Abstract
Context
Visceral adipose tissue (VAT) is a strong predictor of carbohydrate metabolism disorders. Abdominal bioelectrical impedance analysis (A-BIA) is a simple method for the measurement of VAT and is a promising tool in screening and follow-up of abdominal obesity. However the role of A-BIA in dieting individuals has not been evaluated adequately in longitudinal follow-up studies.
Objective
The aim of this study is to determine the role of A-BIA in identifying the changes in metabolic predictors after diet and/or exercise therapy.
Design
All patients who sought weight loss treatment underwent baseline assessment and were prescribed a program of diet. After a mean follow-up of 3.2 months, data were analyzed.
Subjects and Methods
Ultimately, 103 participants who reported adhering to the diet, enrolled to the study. We tested associations between changes in body composition measures and changes in laboratory measures using correlations and multivariate linear regression analysis.
Results
Mean loss of body weight was 3.4±2.8 kg. All but waist-to-hip ratio, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol levels changed significantly (p<0.001). Decreases in body weight, body mass index (BMI), and VAT level significantly correlated with decreases in fasting blood glucose, fasting insulin level, and HOMA-IR score (r=0.230–0.371). In multiple linear regression analysis changes in BMI and VAT significantly correlated with change in HOMA-IR score (F(7.93)=2.283, p=0.034, R2=0.147).
Conclusion
Decreases in BMI and VAT, as determined by A-BIA, were predictors of changes in metabolic laboratory measures. A-BIA is useful for follow-up of patients receiving diet therapy for weight loss.
Keywords: Diet, Abdominal bioelectrical impedance, Anthropometry, Insulin resistance
INTRODUCTION
Studies conducted over the past 20 years have provided abundant evidence for the relationship between obesity, and morbidity and mortality due to numerous diseases (1). Abdominal obesity is associated with impaired glucose and lipid metabolism, insulin resistance (IR) (2), cardiovascular disease (3), and cancer (4).
Previous studies reported that individuals with the same body mass index (BMI) and waist circumference (WC) may have different metabolic and cardiovascular risk profiles (5). Furthermore, normal-weight individuals with a high metabolic and cardiovascular risk profile have a higher amount of adipose tissue (6, 7). The distribution and amount of adipose tissue is affected by numerous factors, including age, gender, and ethnicity (8). Adipose tissue has two components: subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). Increases in SAT and VAT are associated with impaired fasting glucose (9), diabetes mellitus (10), IR (11), hypertension (12, 13), hyperlipidemia (14), and metabolic syndrome (MetS) (15). Several recent studies suggest that the various adipose tissue compartments are related to distinct metabolic risks (16). In particular, although the VAT compartment appears to be a unique pathogenic fat depot, the relationship between peripheral adipose tissue and metabolic impairment is less clear (17). Furthermore, there is some evidence indicating that deep abdominal SAT is strongly associated with insulin sensitivity (18). Nevertheless, the current scientific consensus is that, increases in both components are related to metabolic impairment and that VAT is the most important risk factor (19).
Although WC and BMI are useful and straightforward anthropometric measures for clinical and epidemiological studies, they do not suffice for defining the complex biology of adiposity. However, gold standard methods such as computed tomography (CT) and magnetic resonance imaging (MRI) are difficult to use in the clinical setting because they are time-consuming, expensive, and rely on non-portable devices (8, 20, 21). Therefore, studies are being performed for the development of straightforward, reliable, inexpensive, time-saving, and easily transportable methods for clinical measurement of VAT.
Abdominal bioimpedance analysis (A-BIA) has been investigated in a number of studies. BIA has been used in the clinical setting since the mid-1980s and is accepted as a useful method for clinical studies (22). Although many studies on weight loss have used conventional BIA, very few have utilized A-BIA. Most of the previous studies comparing A-BIA with advanced methods such as CT or MRI found a strong relationship between A-BIA and the imaging techniques (20, 21, 23, 24). However, those studies were correlation studies rather than clinical follow-up studies, as follow-up studies with CT or MRI are challenging and expensive.
An association between decreased adipose tissue volume in obese individuals and improved glucose and lipid metabolism was reported in a previous study (25). Nevertheless, very few longitudinal follow-up studies have used A-BIA to investigate the correlation between changes in the body composition caused by diet and/or exercise and metabolic risk factors. The primary aim of this study is to determine the role of A-BIA in identifying the changes in metabolic predictors after diet and/or exercise therapy. The secondary aim is to determine the predictors of metabolic improvement in dieters.
MATERIALS AND METHODS
This prospective study was conducted in the period between December 2011-June 2013, at the Department of Endocrinology and Metabolism in Başkent University. This study was approved by Başkent University Institutional Review Board and Ethics Committee (Project no: KA12/192), and supported by Başkent University Research Fund. All participants gave their written informed consent before taking part in the study.
Patients and follow-up
A total of 134 participants who were admitted to hospital for weight loss therapy were enrolled in the study. After initial evaluation, all participants were prescribed an exercise program and an appropriate diet with caloric restriction, and underwent a follow-up examination 3±1 months later. All individuals were evaluated by the same dietician and prescribed a low calorie diet to enable weight loss. Each individual was prescribed a specific diet according to his/her lifestyle and level of activity. All participants were re-evaluated by the same dietician after 2 weeks and their prescribed diet was reorganized if needed. On their second visit, all participants were asked by their physician to complete a short questionnaire on diet and exercise adherence. Thirty-one patients were excluded from the analysis as they reported at their second visit that they failed to adhere to the diet. Ultimately, data from 103 participants who reported that they adhered to the diet were included in the analysis. All measurements were repeated in the follow-up visit.
Inclusion and exclusion criteria
Healthy subjects aged 18 to 70 years who are willing to lose weight and do not have any of the criteria for exclusion were included in the study. Exclusion criteria comprised previously known cancer, pacemaker implantation, cardiac valve replacement, malabsorption syndrome, previous history of gastrointestinal surgery, thyroid disease, pregnancy, history of surgery within the last 3 months, usage of oral antidiabetic drugs, insulin, any treatment for obesity, steroids or hormonal drugs, over-the-counter drugs for weight loss, any kind of phytodrug, or any drug that can affect glucose metabolism. Patients who used any of these drugs after the initial evaluation were excluded, as well as patients who reported at their second visit that they failed to adhere to the diet. A participants who lost weight via any of the treatment modalities such as diet and/or exercise, anti-obesity drugs or bariatric surgery in the period before study admission were excluded from the study.
Definitions for BIA methods
Most of the body consists of water, which conducts electrical current. Adipose tissue is much more resistant to electrical current than the bone and muscle. BIA is based on the principle that electrical current passes through different tissue types at varying rates (22). A-BIA is a relatively new type of BIA, in which the VAT was measured by an A-BIA device (AB-140 Viscan, Tanita corp., Tokyo, Japan) and the ranges for VAT measurement are defined as 1–59 arbitrary units by the manufacturer. A-BIA also measures the trunk adipose tissue (TAT) percentage (TAT%) (range: 5%-75%). Measurements were performed with the subject in supine position with arms placed on the chest, with no pillow under the head. The main device is positioned vertically to the subject’s abdomen at the umbilical level and the multi-frequency electrode belt is positioned on the abdomen in direct contact with the skin (Fig. 1). Gender was selected as a parameter by the user from the control panel of the device and therefore, all study results are arranged by gender. The belt consists of four electrodes. Two of them maintain 6.25 and 50 kHz currents into the abdomen and the other two sense the current returning from the body. The obtained data is sent to the main device through a wireless system. Impedance is measured by a software within the device via extrapolation. In contrast to whole-body BIA devices that predict the abdominal fat tissue levels, the AB-140 directly measures abdominal trans-impedance and is therefore expected to reflect the local delivery to tissue compartments better. The current returning from the body, which comes from extracellular (6.25 kHz) and intracellular (50 kHz) fluids, reflects the lean part of the body. By this way, the lean part can be selected more precisely and the fat mass of the body can be calculated. Total body adipose tissue (TBAT) (kg) and TBAT percentage (TBAT%) are measured with another conventional BIA device (TBF-300, Tanita corp., Tokyo, Japan).
Figure 1.
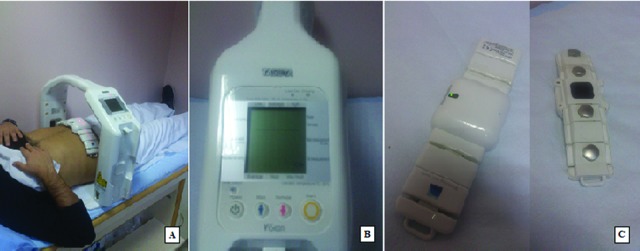
Tanita AB-140 ViScan device. A. Position of the patient during measurement; B. Control panel of the device; C. Top and bottom view of the 4-electrode belt.
All measurements were completed within a mean duration of 60 seconds. Subjects were asked not to consume alcohol during the 24-hour period, and caffeine during the 4-hour period before the assessment (restrictions required for bio-impedance measurements). All accessories on the subjects, such as heavy clothing, rings and earrings, which could affect the measurements, were removed before the assessment. All anthropometric and body composition measurements of the participants, who fasted for at least 8 hours, were performed by a single physician.
The definitions for anthropometric measures, laboratory methods, and metabolic disorders that were used in the study are included in the supplementary materials and methods.
Statistical Analysis
The difference in WC, hip circumference (HC), neck circumference (NC), TBAT, percentage TBAT, VAT, percentage TAT, fasting blood glucose (FBG), fasting insulin level, triglyceride level, low-density lipoprotein cholesterol (LDL-c) level, high-density lipoprotein cholesterol (HDL-c) level, and homeostasis model assessment of insulin resistance (HOMA-IR) score between the first and second evaluations were calculated. The data from the first and second visits were compared. Correlation analysis was used to identify which anthropometric measurements were closely associated with improvements in metabolic measurements. Multivariate linear regression analysis was used to assess any possible independent associations. Statistical analyses were performed using SPSS v16.0 software package for Windows (Statistical Package for Social Sciences; Chicago, IL). Group percentages were compared using chi-square test, means of normally distributed variables were compared using independent sample t-test, and nonparametric data were compared using Mann-Whitney U test. For normally distributed variables, data for the baseline and second visits of participants were compared using paired samples t-test. For non-normally distributed variables, data for the baseline and second visits of participants were compared using Wilcoxon two related samples test. Correlations were analyzed using Pearson and Spearman methods. Multivariable linear regression analysis was used to test whether the observed associations were independent. A p value <0.05 was considered as statistically significant.
RESULTS
Although 31 of 134 (23.1%) participants failed to adhere to the diet, 103 (76.9%) participants reported they did. The mean duration of dieting before the follow-up evaluation was 3.2±0.71 (1.9–4.4) months. The largest proportion of participants, 45.6%, reported a moderate level of compliance with the diet program. In addition, 29% of participants reported that they did not exercise, while 71% reported that they did. Those who exercised reported a mean of 3.1±1.8 (1–9) hours a week of brisk walking or the equivalent amount of other exercise; 46% of participants exercised for ≤2 hours, 35% exercised for >2 to ≤4 hours, 15% exercised for >4 hours to ≤6 hours, and 4% exercised for >6 hours per week.
Of the 103 participants who adhered to the diet, 81.6% (84/103) were female. The mean age was 41.8±10.5 (21-67) years. Of the females, 32.1% (27/84) were postmenopausal and 67.9% were premenopausal. The mean body weight and BMI of the participants were 80.1±15.6 kg and 29.9±5.0 kg/m2, respectively. The percentages of normal-weight (20–25 kg/m2), overweight (25–30 kg/m2), and obese (>30 kg/m2) participants at the first visit were 15%, 41%, and 44%, respectively. The 79% of normal-weight females had a waist circumference greater than 80 cm and only 1 normal-weight male had a waist circumference of 95 cm. Although these patients had normal BMIs, they were complaining about local weight-gain and they wanted to be more fit. Thus, we recommended weight loss to these normal-weight individuals. When the upper limit of WC was set to 102 cm for the males and 88 cm for the females, it was found that 78.6% (81/103) of participants had central obesity. The mean WCs of males and females at the initial evaluation were 110.6±10.3 cm and 94.5±9.6 cm, respectively.
The mean values of all parameters at the initial and second evaluation and changes in the body composition and metabolic measures of 103 participants are shown in Table 1. There were statistically significant reductions in the mean body weight, BMI, NC, WC, HC, VAT, and TAT%, in TBAT and TBAT%, and in FBG, insulin level, HOMA-IR score, triglyceride level, and alanine transaminase (ALT) level. The reduction in LDL-c and elevation in HDL-c levels were not significant. There were no changes in the mean HbA1c levels between the visits (5.95±0.38 vs. 5.97±0.31, p=0.831). Although most participants fulfilled two of the ATPIII criteria at the initial evaluation, most of them satisfied only one criterion at the follow-up evaluation. There was no significant change in waist-to-hip ratio (WHR). Mean weight loss was 3.4 kg (range: 0.1–11.7 kg). No weight change was observed in three participants, while one participant gained 0.6 kg. All other participants lost weight. The amount of reduction in the body weights of normal weight (NW), overweight (OW) and obese (O) subjects were 1.8±1.4, 3.1±2.4, 4.2±3.2 kg, respectively (NW vs. OW, p=0.346; OW vs. O, p=0.188; NW vs. O, p=0.012). The changes in the other measurements are shown in Table 1.
Table 1.
Anthropometric measurements and laboratory measurements of patients at the baseline and second visits, and changes in the body composition and metabolic variables after dieting
| n | Baseline | Second visit | Change* | p | |
| Simple anthropometric measurements | |||||
| Body weight (kg) | 103 | 80.1±15.6 (54.5-132.8) | 76.7±14.9 (51.8-122.9) | -3.4 ±2.8 [(-11.7) − (+0.6)] | <0.001 |
| BMI (kg/m2) | 103 | 29.9±5.0 (21.0-46.9) | 28.6±4.8 (20.6-45.1) | -1.3 ±1.0 [(-4) − (+0.2)] | <0.001 |
| NC (cm) | 102 | 35.9± 3.9 (30-48) | 35.2±3.5 (30-48) | -0.6 ±1.1 [(-5) − (+1.5)] | <0.001 |
| WC (cm) | 103 | 97.5±11.5 (73-130) | 94.8±11.6 (68-133) | -2.7 ±3.6 [(-12) − (+6)] | <0.001 |
| HC (cm) | 103 | 108.3±9.9 (92-141) | 105.5±9.2 (91-140) | -2.8 ±3.3 [(-20) − (+2)] | <0.001 |
| Variables measured by abdominal BIA | |||||
| TAT% | 103 | 43.5±5.9 (27.2-59.2) | 42.0±6.4 (26.1-59.3) | -1.5 ±2.4 [(-10.1) − (+4)] | <0.001 |
| VAT | 103 | 13.6±5.9 (4.0-34.5) | 12.6±5.7 (3.5-31.0) | -1.0 ±1.7 [(-8.0) − (+3.0)] | <0.001 |
| Variables measured by conventional BIA | |||||
| TBAT mass (kg) | 103 | 29.1±9.3 (12.3-57.1) | 26.8±9.1 (11.7-56.5) | -2.3 ±2.2 [(-10.0) − (+2.2)] | <0.001 |
| TBAT% | 103 | 35.9±6.8 (19.3-49.8) | 34.3±6.8 (17.4-50.5) | -1.52 ±1.89 [(-7.1) − (+3.8)] | <0.001 |
| Anthropometric ratios | |||||
| WHR | 103 | 0.90±0.07 (0.74-1.05) | 0.90±0.07 (0.72-1.08) | -0.002±0.03[(-0.05)−(+0.01)] | 0.462 |
| Laboratory measurements and indices | |||||
| Insulin (μU/mL) | 103 | 12.1±6.0 (3.0-41.3) | 9.7±4.4 (3.2-32.1) | -2.38 ±4.6 [(-22.4) − (+6.9)] | <0.001 |
| FBG (mg/dL) | 103 | 97±9.6 (77-118) | 93±8.5 (75-114) | -3.75 ±9.57 [(-31) − (+15)] | <0.001 |
| HOMA-IR score | 103 | 2.94±1.56 (0.57-10.91) | 2.26±1.06 (0.64-7.05) | -0.68 ±1.19 [(-5.8) − (+1.5)] | <0.001 |
| LDL-c (mg/dL) | 103 | 127±35.2 (35-213) | 124±32.2 (37-204) | -2.7 ±23.4 [(-87) − (+60)] | 0.244 |
| HDL-c (mg/dL) | 101 | 47±10.1 (28-80) | 48±11.5 (29-99) | +1.1 ±6.3 [(-14) − (+19)] | 0.085 |
| Triglyceride (mg/dL) | 103 | 131±79.5 (36-440) | 117±59.6 (24-383) | -15 ±44 [(-197) − (+87)] | 0.001 |
| ALT (U/L) | 74 | 26±16.9 (9-114) | 21±11.9 (6-94) | -5 ±17 [(-90) − (+75)] | 0.001 |
| TSH (μIU/mL) | 103 | 1.74±0.83 (0.35-4.86) | 1.68±0.83 (0.15-3.97) | . | 0.276 |
| Metabolic disorders | |||||
| Number of ATPIII criteria | 103 | **2 (0-5) | **1 (0-4) | . | <0.001 |
| Prediabetes | 79 | 41.8% | 30.4% | . | 0.124 |
| HOMA-IR score ≥2.6 | 103 | 55.3% | 31.1% | . | <0.001 |
| MetS according to ATPIII | 103 | 36.9% | 25.2% | . | 0.008 |
BMI: body mass index, NC: neck circumference, WC: waist circumference, HC: hip circumference, TAT: trunk adipose tissue, VAT: visceral adipose tissue, TBAT: total body adipose tissue, WHR: waist-to-hip ratio, FBG: fasting blood glucose, LDL-c: low-density lipoprotein cholesterol, HDL-c: high-density lipoprotein cholesterol, ALT: alanine aminotransferase, TSH: thyroid-stimulating hormone, HOMA-IR: homeostatic model assessment of insulin resistance, ATPIII: adult treatment panel III, MetS: metabolic syndrome. *Values in columns indicate (mean change ± SD [(minimum) – (maximum)]). Minus signs indicate decreased values and plus signs indicate increased values ** Median
After the dietary intervention, the percentage of participants with IR and participants with MetS decreased significantly, from 55% to 31% (p<0.01), and from 37% to 25% (p<0.008), respectively. The percentage of participants with impaired glucose metabolism decreased from 41.8% to 30.4%, but the difference was not statistically significant (p=0.124) (Table 1).
Correlation analysis that was used to identify variables closely associated with improvement in metabolic measurements is shown in Table 2. Multivariate linear regression analysis showed that just the change in BMI and change in VAT were significantly correlated with the change in HOMA-IR score (F(7.93)=3.134, p=0.005, R2 =0.191). However, visceral fat had a greater impact than BMI (β-coefficient for VAT=0.435, p=0.026; β-coefficient for BMI=0.299, p=0.020) (Table 2).
Table 2.
Correlations between mean changes in anthropometric measurements and mean changes in laboratory measurements, and multivariate linear regression analysis to identify the best correlation changes in HOMA-IR score
| Correlation Analysis | Multivariate Linear Regression Analysis | ||||||||
| Variables | ΔFBG | ΔInsulin | ΔHOMA-IR | ΔHOMA-IR* | |||||
| p | r | p | r | p | r | b (SE)* | β* | p* | |
| ΔBW | 0.010 | 0.254 | 0.020 | 0.230 | <0.001 | 0.362 | - | - | - |
| ΔBMI | 0.007 | 0.264 | 0.012 | 0.247 | <0.001 | 0.371 | 0.344 (0.145) | 0.299 | 0.020 |
| ΔTAT% | 0.020 | 0.231 | 0.416 | - | 0.203 | - | -0.152 (0.103) | -0.310 | 0.143 |
| ΔVAT | 0.001 | 0.316 | 0.005 | 0.275 | 0.001 | 0.324 | 0.308 (0.136) | 0.435 | 0.026 |
| ΔTBAT mass | 0.067 | - | 0.226 | - | 0.092 | - | - | - | - |
| ΔTBAT% | 0.240 | - | 0.545 | - | 0.936 | - | -0,103 (0.071) | -0.166 | 0.148 |
| ΔNC | 0.265 | - | 0.230 | - | 0.202 | - | 0.020 (0.111) | 0.066 | 0.523 |
| ΔWC | 0.033 | 0.212 | 0.421 | - | 0.211 | - | 0.071 (0.039) | 0.062 | 0.606 |
| ΔHC | 0.015 | 0.240 | 0.398 | - | 0.122 | - | -0.026 (0.041) | -0.073 | 0.532 |
“Δ”symbol at the front of a variable indicates the mean change in the variable after dieting. “-” sign indicates lack of correlation between variables (p>0.05). *Dependent variable is ΔHOMA-IR score. BW: body weight, BMI: body mass index, TAT: trunk adipose tissue, VAT: visceral adipose tissue, TBAT: total body adipose tissue, NC: neck circumference, WC: waist circumference, HC: hip circumference, FBG: fasting blood glucose, HOMA-IR: homeostatic model assessment of insulin resistance.
DISCUSSION
Similar to the results of our study, previous studies of short- and intermediate-term (2–12 months) dietary interventions reported significant weight loss and decreases in anthropometric measurements and metabolic variables (26, 27). In our study, we found that triglyceride levels were significantly lower after the diet and exercise program, but LDL-c and HDL-c levels did not change. Evidence from numerous studies indicates that diet and exercise decrease triglyceride levels; however, evidence on the effects of dietary intervention on LDL-c and HDL-c is limited (28).
Mean weight loss was 3.4 kg over a mean follow-up of 3.2 months, but three participants did not lose weight and one gained 0.6 kg. All four of these participants reported that they had adhered to the diet. When their metabolic data were analyzed, it was found that although their body weight had increased or remained unchanged, various other metabolic measures decreased or improved, such as the VAT level and HOMA-IR score. In clinical practice, some patients who adhere to a diet are nevertheless unsuccessful at short-term weight loss, which suggests that some individuals may lose faith and motivation during the dieting period, and ultimately stop dieting. Use of anthropometric measurements, body fat analysis, and metabolic measurements like HOMA-IR may thus be a motivational tool for dieters and a useful follow-up protocol for physicians.
After the dietary and exercise intervention, reductions in weight, BMI, and VAT were positively correlated with the reductions in FBG, fasting insulin, and HOMA-IR score. In addition, there were weak correlations between reductions in WC, HC, and TAT, as measured with A-BIA, and fasting glucose. When multiple linear regression analysis was used to identify variables that independently predict the change in HOMA-IR score, changes in BMI and VAT were identified as significant predictors. Thus, BMI and VAT measured with A-BIA appear to be better markers of metabolic improvement than the variables measured with conventional BIA, WC, HC, NC, and body weight.
Previous studies noted that WC correlated with VAT area (as determined by CT) (29), glucose intolerance, and hyperinsulinemia (19, 30). The present study found no correlation between changes in WC and HOMA-IR. Previous reports of low reproducibility and measurement errors for WC (23) may explain this result. These deficiencies may limit the use of WC in monitoring metabolic improvement. During WC measurement, both the attention of the physician and the patient’s cooperation are important. Although all measurements were performed by the same physician in the present study, intraobserver variability is a concern. To decrease intraobserver variability, three separate WC measurements should be performed and the average of these three measurements should be used in the analysis.
To our knowledge, most of the previous studies investigated the correlations between simple measurement methods and advanced measurement methods such as MRI and CT, or the association between cardiovascular risk factors and metabolic variables. These studies were mostly cross-sectional and have produced conflicting results. For example, some studies reported that SAT area measured by CT was strongly correlated with IR, while others found no correlation (18). Furthermore, there have been few follow-up studies, which are important in understanding the cause–effect relationships. In the present study, we found correlations (r=0.196–0.348) between all baseline measurement variables (weight, BMI, VAT, TAT%, TBAT, NC, WC, HC, WHR) and baseline HOMA-IR score (data not reported). This cross-sectional analysis suggests that all these variables reflect IR. However, at the end of the follow-up, only changes in BMI and VAT were independently associated with the change in HOMA-IR score.
A recent study evaluated the very low calorie ketogenic diet and compared dual energy X-ray absorptiometry, air displacement plethysmography and multifrequency BIA (MF-BIA) device for body fat analysis. In the mentioned study, the MF-BIA device that calculates visceral fat area was proposed as an effective and more convenient method for measuring body composition in clinical practice, corroborating our results (31). MF-BIA used in the said study has a technology similar to the A-BIA used in our study. A-BIA device consists of four electrodes that directly contact the abdomen, while MF-BIA device has 8 electrodes that contact the extremities. Although there is no direct correlation study on these two devices, both were validated in the previous studies.
VAT measured using A-BIA is expressed on a scale of 1–59 arbitrary units with no cut-off for visceral adiposity. A higher score indicates greater visceral adiposity. Previously, we reported that a VAT cut-off value of 9.8 arbitrary units for females and a VAT cut-off value of 16 arbitrary units for males were both 90% sensitive for predicting MetS (32). To our knowledge, these cut-off values are the first ones to be reported for the present A-BIA device. Despite the small sample size of the current study, the sensitivity was 87% for a cut-off of 9.8 arbitrary units for females and 80% for a cut-off of 16 arbitrary units for males. More studies with larger sample sizes are needed in order to confirm our findings. However, we hope that, until then, these cut-off values will be useful. Although there is no consensus on cut-off values for WC, data on the cut-off values for VAT measured using A-BIA are not conclusive.
No simple correlations between the change in total body fat percentage and the change in HOMA-IR score were detected in our study. Total body fat was measured by the conventional BIA device. Conventional BIA devices are mostly used to measure the total body fat and fat percentage. However, not all fat storages in the body are pathogenic. Accumulation of fat in the abdominal area, particularly in the viscera, is associated with increased metabolic disorders (5). Our study demonstrated that while the change in VAT measured by A-BIA device was correlated with the change in HOMAIR score, the change in TBAT measured by the conventional BIA device was not. This result indicates that the usefulness of conventional BIA devices in the follow-up of dieters must be questioned.
The present study yielded important data on the use of A-BIA and other anthropometric and metabolic variables in monitoring the dieters. Nevertheless, this study has several limitations. The small sample size and predominance of females in the sample prevents generalization. In addition, the duration of follow-up was short. However, this was intended as a pilot study, as long-term studies on dieting are usually impractical. Finally, classification of diet adherence is subjective and depends on patient reports, but this limitation was not relevant to the aims of our study.
In conclusion, BMI and, to a greater extent, VAT measured using A-BIA appear to be valuable indicators of metabolic improvement in dieters. A-BIA is an efficient, safe, inexpensive, portable, operator-independent, straightforward, and useful technique for clinical measurement of VAT and it may have a future role in the screening and follow-up of abdominal obesity.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339(6116):172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, Stampfer MJ, Manson JE, Grodstein F, Colditz GA, Speizer FE, Willett WC. Trends in the incidence of coronary heart disease and changes in diet and lifestyle in women. N Engl J Med. 2000;343(8):530–537. doi: 10.1056/NEJM200008243430802. [DOI] [PubMed] [Google Scholar]
- 4.Oh TH, Byeon JS, Myung SJ, Yang SK, Choi KS, Chung JW, Kim B, Lee D, Byun JH, Jang SJ, Kim JH. Visceral obesity as a risk factor for colorectal neoplasm. J Gastroenterol Hepatol. 2008;23(3):411–417. doi: 10.1111/j.1440-1746.2007.05125.x. [DOI] [PubMed] [Google Scholar]
- 5.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124(24):e837–e841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 6.Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, Bartels SJ, Somers VK, Lopez-Jimenez F. Normal weight obesity and mortality in United States subjects ≥60 years of age (from the Third National Health and Nutrition Examination Survey) Am J Cardiol. 2013;112(10):1592–1598. doi: 10.1016/j.amjcard.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 7.De Lorenzo A, Martinoli R, Vaia F, Di Renzo L. Normal weight obese (NWO) women: an evaluation of a candidate new syndrome. Nutr Metab Cardiovasc Dis. 2006;16(8):513–523. doi: 10.1016/j.numecd.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Shuster A, Patlas M, Pınthus JH, Mourtzakıs M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. British J Radiol. 2012;85(1009):1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, Fujimoto WY. Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes Care. 2003;26(3):650–655. doi: 10.2337/diacare.26.3.650. [DOI] [PubMed] [Google Scholar]
- 10.Kanaya AM, Harris T, Goodpaster BH, Tylavsky F, Cummings SR. Adipocytokines attenuate the association between visceral adiposity and diabetes in older adults. Diabetes Care. 2004;27(6):1375–1380. doi: 10.2337/diacare.27.6.1375. [DOI] [PubMed] [Google Scholar]
- 11.Tulloch-Reid MK, Hanson RL, Sebring NG, Reynolds JC, Premkumar A, Genovese DJ, Sumner AE. Both subcutaneous and visceral adipose tissue correlate highly with insulin resistance in African Americans. Obes Res. 2004;12(8):1352–1359. doi: 10.1038/oby.2004.170. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, Fujimoto WY. Visceral adiposity is an independent predictor of incident hypertension in Japanese Americans. Ann Intern Med. 2004;140(12):992–1000. doi: 10.7326/0003-4819-140-12-200406150-00008. [DOI] [PubMed] [Google Scholar]
- 13.Ding J, Visser M, Kritchevsky SB, Nevitt M, Newman A, Sutton-Tyrrell K, Harris TB. The association of regional fat depots with hypertension in older persons of white and African American ethnicity. Am J Hypertens. 2004;17(10):971–976. doi: 10.1016/j.amjhyper.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Nicklas BJ, Penninx BW, Ryan AS, Berman DM, Lynch NA, Dennis KE. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care. 2003;26(5):1413–1420. doi: 10.2337/diacare.26.5.1413. [DOI] [PubMed] [Google Scholar]
- 15.Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Shofer JB, Fish BE, Knopp RH, Kahn SE. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53(8):2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 16.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu C-Y, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, Sr, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 17.Wajchenberg BL. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 18.Smith SR, Lovejoy JC, Greenwy F, Ryan D, deJonge L, de la Bretonne J, Volafova J, Bray GA. Contribution of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50(4):426–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 19.Tchkonia T, Karagiannides I, Forse RA, Kirkland JL. Different fat depots are distinct mini-organs. Current Opinion in Endocrinology & Diabetes. 2001;8(5):227–234. [Google Scholar]
- 20.Thomas EL, Collins AL, McCarthy J, Fitzpatrick J, Durighel G, Goldstone AP, Bell JD. Estimation of abdominal fat compartments by bioelectrical impedance: the validity of the ViScan measurement system in comparison with MRI. European Journal of Clinical Nutrition. 2010;64(5):525–533. doi: 10.1038/ejcn.2010.18. [DOI] [PubMed] [Google Scholar]
- 21.Browning LM, Mugridge O, Chatfield MD, Dixon AK, Aitken SW, Joubert I, Prentice AM, Jebb SA. Validity of a new abdominal bioelectrical impedance device to measure abdominal and visceral fat: comparison with MRI. Obesity (Silver Spring) 2010;18(12):2385–2391. doi: 10.1038/oby.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehghan M, Merchant AT. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr J. 2008;7:26. doi: 10.1186/1475-2891-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi I, Sakamoto Y, Kasahara Y, Sato H, Ikeda Y. Study on the measurement of visceral fat using abdominal BIA. J Japan Society for the Study of Obesity. 2006;12:271. (Abstract). [Google Scholar]
- 24.Ryo M, Maeda K, Onda T, Katashima M, Okumiya A, Nishida M, Yamaguchi T, Funahashi T, Matsuzawa Y, Nakamura T, Shimomura I. A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care. 2005;28(2):451–453. doi: 10.2337/diacare.28.2.451. [DOI] [PubMed] [Google Scholar]
- 25.Fujioka S, Matsuzawa Y, Tokunaga K, Kawamoto T, Kobatake T, Keno Y, Kotani K, Yoshida S, Tarui S. Improvement of glucose and lipid metabolism associated with selective reduction of intra-abdominal visceral fat in premenopausal women with visceral fat obesity. Int J Obes. 1991;15(12):853–859. [PubMed] [Google Scholar]
- 26.Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003;148(5):535–542. doi: 10.1530/eje.0.1480535. [DOI] [PubMed] [Google Scholar]
- 27.Calle-Pascula AL, Rodriguez C, Martin-Alvarez PJ, Camacho F, Calle JR, Yuste E, Hidago I, Diaz RJ, Martin-Vaguero P, Santiago M. Effect of weight loss on insulin sensitivity and cardiovascular risk factors in glucose tolerant and intolerant obese subjects. Diabetes Metab. 1991;17(4):404–409. [PubMed] [Google Scholar]
- 28.Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rankinen T, Kim SY, Perusse L, Despres JP, Bouchard C. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord. 1999;23(8):801–809. doi: 10.1038/sj.ijo.0800929. [DOI] [PubMed] [Google Scholar]
- 30.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Arbelaez D, Bellido D, Castro AI, Ordoñez-Mayan L, Carreira J, Galban C, Martinez-Olmos MA, Crujeiras AB, Sajoux I, Casanueva FF. Body composition changes after very low-calorie-ketogenic diet in obesity evaluated by three standardized methods. J Clin Endocrinol Metab. 2016;102(2):488–498. doi: 10.1210/jc.2016-2385. [DOI] [PubMed] [Google Scholar]
- 32.Mousa U, Kut A, Bozkuş Y, Demir Çicek C, Anil C, Tütüncü Bascil N. Performance of abdominal bioelectrical impedance analysis and comparison with other known parameters in predicting the metabolic syndrome. Exp Clin Endocrinol Diabetes. 2013;121(7):391–396. doi: 10.1055/s-0033-1341473. [DOI] [PubMed] [Google Scholar]


