Abstract
Context
Heat Shock Protein 60 (HSP60) is a chaperone protein which is involved in proteins transfer and re-folding of proteins.
Objective
Importance of HSP60 in sperm capacitation and facility of sperm-oocyte membrane binding was confirmed, therefore in this study the effect of HSP60 on the rate of in vitro fertilization and the cleavage rate in mouse embryo was investigated.
Design
Ten male mice and twenty five female mice were involved to collect sperms and oocytes required for this study.
Subjects and Methods
Sperms were collected from the epididymis of male mouse and oocytes were collected from the oviduct of female mouse following ovarian hyperstimulation. Then, capacitated sperms and oocytes were placed together in fertilization medium in four groups in the presence of different concentrations of HSP60 (10, 50 and 100 ng/mL) and in the absence of HSP60. After calculation of the fertilization rate, zygotes were transformed into the other medium for development and the cleavage rate was monitored to blastocyst stage.
Results
There was not a significant difference in the rate of fertilization between 10 ng/mL HSP60 group and the control group. The rate of fertilization and two-cell embryo development decreased significantly (P≤0.05) in 100 ng/mL HSP60 compared to other experimental and control groups. Further, the rate of two-cell embryo development increased significantly (P≤0.05) in 10 ng/mL HSP60 compared to other experimental and control groups.
Conclusions
The present study demonstrated that HSP60 in low dose had a positive effect on two-cell embryo development, however it did not have any significant effect on the fertilization rate. Conversely, HSP60 had adverse effects on the fertilization and cleavage rates at higher doses.
Keywords: Embryo cleavage, Fertilization, Heat shock protein 60 (HSP60), In vitro fertilization (IVF), Pre-implantation embryo
INTRODUCTION
Sperm-oocyte interaction is one of the necessary steps for successful fertilization. Under in vivo conditions, sperm-oocyte interaction occurs in the protected environment of the female genital tract. Sperm surface is heterogeneous and it tolerates continuous remodelling during transition from testis and male genital ducts to female genital tract. In fact, sperm is exposed to secretions of female genital tract, in particular oviduct to get surface remodelling that is essential for the sperm to acquire maximum ability to bind zona pellucida (ZP) and subsequent fertilization (1, 2). However during in vitro fertilization (IVF), sperms are deprived of oviduct secretion that is crucial to induce capacitation. In order to enhance IVF rate, IVF is performed in the presence of epithelial cells of oviduct, or their secretions (3, 4) or IVF media are supplemented with specific proteins or molecules that are present in oviduct secretions (5).
HSP60 (Heat Shock Protein 60) or HSPD1 (Heat Shock Protein Family D Member 1) is a molecular chaperone, a member of HSPs family. HSP60 is a mitochondrial protein that is involved in re-folding of proteins, transport of proteins across membrane and formation of oligomeric protein complexes (6). HSP60 was already found in the middle piece of sperm, on the acrosome of the sperm head, and also in the semen (7-9). Further, it is expressed in the surface of epithelial cells of the oviduct that is transferred to sperms; therefore, it is supposed that HSP60 may have a role in sperm-oocyte interaction (6).
High level of HSP60 in the female genital tract is produced during ovulation and implantation (10-12). HSP60 is translocated to the sperm surface and its tyrosine residue is phosphorylated during capacitation (13). Moreover, studies demonstrated that the incubation of sperm with HSP60 enhance sperm viability, and the rate of capacitation process (14-17). Also during sperm capacitation, phosphorylation of chaperones including HSP60 on the sperm surface may result in forming and/or presenting multiprotein complexes containing ZP receptor proteins on the sperm surface to facilitate sperm binding to zona (18).
In most studies, the role of HSP60 has been demonstrated in the process of spermatogenesis (9) and sperm-egg interaction (18) but there is no report directly addressing the role of HSP60 in fertilization and subsequent pre-implantation embryo development. Considering the presence of HSP60 in oviduct secretions and semen, and its function in sperm capacitation and sperm-oocyte binding facilitation, we wanted to elucidate whether Hsp60 could influence the rate of fertility and embryo development. Therefore in the present study, we investigated the effect of HSP60 as an exogenous chaperone protein in doses of 10, 50 and 100 ng/mL on the rate of IVF and cleavage rate of embryo in mice.
MATERIALS AND METHODS
Reagents
All reagents were obtained from Sigma-Aldrich Company (St. Louis, USA) unless otherwise stated.
Animal care and collection of semen samples
All procedures related to care and use of animals were approved by the Ethics Committee of Zanjan University of Medical Sciences (Ethics code: ZUMS.REC.1394.31). Fertile male NMRI mice and female NMRI mice of 6-8 weeks old weighing 25-30 gr were kept in separate cages and accustomed to the new conditions (23±2°C, 40-60 % humidity, and light-dark cycle for 12 hrs) for two weeks.
After two weeks, each male mouse was sacrificed by cervical dislocation. The abdomen was surgically exposed and the caudal epididymides were removed and chopped. Then, they were transferred to a dish containing T6 medium (473 mg NaCl, 100 mg KCl, 5 mg NaH2PO4, 10 mg MgCl2X, 6H2O, 26 mg CaCl2, X6H2O, 210 mg NaHCO3, 200 μL Na-lactate 100%, 3 mg Na-pyruvate, 100 mg glucose, 6 mg penicillin, 5 mg streptomycin, 1 mg phenol red and 0.6 mg EDTA to make 100 mL T6 medium) plus 10% BSA (Bovine Serum Albumin). Afterwards, the dish containing medium and epididymides was put in a 5% CO2 incubator (New Brunswick, Galaxy 170 S) at 37°C. After 20 min, sperm motility was checked using a light microscope (Motic, BA210, China). The samples which had good motility were used for IVF.
Ovarian induction, oocyte collection and in vitro fertilization (IVF)
In this experimental study, a total of 32 female mice were superovulated using 7.5 IU PMSG (Pregnant Mare Serum Gonadotropin) (Folligon, Intervet, Netherlands, HOR-272) through IP (intraperitoneal) injection at noon (12:00). After 48 hrs, 7.5 IU hCG (Human Chorionic Gonadotropin) (Bioscience, GmbH, Germany) was administered through IP injection. Next morning after injection of hCG (8:00 am), superovulated mice were sacrificed by cervical dislocation. Oviducts were bilaterally dissected and transferred into T6 medium at 37°C. The cumulus-oocyte-complexes (COCs) were released from ampullary region of oviducts into T6 medium using a stereomicroscope (Motic, SMZ-168, China). First the COCs were carefully examined then COCs with fully expanded cumulus and an evenly granulated cytoplasm were considered for IVF. Immature, very mature and atretic COCs were excluded from the study. Then, COCs were transferred to 50 µL/drop of fertilization medium (T6 + BSA 15%), 3 COCs in each drop, and overlaid with mineral oil.
In this study, there were three experimental groups and one control group. For experimental groups, fertilization medium (T6 + BSA 15%) was supplemented by 10 ng/mL or 50 ng/mL and or 100 ng/mL of HSP60, while HSP60 was not added for the control group. Seventy three COCs were used in each group and experiments were carried out in three replicates for all groups (73 COCs in each replicate resulted in 219 COCs in three replicates). In each fertilization drop, sperms suspensions (1 x 106 per mL) were added then incubated in 5% CO2, at 37°C for 6 hrs. Afterwards, the COCs were washed and normal fertilization was confirmed by the presence of two pronuclei and two polar bodies using an inverted microscope (Nikon, eclipse Ts2, Japan). The rate of fertilization was counted in all groups, then zygotes were transferred into a medium containing T6 and BSA 4% and incubated in 5% CO2, at 37°C until blastocyst stage (for four days). Embryo cleavage rate was assessed by checking embryos development to two-cell stage (one day after IVF), four-cell stage (two days after IVF), morula (three days after IVF) and blastocyst (four days after IVF) stage. Also the rate of cleavage-arrested embryos was evaluated and divided into three categories: necrotic, fragmented and fully lysed embryos were classified as type I, partially lysed or fragmented embryos were classified as type II and embryos with some lysed or fragmented blastomeres or cytoplasmic vesicles were classified as type III (19).
Statistical analysis
Statistical analysis was performed using SPSS software (Version 16, IBM, Chicago, USA). Differences among groups were analysed by Student T-test, and One way ANOVA followed by Bonferroni’s post hoc test. A difference with P≤0.05 was statistically considered significant.
RESULTS
Fertilization rate
All data about differences in fertilization and cleavage rates between groups was summarized in Table 1. The number of fertilized oocytes in the experimental group of 100 ng/mL HSP60 was significantly lower than in the control and other experimental groups (P≤0.05) (Table 1). The fertilization rate increased slightly in the experimental group of 10 ng/mL HSP60 compared to the control, although it was not significant (Table 1).
Table 1.
The number and mean of percentage ± standard error of the mean (SEM) of oocytes, fertilized oocytes, embryos in different stages and cleavage-arrested embryos (CAE) with three different types in control and experimental groups
| Groups | Oocytes | Fertilized oocytes | Two-cell | Four-cell | Morulae | Blastocysts | Cleavage-arrested embryos (CAE) | CAE (type I) | CAE (type II) | CAE (type III) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 219 | 200 (91.32 ± 1.2) | 158 (79 ± 2.88) | 121 (76.58 ± 2.8) | 96 (79.33 ± 2.79) | 74 (77.08 ± 10.29) | 126 (63 ± 7.1) | 2 (1.58 ± 0.87) | 9 (7.14 ± 0.79) | 116 (92.06 ± 1.5) |
| HSP60 (10 ng/mL) | 219 | 207 (94.52 ± 0.79) | 182 (87.92 ± 1.38)a | 163 (89.56 ± 0.8) | 142 (87.11 ± 2.56) | 122 (85.91 ± 3.96) | 85 (41.06 ± 5.79)a | 5 (5.88 ± 2.94) | 8 (9.41 ± 1.9) | 72 (84.7 ± 5.98) |
| HSP60 (50 ng/mL) | 219 | 187 (85.38 ± 2.77)b | 129 (68.98 ± 1.5)a, b | 91 (61.07 ± 2.7) | 81 (89.01 ± 2.7) | 65 (80.24 ± 0.48) | 122 (65.24 ± 2.13)b | 11 (9.01 ± 1.1) | 18 (14.75 ± 2.25) | 93 (76.22 ± 3.32) |
| HSP60 (100 ng/mL) | 219 | 154 (70.31 ± 3.56)a, b, c | 97 (62.98 ± 1.87)a, b | 76 (78.35 ± 4.38) | 51 (67.1 ± 6.62) | 36 (70.58 ± 3.96) | 118 (76.62 ± 5.73)a, b | 21 (17.79 ± 4.57)a, b | 20 (16.94 ± 1.03)a, b | 77 (65.25 ± 5.46)a, b |
All data is drawn from three replicates.
- Superscript (a) in each column indicates significant difference (P≤0.05) with control group.
- Superscript (b) in each column indicates significant difference (P≤0.05) with HSP60 (10 ng/mL) group.
- Superscript (c) in each column indicates significant difference (P≤0.05) with HSP60 (50 ng/mL) group.
Cleavage rate and embryonic development
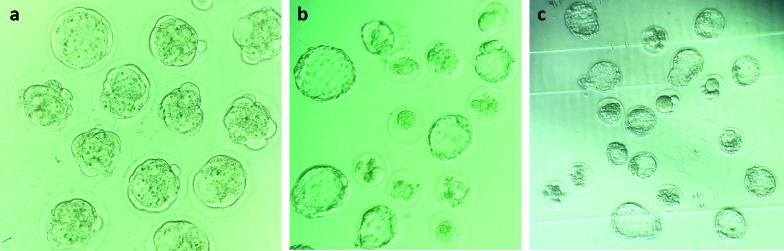
The rate of two-cell embryo development increased significantly (P≤0.05) in 10 ng/mL HSP60 compared to other experimental groups in doses of 50 and 100 ng/mL HSP60 and control group (Table 1). A significant decrease was observed in the rate of two-cell embryo formation in experimental groups of 50 and 100 ng/mL HSP60 in comparison with control and 10 ng/mL HSP60 groups (P≤0.05) (Table 1). The cleavage rate (from two-cell embryo to blastocyst stage) decreased in experimental groups of 50 and 100 ng/mL HSP60 compared to 10 ng/mL HSP60, however it was not significant (Table 1). The total rate of cleavage-arrested embryos and the rate of all three types of that increased significantly (P≤0.05) in 100 ng/mL HSP60 compared to control and 10 ng/mL HSP60 groups, whereas the total rate of cleavage-arrested embryos decreased significantly (P≤0.05) in 10 ng/mL HSP60 experimental group in comparison with control group (Table 1). In vitro produced embryos in the stages of morula and blastocyst and cleavage-arrested embryos are shown in Figure 1.
Figure 1.
Representative image of mice in vitro-produced embryos. (a) Embryos at compaction stage, at 200× magnification. (b) Embryos at blastocyst stage. Also, the image indicates some cleavage-arrested embryos, at 200× magnification. (c) Embryos at blastocyst and hatching-blastocyst stages. Also, the image indicates some cleavage-arrested embryos, at 100× magnification.
DISCUSSION
It was supposed that chaperones including HSP60 were only located in mitochondrion matrix, but today it is known that they are also present on the surface of cells, showing cell surface binding (18, 20). Sperm is transcriptionally inactive and its function depends upon post-translational modifications including phosphorylation (18). During transition of sperms from male genital tract to final location of oviduct, some chaperones including HSP60, HSP90, glucose-regulated protein 78 (GRP78) and endoplasmin (ERP99) are capable to transit to the sperm. During sperm capacitation, tyrosine residues of these chaperones are subjected to phosphorylation. Phosphorylated chaperones can interact with ZP receptor complex, leading to appearance of ZP receptor on the sperm surface (5, 18, 21).
The supplementation of IVF media with antibody against HSP70 declines the rate of IVF in bovine (22), indicating HSP70 important role in fertilization. But in the present study, HSP60 in low dose was not able to increase significantly the fertilization ability of sperms. Perhaps, HSP60 is not able to interact with ZP receptor by itself and it requires collaboration of other chaperones such as ERP99. If other chaperones such as ERP99 or GRP78 were added into the fertilization medium alongside HSP60, they might collaborate with HSP60 in order to enable ZP receptor to be presented on the sperm surface resulting in more subsequent fertilization.
It was interesting that although HSP60 in low dose did not have a certain effect on the fertilization, it affected positively the two-cell embryo development. This positive effect of HSP60 in low dose on the rate of cleavage was observed from two-cell stage to blastocyst stage, however it was not significant. ZP of mouse oocyte and pre-implantation embryo is permeable to macromolecules up to 170 KDa, showing decreased permeability at around 110 KDa (23). Thus, HSP60 with the molecular weight ranging from 57-69 KDa is easily able to pass ZP and affect the newly formed zygote. HSP60 is expressed during final maturation of follicles (Graafian follicles) and it is even expressed in the early stages of embryogenesis in a natural condition and these indicated the positive role of HSP60 during pre-implantation phase (24, 25). Addition of antibodies against HSP60 and HSP70 into the IVF media declined the mouse embryo cleavage rate particularly from morula stage to blastocyst development (20), which strongly supports the importance of these chaperones in mouse pre-implantation development. Indeed, the importance of HSP60 is related to its anti-apoptotic (20, 26) and mitotic activity (27) roles during early embryogenesis. As we know, apoptosis may occur during IVF process because of oxidative stress that is a result of high oxygen concentration in the IVF media (20). Thus, the positive effect of HSP60 on the two-cell embryo development is not surprising. However, if the zygote was sufficiently exposed to HSP60, the embryo development to the blastocyst stage might have been more positively affected by this exogenous chaperone protein. Also, anti-apoptotic role of HSP60 in this study was sensible as there was a decline in the percent of cleavage-arrested embryos when the fertilization medium was supplemented with 10 ng/mL HSP60. Consequently, HSP60 may neutralize Reactive Oxygen Species (ROS) production and prevent apoptosis-induced stress in mice in vitro-produced embryos.
We know that mouse embryo development during first two days (two-cell embryo) is controlled by maternal genome, afterwards embryonic genome is activated and initiates to translate and produce proteins (28). In this period, there is no stress-induced HSP synthesis due to the absence of embryonic genome transcription. Once the embryonic genome is activated, most HSPs become stress inducible (26). So, it may be a reason that HSP60 is effective on cleavage rate just when the zygote achieves its two-cell embryo stage. However, when translational machinery of embryo is switched on and HSPs get inducible by IVF suboptimal conditions, application of more exogenous HSP60 may interfere the activation of embryonic genome.
As we discussed earlier, embryo manipulation in IVF is a stressful procedure. Yet, this stressful condition is able to induce HSPs expression in vitro-produced embryos to cope with adverse effects of oxidative stress (26). Overexpression of HSPs in this stressful situation is definitely in favour of the developing embryo. But, we see a meaningful decline in the rate of fertilization and embryo cleavage and a significant elevation in the rate of cleavage-arrested embryo when the fertilization medium was supplemented with higher doses of HSP60 in particular 100 ng/mL. There is no evidence to explain that HSP60 in high concentration may adversely affect the cell growth and embryo development. However, it seems that increased levels of HSP60 may create an imbalance ratio between HSP60 and other HSPs and proteins related to it. This imbalance may disrupt the collaboration of HSP60 with other chaperones to interact with ZP receptor. Furthermore, the imbalance between HSPs may impact on normal conditions of embryo development leading to ROS and free radicals production that may cause apoptosis or decline the mouse embryo development.
The present study indicates that application of exogenous HSP60 protein in fertilization medium is capable to enhance cleavage rate merely to two-cell embryo development stage in a dose-dependent manner, but it is not effective on acquisition of mouse sperm fertilization ability. Therefore, further investigation is required to unravel the molecular mechanisms involved in HSP60 role in mouse fertilization and pre-implantation embryo development.
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgements
This study was financially supported by a grant from Zanjan University of Medical Sciences (grant number: A-12-537-4) and it is published on behalf of Master Thesis of Sanam Mohsenzadeh.
References
- 1.Coy P, Garcia-Vazquez FA, Visconti PE, Aviles M. Roles of the oviduct in mammalian fertilization. Reproduction. 2012;144(6):649–660. doi: 10.1530/REP-12-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Jonge C. Biological basis for human capacitation. Hum Reprod Update. 2005;11(3):205–214. doi: 10.1093/humupd/dmi010. [DOI] [PubMed] [Google Scholar]
- 3.Barratt CL, Cooke ID. Sperm transport in the human female reproductive tract--a dynamic interaction. Int J Androl. 1991;14(6):394–411. doi: 10.1111/j.1365-2605.1991.tb01268.x. [DOI] [PubMed] [Google Scholar]
- 4.Quintero I, Ghersevich S, Caille A, Munuce MJ, Daniele SM, Morisoli L. Effects of human oviductal in vitro secretion on spermatozoa and search of sperm-oviductal proteins interactions. Int J Androl. 2005;28(3):137–143. doi: 10.1111/j.1365-2605.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 5.Gadella BM. The assembly of a zona pellucida binding protein complex in sperm. Reprod Domest Anim. 2008;43(Suppl 5):9–12. doi: 10.1111/j.1439-0531.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- 6.Boilard M, Reyes-Moreno C, Lachance C, Massicotte L, Bailey JL, Sirard MA, Leclerc P. Localization of the chaperone proteins GRP78 and HSP60 on the luminal surface of bovine oviduct epithelial cells and their association with spermatozoa. Biol Reprod. 2004;71(6):1879–1889. doi: 10.1095/biolreprod.103.026849. [DOI] [PubMed] [Google Scholar]
- 7.Lachance C, Bailey JL, Leclerc P. Expression of Hsp60 and Grp78 in the human endometrium and oviduct, and their effect on sperm functions. Hum Reprod. 2007;22(10):2606–2614. doi: 10.1093/humrep/dem242. [DOI] [PubMed] [Google Scholar]
- 8.Volpe S, Galeati G, Bernardini C, Tamanini C, Mari G, Zambelli D, Seren E, Spinaci M. Comparative immunolocalization of heat shock proteins (Hsp)-60, -70, -90 in boar, stallion, dog and cat spermatozoa. Reprod Domest Anim. 2008;43(4):385–392. doi: 10.1111/j.1439-0531.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 9.Dun MD, Aitken RJ, Nixon B. The role of molecular chaperones in spermatogenesis and the post-testicular maturation of mammalian spermatozoa. Hum Reprod Update. 2012;18(4):420–435. doi: 10.1093/humupd/dms009. [DOI] [PubMed] [Google Scholar]
- 10.Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of Jurkat cells. The EMBO journal. 1999;18(8):2040–2048. doi: 10.1093/emboj/18.8.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92(3):351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 12.Bethke K, Staib F, Distler M, Schmitt U, Jonuleit H, Enk AH, Galle PR, Heike M. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: superiority of HSP60. The Journal of Immunology. 2002;169(11):6141–6148. doi: 10.4049/jimmunol.169.11.6141. [DOI] [PubMed] [Google Scholar]
- 13.Asquith KL, Harman AJ, McLaughlin EA, Nixon B, Aitken RJ. Localization and significance of molecular chaperones, heat shock protein 1, and tumor rejection antigen gp96 in the male reproductive tract and during capacitation and acrosome reaction. Biol Reprod. 2005;72(2):328–337. doi: 10.1095/biolreprod.104.034470. [DOI] [PubMed] [Google Scholar]
- 14.Naaby-Hansen S, Herr JC. Heat shock proteins on the human sperm surface. J Reprod Immunol. 2010;84(1):32–40. doi: 10.1016/j.jri.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volpe S, Galeati G, Bernardini C, Tamanini C, Mari G, Zambelli D, Seren E, Spinaci M. Comparative Immunolocalization of Heat Shock Proteins (Hsp)-60,-70,-90 in Boar, Stallion, Dog and Cat Spermatozoa. Reprod Domestic Anim. 2008;43(4):385–392. doi: 10.1111/j.1439-0531.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Pei Y, Qin Y. Developmental expression of heat shock proteins 60, 70, 90, and A2 in rabbit testis. Cell Tissue Res. 2011;344(2):355–363. doi: 10.1007/s00441-011-1151-4. [DOI] [PubMed] [Google Scholar]
- 17.Neuer A, Spandorfer S, Giraldo P, Dieterle S, Rosenwaks Z, Witkin S. The role of heat shock proteins in reproduction. Hum Reprod Update. 2000;6(2):149–159. doi: 10.1093/humupd/6.2.149. [DOI] [PubMed] [Google Scholar]
- 18.Asquith KL, Baleato RM, McLaughlin EA, Nixon B, Aitken RJ. Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J Cell Sci. 2004;117(Pt 16):3645–3657. doi: 10.1242/jcs.01214. [DOI] [PubMed] [Google Scholar]
- 19.Cebral E, Carrasco I, Vantman D, Smith R. Preimplantation embryotoxicity after mouse embryo exposition to reactive oxygen species. Biocell. 2007;31(1):51–59. [PubMed] [Google Scholar]
- 20.Esfandiari N, Falcone T, Goldberg JM, Agarwal A, Sharma RK. Heat-shock proteins modulate the incidence of apoptosis and oxidative stress in preimplantation mouse embryos. Fertil Steril. 2007;87(5):1214–1217. doi: 10.1016/j.fertnstert.2006.07.1536. [DOI] [PubMed] [Google Scholar]
- 21.Bromfield EG, Nixon B. The function of chaperone proteins in the assemblage of protein complexes involved in gamete adhesion and fusion processes. Reproduction. 2013;145(2):R31–42. doi: 10.1530/REP-12-0316. [DOI] [PubMed] [Google Scholar]
- 22.Matwee C, Kamaruddin M, Betts DH, Basrur PK, King WA. The effects of antibodies to heat shock protein 70 in fertilization and embryo development. Mol Hum Reprod. 2001;7(9):829–837. doi: 10.1093/molehr/7.9.829. [DOI] [PubMed] [Google Scholar]
- 23.Legge M. Oocyte and zygote zona pellucida permeability to macromolecules. J Exp Zool. 1995;271(2):145–150. doi: 10.1002/jez.1402710210. [DOI] [PubMed] [Google Scholar]
- 24.Asquith KL, Baleato RM, McLaughlin EA, Nixon B, Aitken RJ. Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J Cell Sci. 2004;117(16):3645–3657. doi: 10.1242/jcs.01214. [DOI] [PubMed] [Google Scholar]
- 25.Jakus S, Neuer A, Dieterle S, Bongiovanni AM, Witkin SS. Antibody to the Chlamydia trachomatis 60 kDa heat shock protein in follicular fluid and in vitro fertilization outcome. Am J Reprod Immunol. 2008;59(2):85–89. doi: 10.1111/j.1600-0897.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- 26.Neuer A, Spandorfer SD, Giraldo P, Dieterle S, Rosenwaks Z, Witkin SS. The role of heat shock proteins in reproduction. Hum Reprod Update. 2000;6(2):149–159. doi: 10.1093/humupd/6.2.149. [DOI] [PubMed] [Google Scholar]
- 27.Lachance C, Fortier M, Thimon V, Sullivan R, Bailey JL, Leclerc P. Localization of Hsp60 and Grp78 in the human testis, epididymis and mature spermatozoa. Int J Androl. 2010;33(1):33–44. doi: 10.1111/j.1365-2605.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee MT, Bonneau AR, Giraldez AJ. Zygotic genome activation during the maternal-to-zygotic transition. Annu Rev Cell Dev Biol. 2014;30:581–613. doi: 10.1146/annurev-cellbio-100913-013027. [DOI] [PMC free article] [PubMed] [Google Scholar]