediabetes and newly diagnosed type 2 diabetes mellitus. Int J Mol Sci. 2014;15(6):10567–10577. doi: 10.3390/ijms150610567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maegdefessel L, Spin JM, Raaz U, Eken SM, Toh R, Azuma J, Adam M, Nakagami F, Heymann HM, Chernogubova E, Jin H, Roy J, Hultgren R, Caidahl K, Schrepfer S, Hamsten A, Eriksson P, McConnell MV, Dalman RL, Tsao PS. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun. 2014;5:5214. doi: 10.1038/ncomms6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenzen JM, Kaucsar T, Schauerte C, Schmitt R, Rong S, Hübner A, Scherf K, Fiedler J, Martino F, Kumarswamy R, Kölling M, Sörensen I, Hinz H, Heineke J, van Rooij E, Haller H, Thum T. MicroRNA-24 antagonism prevents renal ischemia reperfusion injury. J Am Soc Nephrol. 2014;25(12):2717–2729. doi: 10.1681/ASN.2013121329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiedler J, Jazbutyte V, Kirchmaier BC, Gupta SK, Lorenzen J, Hartmann D, Galuppo P, Kneitz S, Pena JT, Sohn-Lee C, Loyer X, Soutschek J, Brand T, Tuschl T, Heineke J, Martin U, Schulte-Merker S, Ertl G, Engelhardt S, Bauersachs J, Thum T. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124(6):720–730. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 8.Meloni M, Marchetti M, Garner K, Littlejohns B, Sala-Newby G, Xenophontos N, Floris I, Suleiman MS, Madeddu P, Caporali A, Emanueli C. Local inhibition of microRNA-24 improves reparative angiogenesis and left ventricle remodeling and function in mice with myocardial infarction. Mol Ther. 2013;21(7):1390–1402. doi: 10.1038/mt.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui YX, Hua YZ, Wang N, Chen X, Wang F, Liu JY, Wang LL, Yan CY, Ma YG, Cao YH, Zhang XH. MiR-24 suppression of POZ/BTB and AT-hook-containing zinc finger protein 1 (PATZ1) protects endothelial cell from diabetic damage. Biochem Biophys Res Commun. 2016 doi: 10.1016/j.bbrc.2016.10.116. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Chen L, Ding J, Fan Z, Li S, Wu H, Zhang J, Yang C, Wang H, Zeng P, Yang J. MicroRNA-24 inhibits high glucose-induced vascular smooth muscle cell proliferation and migration by targeting HMGB1. Gene. 2016;586(2):268–273. doi: 10.1016/j.gene.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 11.Hu H, Gatti RA. MicroRNAs: new players in the DNA damage response. J Mol Cell Biol. 2011;3(3):151–158. doi: 10.1093/jmcb/mjq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanely Mainzen Prince P, Priscilla H, Devika PT. Gallic acid prevents lysosomal damage in isoproterenol induced cardiotoxicity in Wistar rats. Eur J Pharmacol. 2009;615(1-3):139–143. doi: 10.1016/j.ejphar.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Badavi M, Bazaz A, Dianat M, Sarkaki A. Gallic acid improves endothelium-dependent vasodilatory response to histamine in the mesenteric vascular bed of diabetic rats. J Diabetes. 2016 doi: 10.1111/1753-0407.12513. [DOI] [PubMed] [Google Scholar]
- 14.Ceriello A, Quagliaro L, D’Amico M, Di Filippo C, Marfella R, Nappo F, Berrino L, Rossi F, Giugliano D. Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes. 2002;51(4):1076–1082. doi: 10.2337/diabetes.51.4.1076. [DOI] [PubMed] [Google Scholar]
- 15.Umadevi S, Gopi V, Elangovan V. Regulatory mechanism of gallic acid against advanced glycation end products induced cardiac remodeling in experimental rats. Chem Biol Interact. 2014;208:28–36. doi: 10.1016/j.cbi.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Ramezani-Aliakbari F, Badavi M, Dianat M, Mard SA, Ahangarpour A. Effects of gallic acid on hemodynamic parameters and infarct size after ischemia-reperfusion in isolated rat hearts with alloxan-induced diabetes. Biomed Pharmacother. 2017;96:612–618. doi: 10.1016/j.biopha.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Ramezani-Aliakbari F, Badavi M, Dianat M, Mard SA, Ahangarpour A. Protective effects of gallic acid on cardiac electrophysiology and arrhythmias during reperfusion in diabetes. Iran J Basic Med Sci. 2019;22:515–520. doi: 10.22038/ijbms.2019.27563.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramezani-Aliakbari F, Badavi M, Dianat M, Mard SA, Ahangarpour A. Protective effects of gallic acid on cardiac electrophysiology and arrhythmias during reperfusion in diabetes. Iran J Basic Med Sci. 2019;22:515–520. doi: 10.22038/ijbms.2019.27563.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gargouri M, Magne C, El Feki A. Hyperglycemia, oxidative stress, liver damage and dysfunction in alloxan-induced diabetic rat are prevented by Spirulina supplementation. Nutr Res. 2016;36(11):1255–1268. doi: 10.1016/j.nutres.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 21.Ighodaro OM, Adeosun AM, Akinloye OA. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina (Kaunas) 2017;53(6):365–374. doi: 10.1016/j.medici.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Khanaghaei M, Tourkianvalashani F, Hekmatimoghaddam S, Ghasemi N, Rahaie M, Khorramshahi V, Sheikhpour A, Heydari Z, Pourrajab F. Circulating miR-126 and miR-499 reflect progression of cardiovascular disease; correlations with uric acid and ejection fraction. Heart Int. 2016;11(1):1–9. doi: 10.5301/heartint.5000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorensen CM, Ding J, Zhang Q, Alquier T, Zhao R, Mueller PW. Perturbations in the lipid profile of individuals with newly diagnosed type 1 diabetes mellitus lipidomics analysis of a Diabetes Antibody Standardization Program sample subset. Clin Biochem. 2010;43(12):948–956. doi: 10.1016/j.clinbiochem.2010.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pishavar E, Behravan J. MiR-126 as a therapeutic agent for diabetes mellitus. Curr Pharm Des. 2017;23(22):3309–3314. doi: 10.2174/1381612823666170424120121. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Zhou Q, Pei C, Liu B, Li M, Fang L, Sun Y, Li Y, Meng S. Hyperglycemia and advanced glycation end products regulate miR-126 expression in endothelial progenitor cells. J Vasc Res. 2016;53(1-2):94–104. doi: 10.1159/000448713. [DOI] [PubMed] [Google Scholar]
- 27.Patel SS, Goyal RK. Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats. Pharmacognosy Res. 2011;3(4):239–245. doi: 10.4103/0974-8490.89743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang Y, Cheng J, Wang D, Hu X, Xie Y, Stitham J, Atteya G, Du J, Tang WH, Lee SH, Leslie K, Spollett G, Liu Z, Herzog E, Herzog RI, Lu J, Martin KA, Hwa J. Hyperglycemia repression of miR-24 coordinately upregulates endothelial cell expression and secretion of von Willebrand factor. Blood. 2015;125(22):3377–87. doi: 10.1182/blood-2015-01-620278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel SS, Goyal RK. Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats. Pharmacognosy Res. 2011;3(4):239–245. doi: 10.4103/0974-8490.89743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angel-Morales G, Noratto G, Mertens-Talcott S. Red wine polyphenolics reduce the expression of inflammation markers in human colon-derived CCD-18Co myofibroblast cells: potential role of microRNA-126. Food Funct. 2012;3(7):745–752. doi: 10.1039/c2fo10271d. [DOI] [PubMed] [Google Scholar]
- 31.Kar P, Laight D, Shaw KM, Cummings MH. Flavonoid-rich grapeseed extracts: a new approach in high cardiovascular risk patients? Int J Clin Pract. 2006;60(11):1484–1492. doi: 10.1111/j.1742-1241.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 32.Albinsson S, Skoura A, Yu J, DiLorenzo A, Fernández-Hernando C, Offermanns S, Miano JM, Sessa WC. Smooth muscle miRNAs are critical for post-natal regulation of blood pressure and vascular function. PloS one. 2011;6(4):18869. doi: 10.1371/journal.pone.0018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol. 2010;30(6):1118–1126. doi: 10.1161/ATVBAHA.109.200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang W, Chen M, Zheng D, He J, Song M, Mo L, Feng J, Lan J. A novel damage mechanism: Contribution of the interaction between necroptosis and ROS to high glucose-induced injury and inflammation in H9c2 cardiac cells. Int J Mol Med. 2017;40(1):201–108. doi: 10.3892/ijmm.2017.3006. [DOI] [PubMed] [Google Scholar]